Vetrec-2014-102892 1.12
Cutaneous and renal glomerular vasculopathyas a cause of acute kidney injury in dogsin the UK
L. P. Holm, I. Hawkins, C. Robin, R. J. Newton, R. Jepson, G. Stanzani, L. A. McMahon,P. Pesavento, T. Carr, T. Cogan, C. G. Couto, R. Cianciolo, D. J. Walker
To describe the signalment, clinicopathological findings and outcome in dogs presenting withacute kidney injury (AKI) and skin lesions between November 2012 and March 2014, in whomcutaneous and renal glomerular vasculopathy (CRGV) was suspected and renal thromboticmicroangiopathy (TMA) was histopathologically confirmed. The medical records of dogs withskin lesions and AKI, with histopathologically confirmed renal TMA, were retrospectivelyreviewed. Thirty dogs from across the UK were identified with clinicopathological findingscompatible with CRGV. These findings included the following: skin lesions, predominantlyaffecting the distal extremities; AKI; and variably, anaemia, thrombocytopaenia andhyperbilirubinaemia. Known causes of AKI were excluded. The major renal histopathogicalfinding was TMA. All thirty dogs died or were euthanised. Shiga toxin was not identified in thekidneys of affected dogs. Escherichia coli genes encoding shiga toxin were not identified infaeces from affected dogs. CRGV has previously been reported in greyhounds in the USA,a greyhound in the UK, without renal involvement, and a Great Dane in Germany. This is thefirst report of a series of non-greyhound dogs with CRGV and AKI in the UK. CRGV is a disease ofunknown aetiology carrying a poor prognosis when azotaemia develops.
). CRGV is the only canine disease previously
Cutaneous and renal glomerular vasculopathy (CRGV) is a
reported to cause vasculopathy preferentially affecting the small
disease of unknown aetiology, reported to cause ulceration of the
vessels of the kidney and skin.
distal extremities in dogs. It is variably associated with clinically
AKI can result from pre-renal (), renal
relevant acute kidney injury (AKI). CRGV has been reported in
greyhounds in the USA a Great
and post-renal events ().
Dane in Germany () and a grey-
Skin lesions are not commonly associated with AKI in dogs,
hound in the UK ().
unless the AKI has resulted from immune-mediated disease
The major renal histopathological lesion reported in CRGV is
(certain neoplasms
thrombotic microangiopathy (TMA). Thrombotic microangiopa-
infectious diseases (or vascular
thies are characterised by inflammation and damage to vascular
events, such as vasculopathy ).
endothelium, leading to widespread formation of microthrombi
The purpose of this report is to summarise the signalment,
and resultant consumptive thrombocytopaenia, microangio-
clinicopathological findings and outcome in 30 dogs presenting
pathic haemolytic anaemia and multiorgan dysfunction (
between November 2012 and March 2014, in which CRGV wassuspected clinically and TMA was identified histopathologically.
Veterinary Record (2015)
doi: 10.1136/vr.102892
L. P. Holm, BVM&S, CertSAM,
C. Robin, MSc, BSc,
Department of PMI, University of
Couto Veterinary Consultants, Hilliard,
R. J. Newton, BVSc, MSc, PhD,
California, Davis, School of Veterinary
L. A. McMahon, BVetMed (Hons),
Medicine, 4206 VM3A, 1 Shields
R. Cianciolo, VMD, PhD, DACP,
DipACVIM, DipECVIM-CA, MRCVS,
Animal Health Trust, Lanwades Park,
Avenue, Davis, California 95616, USA
International Veterinary Renal Pathology
D. J. Walker, BVetMed (Hons),
Kentford, Newmarket, Suffolk CB8
T. Carr, BVMS GPcert (SAM),
Service, Department of Veterinary
DipACVIM, DipECVIM-CA, MRCVS,
Biosciences, The Ohio State University,
Anderson Moores Veterinary
R. Jepson, BVSc(Dist), MVetMed,
Martin and Carr, The Old Wel , Station
301 Goss Laboratory, 1925 Coffey Rd,
Specialists, The Granary, Bunstead
PhD, DipACVIM, DipECVIM, MRCVS,
Road, Pershore, Worcestershire WR10
Columbus, Ohio, USA
Barns, Poles Lane, Hursley,
G. Stanzani, DVM, MRCVS,
E-mail for correspondence:
Winchester, Hampshire SO21 2LL, UK
Department of Clinical Science and
T. Cogan, BSc (Exon), PhD (Exon),
I. Hawkins, DVM, MRCVS, Dip ACVP,
Services, Royal Veterinary College,
School of Veterinary Sciences,
Bridge Pathology, Horner Court, 637
Hawkshead Lane, North Mymms,
University of Bristol, Langford, Bristol
Provenance: Not commissioned;
Gloucester Road, Horfield, Bristol BS7
Hertfordshire AL9 7TA, UK
externally peer reviewed
P. Pesavento, DVM, PhD, Dip ACVP,
C. G. Couto, DVM, DipACVIM,
Accepted January 21, 2015
10.1136/vr.102892 Veterinary Record 1 of 12
Materials and methods
sections, which were then washed three times and incubated
Cases were identified by comprehensive search of computerised
with goat anti-mouse G1 FITC-conjugated secondary antibody.
record systems (using keywords) at two referral practices. Case
Slides were viewed using a fluorescence microscope.
records were searched for the diagnosis of AKI, with subsequent
PCR for Shiga toxin on renal tissue:iii DNA was extracted
review of clinical case files and renal/dermal histopathology in
from paraffin-embedded samples using the QIAamp®DNA FFPE
order to identify cases compatible with CRGV. Further case sub-
Tissue Kit (Qiagen, Hilden, Germany). PCR for verotoxin 1 and
missions came from two other referral practices and 49 first
2 was then performed as previously described
opinion practices. Practices became aware of CRGV through a
combination of: a letter in the veterinary literature (
PCR for E. coli virulence genes on faeces:iv DNA was
media reports and information on a specialist
extracted from colonies of E. coli cultured from faeces (Wizard
practice website. Records from suspected cases were thus
Miniprep DNA purification System, Promega). Multiplex PCRs
selected by the additional 51 practices both prospectively and
for eaeA, stx 1 and 2, LT1 and ST1 and 2 genes were performed,
retrospectively (from memory rather than via computerised
as previously described (
record system searches) and were subsequently reviewed by twoof the authors (LPH, DJW). Dogs were included if they presented
between November 1, 2012 and March 31, 2014 with skin
Seventy-one cases of AKI with skin lesions were identified
lesions and AKI with no known identifiable cause, and with
within the defined time period for which there was clinical sus-
renal histopathological evidence of TMA. Animals were defined
picion of CRGV. Of these, 41 cases were excluded due to limited
as having AKI if they had historical and laboratory evidence of
investigation and/or incomplete medical records. Thirty cases
kidney injury with or without clinical oligoanuria (International
met the inclusion criteria as affected cases with confirmed TMA
on renal histopathology.
Clinicopathological data are reported as median and range.
Signalment, history and clinical signs
Breeds represented were English springer spaniel (n=5), cross-
Representative sections from skin, kidney and other organs,
breed above 20 kg (n=4), flat coated retriever (n=4), whippet
where possible, were paraffin embedded. Sections of 3–4 μm
(n=3), border collie (n=2), Jack Russell terrier (n=2), Doberman
were prepared from each of the tissues via microtome and
(n=2) and one each of, Labrador retriever, cocker spaniel,
affixed to lysine-charged glass slides. These were stained by
standard technique with haematoxylin and eosin. In addition,
Dalmatian, Tibetan terrier and crossbreed below 20 kg. Median
sections of the kidney were also stained with Warthin-Starry
age was 4.90 years (1.00–11.75 years). Ten were male neutered,
silver stain and Periodic Acid Schiff. Kidneys from three dogs
seven were female neutered, six were male entire and seven were
were also stained with Masson's trichrome and Jones
female entire. Median weight was 23.2 kg (7.3–40.4 kg, n=28).
Methenamine silver method.
Affected cases were identified from multiple areas of north-
All of the renal histopathology was reviewed by a single vet-
ern and southern England (). Ten dogs had been in the New
erinary pathologist (IH). Renal samples from three dogs were
Forest National Park shortly (four hours to 14 days) before devel-
also submitted to a second veterinary pathologist (RC).
oping skin lesions and/or becoming unwell.
PCR for pathogenic Leptospira spp.i was carried out on sec-
Over the first 12 months of the study period (November 1,
tions of kidney and liver using an amplification mixture as previ-
2012–October 31, 2013), confirmed cases presented in November
ously described ). DNA was amplified
(n=2), December (n=2), February (n=4), March (n=1) and May
and detected on a Stratagene Mx3005P qPCR system, using a
(n=1). The remaining 20 confirmed cases presented between
program of 95°C for 10 minutes followed by 50 cycles of 95°C
November 1, 2013 and March 31, 2014.
for 30 seconds and 57°C for 60 seconds.
Twenty dogs were vaccinated within the past year (vaccines
Fluorescence in situ hybridisation (FISH) for pathogenic bac-
used included distemper, D; hepatitis, H; leptospirosis, L; parvo-
teriai was performed on de-waxed tissue sections (kidney and
virus, P; and parainfluenza, Pi: DHLPPi n=10; DHPPi n=1; LP
liver) using fluorescently labelled probes. Eubacteria were
n=1; DHLP n=2; L n=3; LPi n=2; type not recorded n=1), eight
detected using a mixture of three probes: GCTGCCTCCCG
were unvaccinated and vaccinal status was unknown in two dogs.
TAGGAGT, GCAGCCACCCGTAGGTGT and GCTGCCACCCG
Skin lesions commonly appeared before signs of systemic
TAGGTGT. Leptospira were detected using probe CGGGTGCT
illness (lethargy, malaise, anorexia, vomiting, pyrexia; n=19).
CCCCACTCAG. Escherichia coli were detected using probe
Median time from development of skin lesions to diagnosis of
AKI was four days (1–9 days). Nine dogs had systemic signs con-
Viral metagenomicsii was performed on fresh kidney tissue,
current with skin lesions and two dogs were systemically ill
liver and lymph node by random nucleic acid amplification after
before developing skin lesions. The management of skin lesions
enrichment for viral particles, followed by DNA sequencing and
before the development of AKI was variable: no medication
similarity searches (Illumina MiSeq library) for sequences related
(n=7), NSAIDs alone (n=3), antibiotic alone (amoxicillin-
to those of known viruses (
clavulanate n=4; marbofloxacin n=1) or a combination of
PCR for Dog Circovirus was performed on splenic tissueii ( par-
NSAIDs or dexamethasone, and antibiotic (n=12). Information
affin embedded samples and fresh frozen tissue) as previously
regarding previous medications was unavailable for three cases.
described ().
With the exception of NSAIDs, none of the dogs had known
FISH for Dog Circovirusi was performed on kidney tissue
access to nephrotoxins before initial presentation.
using probe CTCAGACAGAGACACCGTTGCTATG as previ-
Distribution of skin lesions was: distal limbs (n=28),
ously described Identification of bacteria
ventrum (n=9) and oral cavity/muzzle (n=10). Sixteen dogs had
was made against both unstained and organism-negative
more than one lesion. Fourteen had lesions in multiple locations.
The appearance of the skin lesions was highly variable, ranging
FISH for Shiga toxin:i mouse anti-Shiga toxin antibody
from superficial erosion through to full thickness ulceration,
(diluted 1/100) was incubated overnight at 4°C on renal tissue
iiiCenter for Zoonotic Diseases, Bacterial Diseases, and Antimicrobial
iUniversity of Bristol Veterinary Diagnostics, School of Veterinary
Resistance, Institute for Veterinary Bacteriology, University of Bern,
Sciences, University of Bristol, Langford, Bristol BS40 5DU, UK.
Hochschulstrasse 4, Bern CH-3012, Switzerland.
iiPesavento Research Group, University of California, Davis, School of
ivBatt Lab, University of Warwick Science Park, The Venture Centre, Sir
Veterinary Medicine, 1 Shields Avenue, Davis, CA 95616, USA.
William Lyons Road, Coventry CV4 7EZ, UK.
2 of 12 Veterinary Record 10.1136/vr.102892
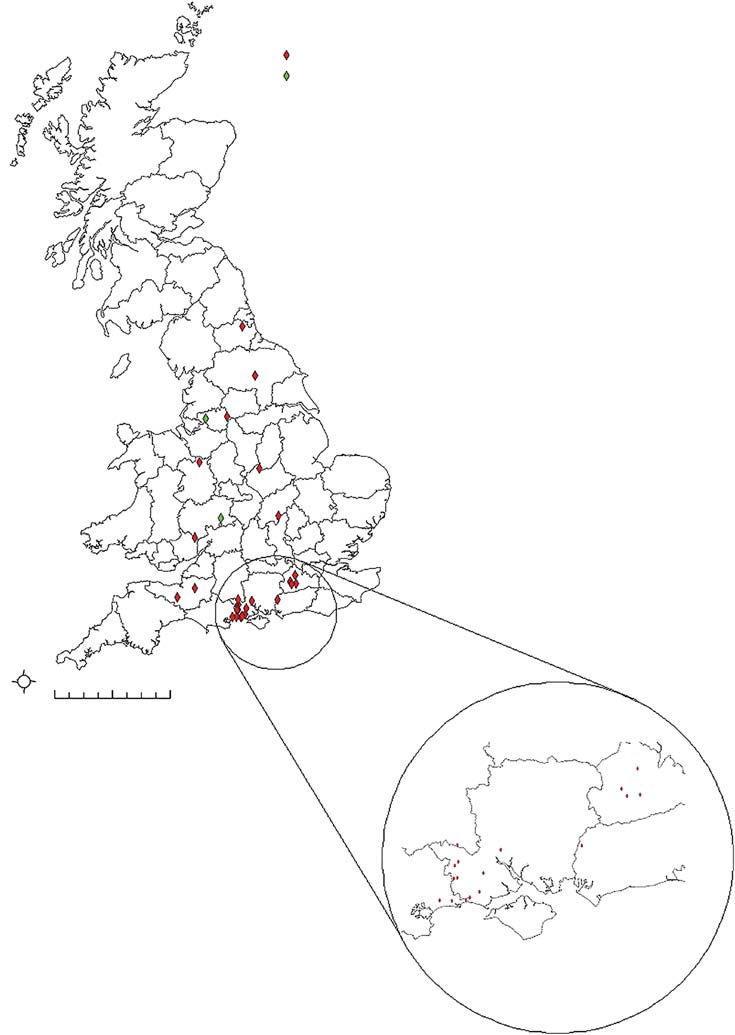
Location with single case
Location with 2 cases
FIG 1: Map to show distribution of where confirmed cases lived. (Zoomed in view shows distribution of cases in the South of England asthere were proportionally more cases from this area)
with erythema, oedema and exudation ). Early lesions were
days before and six days after the confirmed CRGV case that
often erythematous and focal; they occasionally appeared vesicu-
they lived with. All eight dogs were related to a confirmed
lar, with ulceration and necrosis developing subsequently. The
CRGV case and/or each other.
skin lesions were often attributed to wounds, bites, stings orfocal dermatitis. Lesion size ranged from 0.5 to 5 cm in diameter.
Six dogs developed new limb and/or oral lesions while hospita-
Selected haematology, serum biochemistry, urinalysis and sys-
lised. Lesions were typically painful on palpation and digital
tolic blood pressure measurement results for the 30 dogs are pre-
lesions often caused lameness. Oral lesions were variable but
sented in , with further detail of clinicopathological
were most often focal erosions or ulcers ().
abnormalities presented in . Seven dogs demonstrated
The dogs' clinical signs are summarised in Pyrexia
normocytic, normochromic anaemia at presentation, with a
generally occurred early in the course of illness (39.8°C;
further eight dogs becoming anaemic after presentation.
39.4–40.1°C) and hypothermia typically developed later in the
Absolute reticulocyte count was only available for two dogs
disease course (37.4°C; 36.0–37.7°C).
which were anaemic at presentation and one dog subsequently.
Fourteen dogs lived in the same household as one of the 30
The anaemia was pre- or non-regenerative (median reticulocyte
confirmed CRGV cases. Of these 14 dogs, two developed skin
count 22.58×109/l). Blood smear examination was performed in
lesions and AKI and six developed skin lesions without AKI
13 dogs and revealed schistocytes, burr cells and/or acanthocytes
(n=8). Thirteen of these 14 dogs were not part of the study
in five dogs and was unremarkable in the remainder. Fifteen dogs
population and the information on these dogs was obtained by
were thrombocytopaenic at presentation and four became
questioning owners. The eight dogs became unwell between 20
10.1136/vr.102892 Veterinary Record 3 of 12
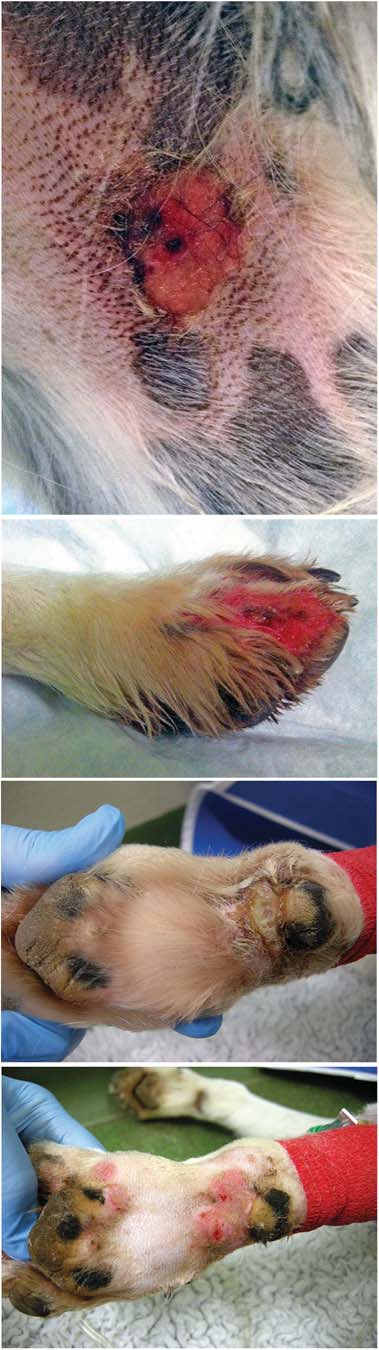
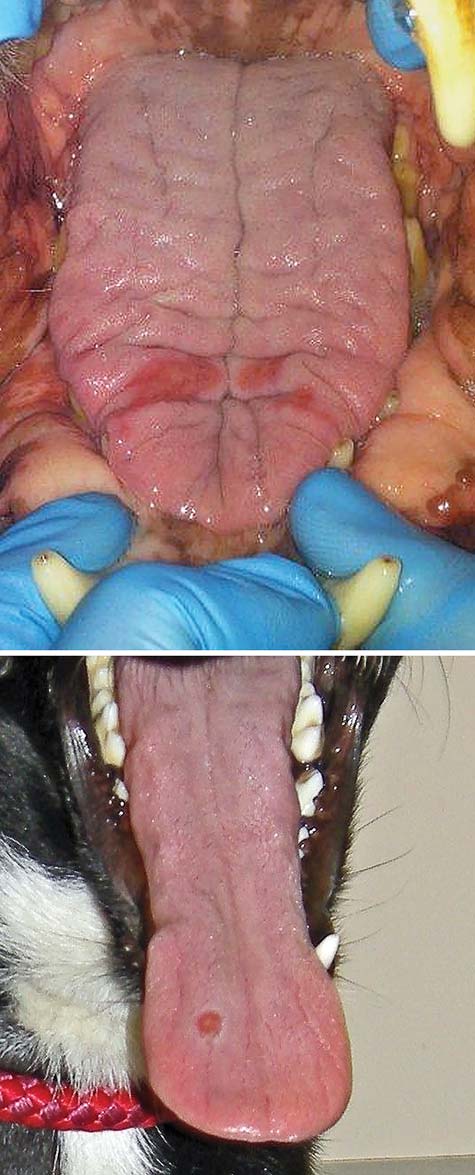
FIG 3: (a) Tongue lesions. (b) Tongue ulcer
dogs and excluded glucocorticoid deficient hypoadrenocorticism inall. Clotting times, measured in eight dogs, were not consistentwith disseminated intravascular coagulation (DIC). Six dogs hadcanine-specific pancreatic lipase immunoreactivity measured (Idexxsnap test n=4; quantitative cPLi n=2) and all had abnormal results(680 and above 1000 mg/l—reference range less than 200 mg/l).v
FIG 2: All images are photographs of lesions affecting confirmed
Urine dipstick and sediment examination was performed in 17
cases. (a) Superficial ulcer affecting medial thigh. (b) Deep
dogs revealing: haemoglobinuria or myoglobinuria (n=16), pro-
ulceration, erythema and exudation on a digit. (c) Erosion to carpal
teinuria (n=11, see and glucosuria (n=6). Sediment
pad, before cleaning. (d) The same lesion after clipping and cleaning
At presentation, 26 dogs were azotaemic. The non-azotaemic
dogs became azotaemic after initial presentation (less than
vDogs were seen at numerous different veterinary practices using multiple
24 hours to six days later). Basal cortisol was measured in eight
laboratories. Biochemistry reference ranges were therefore variable.
4 of 12 Veterinary Record 10.1136/vr.102892
Four dogs were tested for Dog Circovirus via PCR on splenic
TABLE 1: Summary of presenting clinical signs of the 30
tissue, three via PCR on peripheral EDTA blood and six via FISH
on renal tissue and all results were negative. Viral metagenomics
Number of dogs affected
was performed on renal tissue from two dogs; and on liver,
spleen and lymph node from one of those dogs. No match was
identified between viral nucleic acids enriched from those
samples to known viruses.
Borrelia PCR (n=5) and Borrelia serology (n=2) results were
negative. Renal heavy metal concentrations (lead, arsenic and
cadmium) were measured in two dogs (Animal Health and
Veterinary Laboratories Agency, Winchester) and were below
reported reference intervals in both. Routine aerobic and anaer-
obic bacterial culture was performed on renal tissue from three
dogs and results were negative.
Faecal culture, performed in seven dogs, yielded E. coli. PCRs
for E. coli virulence genes (eaeA, stx 1 and 2, LT1 and ST1 and 2)
were negative in all seven, providing no evidence for infection
with enteropathogenic E. coli, verotoxigenic E. coli or enterotoxi-
genic E. coli.
Skin lesion cultures, performed in 11 dogs, were positive in 7,
yielding Staphylococcus intermedius (n=1), Staphylococcus aureus(n=1), coagulase positive Staphylococcus (n=1), non-haemolytic
examination revealed casts in nine dogs: granular (n=5), fatty
Streptococcus (n=1), β haemolytic Streptococcus (n=1), Enterococcus
(n=1), hyaline (n=2) and not described (n=1). Urine culture was
(n=1), E. coli (n=3), Pseudomonas aeruginosa and Corynebacterium
negative in 11 of 12 dogs (faecal contaminants were cultured from
(n=1). Three dogs cultured more than one bacterium. Blood
the remaining case). Urine toxicology was negative in five of six
culture was performed in one dog and was negative.
Laboratory). Pentaethylene glycol (trace) was detected in one dog.
At presentation, nine dogs were oliguric, two were anuric
Full post mortem examination was performed in five dogs by
and seven had normal urine output. Urine output was unknown
pathologists with a Diploma of the American or European
in 13 dogs. International Renal Interest Society (IRIS) AKI
College of Veterinary Pathologists, or equivalent. Tissue samples
grading at presentation was: grade I (n=3), II (n=4), III (n=8),
for histopathology were obtained post mortem by the case veter-
IV (n=14) and V (n=1) 2013).
inarians in the remaining 25 cases. Renal histopathology was
Abdominal ultrasonography ( performed by a European
available for all 30 dogs and skin histopathology for 24 dogs. The
diploma holder in Diagnostic Imaging in 11 dogs and a general
most prominent histological changes were noted in the kidney
practice veterinary surgeon in 2 dogs) revealed no evidence for
and skin. For the kidney, the most striking changes involved the
chronic kidney disease (CKD) or pyelectasia (n=13). The
glomeruli, with frequent fibrinoid necrosis of glomerular arter-
kidneys appeared unremarkable in eight dogs and the remaining
ioles, characterised by distortion of vessel walls with an eosino-
five had bilateral hyperechoic renal cortices. Three dogs had pos-
philic, hyalinised, smudgy material, intermingled with low
sible evidence for gastritis (thickened hypoechoic gastric rugal
numbers of degenerate and viable neutrophils, fragmented red
folds n=1; thickened hyperechoic rugal folds n=1; reduced
blood cells and mild amounts of karyorrhectic debris ().
gastric wall layer definition n=1); one dog had hyperechoic
Frequent vessels were occluded by thrombi. The majority of the
small intestinal mucosal striations and thickening and uneven-
glomeruli were affected and for individual glomeruli these
ness of the colonic mucosa which may have suggested entero-
changes ranged from mild and segmental to global and severe.
colitis. One additional dog had evidence for hepatopathy
Also, frequent glomerular tufts were congested and partially
(hypoechoic hepatic parenchyma with cuffing of the portal
occluded by haemorrhage. Increased cellularity of the glomerular
veins). No other relevant abnormalities were identified on
tufts consistent with endothelial cell hypertrophy and swelling
was identified in 17 cases. Fibrinoid necrosis of intralobular and
Leptospirosis microscopic agglutination testing (MAT) was
arcuate arteries was occasionally observed Twenty-nine
performed in 15 cases. Ten had negative titres, obtained a
dogs had concurrent evidence of tubular necrosis, ranging from
median of three days (1–8 days) after the development of sys-
mild to marked, often with concurrent evidence of tubular resti-
temic signs. Five dogs had positive titres; their results and the
tution. In affected kidneys, micro-organisms, viral cytopathic
results of additional leptospirosis testing are summarised in
effects and metazoan parasites were not identified. When per-
FISH for Leptospires was performed on renal tissue from
formed, Warthin-Starry stains did not reveal argyrophilic organ-
seven dogs and liver tissue from one dog.vi A positive result was
isms (Leptospires) within the tissue sections.
obtained from six renal samples; Leptospires were seen in small
In the skin samples, the epidermis was focally to diffusely
localised clusters in three dogs (one of whom had a negative
ulcerated. The subjacent dermis was often undergoing coagula-
MAT titre) and diffusely throughout the renal tissue in three
tive necrosis. At the level of the adnexa, the hair follicles had
dogs (one of whom was not tested for Leptospirosis by MAT).
reduced to absent sebaceous glands, reduced cellularity and were
The remaining results were negative. Leptospirosis PCR was per-
separated by increased fibrous tissue and an attenuated follicular
formed on the renal tissue of three of the dogs with positive
epithelium. The affected follicles were often bordered by variable
FISH results; one dog had a negative result and one had a posi-
numbers of neutrophils, foamy macrophages and karyorrhectic
tive result; one dog had PCR performed at two laboratoriesvi vii
debris ); this often obscured the follicular epithelium inter-
and discordant results were obtained. Leptospirosis PCR was per-
face and sebaceous gland units. In most cases, the deep dermis
formed on liver tissue from one dogvi and the result was
and subcutis were thickened by a layer of maturing fibrovascular
tissue. Occasionally this fibrovascular tissue replaced portions ofthe adnexa. In a few cases (n=6), fibrinoid necrosis was observedin the small dermal arterioles Rarely, thrombi were iden-tified in such vessels. In one case, similar necro-ulcerative
viUniversity of Bristol Veterinary Diagnostics.
changes were identified in skin from the lip. In samples from the
oral cavity lesions, similar ulceration of the mucosa was observed
10.1136/vr.102892 Veterinary Record 5 of 12
TABLE 2: Selected data for the 30 confirmed cases
Last available result
Reference interval
Platelet count (×109/l)
Neutrophil count (×109/l)
Creatinine (mmol/l)
40–106 mmol/l (13 dogs)44–159 mmol/l (16 dogs)
Phosphate (mmol/l)
0.8–1.6 mmol/l (14 dogs)0.81–2.20 mmol/l (12 dogs)
0–25 U/L (10 dogs)10–100 U/L (15 dogs)
0–50 U/L (11 dogs)23–212 U/L (17 dogs)
Total bilirubin (mmol/l)
Potassium (mmol/l)
Prothrombin time (seconds)
No further results available
Activated partial thromboplastin time (seconds)
No further results available
Urine specific gravity
No further results available
Urine protein: creatinine ratio
No further results available
Systolic blood pressure (mmHg)
with associated necrosis, inflammation and fibrovascular change
was identified in the tissue of one dog and a faint band in the
of the submucosa.
other. Staphylococcaceae were identified in both samples. The
The majority of other tissues evaluated (stomach, n=2;
other laboratory identified solely Leptospires in both samples.
small intestine, n=6; colon, n=2; liver, n=14; pancreas, n=4;
PCR for verotoxin 1 and 2, performed on the kidneys from
heart, n=2; spleen, n=9; lung, n=1; brain, n=1; eye, n=1; saliv-
four dogs, gave negative results. FISH for shiga toxin, performed
ary gland, n=1; urinary bladder, n=3; tongue, n=2; soft palate,
on the kidneys from six dogs, was also negative.
n=1; bone marrow, n=2; adrenal gland, n=2; tonsil, n=1; skel-etal muscle, n=1 and lymph node, n=2) appeared unremarkable,
but occasionally exhibited mild, non-specific changes (see online
Ten cases were managed at referral centres and 20 in primary
supplementary appendix 1).
practice. Initial management typically consisted of intravenous
Electron microscopy was performed in three dogs and
fluid therapy (n=26) and antibiotic therapy (n=26) and is sum-
revealed distension of the glomerular capillary loops by erythro-
marised in Ten cases were managed with an indwelling
cytes, occasional schistocytes and rare polymorphonuclear cells.
urinary catheter for measurement of urine output. Three cases
Endothelial cells, when identifiable, were severely swollen.
underwent continuous renal replacement therapy.
Podocyte foot processes were globally effaced. Occasionally,mesangiolysis (dissolution of mesangium) was noted. Immunecomplexes were not identified. Immunostaining was negative for
IgG, IgM, IgA, C3, C1q, kappa light chain (KLC) and lambda
Twenty-four dogs died, or were euthanised, solely due to their
light chain (LLC)
disease and six were euthanised at the owners' request. IRIS AKI
Renal tissue from two dogs was submitted to two separate
grade progressed in 10 dogs, reduced in 2 and was unchanged in
laboratories with both laboratories receiving both samples
12. Terminally, IRIS grades were: II (n=1), III (n=8), IV (n=13),
(Department of Medical Microbiology and Immunology, School
V (n=3) and unknown (n=5). Causes of death/euthanasia were:
of Medicine, University of California, Davis, USA; University of
oligoanuria (n=9), anaemia and thrombocytopaenia (n=2), pro-
Bristol Veterinary Diagnostics, School of Veterinary Sciences,
gressive azotaemia (n=6), unspecified clinical deterioration
University of Bristol, Langford, Bristol, UK) for evaluation with
(n=3), suspected DIC (n=1), dyspnoea (n=1), collapse (n=1),
a broad spectrum set of 16S rRNA-directed probes (to detect bac-
ascites (n=1) and owners' request due to concurrent disease(s),
terial genetic material). From one laboratory, a clear 16S band
financial constraints or concern regarding prognosis (n=6).
TABLE 3: Frequency of clinicopathological abnormalities
Results for additional patients identified asdeveloping the abnormality during
Results at presentation
Number of patients
Number of patients
Reference interval
Elevated serum urea concentration
Elevated serum creatinine concentration
(40–106 mmol/l 13 dogs;44–159 mmol/l, 16 dogs)
6 of 12 Veterinary Record 10.1136/vr.102892
Median time from onset of clinical signs to death or euthanasiawas seven days (1–16 days).
CRGV has been recognised in the USA for almost 30 years
(), but has only sporadically beenreported in individual dogs elsewhere. This is the first case series
of dogs with CRGV in the UK.
Most of the dogs in this case series were initially evaluated at
their primary practice for a skin lesion (or lesions) which was
considered consistent with pyoderma, pododermatitis, a bite/
sting, or a wound. Systemic signs developed a median of fourdays later, but some dogs were unwell concurrently. Initial inves-
tigations revealed renal azotaemia attributable to AKI. Pre-renal
causes were excluded via assessment of urine specific gravity,
lack of response to intravenous fluid therapy and exclusion of
hypoadrenocorticism. Abdominal imaging, where available, was
used to exclude post-renal causes. CKD was excluded via a com-bination of clinical history and imaging findings. Eleven of the
dogs (36.7 per cent) received NSAIDs before the diagnosis of
AKI; although it is possible that their use exacerbated AKI, the
histopathologic lesions were not consistent with NSAIDs beingthe sole cause of the AKI.
Other known causes of AKI were explored as thoroughly as
possible in most of the dogs. Leptospirosis, which can cause
similar clinical and laboratory signs to those seen in this caseseries (,, ), was furtherinvestigated with serology, PCR on peripheral blood, renal and
hepatic tissue, and FISH on renal and hepatic tissue. The five
dogs with positive Leptospirosis titres (1:100–1:800) had been
vaccinated less than 1 year before testing and although vaccinal
titres often decline by four months post-vaccination, they can
sometimes persist for longer (leading to
false-positive results. Additionally, only single titres above 1:1600are considered significant for indicating infection in vaccinated
dogs (whereas the positive titre
results obtained in the five dogs in this study were relatively low.
It is possible that the titres would have been higher if MAT
testing had not been performed so early in the disease process.
Only 55 per cent of dogs with leptospirosis were diagnosed by a
single MAT titre obtained within 72 hours of initial presentation
in one study Convalescent phase
samples could not, however, be obtained in the dogs in this
study given the short survival time.
In cases of acute leptospirosis, histopathology often reveals
mild renal tubular necrosis and interstitial oedema
with focal areas of hepatic necrosis characterised by
cellular disassociation of the hepatic cords
This contrasts with the histopathological findings
in this case series; no typical hepatic lesions were identified and
the predominant renal lesion was TMA. Acute to peracute, peri-
acinar hepatocellular loss with associated haemorrhage was
observed in one dog in this series. This raised concern for lepto-spirosis, however, this case exhibited fibrinoid necrosis of small
portal vessels, and consequently the periacinar changes may also
have represented ischaemic change associated with the fibrinoid
necrosis. In addition, both PCR and FISH on paraffin embedded
sections of the affected liver were negative for Leptospira sp.,
making acute leptospirosis less likely. There was no evidence of
argyrophilic Leptospires with silver staining, although this tech-
nique can fail to identify Leptospires during acute leptospirosis
(With the exception of calcinosis cutis,
which was not identified in any dog in this series, skin lesions
have not previously been reported in association with canine
leptospirosis ).
FISH and PCR detected Leptospires in the renal tissues of
some of the CRGV affected dogs reported in this series; however,
this does not confirm clinical infection, as some dogs are asymp-tomatic maintenance hosts for Leptospires
Although a causal relationship between leptospirosis and
10.1136/vr.102892 Veterinary Record 7 of 12
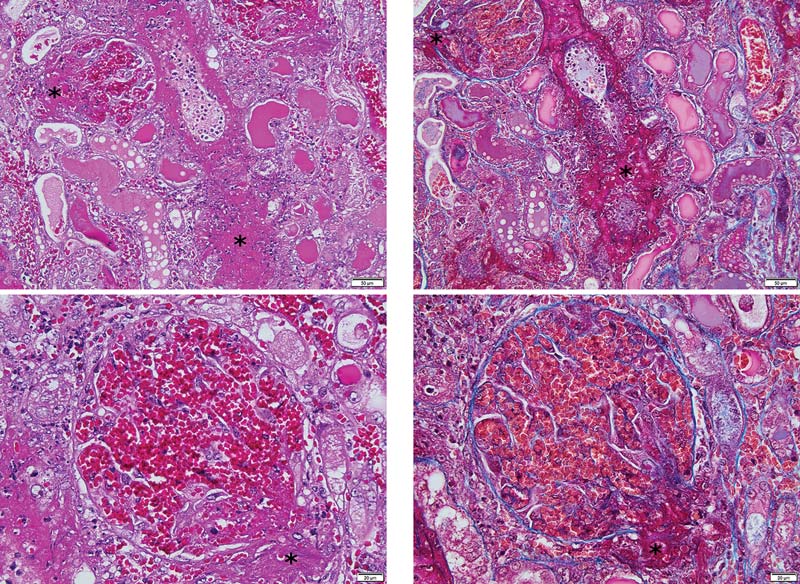
FIG 4: Photomicrographs of a glomerulus and an intralobular artery from a dog with CRGV, stained with haematoxylin and eosin [H&E] (a andc) and Masson's trichrome (b and d). There is fibrinoid vascular necrosis (asterisks) of intralobular arteries and arterioles. Glomerularcapillaries are severely distended and contain red blood cells, many of which are fragmented. Distension of glomerular capillaries is due todissolution of the mesangial matrix (mesangiolysis). Tubules are undergoing degeneration and necrosis; most tubules contain protein castswhereas some contain red blood cell casts.
CRGV cannot be fully excluded, the low number of positive
Similarly, many breeds were represented in this case series. It is
leptospirosis test results in this case series was felt unlikely to
unclear at this time whether canine HUS and CRGV are truly
support a diagnosis of acute leptospirosis as the clinical picture,
two distinct disease processes. Dogs with CRGV typically
outcome and histopathological findings differed significantly
present with acute onset skin lesions affecting the distal limbs;
from those previously reported with acute leptospirosis.
kidney injury and haematological abnormalities are variably
Histopathologically, AKI in the dogs in this case series was
reported In contrast skin lesions
found to be attributable to TMA. A search of the International
have not previously been reported in dogs with HUS
Veterinary Renal Pathology Service database of more than 1000
renal biopsies from small animals, revealed TMA to account for
). The proportion of dogs in the UK that develop
below 1 per cent of all diagnoses reached (Cianciolo R, unpub-
CRGV without developing AKI is unknown at this stage;
lished data). TMAs are characterised by inflammation and damage
however, 42.9 per cent of dogs in contact with those reported in
to vascular endothelium, leading to widespread formation of
this study, developed skin lesions without biochemical evidence
microthrombi, consumptive thrombocytopaenia, microangio-
of AKI and it would have been interesting to review dermal and
pathic haemolytic anaemia and multiorgan dysfunction (
renal histopathology in these dogs had it been available. Previous
). Five cases in this series (38.5 per cent of dogs in
reports indicate that non-azotaemic dogs with CRGV tend to
whom a blood smear was examined) had evidence for burr cells,
have reduced glomerular filtration rates and renal histopathology
schistocytes or acanthocytes; additionally 19 dogs were thrombo-
showing mild, multifocal, endothelial glomerular changes
cytopaenic and 13 dogs were hyperbilirubinaemic by the time of
death. All of these findings can be the result of microangiopathy
Clinicopathologic findings previously reported in dogs with
CRGV include anaemia, thrombocytopaenia, azotaemia, high
other mechanisms could also have been contributing to the
serum liver enzyme activity, high muscle enzyme activity,
anaemia, thrombocytopaenia and hyperbilirubinaemia.
Known differential diagnoses for canine TMA include CRGV
) and haemolytic uraemic syndrome
the abnormalities identified in the dogs in this case series.
(HUS). HUS has previously been reported in five dogs
Previous reports have not further classified the anaemia
three cats (and a
case series the anaemia appeared pre- or non-regenerative and
number of other species
the former was considered most likely. Possible aetiologies con-
sidered included gastrointestinal haemorrhage secondary to
). CRGV has been almost exclusively reported in grey-
uraemia or microangiopathic red cell injury. Hypoalbuminaemia
hounds (, although
(identified in 63.3 per cent of the confirmed cases in this series)
there is one report of an affected Great Dane (
may support gastrointestinal haemorrhage, without excluding
In contrast, HUS in dogs has been reported in a
other possible causes.
variety of breeds: Yorkshire terrier, miniature poodle, Labrador
Histopathological findings previously reported with CRGV
retriever, German shepherd dog and boxer (
with those seen in this case series. The majority of the skin
8 of 12 Veterinary Record 10.1136/vr.102892
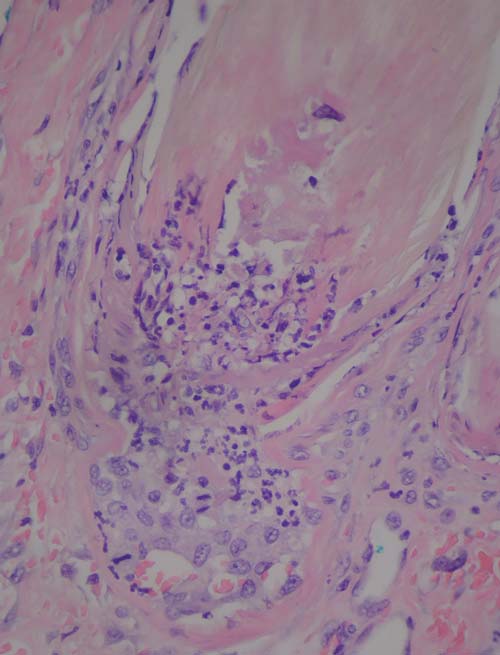
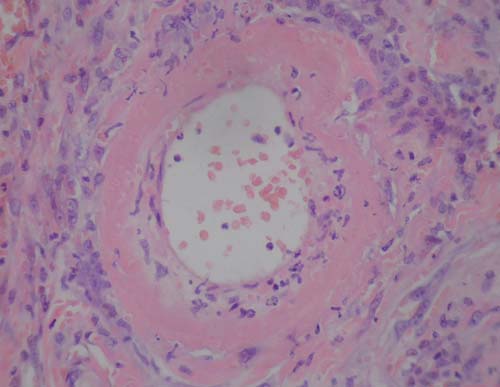
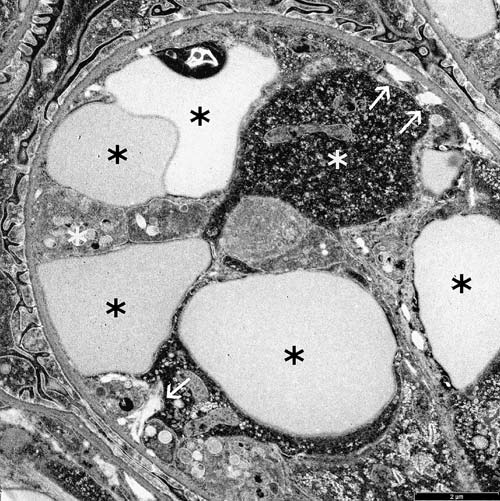
FIG 7: Electron micrograph of a glomerular capillary loop from a dogwith cutaneous and renal glomerular vasculopathy (CRGV). Thereare multiple deformed red blood cells (white asterisks) and swollenendothelial cells (black asterisks). A few small aggregates of fibrintactoids are present at the periphery of the capillary loop (arrows)
Glomerular ultrastructural changes previously reported with
FIG 5: Photomicrograph of a necrotic hair follicle with neutrophilic
CRGV ) were similar to the changes
identified in this case series. Immune complexes, complementand immunoglobulins have not previously been identified in the
lesions in this case series, as in previous reports of CRGV,
kidneys of dogs affected by CRGV ,
involved the distal extremities. This could be attributable to the
and were not identified in this popula-
increased number of smaller calibre vessels in this location and
tion of UK dogs.
an increased propensity to infarction.
Microscopic lesions in abdominal organs other than the
kidneys in dogs with CRGV were reported as being consistentwith uraemia and hypovolaemia in one report (
TABLE 5: Summary of case management
). Hyalinisation and rare thrombi were identified in
the submucosa of the stomach, and small and large intestine inanother report (). Fibrinoid necrosis of
smaller vessels was identified in this case series but thrombi
were not identified in abdominal organs other than the kidney.
Route of antibiotic administration
Other medications
Corticosteroids (anti-inflammatory dose)
Corticosteroids (immunosuppressive dose)
Fresh frozen plasma
FIG 6: Photomicrograph of a small dermal artery with fibrinoid
CRRT, continuous renal replacement therapy
necrosis of the vessel wall
10.1136/vr.102892 Veterinary Record 9 of 12
As discussed, canine TMAs can be the result of CRGVor HUS,
considered unlikely in this case series: PCR, FISH and viral meta-
which may represent two variants of the same disease with the
genomics ( performed in an effort to detect any encapsulated
same aetiology, or which may be two separate diseases. The most
virus potentially present in kidney tissue) results were negative,
common form of HUS in human beings, termed STEC-HUS or D
and histopathologically there was no evidence of viral cytopathic
+HUS, is associated with E. coli or Shigella dysenteriae shiga toxin
effect (cytoplasmic inclusion bodies) in any of the tissues exam-
ined. Negative results for viral metagenomics do not completely
Co-infection with Salmonella or Campylobacter may
exclude a viral aetiology, however. The results could indicate that
also be important STEC-HUS typic-
virus was present in low copy number, or that the virus was too
ally starts with watery, then haemorrhagic, diarrhoea followed by
remotely related to known viruses used for sequence alignment,
thrombocytopaenia, haemolysis and azotaemia
or that the sample used was too autolysed to preserve the virus.
). STEC-HUS appears a median
The significance of the Staphylococcaceae detected by 16S
of seven days after the onset of diarrhoea (
rRNA-directed probe in two dogs in this case series is unclear
In this case series, a diarrhoeic prodrome was only reported in four
but, contamination with commensal skin bacteria is considered
dogs; however, if owners were not specifically questioned about
more likely than disease-causing infection, as the kidneys were
prodromal diarrhoea, this information may have been missed. In
not kept sterile before the DNA extraction phase. The negative
contrast, four of the five dogs previously reported with HUS had
urine and renal tissue culture results obtained support this
diarrhoeic prodromes ,
It is currently unknown if CRGV is a novel canine disease or
E. coli shiga toxin has not been identified in dogs with HUS
if it is a variant of HUS, aHUS or indeed one of the other TMA's
reported in man. These include ‘HUS of unknown aetiology'
and thrombotic thrombocytopaenic purpura (TTP)
). Shiga toxin has been identified in one horse (
). Evaluation of the canine complement system may
and two of three rabbits previously reported
provide further information regarding the aetiology of CRGV.
with HUS Shiga toxin producing
Management of human TMA's is dependent upon the under-
E. coli, Salmonella and Campylobacter were not identified in the
lying cause. Plasma therapy, antibiotic administration, monoclo-
faeces or kidneys of dogs in this study. Reasons for failing to
nal shiga toxin antibodies and renal transplantation have all
identify toxin, or causative bacteria, may have included previous
been used in STEC-HUS. A recombinant, anti-C5 antibody (ecu-
antibiotic administration, inappropriate sample handling or late
lizumab) is the treatment of choice for human aHUS (
collection of samples. In human beings, recovery of toxin-
, ) but the cost has
producing E. coli is highly dependent upon faecal culture being
prohibited its evaluation in dogs. Plasma exchange remains the
performed within six days of the onset of diarrhoea (
treatment of choice for human TTP
and is a useful therapy for aHUS ().
In one case series of 18 dogs with CRGV in the USA, season-
Monoclonal antibody therapy to CD20 and classical immuno-
ality was reported as early winter and early summer (
suppressive drug therapy have also been reported for manage-
in this case series, dogs presented over winter and
ment of human TTP (). One dog with
early spring. In contrast, STEC-HUS in people is more com-
CRGV was reportedly ineffectively managed with immunosup-
monly reported in the summer
pressive therapy ). The efficacy of
). Seasonality of canine HUS has not been
plasma therapy and monoclonal antibody therapy has yet to be
reported (Any potential seasonal dis-
evaluated in CRGV.
tribution of CRGV may become apparent with time, provided
Case selection bias could have been introduced in this case
disease surveillance continues.
series. Fifty one of the 53 practices involved identified cases
Human STEC-HUS tends to occur either sporadically or in
based on their awareness of CRGV and the presenting signs.
small geographical clusters (,
Cases may have been missed in these practices without compre-
), which may be similar
hensive searching of computerised record systems. The actual
to the findings in this case series and epidemiological investiga-
number of dogs affected by CRGV may therefore be higher than
tions are ongoing. Although cases were reported from across the
reported in this case series.
north and south of England, 36.7 per cent came from The New
Six surviving dogs were strongly suspected, by the authors,
Forest, Hampshire. This high percentage could, however, be
to be suffering from CRGV. Renal histopathology was not avail-
attributed to the geographical location of the primary investiga-
able to confirm the diagnosis as, invasive procedures, like renal
tors in Hampshire and increased awareness amongst local
biopsy in patients with AKI showing apparent improvement to
symptomatic management, are considered clinically difficult to
In people, genetic or acquired conditions causing comple-
justify. This may suggest that CRGV is not an invariably fatal
ment dysregulation can cause another TMA, known as atypical
HUS (aHUS) (Skin lesions have beenreported alongside haemolysis and AKI with aHUS (which isnot the case with STEC-HUS), but the incidence is rare
Even in patients with multiple
CRGV is a TMA of unknown aetiology which, when azotaemia
genetic defects, aHUS may not develop until adulthood and an
develops, currently appears to carry a grave prognosis.
environmental trigger is considered likely for the development of
Vasculopathy, preferentially affecting the small vessels of the
disease (). Atypical HUS has not been
skin and kidneys in dogs, as identified in this case series, appears
reported in dogs; however, CRGV may bear some resemblance to
to be unique to CRGV and has not, to the authors' knowledge,
this disease, especially given the concurrent findings of skin
been reported associated with any other canine disease.
lesions and AKI identified in both diseases. An infectious or
Although this case series provides useful initial information
environmental trigger for CRGV may be suspected, given the
about CRGV in the UK, the retrospective multicentre nature of
number of in-contact dogs in this case series that developed skin
the study is a limitation. Continued detailed clinical, clinico-
lesions with or without AKI. It was also interesting to note that
pathological and epidemiological evaluation will further enhance
all of the affected in-contact dogs were related either to each
the understanding of the disease and will hopefully help to iden-
other and/or to a confirmed case.
tify possible triggers, define prognostic indicators and determine
Dog Circovirus has recently been isolated from the tissues of
the most appropriate management for these patients. The ques-
dogs with vascular and granulomatous disease of unknown
tion remains as to whether this is an emerging disease or, one
origin (); however, a viral aetiology was
that was previously present but unrecognised.
10 of 12 Veterinary Record 10.1136/vr.102892
DICKINSON, C. E., GOULD, D. H., DAVIDSON, A. H., AVERY, P. R., LEGARE,
The authors would like to thank the following veterinary sur-
M. E., HYATT, D. R. & DEBROY, C. (2008) Hemolytic-uremic syndrome in apostpartum mare concurrent with encephalopathy in the neonatal foal.
geons: Roger Stobbs BVSc, MRCVS; Monica Augusto DVM
CertSAM DipECVIM-CA MRCVS; Aran Mas DVM DipECVIM-
EUBIG, P. A., BRADY, M. S., KHAN, S. A., MAZZAFERRO, E. M. & MORROW,
CA MRCVS; Fabio Procoli DMV, MVetMed, DACVIM,
C. M. K. (2005) Acute renal failure in dogs after the ingestion of grapes or raisins:
DECVIM-CA, MRCVS; Stephen C B Perkins BVSc, BSc,
a retrospective evaluation of 43 dogs (1992–2002). Journal of Veterinary InternalMedicine 19, 663–674
MRCVS; Louise Ketteridge BVetMed, MRCVS; Ken C Smith
FERRER, L., RABANAL, R., FONDEVILA, D., RAMOS, J. A. & DOMINGO, M. (1988)
Skin lesions in canine leishmaniasis. 29, 381–388
Harrington BSc Vet Path, MVB, MVetMed, DipACVP, MRCVS;
FISCHER, J. R., LANE, I. F. & STOKES, J. (2009) Acute postrenal azotaemia: eti-
Nicholas G A Woodger BVetMed, BSc, MRCVS; Clare James
ology, clinicopathology, and pathophysiology. Compendium: Continuing Educationfor Veterinarians 31, 520–530
BVSc, BSc, DipACVP, MRCVS; Kerstin Baiker Dr med vet,
FOURNEL, C., CHABANNE, L., CAUX, C., FAURE, J. R., RIGAL, D., MAGNOL,
DVM, DiplECVP; Kerstin Erles DrMedVet, FRCPath, MRCVS;
J. P. & MONIER, J. C. (1992) Canine systemic lupus erythematosus. I: a study of
Stefanie B Gobelli Dr med vet, FVH; Isabelle Brodard; Darren J
75 cases. 1, 133–139
Lucas VetMB, MA, MRCVS; Edward M Roberts BVetMed,
GARCIA, A., MARINI, R. P., FENG, Y., VITSKY, A., KNOX, K. A., TAYLOR, N. S.,
MRCVS; Roisin Dickinson BVetMed, MRCVS; Tara C Zilic
SCAUER, D. B. & FOX, J. G. (2002) A naturally occurring rabbit model of entero-hemorrhagic Escherichia coli-induced disease. 186,
BVetMed, MRCVS; Louise C Melling BVetMed, MRCVS; and
Kobus Engelbrecht BVSc, MRCVS along with all of the other
GOLDFARB, S. & ADLER, S. H. (2001) Acute renal failure: pathophysiology and
veterinary surgeons who very kindly submitted clinical records
treatment. Nephrology 4, 1–12
and samples for histopathology to Anderson Moores Veterinary
GREENE, C. E., SYKES, J. E., BROWN, C. A. & HARTMAN, K. (2006)
Leptospirosis. In Infectious Diseases of the Dog and Cat. 3rd edn. Ed. C. E. GREENE.
Specialists. The authors would also like to thank owners of
Saunders Elsevier Inc. Chapter 44. pp 402–417
affected dogs, owners that returned questionnaires, and the New
HENDRICKS, A. (2000) Akute Ulzerative Dermatitis Bei Einem Greyhound.
Forest District Council, Forestry Commission, New Forest Dog
Proceedings of the 46th Annual Congress of the Small Animal Veterinary Association,
Owners Group and Anderson Moores Veterinary Specialists who
Dusseldorf, Germany. pp 62–63
HERTZKE, D. M., COWAN, L. A., SCHONING, P. & FENWICK, B. W. (1995)
provided funding for diagnostic testing. The authors would also
Glomerular Ultrastructural Lesions of Idiopathic Cutaneous and Renal
like to thank Dr Paul S Bass BSc MD FRCPath for his valuable
Glomerular Vasculopathy of Greyhounds. 32, 451–459
insights regarding the renal histopathology.
HOLLOWAY, S., SENIOR, D., ROTH, L. & TISHER, C. C. (1993) Hemolytic
uremic syndrome in dogs. 7, 220–227
▸ Additional material is available. To view please visit the journal
KAVANAGH, D., GOODSHIP, T. H. & RICHARDS, A. (2013) Atypical hemolytic
uremic syndrome. 33, 508–530
LI, L., MCGRAW, S., ZHU, K., LEUTENEGGER, C. M., MARKS, S. L., KUBISKI,
S., GAFFNEY, P., DELA CRUZ, F. N. Jr, WANG, C., DELWART, E. & PESAVENTO,
Open Access This is an Open Access article distributed in accordance with the
P. A. (2013) Circovirus in tissues of dogs with vasculitis and hemorrhage.
Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits
others to distribute, remix, adapt, build upon this work non-commercially, and license
MILFORD, D. V., TAYLOR, C. M., GUTTRIDGE, B., HALL, S. M., ROWE, B. &
KLEANTHOUS, H. (1990) Haemolytic uraemic syndromes in the British Isles
their derivative works on different terms, provided the original work is properly cited
1985–8: association with Verocytotoxin producing Escherichia coli. Part 1: clinical
and the use is non-commercial. See:
and epidemiological aspects. 65, 716–721
MONAHAN, A. M., CALLANAN, J. J. & NALLY, J. E. (2009) Review paper:
host-pathogen interactions in the kidney during chronic leptospirosis. 46, 792–799
MOORE, P. F., OLIVRY, T. & NAYDAN, D. (1994) Canine cutaneous epithelio-
ARDISSINO, G., POSSENTI, I., SALARDI, S., TEL, F., COLOMBO, E., TESTA, S.,
trophic lymphoma (mycosis fungoides) is a proliferative disorder of CD8+ T
DAPRAI, L., PICICCO, D., COLOMBO, R. M. & TORRESANI, E. (2014a)
cells. American Journal of Pathology 144, 421–429
Co-infection in children with bloody diarrhea caused by Shigatoxin-producing
MORRIS, C. F., ROBERTSON, J. L., MANN, P. C., CLARK, S. & DIVERS, T. J.
Escherichia coli: data of the North Italian HUS network.
(1987) Hemolytic uremic-like syndrome in two horses. Journal of the American
Veterinary Medical Association 191, 1453–1454
ARDISSINO, G., TEL, F., TESTA, S., MARZANO, A. V., LAZZARI, R., SALARDI,
MUNDAY, J. S., BERGEN, D. J. & ROE, W. D. (2005) Generalised calcinosis cutis
S. & EDEFONTI, A. (2014b) Skin involvement in atypical hemolytic uremic syn-
associated with probable leptospirosis in a dog. 16, 401–406
drome. 63, 652–655
NORIS, M., MESCIA, F. & REMUZZI, G. (2012) STEC-HUS, atypical HUS and TTP
ARONSON, L. R. & GREGORY, C. (1999) Possible hemolytic uremic syndrome in
are all diseases of complement activation. 8, 622–633
three cats after renal transplantation and cyclosporine therapy.
PASS, M. A., ODEDRA, R. & BATT, R. M. (2000) Multiplex PCRs for identification
of Escherichia coli virulence genes. Journal of Clinical Microbiology 38, 2001–2004
BIRNBAUM, N., BARR, S. C., CENTER, S. A., SCHERMERHORN, T.,
REBAR, A. H., LEWIS, H. B., DENICOLA, D. B., HALLIWELL, W. H. & BOON,
RANDOLPH, J. F. & SIMPSON, K. W. (1998) Naturally acquired Leptospirosis in
G. D. (1981) Red cell fragmentation in the dog: an editorial review. Veterinary
36 dogs: serological and clinicopathological features.
Pathology 18, 415–426
RENTKO, V. T., CLARK, N., ROSS, L. A. & SCHELLING, S. H. (1992) Canine
BLOMBERY, P. & SCULLY, M. (2014) Management of thrombotic thrombocytope-
leptospirosis. A retrospective study of 17 cases.
nic purpura: current perspectives. Journal of Blood Medicine 5, 15–23
BOURHY, P., BREMONT, S., ZININI, F., GIRY, C. & PICARDEAU, M. (2011)
ROBY, K. A. W., BLOOM, J. C. & BECHT, J. L. (1987) Postpartum hemolytic-uremic
Comparison of real-time PCR assays for detection of pathogenic Leptospira spp.
syndrome in a cow. Journal of the American Veterinary Medical Association 190, 187–190
in blood and identification of variations in target sequences.
ROTERMUND, A., PETERS, M., HEWICKER-TRAUTWEIN, M. & NOLTE, I.
(2002) Cutaneous and renal glomerular vasculopathy in a Great Dane resembling
BOYCE, T. G., SWERDLOW, D. L. & GRIFFIN, P. M. (1995) Escherichia coli 0157:H7
‘Alabama rot' of greyhounds. 151, 510–512
and the hemolytic-uremic syndrome. 333,
RUGGENENTI, P., NORIS, M. & REMUZZI, G. (2001) Thrombotic microangiopa-
thy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura.
BURNENS, A. P., FREY, A., LIOR, H. & NICOLET, J. (1995) Prevalence and clinical
significance of vero-cytotoxin-producing Escherichia coli (VTEC) isolated from
SALVADORI, M. & BERTONI, E. (2013) Update on hemolytic uremic syndrome:
cattle in herds with and without calf diarrhoea. Zentralblatt fur Veterinarmedizin
diagnostic and therapeutic recommendations. 2, 56–76
( Journal of Veterinary Medicine) series B 42, 311–318
SYKES, J. E., HARTMANN, K., LUNN, K. F., MOORE, G. E., STODDARD, R. A.
CARPENTER, J. L., ANDELMAN, N. C., MOORE, F. M. & KING, N. W. Jr (1988)
& GOLDSTEIN, R. E. (2011) 2010 ACVIM Small animal consensus statement on
Idiopathic cutaneous and renal glomerular vasculopathy of greyhounds.
leptospirosis: diagnosis, epidemiology, treatment and prevention.
CHANTREY, J., CHAPMAN, P. S. & PATTERSON-KANE, J. C. (2002) Haemolytic-
TANGEMAN, L. E. & LITTMAN, M. P. (2013) Clinicopathologic and atypical fea-
uraemic syndrome in a dog. 49, 470–472
tures of naturally occurring leptospirosis in dogs: 51 cases (2000–2010).
COWAN, L. A., HERTZKE, D. M., FENWICK, B. W. & ANDREASEN, C. B. (1997)
Clinical and clinicopathologic abnormalities in greyhounds with cutaneous and
TARR, P. I., GORDON, C. A. & CHANDLER, W. L. (2005) Shiga-toxin-producing
renal glomerular vasculopathy: 18 cases (1992–1994). Journal of the American
Escherichia coli and haemolytic uraemic syndrome. Lancet 365, 1073–1086
Veterinary Medical Association 210, 789–793
TARR, P. I., NEILL, M. A., CLAUSEN, C. R., WATKINS, S. L., CHRISTIE, D. L. &
DELL'ORCO, M., BERTAZZOLO, W., PAGLIARO, L., ROCCABIANCA, P. &
HICKMAN, R. O. (1990) Escherichia coli 0157:H7 and the hemolytic uremic syn-
COMAZZI, S. (2005) Hemolytic-uremic syndrome in a dog.
drome: importance of early cultures in establishing the etiology.
10.1136/vr.102892 Veterinary Record 11 of 12

UCHIDA, H., KIYOKAWA, N., HORIE, H., FUJIMOTO, J. & TAKEDA, T. (1999)
VICTORIA, J. G., KAPOOR, A., LI, L., BLINKOVA, O., SLIKAS, B. & WANG, C.
The detection of shiga toxins in the kidney of a patient with hemolytic uremic
(2009) Metagenomic analyses of viruses in stool samples from children with
syndrome. 45, 133–137
acute flaccid paralysis. 83, 4642–4651
VAN DEN INGH T. S. G. A. M., VAN WINKLE T., CULLEN J M., CHARLES J A. &
WAIKAR, S. S., LIU, K. D. & CHERTOW, G. M. (2008) Diagnosis, epidemiology
DESMET V. J. (2006). Morphological Classification of Parenchymal Disorders of
and outcomes of acute kidney injury.
the Canine and Feline Liver. In: ROTHUIZEN J., BUNCH S. E., CHARLES J. A.,
et al. Chronic Hepatitis. WSAVA Standards for Clinical and Histological Diagnosis of
WALKER, D. & MCMAHON, L. (2013) Acute kidney injury in dogs.
Canine and Feline Liver Diseases. Philadelphia PA: Saunders Elsevier Ltd. Chapter
7. pp, 93-95.
VAN DEN INGH, T. S. G. A. M., VAN WINKLE, T., CULLEN, J. M., CHARLES,
J. A. & DESMET, V. J. (2006) Morphological classification of parenchymal disor-ders of the canine and feline liver. WSAVA Standards for Clinical and HistologicalDiagnosis of Canine and Feline Liver Diseases 93–95
12 of 12 Veterinary Record 10.1136/vr.102892
Cutaneous and renal glomerular
vasculopathy as a cause of acute kidney
injury in dogs in the UK
L. P. Holm, I. Hawkins, C. Robin, R. J. Newton, R. Jepson, G. Stanzani,
L. A. McMahon, P. Pesavento, T. Carr, T. Cogan, C. G. Couto, R.
Cianciolo and D. J. Walker
published online March 23, 2015
Veterinary Record
Updated information and services can be found at:
These include:
Supplementary Supplementary material can be found at:
This article cites 44 articles, 15 of which you can access for free at:
Open Access
This is an Open Access article distributed in accordance with the CreativeCommons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this worknon-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use isnon-commercial. See:
Receive free email alerts when new articles cite this article. Sign up in the
box at the top right corner of the online article.
Articles on similar topics can be found in the following collections
To request permissions go to:
To order reprints go to:
To subscribe to BMJ go to:
Source: http://alabamarot.co.uk/wp-content/uploads/2015/03/Veterinary-Record-2015-Holm-vr.102892.pdf
For examination in June and November 2015 This syllabus is approved for use in England, Wales and Northern Ireland as a Cambridge International Level 1/Level 2 Certificate (QN: ###/###/#). Cambridge Secondary 2 Changes to syllabus for 2015 This syllabus has been updated, but there are no significant changes. Cambridge International Examinations retains the copyright on all its publications. Registered Centres are permitted to copy material from this booklet for their own internal use. However, we cannot give permission to Centres to photocopy any material that is acknowledged to a third party even for internal use within a Centre.
Evidence for Dose-Additive Effects of Pyrethroids on Motor Activity in RatsMarcelo J. Wolansky,1 Chris Gennings,2 Michael J. DeVito,3 and Kevin M. Crofton41Departamento de Química Biológica (Área Toxicología), Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Ciudad Universitaria, Buenos Aires, Argentina; 2Solveritas, LLC, Richmond, Virginia, USA; 3Division of Experimental Toxicology, and







