Chemistry and applications of nanocrystalline cellulose and its derivatives: a nanotechnology perspective
Chemistry and Applications of Nanocrystalline
Cellulose and its Derivatives: a
B. L. Peng,1,2 N. Dhar,1 H. L. Liu2 and K. C. Tam1*
1. Department of Chemical Engineering, Waterloo Institute for Nanotechnology, University of Waterloo, 200 University Avenue
West, Waterloo, ON, Canada N2L 3G1
2. State Key Laboratory of Chemical Engineering and Department of Chemistry, East China University of Science and
Technology, Shanghai 200237, P.R. China
Nanocrystalline cellulose (NCC) is an emerging renewable nanomaterial that holds promise in many different applications, such as in personalcare, chemicals, foods, pharmaceuticals, etc. By appropriate modification of NCC, various functional nanomaterials with outstanding properties,or significantly improved physical, chemical, biological, as well as electronic properties can be developed. The nanoparticles are stabilised inaqueous suspension by negative charges on the surface, which are produced during the acid hydrolysis process. NCC suspensions can form achiral nematic ordered phase beyond a critical concentration, i.e. NCC suspensions transform from an isotropic to an anisotropic chiral nematicliquid crystalline phase. Due to its nanoscale dimension and intrinsic physicochemical properties, NCC is a promising renewable biomaterial thatcan be used as a reinforcing component in high performance nanocomposites. Many new nanocomposite materials with attractive properties wereobtained by the physical incorporation of NCC into a natural or synthetic polymeric matrix. Simple chemical modification on NCC surface canimprove its dispersability in different solvents and expand its utilisation in nano-related applications, such as drug delivery, protein immobilisation,and inorganic reaction template. This review paper provides an overview on this emerging nanomaterial, focusing on the surface modification,properties and applications of NCC.
Keywords: nanocrystalline cellulose, chemical modification, applications
materials. Compared to cellulose fibres, NCC possesses manyadvantages, such as nanoscale dimension, high specific strength
which is renewable, biodegradable, as well as non-toxic.
These amazing physicochemical properties and wide application
It is a carbohydrate polymer made up of repeating ˇ-D-
prospects have attracted significant interest from both research
glucopyranose units and consists of three hydroxyl groups per
scientists and industrialists. Gray and co-workers have conducted
anhydroglucose unit (AGU) giving the cellulose molecule a high
extensive research on NCC, and they have made important contri-
degree of functionality. The knowledge of the molecular structure
butions to the understanding of NCC. A recent review on NCC was
of cellulose is of prime importance as it explains the characteristic
reported by Habibi et al. (2010a), where the chemical structure,
properties of cellulose, such as hydrophilicity, chirality, biodegrad-
composition and their relationship to the optical and mechanical
ability and high functionality. As a renewable material, cellulose
properties of NCC were discussed. At present, nanoscience and
and its derivatives have been widely studied, focusing on theirbiological, chemical, as well as mechanical properties. The mate-rials based on cellulose and its derivatives have been used for
∗
Author to whom correspondence may be addressed.
more than 150 years in a wide variety of applications, such as
E-mail address: [email protected]
food, paper production, biomaterials and pharmaceuticals (Coffey
Can. J. Chem. Eng. 9999:1–16, 2011
et al., 1995; de Souza Lima and Borsali, 2004).
2011 Canadian Society for Chemical Engineering
DOI 10.1002/cjce.20554
Nanocrystalline cellulose (NCC) obtained from acid hydrolysis
Published online in Wiley Online Library
of cellulose fibres, has been realised as a new class of nano-
VOLUME9999,2011
THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING 1
nanotechnology are disciplines that have been highlighted for spe-
of crystallinity and the geometrical aspect ratio, i.e. the length-
cial focus by many funding agencies and government. Many novel
to-diameter (L/d) are very important parameters controlling the
products have been created and developed based on nanotechnol-
properties of NCC-based materials. An important characteristic
ogy. Undoubtedly, the exploitation of NCC will become a bridge
of NCCs, when prepared in sulphuric acid, is that they possess
between nanoscience and natural resource products, which could
negative charges on their surface due to the formation of sul-
play a major role in reviving the forest industry in Canada and
phate ester groups during acid treatment, which enhances their
countries with abundant forest resources.
stability in aqueous solutions. In order to characterise the mor-
In this review, recent developments on surface modification,
phology of NCC, various types of instruments can be used. The
physicochemical properties, and applications of NCC will be con-
most conventional and common one is the transmission electron
sidered and discussed. Above all, the production and morphology
microscopy (TEM) (Araki et al., 1998), which can directly provide
of NCC are described, and some of their unique solution prop-
high-resolution images. Moreover, scanning electron microscopy
erties highlighted. A variety of surface functionalisation routes
(SEM) (Miller and Donald, 2003), atomic force microscopy (AFM)
will be considered, where the focus is on improving the dis-
(Pranger and Tannenbaum, 2008), small angle neutron scatter-
persability of NCC in different solvents, thereby expanding their
ing (SANS) (Terech et al., 1999) and polarised and depolarised
applications in various market sectors. Some potential applica-
dynamic light scattering (DLS, DDLS) (de Souza Lima and Bor-
tions of NCC include nanocomposite films, drug delivery, protein
sali, 2002; de Souza Lima et al., 2003) were also employed to
immobilisation and metallic reaction template.
study the morphology of NCC.
GENERAL ASPECT OF NANOCRYSTALLINE
PRODUCTION OF NCC
Acid treatment (acid hydrolysis) is the main process used to pro-
Nanocrystalline cellulose derived from acid hydrolysis of native
duce nanocrystalline cellulose, which are the smaller building
cellulose possesses different morphologies depending on the ori-
blocks released from the original cellulose fibres. Native cellulose
gin and hydrolysis conditions. NCCs are rigid rod-like crystals
consists of amorphous and crystalline regions, and the amorphous
with diameter in the range of 10–20 nm and lengths of a few
regions have lower density compared to the crystalline regions,
hundred nanometers (Figure 1); e.g. crystallites from tunicates
so when cellulose fibres were subjected to harsh acid treatment,
and green algae have lengths in the range of a few microm-
the amorphous regions break up, releasing the individual crys-
eters and crystallites from wood and cotton have lengths of
tallites. The properties of NCC depend on various factors, such
the order of a few hundred nanometers, while some spherical
as cellulose sources, reaction time and temperature, and types of
shape NCCs were also produced during the acid treatment (Zhang
acid used for hydrolysis. Since R˚anby (1951) first reported that
et al., 2007; Wang et al., 2008). Therefore, the relative degree
colloidal sulphuric acid-catalysed degradation of cellulose fibres,
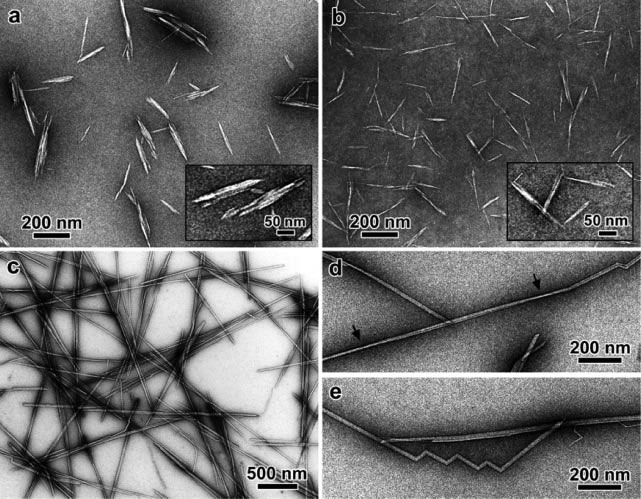
Figure 1. TEM micrographs of nanocrystals obtained by sulfuric acid hydrolysis of (a) cotton (b) avicel (c-e) tunicate cellulose. The insets of (a) and (b)
provide higher resolution images of some characteristic particles (Elazzouzi-Hafraoui et al., 2008).
2 THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING
VOLUME 9999, 2011
Table 1. Examples of the length (L) and diameter (d) of NCCs from various sources obtained via different techniques
Aspect ratio (L/d)
Araki et al. (2001)
Araki et al. (2001)
Miller and Donald (2003)
Pranger and Tannenbaum (2008)
de Menezes et al. (2009)
de Rodriguez et al. (2006)
de Souza Lima et al. (2003)
100–several 1000
Kimura et al. (2005)
Beck-Candanedo et al. (2005)
a series of NCC products were produced from a variety of sources,
they demonstrated that shorter NCC was obtained by increasing
such as wood (Table 1) (Beck-Candanedo et al., 2005), cotton
the temperature. Sulphuric and hydrochloric acids are extensively
(Araki et al., 2001; Miller and Donald, 2003), sisal (de Rodriguez
used in the preparation of NCC, however, the dispersability of NCC
et al., 2006), tunicate (de Souza Lima et al., 2003; Kimura et al.,
derived from these two kinds of acids is different. Due to the abun-
2005), bacterial (Roman and Winter, 2004), microcrystalline cel-
dance of charged sulphate groups on its surface, NCC obtained
lulose (Pranger and Tannenbaum, 2008), ramie (de Menezes et
from sulphuric acid hydrolysis dispersed readily in water, while
al., 2009) and Valonia cellulose (Revol, 1982).
those produced from hydrochloric acid hydrolysis do not dis-
The geometrical dimensions (length, L and diameter, d) of NCC
perse as readily, and their aqueous suspensions tend to flocculate.
were found to vary widely, in accordance to the examples shown
In addition, differences in the thermal stability and rheologi-
in Table 1. The varieties of dimensions depend on the source of
cal behaviour between the NCC produced from sulphuric acid
cellulosic material and conditions under which the hydrolysis is
and those from hydrochloric acid were observed (Araki et al.,
performed (Habibi et al., 2010a). It was reported that nanocrys-
1998). NCC has also been produced from recycled pulp using
talline cellulose derived from tunicate and bacterial cellulose is
microwave assisted enzymatic hydrolysis. Filson et al. (2009)
usually larger in dimension compared to those obtained from
reported a method to produce NCC using endoglucanase enzyme,
wood and cotton (Heux et al., 2000; de Souza Lima et al., 2003).
a constituent of cellulases. They observed that microwave heat-
This is because tunicate and bacterial cellulose are highly crys-
ing produced NCC with greater yield compared to conventional
talline, hence there are lower fractions of amorphous regions that
heating since microwave heating is more selective and reduces
need to be cleaved resulting in the production of larger nanocrys-
the reaction time as well.
tals. Beck-Candanedo et al. (2005) compared the properties ofNCC from softwood (black spruce) and hardwood (eucalyptus)produced at the same reaction time, temperature and acid-to-pulp
PROPERTIES OF NCC SUSPENSIONS
ratios. They found that these NCC suspensions exhibited simi-
Nanocrystalline cellulose derived from acid hydrolysis using var-
lar dimensions, surface charge, as well as critical concentrations
ious forest product sources can disperse in water due to their
required to form anisotropic liquid phases. It was also observed
negative charged surfaces. At low concentration, NCC particles
that at a higher acid-to-pulp ratio and longer reaction time, shorter
are randomly oriented in aqueous suspension as an isotropic
nanocrystals with narrow polydispersity index (PDI) were pro-
phase, and when the concentration reaches a critical value, they
duced and the critical concentration to form an anisotropic phase
form a chiral nematic ordering, where NCC suspensions transform
was increased. As the cleaving on cellulose chains occurred ran-
from an isotropic to an anisotropic chiral nematic liquid crys-
domly during the acid hydrolysis process, the dimensions of NCC
talline phase (Revol et al., 1992). As the concentration increased
are not uniform. Bai et al. (2009) proposed a method to obtain
further, aqueous NCC suspensions showed a shear birefringence
NCC with a narrow size distribution via the differential centrifu-
phenomenon. The critical concentration for sulphated NCC typ-
gation technique. Six different NCC fractions with narrow PDI
ically ranges between 1 and 10% (w/w), which is a function
were produced using differential angular velocities that gener-
of aspect ratio of NCC, charge density and osmolarity. Theories
ate relative centrifugal force (RCF) for each fraction at a constant
based on different parameters have been studied to explain the
phenomena (Stroobants et al., 1986).
By using response surface methodology, Bondeson et al. (2006)
The phase behaviour of NCC is sensitive to the presence of elec-
optimised the reaction conditions for sulphuric acid hydrolysis
trolytes and their counter ions, as well as macromolecules. The
of MCC from Norway spruce (Picea abies). The concentration
effect of added electrolyte on the phase separation of NCC was
of MCC and sulphuric acid, hydrolysis time, temperature and
studied by Dong et al. (1996), and they found that the addition of
ultrasonic treatment time were varied during the process. It was
electrolytes, e.g. HCl, NaCl and KCl significantly reduced the vol-
found that the reaction time, temperature and acid concentration
ume fraction of the anisotropic phase, indicating that addition of
were critical factors for the production of NCC. It was possible
electrolytes reduces the anisotropic phase. Dong and Gray (1997)
to produce cellulose whiskers with a yield of 30% (of initial
also studied the effect of counter ions on the phase separation
weight) with a sulphuric acid concentration of 63.5% (w/w) in
behaviour and stability of NCC suspensions by adding inor-
approximately 2 h to yield NCC with length of between 200 and
ganic counterions, weakly basic organic counterions and highly
400 nm and diameter of less than 10 nm. Elazzouzi-Hafraoui et al.
basic organic tetraalkylammonium salts. It was observed that the
(2008) studied the effect of temperature on the size distribution
types of counter ions had a significant effect on phase separation
of NCC produced from sulphuric acid hydrolysis of cotton, and
behaviour of NCC suspensions. Similar to electrolytes, the phase
VOLUME9999,2011
THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING 3
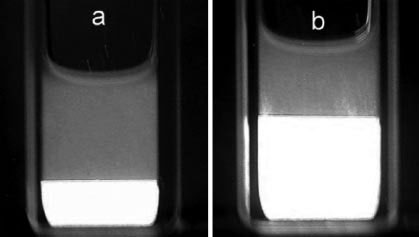
Figure 3. Numbering system for carbon atoms in anhydroglucose unit
of cellulose.
Figure 2. Phase separation observed between cross polars for different
concentrations of NCC (cotton fibers) suspensions at total concentrations
ination of the chiral nematic structure (Figure 2) was recently
of (a) 19.8 wt% and (b) 25.0wt% (Elazzouzi-Hafraoui et al., 2009).
reported (Elazzouzi-Hafraoui et al., 2009). Zhou et al. (2009)illustrated that NCC with adsorbed xyloglucan oligosaccharide-
separation of NCC suspensions is strongly affected by the addi-
poly(ethylene glycol)-polystyrene showed excellent dispersability
tion of macromolecules. Gray and co-workers (Edgar and Gray,
in non-polar solvents. In addition, the addition of surfactants
2002; Beck-Candanedo et al., 2006, 2007) conducted a detailed
enhanced the dispersion of NCC in polystyrene matrix (Kim
study on the effect of dextran and ionic dyes on the phase equi-
et al., 2009).
librium of NCC suspensions. Surfactant coating used to disperse
Recently, the assembling behaviours of NCC under external
NCC whiskers in non-polar solvents was first reported by Heux
field, such as AC electric field and magnetic field were investigated
et al. (2000), where NCC whiskers from cotton and tunicate were
(Sugiyama et al., 1992; Fleming et al., 2000; Bordel et al., 2006).
mixed with Beycostat NA (BNA) surfactant. The authors demon-
The effect of AC electric field on the alignment and orientation
strated that the chiral nematic phases were formed in spite of
of NCC was investigated by Habibi et al. (2008a). They observed
a layer of surfactants around the NCC whiskers. Detailed exam-
that the application of an AC electric field to NCC suspensions
Figure 4. Schematic diagram illustrating the various types of chemical modifications on NCC surface (Braun and Dorgan, 2009; Hasani et al., 2008;
Dong and Roman, 2007; Gouss´e et al., 2002; Habibi et al., 2006; Morandi et al., 2009).
4 THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING
VOLUME 9999, 2011
deposited between two metallic electrodes resulted in the homo-
ACETYLATION AND ESTERIFICATION
geneous alignment of NCC molecules. Moreover, the alignment of
Several methods had been employed to achieve acetylation and
cellulose nanocrystals generated films was greatly influenced by
esterification of NCC. Based on a non-swelling reaction mecha-
the frequency and strength of the applied electric field, while the
nism, the reaction only occurred on the cellulose chains located on
orientation of cellulose nanocrystals became more homogeneous
NCC surface. The limitation on the extent of acetylation lies in the
with increasing electric field greater than 2000 V/cm with a fre-
susceptibility and accessibility of hydrolysed NCC on the surface.
quency ranging between 104 and 106 Hz. Previous studies (Revol
Sassi and Chanzy (1995) employed acetic anhydride and acetic
et al., 1994) have shown that NCC suspensions exhibited nega-
acid to modify the fibrous and homogenous cellulose. They
tive diamagnetic susceptibility anisotropy as they dried under the
showed that acetylation commenced from the surface and pro-
influence of a magnetic field. The authors also demonstrated that
ceeded through a non-swelling mechanism. Due to the low
for NCC films, the presence of magnetic field did not facilitate the
solubility, the acetylated cellulose on fibrous NCC was observed
formation of chiral nematic phase but it only increased the chiral
to surround the un-reacted NCC core; compared to homogenous
nematic pitch of the suspensions. Similar study was recently con-
acetylation that proceeded through dissolvation of the surface
ducted by Pan et al. (2010), where they examined various factors
acetylated cellulose. Yuan et al. (2006) used straightforward freeze
affecting the chiral nematic properties of NCC films.
drying and heating of mixtures of alkyenyl succinic anhydride(ASA) aqueous emulsions and NCC suspensions to obtain acety-
CHEMICAL MODIFICATION OF
lated NCC. This process imparted hydrophobicity to the NCC andrendered it soluble in a solvent with low polarity, i.e. DMSO and
1,4-dioxane. Surface acetylation of NCC whiskers (obtained from
According to its structure, NCC possesses an abundance of
MCC) was undertaken by reacting it with vinyl acetate in the
hydroxyl groups on the surface, where chemical reactions can
presence of potassium carbonate as catalyst (C
¸ etin et al., 2009).
be conducted. Among the three kinds of hydroxyl groups (Figure
By progressively increasing the reaction times, it was observed
3), the OH group on the sixth position acts as a primary alcohol,
that crystalline structure of the NCC whiskers was destroyed.
where most of the modification predominantly occurs (Roy et al.,
In an attempt to avoid complex surface functionalisation routes,
2009). Various chemical modifications of NCC, such as esterifica-
Braun and Dorgan (2009) recently combined the synthesis and
tion, cationisation, carboxylation, silylation and polymer grafting
functionalisation of NCC in a single step. By utilising a mixture
have been reported (Figure 4) (Gouss´e et al., 2002; Habibi et al.,
of acetic acid, HCl and organic acids, NCC whiskers were synthe-
2006; Hasani et al., 2008; Braun and Dorgan, 2009; Morandi et al.,
sised and functionalised using the Fischer esterification process
2009). Most of these focused on the improvement of its dis-
(Figure 5). The presence of acetate and butyrate groups affects
persability and compatibility in different solvents or matrices that
the hydrophilicity of NCC whiskers making their aqueous solu-
are suitable in the production of nanocomposites. There is increas-
tions unstable but they possessed better dispersibaility in ethyl
ing research focus on modification of NCC because of the increas-
acetate and toluene. Apart from the solution process, Berlioz et al.
ing potential applications of modified NCC in various industrial
(2009) demonstrated a gas phase process that makes use of evap-
sectors, such as personal care, nanocomposites, biomedical, etc.
oration of large excess of palmitoyl chloride to achieve a surface
Figure 5. Single step process for cellulose hydrolysis and esterification of hydroxyl groups using a mixture of acetic and hydrochloric acid (Braun and
Dorgan, 2009).
VOLUME9999,2011
THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING 5
Figure 6. Reaction route for surface fluorescently labeled NCC with FITC (Dong and Roman, 2007).
to core esterification. This method can be extended to esterifica-
surface of NCC, they developed a simple method involving a three-
tion of different fatty acid chlorides. The nature and treatment of
step reaction pathway described by the reaction route shown in
the cellulose were found to be an important factor controlling the
Figure 6. First, the surface of NCC was decorated with epoxy
extent of esterification and the final morphology of NCC. Simi-
functional groups via reaction with epichlorohydrin, and then
lar concept was applied to esterification by refluxing hydrolysed
the epoxy ring was opened with ammonium hydroxide to intro-
NCC in organic acid chloride (de Menezes et al., 2009). Graft-
duce primary amino groups. Finally, the primary amino group
ing organic fatty acids of different aliphatic chain length (up to
was reacted with isothiocyanate group of FITC to form a thiourea.
18) was achievable; while the crystalline core was found to be
They compared the UV/vis absorption spectrum of unlabelled and
un-affected. Improved homogeneity of the extruded LDPE was
FITC-labelled NCC in their suspensions, and found the absorp-
reported with modified NC grafted with longer aliphatic chain.
tion peaks of FITC in the wavelength range of 450–500 nm in thespectrum of the FITC-labelled NCC.
Gray and co-workers (Hasani et al., 2008) described a one stepmethod to introduce positive charges on the surface of NCC
Cellulose whiskers resulting from the acid hydrolysis of tunicate
through the grafting of epoxypropyltrimethylammonium chloride
were partially silylated by a series of alkyldimethylchlorosilanes,
(EPTMAC) onto NCC surfaces. Such surface cationisation proce-
with alkyl moieties ranging from isopropyl to n-butyl, n-octyl and
dure was conducted via a nucleophilic addition of alkali-activated
n-dodrecyl (Gouss´e et al., 2002). The partially silylated whiskers
cellulose hydroxyl groups to the epoxy moiety of EPTMAC. This
with degree of substitution (DS) of between 0.6 and 1 can readily
modification process reversed the surface charge and led to stable
redispersed in medium polarity organic solvents, such as ace-
aqueous suspensions of NCC with unexpected thixotropic gelling
tone and THF. At DS of less than 0.6, the morphological integrity
properties. It seems that thixotropic gels inhibit the formation of
of the whiskers was preserved, however, it was disrupted when
chiral nematic liquid crystalline phase, because no liquid crys-
the DS was greater than 1. The authors developed a model with
talline chiral nematic phase separation was detected, while shear
four silylating agents to describe the experimental observations
birefringence was observed. However, mild alkaline cationisation
at different DS. Figure 7a shows the NCC whisker at low DS that
conditions preserve the original morphology and maintain the
is still hydrophilic, where its structure integrity is maintained.
integrity of the crystal.
Such NCC was found to flocculate in THF. Figure 7b shows NCCwhisker with moderate DS rendering the surface hydrophobic.
Some of the chains are derivatised, where the soluble chain ends
FLUORESCENTLY LABELED NCC
stabilise the whiskers in organic solvents of low polarity. Figure 7c
Fluorescence techniques were extensively used to study the
shows highly silylated whiskers where the surface chains are sol-
cellular uptake and biodistribution of nanoparticulate deliv-
ubilised in the reaction medium and the inner core is exposed to
ery systems, by tracking the localisation of the fluorophores.
more silylation thereby compromising its structural integrity and
Dong and Roman (2007) described a method to label NCC with
crystalline nature. Moreover, the partially silylated whiskers were
fluorescein-5�-isothiocyanate (FITC) for fluorescence bioassay and
found to be more swollen compared to the needle-like images
bioimaging applications. To covalently attach FITC moieties to the
of unmodified whiskers, indicating the occurrence of slight
6 THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING
VOLUME 9999, 2011
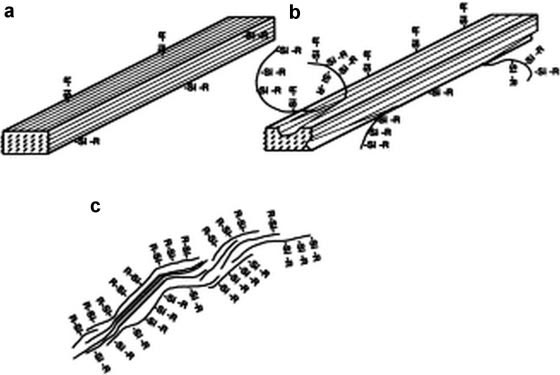
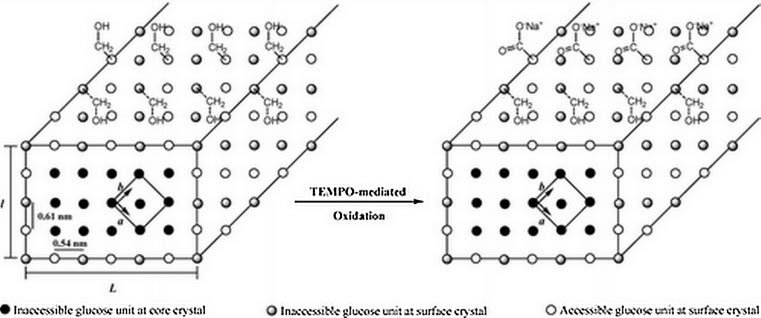
Figure 7. Model explaining the silylation of NCC whiskers (a) Onset of surface silylation. (b) Silylated with silylating agents (c) Partially silylated NCC
whiskers (Gouss´e et al. 2002).
silylation of the NCC core. In addition, Grunert and Winter (2002)
tion. Figure 8 shows a schematic of NCC whiskers and how the
also studied the surface trimethyl silylation of NCC from bacterial
surface hydroxyl groups become oxidised to carboxylic functional
cellulose, and investigated their reinforcement characteristics in
groups using TEMPO-mediated oxidation.
After TEMPO-mediated oxidation, NCC suspension will adopt
a birefringence pattern indicating a liquid-crystal like behaviour.
The NCC was found to be better dispersed in an aqueous envi-
ronment; attributed to electrostatic stabilisation from negatively
Conversion of hydroxymethyl groups into carboxylic groups
charged carboxylic groups. This is beneficial for further grafting of
can be conducted using 2,2,6,6-tetramethylpiperidine-1-oxyl
various types of functionalities. Using a carboxylation-amidation
(TEMPO) reagent (Saito and Isogai, 2004; Saito et al., 2010). This
procedure, Araki et al. (2001) prepared ‘brush polymer' by graft-
is a simple oxidative route that uses TEMPO as nitroxyl free radial
ing PEG with amide linkage onto TEMPO oxidised NCC. Habibi
to specifically oxidise primary hydroxymethyl in an environment
et al. (2006) reported that by using different molar ratio of NaOCl
of NaBr and NaOCl; leaving the secondary hydroxyl groups intact
over anydroglucose unit of hydrolysed cellulose, the degree of
(Habibi et al., 2006). The morphology and crystal axis of the
oxidation and solubility could be varied. Higher NaOCl resulted
NCC are critical in determining the accessibility of the hydrox-
in an increase of carboxyl content. The degree of oxidation was
ymethyl group. It is generally accepted that only 50% of the
determined from conductometric titration that measures the con-
surface hydroxymethyl groups are accessible for the TEMPO reac-
centration of carboxylic acid. Importantly, the structural integrity
Figure 8. Schematic of the NCC whiskers before and after TEMPO-mediated oxidation (Habibi et al., 2006).
VOLUME9999,2011
THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING 7
Figure 9. Formation of PDMAEMA grafted on rod-like NCC by surface initiated ATRP (Yi et al., 2009).
of NCC was retained after hydrolysis and TEMPO-mediated oxi-
ciencies and molecular weights. Yi et al. (2008) also investigated
dation. However, excessive oxidation did affect the structural
the surface grafting of NCC using styrene as a monomer, while
integrity of the original NCC; since the amorphous region of the
another hydrophilic monomer, namely, N,N-dimethylaminoethyl
NCC would be degraded (Montanari et al., 2005).
methacrylate (DMAEMA) was also used to study the temperature-induced chiral nematic phase behaviour of suspensions ofPDMAEMA-grafted NCC (Yi et al., 2009) (Figure 9). Similar
to these reported studies, Xu et al. (2008) synthesised NCC
The methods for polymer grafting onto NCC surface are based
whiskers grafted with azobenzene polymers to produce a novel
on two approaches, i.e. ‘grafting-to' and ‘grafting-from'. Many
amphotropic hairy rod-like system. This amphotropic system
techniques for surface modification of NCC whiskers involve
exhibited thermotropic and lyotropic liquid crystalline properties.
the ‘grafting-to' approach, where a polymer chain is grafted
Ring-opening polymerisation was also used in the ‘grafting-from'
to the NCC surface. The ‘grafting-to' approach was adopted by
approach to graft polycaprolactone (PCL) polymers onto NCC sur-
Habibi and Dufresne (2008) to graft various molecular weights
face (Habibi et al., 2008b), where stannous octoate (Sn(Oct)2) was
polycaprolactone (PCL) to the NCC via the isocyanate-mediated
used as a grafting and polymerising agent. To enhance the grafting
coupling reaction. They found that the grafted PCL chains were
efficiency, Chen et al. (2009); and Lin et al. (2009) studied simi-
able to crystallise at the NCC surface, when the grafting density
lar grafting process via microwave irradiation. Recently, Siqueira
was sufficiently high. Similar efforts were reported in the graft-
et al. (2010) grafted long chain isocyanate onto NCC whiskers
ing process of presynthesised waterborne polyurethane onto the
using two new novel methods of in situ solvent exchange in
surface of NCC (Cao et al., 2009). Peptide coupling reaction was
order to prevent dispersion problems in the reaction medium. A
also used in the polymer grafting process of NCC. HCl-hydrolyzed
summary of various types of modifications on NCC whiskers is
NCC was carboxylated by TEMPO-mediated oxidation, and then
documented in Table 2.
EDC/NHS carbodiimide chemistry was used to conduct a roomtemperature reaction between the –COOH groups on carboxylated
APPLICATIONS OF NANOCRYSTALLINE
NCC and –NH2 groups on PEG-NH2 (Araki et al., 2001). The sameapproach was implemented by Mangalam et al. (2009), who suc-
cessfully grafted DNA oligomers on the surface of TEMPO oxidised
As a result of their distinctive properties, nanocrystalline cellulose
NCC. The temperature dependence and structural morphology of
has the potential of becoming an important class of renewable
DNA-g-NCC composite on the nanoscale were also reported. Graft-
nanomaterials, which could find many useful applications. The
ing of DNA to NCC extends the application of NCC to nucleic acid
main application of NCC is for the reinforcement of polymeric
research. Ljungberg et al. (2005) investigated the process of graft-
matrix in nanocomposite materials. Favier et al. (1995) was the
ing maleated polypropylene onto the surface of NCC using the
first to report the use of NCC as reinforcing fillers in poly(styrene-
‘grafting-to' approach. Recently, Azzam et al. (2010) reported the
co-butyl acrylate) (poly(S-co-BuA))-based nanocomposites. Since
grafting of thermo-responsive polymers onto NCC via a peptidic
then, numerous nanocomposite materials were developed by
coupling reaction. The modified NCC possesses unusual prop-
incorporating NCC into a wide range of polymeric matrices. The
erties, such as colloidal stability at high ionic strength, surface
properties of these cellulosic nanocomposites depend on the types
activity and thermoreversible aggregation. They also reported a
and characteristics of NCC and polymeric matrices (which could
thermoreversible aggregation, which could pave the way for the
be both natural and synthetic polymers) (Samir et al., 2005).
design of stimuli–responsive biobased nanocomposite materials.
Chemical functionalisation of NCC improves its dispersability in
The ‘grafting-from' approach has been used to grow poly-
organic solvents and this greatly expands its potential applications
meric chains from the NCC surface via the atom transfer radical
in various sectors. The following section highlights some recent
polymerisation (ATRP). This technique allows for very precise
studies on potential applications of NCC from a nanotechnological
control over the grafting process that produces well-defined
monodispersed particles (Wang and Matyjaszewski, 1995). Sur-face initiated ATRP is a two-step process: the first step is
the esterification of hydroxyl groups on NCC surface with 2-bromoisobuturyl bromide (BIBB), which is followed by the
The mechanical properties of nanocomposite films mainly depend
polymerisation of monomers. Styrene was used as the monomer
on the morphology and dimensions of the two constituents, i.e.
for surface initiated ATRP by Morandi et al. (2009), and they
NCC and polymeric matrix, as well as the processing techniques.
reported a series of grafting products with different grafting effi-
Any other factor that interferes or controls the formation of the
8 THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING
VOLUME 9999, 2011
Table 2. Summary of various types of modifications on NCC whiskers
Type of modification
Alkyenyl succinic anhydride (ASA)
Yuan et al. (2006)
Surface acetylation
Vinyl acetate in the presence of potassium carbonate as catalyst
¸ etin et al. (2009)
Fischer esterification process
Mixture of acetic acid, HCl and organic acids
Braun and Dorgan (2009)
Surface to core esterification
Gas phase evaporation of large excess of palmitoyl chloride
Berlioz et al. (2009)
Grafting epoxypropyltrimethylammonium chloride (EPTMAC)
Hasani et al. (2008)
Fluorescently labelled NCC
Dong and Roman (2007)
Series of alkyldimethylchlorosilanes
Gouss´e et al. (2002)
TEMPO-media oxidation
Oxidation of –OH groups to –COOH using
Habibi et al. (2006)
Grafting of PEG-NH2
Reaction of PEG-NH2 and TEMPO oxidised NCC
Araki et al. (2001)
Grafting-to approach using isocyanate-mediated coupling reaction
Habibi and Dufresne (2008)
(PCL)Grafting of styrene, DMAEMA or
Grafting-from approach using surface initiated ATRP
Yi et al. (2008); Morandi et al.
azobenzene polymers
(2009); Xu et al. (2008)
percolating whiskers network will also change the mechanical
mer grafting is beneficial for dispersing NCC and to formulate
performance of the nanocomposite (Dufresne, 2008).
nanocomposites in non-polar solvents (Ljungberg et al., 2005).
The geometrical aspect ratio, defined as the length-to-diameter
However, drying and redispersion of NCC without aggregation
(L/d), is a major factor that controls the mechanical properties of
in casting-evaporation processing is still challenging. Habibi and
nanocompsites and determines the percolation threshold value.
Dufresne (2008) prepared nanocomposite films using unmodi-
This factor is related to the original cellulose fibres and production
fied and PCL-grafted NCC nanoparticles as filler and PCL as the
conditions, which was previously discussed. Fillers with a high
matrix, and they that found PCL-grafted nanoparticles were eas-
aspect ratio give the best reinforcing effect. It was reported that
ily dispersed when compared to the unmodified system. They
the highest modulus increase in the rubbery state of the poly(S-
demonstrated that the transformation of NCC nanoparticles into a
co-BuA) matrix and thermal stability were obtained with tunicin
cocontinuous material through long chain surface chemical mod-
whiskers (L/d ∼ 67) in comparison with bacterial (L/d ∼ 60) or
ification represents a new and promising way for the processing
Avicel whiskers (L/d ∼ 10) (Samir et al., 2005). de Rodriguez and
of nanocomposite material.
co-workers (2006) studied sisal nanowhiskers with high aspectratio as filler in the nanocomposites with polyvinyl acetate (PVAc)
as the matrix. They found that the high aspect ratio could ensure
Electrostatic fibre spinning or ‘electrospinning' is a versatile
percolation, resulting in mechanical improvements and thermal
method for preparing fibres with diameters ranging from sev-
stability at lower fibre loadings. Dubief et al. (1999) also reported
eral microns down to 100 nm through the action of electrostatic
the mechanical behaviour of composites based on amorphous
forces (Dufresne, 2010). Electrospinning is a fast and simple pro-
poly(ˇ-hydroxyoctanoate) (PHO) when reinforced with tunicin
cess to produce polymeric filaments, and this approach has been
microcrystals. They proved that tunicin whiskers with high aspect
widely studied. Park et al. (2007) reported on the incorporation of
ratio led to higher mechanical properties through the formation
bacterial cellulose whiskers into polyethylene oxide (PEO) nanofi-
of a rigid filler network. Similar effects on the dependence of
bres via the electrospinning process. The authors demonstrated
mechanical properties of nanocomposites on aspect ratio were
that electrospun PEO fibres with a diameter of less than 1 m
also reported using carbon nanotubes as fillers (Jiang et al., 2007;
were successfully prepared, and the mechanical properties were
Wong et al., 2009).
enhanced by the cellulose whiskers. Nanocomposite films from
In this section, different processing methods, such as casting
polystyrene and cellulose nanowhiskers were also produced by
evaporation, electrospinning, as well as extrusion and impregna-
Rojas et al. (2009) using electrospinning. They found that sorbitan
tion, were evaluated for the preparation of nanocomposite films
monostearate, a nonionic surfactant could be used to improve the
using water and organic solvents. The resulting thermal prop-
dispersion of hydrophilic reinforcing nanoparticle in hydropho-
erties, mechanical properties and applications of nanocomposite
bic matrix. Electrospun poly (vinyl alcohol) (PVA) (Peresin et al.,
films will be discussed.
2010) and polycaprolactone (PCL) (Zoppe et al., 2009) nanofi-bres reinforced with NCC were also obtained by electrospinning.
Casting Evaporation
The new composites from PCL and NCC showed a significant
Casting-evaporation is one of the most common techniques used
increase in the storage modulus at all temperatures and possessed
to produce nanocomposite films, where the solvent is evapo-
a nonlinear stress–strain deformation behaviour. Fibre webs from
rated after dispersion casting. To achieve good reinforcement,
PCL reinforced with 2.5% unmodified NCC showed ca. 1.5-fold
the dispersability of the filler, such as NCC in both the poly-
increase in Young's modulus and the ultimate strength compared
meric matrix and processing solvents is critical. The water-soluble
to PCL webs. The reason was explained in terms of the difference
matrix is the simplest system for incorporating NCC as filler,
in the fibre diameter, NCC loading, and crystallisation processes.
due to the hydrophilic character of NCC. Schroers et al. (2004)
A co-electrospinning technique to produce a core-in-shell nano-
studied the properties of nanocomposite films based on ethy-
material consisting of a cellulose shell and a core containing
lene oxide/epichlorohydrin and NCC, which were produced by
the cellulose nanocrystals was first reported by Magalh˜aes et al.
dispersion-casting of NCC fillers in THF/water mixtures. The poly-
VOLUME9999,2011
THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING 9
Extrusion and Impregnation
the air/liquid interface in the presence of dioctadecyldimethy-lammonium (DODA), a cationic amphiphilic molecule. Dioctade-
Extrusion and impregnation are two methods used to prepare
cyldimethylammonium (DODA) was used to produce NCC-DODA
nano-composites comprising of a polymeric matrix and modified
complexes that allowed the transfer of NCC from the air/liquid
NCC filler. The main challenge lies in the poor dispersion and
interface in an aqueous suspension to hydrophobic solid sub-
agglomeration of NCC inside the polymeric matrix, which is due to
strates. Atomic force microscopy (AFM) and X-ray photoelectron
the hydrophilic nature of NCC and the formation of inter-chain H-
spectroscopy (XPS) were employed to characterise the morphol-
bonding. These non-optimised conditions thus limit the mechani-
ogy and chemical composition of NCC films. It was demonstrated
cal properties of the prepared nano-composite. One way to address
that both of these two techniques could be used to prepare sta-
the agglomeration problem previously reported is by introduc-
ble, smooth and robust monolayers of NCC. Hence, these layers
ing microcrystalline cellulose at the intermediate stage during the
offer an opportunity to investigate the interfacial properties rele-
extrusion of poly (lactic acid) (PLA), where the suspension of
vant to the chemical and biological transformations of cellulose.
whiskers was pumped into the polymer melt during the extrusion
Alternatively, these films can be used as a coating technology to
process (Oksman et al., 2006). N,N-dimethylacetamide (DMAc)
modify the surface of other materials to achieve unique properties
containing lithium chloride (LiCl) was used to better disperse the
(Habibi et al., 2010b).
microcrystalline cellulose (MCC); however, it appeared to result
The self-assembly of NCC in suspension can produce iridescent
in the degradation of the composites at high temperature. The
chiral nematic films upon drying (Beck et al., 2011) The irides-
mechanical properties did not show improvements compared to
cence colour exhibited by NCC film is attributed to the ability
pure PLA, due to combined effect of additives and high processing
of the NCC to reflect circularly polarised light at specific wave-
temperature. Wide angle X-ray diffraction showed that the crystal
lengths. It was reported that ultrasound treatment increased the
integrity of the cellulose was retained after extrusion. A similar
chiral nematic pitch in the suspension resulting in the red-shift
process was performed by employing poly (vinyl alcohol) (PVA)
of the reflection wavelength of NCC films as the applied energy
as the dispersing agent; and the MCC was distributed in the PVA
phase (Bondeson and Oksman, 2007). Another method employedis the modification of surface functional groups on NCC prior toextrusion. Hydrolysis followed by grafting of organic aliphatic acid
chain of different lengths was used (de Menezes et al., 2009). The
The layer-by-layer assembly (LBL) is a unique and facile method
mixture of modified NCC and low density polyethylene (LDPE)
for fabricating composite films with nanometer precision. The
was extruded and improvement in the dispersion resulting in a
advantages of this approach are simplicity, universality and thick-
more homogeneous mixture was observed; where better disper-
ness control at the nano length scale. Two components are
sion was reported in formulations with longer aliphatic chain.
alternately deposited by solution-dipping or spin-coating, and
Impregnation is another technique that could be used to prepare
usually there are strong interactions between the alternative lay-
dried film or a mat comprising of NCC, before immersing the thin
ers. The self-assembling systems obtained from the LBL technique
film in the thermosetting resin (e.g. epoxy or melamine formalde-
could be used for a wide range of applications.
hyde). The cellulose thin film can be prepared via membrane
LBL assembly has already been applied to prepare multilay-
filtration of cellulose solution or by pressing the dried cellulose. At
ered films based on nanocrystalline cellulose. Podsiadlo et al.
low pressure, the resin impregnates and fills the cavities within the
(2005) first reported the preparation of NCC multilayer composites
NCC; followed by curing to produce a composite. This technique is
with poly (diallyldimethylammonium chloride) using LBL assem-
mostly used for sample preparation for evaluating the mechanical
bly, and they observed uniform coverage and densely packed
(Henriksson and Berglund, 2007; Iwamoto et al., 2008), thermal
NCC surface from AFM and SEM analyses. The feasibility of
(Shimazaki et al., 2007), and optical (Nogi et al., 2005) properties
LBL assembly of NCC was demonstrated with poly-(allylamine
of cellulose-filled composites. Higher degree of fibrillation of cel-
hydrochloride) (PAH). The formation of a well-ordered alignment
lulose was found to be beneficial for composite strength; due to the
from anisotropic NCC suspensions and the smoothening effect of
elimination of weaker fibres acting as crack initiator. Nakagaito
flexible PAH macromolecules was reported by Jean et al. (2008).
and Yano (2008) used NaOH treated cellulose micro-fibres impreg-
Layer-by-layer orientated self-assembled multilayer films contain-
nated with phenol–formaldehyde resin and a 20% increase in
ing NCC and PAH were also prepared by Cranston and Gray using
fracture strain compared to non-treated cellulose micro-fibres was
a strong magnetic film (Cranston and Gray, 2006a) and spin
observed. Composite made from the impregnation of bis-phenol
coating techniques (Cranston and Gray, 2006b, 2008). The LBL
A-type epoxy resin into cellulose thin film prepared from mem-
technique was also used to prepare self-organised films composed
brane filtration was found to have a high thermal conductivity and
solely of anionically and cationically modified NCC (Aulin et al.,
low thermal expansion (Shimazaki et al., 2007). Improvement of
2010). The authors demonstrated that separation of surfaces cov-
thermal stability was reported for composites consisting of lay-
ered by PEI/anionic microfibrillated cellulose (MFC) multilayers
ers of micro-size or nano-size cellulose fibres and polyurethane
required more energy than surfaces covered by cationic/anionic
Ozg ¨ur Seydibeyo ˘glu and Oksman, 2008). The cellulose was pre-
MFC multilayers, because the fibrils in the PEI/anionic MFC sys-
pared by filtering the water-cellulose slurry and the H-bonding
tem required more energy to be extracted from the multilayer.
enhanced the thin film integrity.
Biobased ‘green' nanocomposite films based on alternating lay-ers of two renewable bio-based materials (i.e. NCC and Chitosan)
were prepared using the LBL technique (de Mesquita et al., 2010).
Interfacial behaviours of NCC were studied by Habibi et al. using
The driving forces for the growth of the multilayered films are
the Langmuir–Blodgett (2007) and Langmuir–Schaeffer (2010)
hydrogen bonds and electrostatic interactions between the two
techniques. Smooth monolayer films based on NCC were pre-
layers. In addition, LBL assembly of multilayers using two bio-
pared by Langmuir–Blodgett vertical and Langmuir–Schaeffer
materials driven by non-electrostatic interactions was recently
horizontal deposition. NCC was found to form stable layers at
reported by Jean et al. (2009).
10 THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING
VOLUME 9999, 2011
Table 3. Protein binding capacities of magnetised Avicel samples
The materials for drug delivery seem to be one of the most inter-
(Marchessault et al., 2006)
esting research fields. Abundant researches were conducted toinvestigate various drug delivery systems, such as liposomes,
% Bound proteins (w/w)
micelles, microgels (Ha and Gardella, 2005). Considering thesafety and efficacy, nanocrystalline cellulose has attracted increas-
ing attention in biomedical applications (such as a drug carrier),
due to its attractive properties. The toxicity assessment of NCC inhuman brain microvascular endothelial cells was conducted andNCC was non-toxic to cells and could be used as carriers in the
activity, and it is anticipated that the approach can be extended
targeted delivery of therapeutics (Roman et al., 2010). Recently,
to other enzymes.
a comprehensive assessment involving toxicity tests with rain-bow trout hepatocytes and nine aquatic species were conducted
NANOSTRUCTURES VIA TEMPLATING WITH NCC
by a team of Canadian researchers (Kovacs et al., 2010). From theinitial ecotoxicological characterisation of NCC, no serious envi-
Since Mobil researchers reported the first synthesis of mesoporous
ronmental concerns were observed. However, further testings will
material in 1992 (Kresge et al., 1992), this approach has attracted
be necessary, such as the evaluation on the fate, potential NCC
significant attention in fundamental and applied fields. Due to its
uptake and exposure studies, so that a detailed risk assessment of
attractive properties, NCC has already been used as a template in
NCC can be determined.
the synthesis of mesoporous materials. Porous titania with anatase
Recently, Dong and Roman (2007) reported a method to label
structure was prepared using NCC as a template and aqueous
NCC with fluorescein-5'-isothiocyanate (FITC) for fluorescence
Tyzor-LA solution as a cheap and stable titania precursor (Shin
bioassay and bioimaging applications. The interaction of labelled
and Exarhos, 2007). The titania material possesses high specific
NCC with cells and its biodistribution in vivo by fluorescence
surface area, and may be applied to many fields, such as catalysis,
techniques were evaluated. Shi et al. (2003) used microcrystalline
catalyst support and photovoltaics.
cellulose as host beads, where drug nanoparticles dispersion
A new NCC-inducing route was proposed for the synthe-
was spray coated onto them, and they examined the morphology
sis of shape-controlled nanoparticles (Zhou et al., 2007). The
using scanning electron and AFM. NCC particles were incorpo-
novel cubic-shaped TiO2 nanoparticles (Figure 10) with high
rated into hydrogels based on cyclodextrin/polymer inclusion
crystallinity and uniform size were prepared using NCC as
(Zhang et al., 2010), and they found that the new nanocomposite
morphology-inducing and coordinate agent at low temperature.
hydrogels can be used as a controlled delivery vehicle.
Thermal gravimetric analysis (TGA) suggests that NCC is probably
Extrusion-spheronisation is one of several methods used to
embedded in the TiO2 nanoparticles to promote the development
produce pellets, where the excipient plays an important role as
of regular anatase nanocubes.
spheronisation aid. Dukic-Ott et al. (2009) discussed the prop-
Some metal nanoparticles have been synthesised on NCC sur-
erties of different materials, and they proposed alternatives for
face via a reduction method, such as Ni nanoparticles (Shin et al.,
MCC, which is considered as a golden standard to produce pel-
2007) and Au–Ag alloy nanoparticles (Shin et al., 2008). In these
lets via extrusion-spheronisation. The behaviour of MCC during
processes, NCC serves as a dual role, as a matrix and a stabilis-
extrusion-spheronisation process could be explained by two pro-
ing template, to produce stable dispersions of nanoparticles on
posed models: ‘molecular sponge model' (Fielden et al., 1988;
NCC surface, and the crystallinity of NCC was maintained during
Ek and Newton, 1998) and ‘crystallite-gel model' (Kleinebudde,
the alloy formation (Shin et al., 2008). These reducing processes
1997). In another case study with itraconazole, Van Eerden-
could be recognised as ‘green' processes ascribed to the use of NCC
brugh et al. (2008) demonstrated the efficiency of MCC as a
and applied to the preparation of transition metal nanoparticles,
viable alternative for the preservation of the dissolution-rate of
which have high oxidising property without additional reducing
the nanoparticles upon freeze-drying.
A recent discovery of free-standing mesoporous silica films with
tunable chiral nematic structures was made by the research groupof MacLachlan at the University of British Columbia (Shopsowitz
et al., 2010). Various types of mesoporous silica films were pro-
Marchessault et al. (2006) provided a description of a ‘proteins
duced by calcinating the NCC/silica composite systems, and the
fishing' phenomenon for magnetic MCC. The first step is the
transmission spectra of the mesoporous silica films are shown in
ferrite synthesis that yields predominantly magnetite. Two dif-
Figure 11(left panel). Photograph of the different colours of meso-
ferent approaches have been envisaged for preparing magnetic
porous silica films S1–S4 are shown in Figure 11(right panel). The
MCC, where the order of magnetisation and oxidation of MCC
colours in these silica films arise only from the chiral nematic pore
was altered, producing Mag-Oxy-MCC (oxidation first) and Oxy-
structure present in the materials. This discovery could lead to the
Mag-MCC (magnetisation first). The results of protein binding
development of novel materials for applications, such as tuneable
capacities of magnetic MCC are summarised in Table 3, which
reflective filters and sensors. In addition, NCC could be used as a
were determined using bovine serum albumin (BSA) as a model
hard template to produce other new materials with chiral nematic
protein ligand.
A novel nanocomposite consisting of NCC and gold nanopat-
icle was recently investigated as a matrix for enzyme/protein
OTHER POSSIBLE APPLICATIONS
immobilisation (Mahmoud et al., 2009). Cyclodextrin glycosyltransferase (CGTase) and alcohol oxidase were used as test mod-
As more researchers become interested in NCC, many applica-
els, and they showed a phenomenally high loading rate in the
tions in diverse fields have been proposed and explored, ranging
matrix. The novel matrix also exhibits significant biocatalytic
from iridescent pigments to biomolecular NMR contrast agents
VOLUME9999,2011
THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING 11
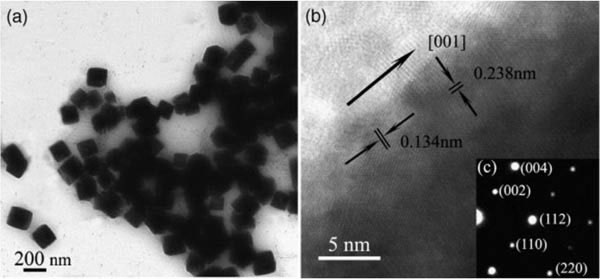
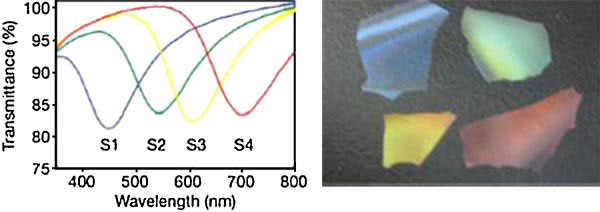
Figure 10. (a) Transmission electron microscopy (TEM) image, (b) High-resolution transmission electron microscopy (HRTEM) image, (c) selected area
electron diffraction (SAED) pattern of TiO2 nanocubes (Zhou et al., 2007).
Figure 11. Optical characterization and properties of NCC/silica mesoporous films (a) Transmission spectra; (b) Photograph showing different colours
of mesoporous silica films S1 to S4 (Shopsowitz et al., 2010).
(Fleming et al., 2001). In addition, NCC may be used in security
to explore various modification processes that enhance the prop-
paper (Revol et al., 1997, 1998), based on the solidified liquid crys-
erties of NCC, making it attractive for use in a wide range of
tals property. It is believed that NCC may be used in lithium battery
industrial sectors. Thus, the recent decision by Domtar and FP
products as a mechanical reinforcing agent for low-thickness poly-
Innovations to build a demonstration pilot plant to produce larger
mer electrolytes (Samir et al., 2004a, b, c; Schroers et al., 2004).
amounts NCC will accelerate the adoption of this new renewable
However, due to the duration of the preparation technique, many
industrial applications of NCC have focused on nanocomposites
Thus far, most of the studies focus on the mechanical and chiral
(Samir et al., 2005).
nematic liquid properties of NCC nanocomposites, however, otherresearch directions are also being explored. In nanocomposite sys-tems, the homogeneous dispersion of NCC in a polymer matrix is
CONCLUSIONS AND OUTLOOK
still a challenging issue, as aggregation or agglomeration of NCC is
The paper reviews the recent development on the modification
commonly encountered. We believe that appropriate modification
and applications of nanocrystalline cellulose from a nanotechnol-
of NCC to impart functional characteristics to this nanomaterials
ogy perspective. It is shown that NCC has a distinct advantage for
will be necessary if NCC is to be successfully incorporated into
preparing nanocomposites with outstanding thermal and mechan-
a specific product system. It is anticipated that nanotechnology
ical properties. Moreover, NCC may be used in drug delivery,
innovations in renewable resources, such as NCC will not only
protein immobilisation and metallic reaction template due to its
improve the future viability of the forest industry in Canada, but it
attractive properties. The feasibility of its potential applications is
will also create a larger market for future products based on NCC.
also discussed.
Nanocrystalline cellulose is an environmentally-friendly forest-
based material that could serve as a valuable renewable resourcefor rejuvenating the beleaguered forest industry. New and emerg-
B. L. Peng thanks the China Scholarship Council for support-
ing industrial extraction processes need to be optimised to achieve
ing his research at University of Waterloo, Canada. K. C. Tam
more efficient operations and this will require active research
would like to acknowledge the support from CFI and NSERC.
participations from the academic and industrial sectors. The
H. L. Liu would like to acknowledge the financial support from the
application of nanotechnology in developing NCC from the for-
National Natural Science Foundation of China (20736002) and the
est industry to more valuable products is required, because the
111 Project of China (B08021). We wish to thank Dr. Richard Berry
availability of materials based on NCC is still limited. Increas-
(Domtar-FPInnovations Joint Venture Company) for his support
ing attention is devoted to produce NCC in larger quantities, and
and encouragement in the research on NCC.
12 THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING
VOLUME 9999, 2011
Polysaccharides and Their Applications," Marcel Dekker, NewYork (1995), pp. 124.
Araki, J., M. Wada and S. Kuga, "Steric Stabilisation of a
Cranston, E. D. and D. G. Gray, "Formation of Cellulose-Based
Cellulose Microcrystal Suspension by Poly(ethylene glycol)
Electrostatic Layer-by-Layer Films in a Magnetic Field," Sci.
Grafting," Langmuir 17, 21–27 (2001).
Technol. Adv. Mat. 7, 319–321 (2006a).
Araki, J., M. Wada, S. Kuga and T. Okano, "Flow Properties of
Cranston, E. D. and D. G. Gray, "Morphological and Optical
Microcrystalline Cellulose Suspension Prepared by Acid
Characterisation of Polyelectrolyte Multilayers Incorporating
Treatment of Native Cellulose," Colloids Surf. Physicochem.
Nanocrystalline Cellulose," Biomacromolecules 7, 2522–2530
Eng. Aspects 142, 75–82 (1998).
Aulin, C., E. Johansson, L. W˚agberg and T. Lindstr ¨om,
Cranston, E. D. and D. G. Gray, "Birefringence in Spin-Coated
"Self-Organised Films from Cellulose I Nanofibrils Using the
Films Containing Cellulose Nanocrystals," Colloids Surf.
Layer-by-Layer Technique," Biomacromolecules 11, 872–882
Physicochem. Eng. Aspects 325, 44–51 (2008).
de Menezes A. Jr., G. Siqueira, A. A. S. Curvelo and A. Dufresne,
Azzam, F., L. Heux, J. L. Putaux and B. Jean, "Preparation By
"Extrusion and Characterisation of Functionalised Cellulose
Grafting Onto, Characterisation, and Properties of Thermally
Whiskers Reinforced Polyethylene Nanocomposites," Polymer
Responsive Polymer-Decorated Cellulose Nanocrystals,"
50, 4552–4563 (2009).
Biomacromolecules 11, 3652–3659 (2010).
de Mesquita, J. P., C. L. Donnici and F. V. Pereira, "Biobased
Bai, W., J. Holbery and K. Li, "A Technique for Production of
Nanocomposites From Layer-by-Layer Assembly of Cellulose
Nanocrystalline Cellulose With a Narrow Size Distribution,"
Nanowhiskers with Chitosan," Biomacromolecules 11,
Cellulose 16, 455–465 (2009).
473–480 (2010).
Beck, S., J. Bouchard and R. Berry, "Controlling the Reflection
de Rodriguez, N. L. G., W. Thielemans and A. Dufresne, "Sisal
Wavelength of Iridescent Solid Films of Nanocrystalline
Cellulose Whiskers Reinforced Polyvinyl Acetate
Cellulose," Biomacromolecules 12, 167–172 (2011).
Nanocomposites," Cellulose 13, 261–270 (2006).
Beck-Candanedo, S., D. Viet and D. G. Gray, "Triphase Equilibria
de Souza Lima, M. M. and R. Borsali, "Rodlike Cellulose
in Cellulose Nanocrystal Suspensions Containing Neutral and
Microcrystals: Structure, Properties, and Applications,"
Charged Macromolecules," Macromolecules 40, 3429–3436
Macromol. Rapid Commun. 25, 771–787 (2004).
de Souza Lima, M. M. and R. Borsali, "Static and Dynamic Light
Beck-Candanedo, S., D. Viet and D. G. Gray, "Induced Phase
Scattering from Polyelectrolyte Microcrystal Cellulose,"
Separation in Cellulose Nanocrystal Suspensions Containing
Langmuir 18, 992–996 (2002).
Ionic Dye Species," Cellulose 13, 629–635 (2006).
de Souza Lima, M. M., J. T. Wong, M. Paillet, R. Borsali and R.
Beck-Candanedo, S., M. Roman and D. G. Gray, "Effect of
Pecora, "Translational and Rotational Dynamics of Rodlike
Reaction Conditions on the Properties and Behaviour of Wood
Cellulose Whiskers," Langmuir 19, 24–29 (2003).
Cellulose Nanocrystal Suspensions," Biomacromolecules 6,
Dong, S. P. and M. Roman, "Fluorescently Labeled Cellulose
1048–1054 (2005).
Nanocrystals for Bioimaging Applications," J. Am. Chem.
Berlioz, S., S. Molina-Boisseau, Y. Nishiyama and L. Heux,
Soc. 129, 13810–13811 (2007).
"Gas-Phase Surface Esterification of Cellulose Microfibrils and
Dong, X. M. and D. G. Gray, "Effect of Counterions on Ordered
Whiskers," Biomacromolecules 10, 2144–2151 (2009).
Phase Formation in Suspensions of Charged Rodlike Cellulose
Bondeson, D. and K. Oksman, "Polylactic Acid/Cellulose
Crystallites," Langmuir 13, 2404–2409 (1997).
Whisker Nanocomposites Modified by Polyvinyl Alcohol,"
Dong, X. M., T. Kimura, J.-F. Revol and D. G. Gray, "Effects of
Compos. A: Appl. Sci. Manufact. 38, 2486–2492 (2007).
Ionic Strength on the Isotropic-Chiral Nematic Phase
Bondeson, D., A. Mathew and K. Oksman, "Optimisation of the
Transition of Suspensions of Cellulose Crystallites," Langmuir
Isolation of Nanocrystals From Microcrystalline Cellulose by
12, 2076–2082 (1996).
Acid Hydrolysis," Cellulose 13, 171–180 (2006).
Dubief, D., E. Samain and A. Dufresne, "Polysaccharide
Bordel, D., J. Putaux and L. Heux, "Orientation of Native
Microcrystals Reinforced Amorphous
Cellulose in an Electric Field," Langmuir 22, 4899–4901
Macromolecules 32, 5765–5771 (1999).
Braun, B. and J. R. Dorgan, "Single-Step Method for the Isolation
Dufresne, A., "Polysaccharide Nanocrystal Reinforced
and Surface Functionalisation of Cellulosic Nanowhiskers,"
Nanocomposites," Can. J. Chem. 86, 484–494 (2008).
Biomacromolecules 10, 334–341 (2009).
Dufresne, A., "Processing of Polymer Nanocomposites
Cao, X., Y. Habibi and L. A. Lucia, "One-Pot Polymerisation,
Reinforced With Polysaccharide Nanocrystals," Molecules 15,
Surface Grafting, and Processing of Waterborne
4111–4128 (2010).
Polyurethane-Cellulose Nanocrystal Nanocomposites," J.
Dukic-Ott, A., M. Thommes, J. P. Remon, P. Kleinebudde and C.
Mater. Chem. 19, 7137–7145 (2009).
Vervaet, "Production of Pellets Via Extrusion-Spheronisation
¸ etin, N. S., P. Tingaut, N. ¨
Ozmen, N. Henry, D. Harper, M.
Without the Incorporation of Microcrystalline Cellulose: A
Dadmun and G. S ebe, "Acetylation of Cellulose Nanowhiskers
Critical Review," Eur. J. Pharm. Biopharm. 71, 38–46 (2009).
With Vinyl Acetate under Moderate Conditions," Macromol.
Edgar, C. D. and D. G. Gray, "Influence of Dextran on the Phase
Biosci. 9, 997–1003 (2009).
Behaviour of Suspensions of Cellulose Nanocrystals,"
Chen, G., A. Dufresne, J. Huang and P. R. Chang, "A Novel
Macromolecules 35, 7400–7406 (2002).
Thermoformable Bionanocomposite Based on Cellulose
Ek, R. and J. M. Newton, "Microcrystalline Cellulose as a
Sponge as an Alternative Concept to the Crystallite-Gel Model
Eng. 294, 59–67 (2009).
for Extrusion and Spheronisation," Pharm. Res. 15, 509–512
Coffey, D. G., D. A. Bell and A. Henderson, "Cellulose and
Cellulose Derivatives," in A. M. Stephen, Ed., "Food
VOLUME9999,2011
THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING 13
Elazzouzi-Hafraoui, S., J. Putaux and L. Heux, "Self-Assembling
Henriksson, M. and L. A. Berglund, "Structure and Properties of
and Chiral Nematic Properties of Organophilic Cellulose
Cellulose Nanocomposite Films Containing Melamine
Nanocrystals," J. Phys. Chem. B 113, 11069–11075
Formaldehyde," J. Appl. Polym. Sci. 106, 2817–2824 (2007).
Heux, L., G. Chauve and C. Bonini, "Nonflocculating and
Elazzouzi-Hafraoui, S., Y. Nishiyama, J. Putaux, L. Heux, F.
Chiral-Nematic Self-ordering of Cellulose Microcrystals
Dubreuil and C. Rochas, "The Shape and Size Distribution of
Suspensions in Nonpolar Solvents," Langmuir 16, 8210–8212
Crystalline Nanoparticles Prepared by Acid Hydrolysis of
Native Cellulose," Biomacromolecules 9, 57–65 (2008).
Iwamoto, S., K. Abe and H. Yano, "The Effect of Hemicelluloses
Favier, V., H. Chanzy and J. Y. Cavaille, "Polymer
on Wood Pulp Nanofibrillation and Nanofiber Network
Nanocomposites Reinforced by Cellulose Whiskers,"
Characteristics," Biomacromolecules 9, 1022–1026 (2008).
Macromolecules 28, 6365–6367 (1995).
Jean, B., F. Dubreuil, L. Heux and F. Cousin, "Structural Details
Fielden, K. E., J. M. Newton, P. O'Brien and R. C. Rowe,
of Cellulose Nanocrystals/Polyelectrolytes Multilayers Probed
"Thermal Studies on the Interaction of Water and
by Neutron Reflectivity and AFM," Langmuir 24, 3452–3458
Microcrystalline Cellulose," J. Pharm. Pharmacol. 40,
674–678 (1988).
Jean, B., L. Heux, F. Dubreuil, G. Chambat and F. Cousin,
Filson, P. B., B. Dawson-Andoh and D. Schwegler-Berry,
"Non-Electrostatic Building of Biomimetic
"Enzymatic-Mediated Production of Cellulose
Cellulose–Xyloglucan Multilayers," Langmuir 25, 3920–3923
Nanocrystals From Recycled Pulp," Green Chem. 11,
1808–1814 (2009).
Jiang, B., C. Liu, C. Zhang, B. Wang and Z. Wang, "The Effect of
Fleming, K., D. G. Gray and S. Matthews, "Cellulose
Non-Symmetric Distribution of Fiber Orientation and Aspect
Crystallites," Chem. Eur. J. 7, 1831–1836 (2001).
Ratio on Elastic Properties of Composites," Composites B 38,
Fleming, K., D. G. Gray, S. Prasannan and S. Matthews,
24–34 (2007).
"Cellulose Crystallites: A New and Robust Liquid Crystalline
Kim, J., G. Montero, Y. Habibi, J. P. Hinestroza, J. Genzer, D. S.
Medium for the Measurement of Residual Dipolar Couplings,"
Argyropoulos and O. J. Rojas, "Dispersion of Cellulose
J. Am. Chem. Soc. 122, 5224–5225 (2000).
Crystallites by Nonionic Surfactants in a Hydrophobic
Gouss´e, C., H. Chanzy, G. Excoffier, L. Soubeyrand and E.
Polymer Matrix," Polym. Eng. Sci. 49, 2054–2061 (2009).
Fleury, "Stable Suspensions of Partially Silylated Cellulose
Kimura, F., T. Kimura, M. Tamura, A. Hirai, M. Ikuno and F.
Whiskers Dispersed in Organic Solvents," Polymer 43,
Horii, "Magnetic Alignment of the Chiral Nematic Phase of a
2645–2651 (2002).
Cellulose Microfibril Suspension," Langmuir 21, 2034–2037
Grunert, M. and W. T. Winter, "Nanocomposites of Cellulose
Acetate Butyrate Reinforced With Cellulose Nanocrystals," J.
Kleinebudde, P., "The Crystallite-Gel-Model for Microcrystalline
Polym. Environ. 10, 27–30 (2002).
Cellulose in Wet Granulation, Extrusion, and
Ha, C. S. and J. A. Gardella, "Surface Chemistry of
Spheronisation," Pharm. Res. 14, 804–809 (1997).
Biodegradable Polymers for Drug Delivery Systems," Chem.
Kovacs, T., V. Naish, B. O'Connor, C. Blaise, F. Gagne, L. Hall, V.
Rev. 105, 4205–4232 (2005).
Trudeau and P. Martel, "An Ecotoxicological Characterisation
Habibi, Y. and A. Dufresne, "Highly Filled Bionanocomposites
of Nanocrystalline Cellulose (NCC)," Nanotoxicol. 4(3),
from Functionalised Polysaccharide Nanocrystals,"
255–270 (2010).
Biomacromolecules 9, 1974–1980 (2008).
Kresge, C. T., M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S.
Habibi, Y., H. Chanzy and M. Vignon, "TEMPO-Mediated
Beck, "Ordered Mesoporous Molecular Sieves Synthesised by
Surface Oxidation of Cellulose Whiskers," Cellulose 13,
a Liquid-Crystal Template Mechanism," Nature 359, 710–712
679–687 (2006).
Habibi, Y., L. Foulon, V. Agui´e-B´eghin, M. Molinari and R.
Lin, N., G. Chen, J. Huang, A. Dufresne and P. R. Chang,
Douillard, "Langmuir-Blodgett Films of Cellulose
"Effects of Polymer-Grafted Natural Nanocrystals on the
Nanocrystals: Preparation and Characterisation," J. Colloid
Structure and Mechanical Properties of Poly(lactic acid): A
Interface Sci. 316, 388–397 (2007).
Case of Cellulose Whisker-Graft-Polycaprolactone," J. Appl.
Habibi, Y., A. Goffin, N. Schiltz, E. Duquesne, P. Dubois and A.
Polym. Sci. 113, 3417–3425 (2009).
Dufresne, "Bionanocomposites Based on
Ljungberg, N., C. Bonini, F. Bortolussi, C. Boisson, L. Heux and
Poly(ε-caprolactone)-Grafted Cellulose Nanocrystals by
J. Y. Cavaill´e, "New Nanocomposite Materials Reinforced
Ring-Opening Polymerisation," J. Mater. Chem. 18,
with Cellulose Whiskers in Atactic Polypropylene: Effect of
Surface and Dispersion Characteristics," Biomacromolecules
Habibi, Y., T. Heim and R. Douillard, "AC Electric Field-Assisted
6, 2732–2739 (2005).
Assembly and Alignment of Cellulose Nanocrystals," J.
Magalh˜aes, W. L. E., X. Cao and L. A. Lucia, "Cellulose
Polym. Sci. B: Polym. Phys. 46, 1430–1436 (2008a).
Habibi, Y., I. Hoeger, S. S. Kelley and O. J. Rojas, "Development
Assemblies," Langmuir 25, 13250–13257 (2009).
of Langmuir–Schaeffer Cellulose Nanocrystal Monolayers and
Mahmoud, K. A., K. B. Male, S. Hrapovic and J. H. T. Luong,
Their Interfacial Behaviours," Langmuir 26, 990–1001
"Cellulose Nanocrystal/Gold Nanoparticle Composite as a
Matrix for Enzyme Immobilisation," ACS Appl. Mater.
Habibi, Y., L. A. Lucia and O. J. Rojas, "Cellulose Nanocrystals:
Interfaces 1, 1383–1386 (2009).
Chemistry, Self-Assembly, and Applications," Chem. Rev. 110,
Mangalam, A. P., J. Simonsen and A. S. Benight,
"Cellulose/DNA Hybrid Nanomaterials," Biomacromolecules
Hasani, M., E. D. Cranston, G. Westman and D. G. Gray,
10, 497–504 (2009).
"Cationic Surface Functionalisation of Cellulose
Marchessault, R. H., G. Bremner and G. Chauve, "Fishing for
Nanocrystals," Soft Matter 4, 2238–2244 (2008).
Proteins with Magnetic Cellulosic Nanocrystals," in
14 THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING
VOLUME 9999, 2011
"Polysaccharides for Drug Delivery and Pharmaceutical
Revol, J.-F., L. Godbout, X. M. Dong, D. G. Gray, H. Chanzy and
Applications," American Chemical Society, Washington DC
G. Maret, "Chiral Nematic Suspensions of Cellulose
(2006), pp. 3–17.
Crystallites; Phase-Separation and Magnetic-Field
Miller, A. F. and A. M. Donald, "Imaging of Anisotropic
Orientation," Liquid Crystals 16, 127–134 (1994).
Cellulose Suspensions Using Environmental Scanning
Rojas, O. J., G. A. Montero and Y. Habibi, "Electrospun
Electron Microscopy," Biomacromolecules 4, 510–517 (2003).
Nanocomposites From Polystyrene Loaded With
Montanari, S., M. Roumani, L. Heux and M. R. Vignon,
Cellulose Nanowhiskers," J. Appl. Polym. Sci. 113, 927–935
"Topochemistry of Carboxylated Cellulose Nanocrystals
Resulting From TEMPO-Mediated Oxidation,"
Roman, M. and W. T. Winter, "Effect of Sulfate Groups from
Macromolecules 38, 1665–1671 (2005).
Sulfuric Acid Hydrolysis on the Thermal Degradation
Morandi, G., L. Heath and W. Thielemans, "Cellulose
Behaviour of Bacterial Cellulose," Biomacromolecules 5,
Nanocrystals Grafted With Polystyrene Chains Through
1671–1677 (2004).
Surface-Initiated Atom Transfer Radical Polymerisation
Roman, M., S. P. Dong, H. Anjali and Y. W. Lee, "Cellulose
(SI-ATRP)," Langmuir 25, 8280–8286 (2009).
Nanocrystals for Drug Delivery," in "Polysaccharide
Nakagaito, A. and H. Yano, "Toughness Enhancement of
Materials: Performance by Design," American Chemical
Cellulose Nanocomposites by Alkali Treatment of the
Society, Washington DC (2010), pp. 81–91.
Reinforcing Cellulose Nanofibers," Cellulose 15, 323–331
Roy, D., M. Semsarilar, J. T. Guthrie and S. Perrier, "Cellulose
Modification by Polymer Grafting: A Review," Chem. Soc.
Nogi, M., K. Handa, A. N. Nakagaito and H. Yano, "Optically
Rev. 38, 2046–2064 (2009).
Transparent Bionanofiber Composites With Low Sensitivity to
Saito, T. and A. Isogai, "TEMPO-Mediated Oxidation of Native
Refractive Index of the Polymer Matrix," Appl. Phys. Lett. 87,
Cellulose. The Effect of Oxidation Conditions on Chemical
and Crystal Structures of the Water-Insoluble Fractions,"
Oksman, K., A. P. Mathew, D. Bondeson and I. Kvien,
Biomacromolecules 5, 1983–1989 (2004).
"Manufacturing Process of Cellulose Whiskers/Polylactic
Saito, T., M. Hirota, N. Tamura and A. Isogai, "Oxidation of
Acid Nanocomposites," Composites Sci. Technol. 66,
Bleached Wood Pulp by TEMPO/NaClO/NaClO2 System:
2776–2784 (2006).
Effect of the Oxidation Conditions on Carboxylate Content
Ozg ¨ur Seydibeyo ˘glu, M. and K. Oksman, "Novel
and Degree of Polymerisation," J. Wood Sci. 56, 227–232
Nanocomposites Based on Polyurethane and Micro Fibrillated
Cellulose," Composites Sci. Technol. 68, 908–914 (2008).
Samir, M. A. S. A., F. Alloin and A. Dufresne, "Review of Recent
Pan, J., W. Hamad and S. K. Straus, "Parameters Affecting the
Research into Cellulosic Whiskers, Their Properties and Their
Chiral Nematic Phase of Nanocrystalline Cellulose Films,"
Application in Nanocomposite Field," Biomacromolecules 6,
Macromolecules 43, 3851–3858 (2010).
612–626 (2005).
Park, W.-I., M. Kang, H.-S. Kim and H.-J. Jin, "Electrospinning
Samir, M. A. S. A., F. Alloin, J. Sanchez and A. Dufresne,
of Poly(ethylene oxide) with Bacterial Cellulose Whiskers,"
"Cross-Linked Nanocomposite Polymer Electrolytes
Macromol. Symp. 249–250, 289–294 (2007).
Reinforced With Cellulose Whiskers," Macromolecules 37,
Peresin, M. S., Y. Habibi, J. O. Zoppe, J. J. Pawlak and O. J.
Rojas, "Nanofiber Composites of Polyvinyl Alcohol and
Samir, M. A. S. A., F. Alloin, W. Gorecki, J. Sanchez and A.
Cellulose Nanocrystals: Manufacture and Characterisation,"
Dufresne, "Nanocomposite Polymer Electrolytes Based on
Biomacromolecules 11, 674–681 (2010).
Poly(oxyethylene) and Cellulose Nanocrystals," J. Phys.
Podsiadlo, P., S. Choi, B. Shim, J. Lee, M. Cuddihy and N. A.
Chem. B 108, 10845–10852 (2004b).
Kotov, "Molecularly Engineered Nanocomposites:
Samir, M. A. S. A., A. M. Mateos, F. Alloin, J. Sanchez and A.
Layer-by-Layer Assembly of Cellulose Nanocrystals,"
Dufresne, "Plasticized Nanocomposite Polymer Electrolytes
Biomacromolecules 6, 2914–2918 (2005).
Based on Poly(oxyethylene) and Cellulose Whiskers,"
Pranger, L. and R. Tannenbaum, "Biobased Nanocomposites
Electrochim. Acta 49, 4667–4677 (2004c).
Prepared by In Situ Polymerisation of Furfuryl Alcohol with
Sassi, J. and H. Chanzy, "Ultrastructural Aspects of the
Cellulose Whiskers or Montmorillonite Clay,"
Acetylation of Cellulose," Cellulose 2, 111–127 (1995).
Macromolecules 41, 8682–8687 (2008).
Schroers, M., A. Kokil and C. Weder, "Solid Polymer Electrolytes
R˚anby, B.G., "The Colloidal Properties of Cellulose Micelles,"
Based on Nanocomposites of Ethylene Oxide-Epichlorohydrin
Discussions. Faraday Soc. 11, 158–164 (1951).
Copolymers and Cellulose Whiskers," J. Appl. Polym. Sci. 93,
Revol, J.-F., "On the Cross-Sectional Shape of Cellulose
2883–2888 (2004).
Crystallites in Valonia Ventricosa," Carbohydr. Polym. 2,
Shi, H. G., L. Farber, J. N. Michaels, A. Dickey, K. C. Thompson,
123–134 (1982).
S. D. Shelukar, P. N. Hurter, S. D. Reynolds and M. J.
Revol, J.-F., H. Bradford, J. Giasson, R. H. Marchessault and D.
Kaufman, "Characterisation of Crystalline Drug Nanoparticles
G. Gray, "Helicoidal Self-Ordering of Cellulose Microfibrils in
Using Atomic Force Microscopy and Complementary
Aqueous Suspension," Int. J. Biol. Macromol. 14, 170–172
Techniques," Pharm. Res. 20, 479–484 (2003).
Shimazaki, Y., Y. Miyazaki, Y. Takezawa, M. Nogi, K. Abe, S.
Revol, J.-F., L. Godbout and D. G. Gray, "Solid Films of Cellulose
Ifuku and H. Yano, "Excellent Thermal Conductivity of
With Chiral Nematic Order and Optically Variable Properties,"
Transparent Cellulose Nanofiber/Epoxy Resin
PPR 1331 report (1998).
Nanocomposites," Biomacromolecules 8, 2976–2978 (2007).
Revol, J.-F., L. Godbout and D. G. Gray, "Solidified Liquid
Shin, Y. and G. J. Exarhos, "Template Synthesis of Porous Titania
Crystals of Cellulose with Optically Variable Properties," U.S.
Using Cellulose Nanocrystals," Mater. Lett. 61, 2594–2597
Patent No. 5,629,055 (1997).
VOLUME9999,2011
THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING 15
Shin, Y., I. Bae, B. W. Arey and G. J. Exarhos, "Facile
Zhou, Q., H. Brumer and T. T. Teeri, "Self-Organisation of
Stabilisation of Gold–Silver Alloy Nanoparticles on Cellulose
Cellulose Nanocrystals Adsorbed with Xyloglucan
Nanocrystal," J. Phys. Chem. C 112, 4844–4848 (2008).
Shin, Y., I. Bae, B. W. Arey and G. J. Exarhos, "Simple
Copolymer," Macromolecules 42, 5430–5432 (2009).
Preparation and Stabilisation of Nickel Nanocrystals on
Zhou, Y., E. Ding and W. Li, "Synthesis of TiO2 Nanocubes
Cellulose Nanocrystal," Mater. Lett. 61, 3215–3217 (2007).
Induced by Cellulose Nanocrystal (CNC) at Low
Shopsowitz, K. E., H. Qi, W. Y. Hamad and M. J. MacLachlan,
Temperature," Mater. Lett. 61, 5050–5052 (2007).
"Free-Standing Mesoporous Silica Films With Tunable Chiral
Zoppe, J. O., M. S. Peresin, Y. Habibi, R. A. Venditti and O. J.
Nematic Structures," Nature 468, 422–426 (2010).
Rojas, "Reinforcing Poly(ε-caprolactone) Nanofibers With
Siqueira, G., J. Bras and A. Dufresne, "New Process of Chemical
Cellulose Nanocrystals," ACS Appl. Mater. Interfaces 1,
Grafting of Cellulose Nanoparticles with a Long Chain
1996–2004 (2009).
Isocyanate," Langmuir 26, 402–411 (2010).
Stroobants, A., H. N. W. Lekkerkerker and T. Odijk, "Effect of
Electrostatic Interaction on the Liquid Crystal Phase
Manuscript received October 13, 2010; revised manuscript
Transition in Solutions of Rodlike Polyelectrolytes,"
received January 25, 2011; accepted for publication February 3,
Macromolecules 19, 2232–2238 (1986).
Sugiyama, J., H. Chanzy and G. Maret, "Orientation of Cellulose
Microcrystals by Strong Magnetic Fields," Macromolecules
25, 4232–4234 (1992).
Terech, P., L. Chazeau and J. Y. Cavaille, "A Small-Angle
Scattering Study of Cellulose Whiskers in Aqueous
Suspensions," Macromolecules 32, 1872–1875 (1999).
Van Eerdenbrugh, B., S. Vercruysse, J. A. Martens, J. Vermant, L.
Froyen, J. van Humbeeck, G. van den Mooter and P.
Augustijns, "Microcrystalline Cellulose, a Useful Alternative
for Sucrose as a Matrix Former During Freeze-Drying of Drug
Nanosuspensions – A Case Study With Itraconazole," Eur. J.
Pharm. Biopharm. 70, 590–596 (2008).
Wang, J. and K. Matyjaszewski, "Controlled/Living Radical
Polymerisation. Atom Transfer Radical Polymerisation in the
Presence of Transition-Metal Complexes," J. Am. Chem. Soc.
117, 5614–5615 (1995).
Wang, N., E. Ding and R. Cheng, "Preparation and Liquid
Crystalline Properties of Spherical Cellulose Nanocrystals,"
Langmuir 24, 5–8 (2008).
Wong, K. K. H., M. Zinke-Allmang, J. L. Hutter, S. Hrapovic, J.
H. T. Luong and W. Wan, "The Effect of Carbon Nanotube
Aspect Ratio and Loading on the Elastic Modulus of
Electrospun Poly(vinyl alcohol)-Carbon Nanotube Hybrid
Fibers," Carbon 47, 2571–2578 (2009).
Xu, Q., J. Yi, X. Zhang and H. Zhang, "A Novel Amphotropic
Polymer Based on Cellulose Nanocrystals Grafted With Azo
Polymers," Eur. Polym. J. 44, 2830–2837 (2008).
Yi, J., Q. Xu, X. Zhang and H. Zhang, "Chiral-Nematic
Self-Ordering of Rodlike Cellulose Nanocrystals Grafted With
Poly(styrene) in Both Thermotropic and Lyotropic States,"
Polymer 49, 4406–4412 (2008).
Yi, J., Q. Xu, X. Zhang and H. Zhang, "Temperature-Induced
Chiral Nematic Phase Changes of Suspensions of
Poly(N,N-dimethylaminoethyl methacrylate)-Grafted
Cellulose Nanocrystals," Cellulose 16, 989–997 (2009).
Yuan, H., Y. Nishiyama, M. Wada and S. Kuga, "Surface
Acylation of Cellulose Whiskers by Drying Aqueous
Emulsion," Biomacromolecules 7, 696–700 (2006).
Zhang, J., T. J. Elder, Y. Pu and A. J. Ragauskas, "Facile
Synthesis of Spherical Cellulose Nanoparticles," Carbohydr.
Polym. 69, 607–611 (2007).
Zhang, X. L., J. Huang, P. R. Chang, J. L. Li, Y. M. Chen, D. X.
Wang, J. H. Yu and J. H. Chen, "Structure and Properties of
Polysaccharide Nanocrystal-Doped Supramolecular Hydrogels
Based on Cyclodextrin Inclusion," Polymer 51, 4398–4407
(2010).
16 THE CANADIAN JOURNAL OF CHEMICAL ENGINEERING
VOLUME 9999, 2011
Source: http://www.arboranano.ca/pdfs/chemistry%20and%20applications%20of%20nanocrystalline%20cellulose%20and%20its%20derivatives%20a%20nanotechnology%20perspective-2011.pdf
Fax: 1-877-448-5539 359 Johnson Ave. West Unit E Winnipeg, MB, Canada, R2L 0J2 www.youdrugstore.com [email protected] Patient Order Form YOUDRUGSTORE.COM PATIENT MEDICAL PROFILE Please fax these forms toll free to 1-877-448-5539- *** Note: Your original prescription(s) must be mailed to us unless they are faxed from your
F-MELT series, Issue2, February 2011 Formulating Taste Masked and High Quality ODT of Poorly Water Soluble Drugs In this paper, we present case studies with taste masking technology and application of wet granulation technologies to develop ODTs of Loratadine, a water insoluble drug, and Famotidine, a slightly water





