Effectiveness of topical corticosteroids in addition to antiviral therapy in the management of recurrent herpes labialis: a systematic review and meta-analysis
Arain et al. BMC Infectious Diseases (2015) 15:82 DOI 10.1186/s12879-015-0824-0
Effectiveness of topical corticosteroids inaddition to antiviral therapy in the managementof recurrent herpes labialis: a systematic reviewand meta-analysis
Nasira Arain1, Sharath CV Paravastu1 and Mubashir A Arain2*
Background: Recurrent herpes labialis (RHL) is one of the most common viral infections worldwide. The availabletreatments have limited efficacy in preventing the recurrence of ulcerative lesions and reducing the duration ofillness. The objective of this review was to identify the effectiveness of topical corticosteroids in addition to antiviraltherapy in the treatment of RHL infection.
Methods: A systematic review of randomized clinical trials comparing the efficacy of combined therapy (topicalcorticosteroids with antiviral) with placebo or antiviral alone in the management of RHL was conducted. MEDLINE,EMBASE, CINAHL, Web of Science, the Cochrane library, and Google Scholar databases were searched. We usedRevMan software to conduct the meta-analysis. A fixed-effects model was used for mild to moderate heterogeneity,whereas a random-effects model was used for significant heterogeneity. Heterogeneity among trials was establishedusing I2 and chi-square test for heterogeneity.
Results: Four studies that fulfilled the selection criteria were included in this review. The total number ofparticipants across included studies was 1,891 (range, 29 to 1,443). The antiviral drugs used were acyclovir,famciclovir, and valacyclovir. Corticosteroids used were 1% hydrocortisone and 0.05% fluocinonide. Pooled resultsshowed that patients receiving combined therapy had a significantly lower recurrence rate of ulcerative lesionscompared to those in both the placebo group (OR, 0.50; 95% CI, 0.39-0.66; P < .001) and the antiviral treatmentalone group (OR, 0.73, 95% CI, 0.58–0.92; P = .007). The healing time was also significantly shorter in combinedtherapy in comparison to placebo (P < .001). However, there were no significant differences in healing timebetween combined therapy and antiviral alone. The adverse reactions in combined therapy were not significantlydifferent than the placebo group (OR, 1.09; 95% C, 0.75-1.59; P = .85).
Conclusion: Treatment with combined therapy is safe and more effective than placebo or antiviral alone forpreventing the recurrence of ulcerative lesions in RHL infection.
Keywords: Herpes labialis, Recurrent herpes infection, Cold sores, Corticosteroids and Herpes infection,Meta-analysis
* Correspondence: 2Faculty of Nursing, University of Calgary, 2500 University Drive NW, T2N1 N4 Calgary, AB, CanadaFull list of author information is available at the end of the article
2015 Arain et al.; licensee BioMed Central. This is an Open Access article distributed under the terms of the CreativeCommons Attribution License which permits unrestricted use, distribution, andreproduction in any medium, provided the original work is properly credited. The Creative Commons Public DomainDedication waiver applies to the data made available in this article,unless otherwise stated.
Arain et al. BMC Infectious Diseases (2015) 15:82
yet in other countries such as the UK. Therefore, it is im-
Herpes labialis infection is a global public health prob-
portant to understand the role of topical corticosteroid
lem, with 15 to 40% of the population who experience
therapy plus anti-viral agent in the management of RHL
symptomatic outbreak Infection rates are high
infection. The purpose of this review was to determine the
among HIV positive patients; about 95% of patients are
effectiveness of topical corticosteroids in addition to anti-
seropositive to herpes simplex type I antigen [In de-
viral therapy (combined therapy) compared to antiviral
veloped countries, one third of the population suffer
therapy alone or placebo in the management of RHL infec-
from recurrent herpes labialis. (RHL) Between 20 to 40%
tion using a systematic review and meta-analysis.
of adults become infected with herpes simplex infectionat some point during their lifetime Detectable serum
antibodies against herpes simplex virus are more preva-
Search methods for identification of studies
lent in lower socioeconomic groups Over the last
We searched electronic databases including MEDLINE
20 years, prevalence has increased globally and the pre-
via ovid, EMBASE, CINAHL, Web of Science, and
vention of RHL poses a big challenge for the 21st cen-
Cochrane Library. Searches were limited to English-
tury. The infection is difficult to eradicate and treatment
language reports of human studies from 2000, which
has minimal impact on reduction and prevention of
marked the first clinical trial conducted on topical corti-
herpes infection. To date, no vaccination has been suc-
costeroids and antiviral therapy on humans, to 2013.
cessful so far in humans to prevent the primary infection
A combination of medical subject headings (MeSH)
and key word searches were used to retrieve the relevant
Herpes simplex labialis (HSL) is a contagious infection
literature in this review. All keywords were entered ei-
that appears as a rash of the skin, usually involving the
ther with "OR" and "AND" boolean operators and were
lips but can affect oral membranes, and is characterized
used with $/* where appropriate.
by blisters with pain and occasional itching There
The following MeSH search terms were used:
are different sequential stages of lesion including pro-
"herpes simplex"; "herpes simplex virus"; "herpes labia-
drome, redness, papule, vesicular, ulcer, hard crust, dry
lis"; herpes labialis viruses"; "recurrences"; "recurrent
flaking, and normal skin (complete epithelisation). Ul-
herpes"; "topical administration"; "anti-infective agents";
cerative lesions take longer time to heal, which can
"topical drug administration"; "corticosteroids"; "steroid";
adversely impact quality of life The diagnosis is typ-
"antiviral agents"; antiviral drugs".
ically based on clinical history and examination; in some
The following key words were used:
cases, however, specific laboratory tests may be required
herpes simplex; herpes labialis; herpes virus; recurrent
The condition is usually mild, but some patients
herpes labialis; prevention of herpes; treatment of herpes
may have severe disease that may affect internal organs
labialis; antiviral for herpes; antiviral + corticosteroid +
or lead to secondary bacterial infections [
herpes; topical antiviral for herpes; topical treatment + her-
Current treatment options for HSL include oral anti-
pes; herpes labialis treatment + antiviral + corticosteroid.
viral drugs, antiviral ointment or other topical applica-
Additional studies were sought through a review of
tions (e.g., zinc oxide, zinc sulphate), and anesthetic
the reference lists of obtained reports and other relevant
creams for symptomatic improvement Treatment
reviews on the topic. Grey literature was also searched
needs to be initiated promptly to achieve favorable re-
using Google Scholar and the ClinicalTrials.gov website
sults. Despite the above mentioned available treatment
options, antivirals are commonly used and can slightlyreduce the duration of herpes lesions by restraining the
Inclusion and exclusion criteria
multiplication of the virus However, treatment with
Original research articles of randomized controlled clin-
antiviral alone is not very effective and in most cases
ical trials (RCTs) or controlled clinical trials comparing
only has a minor effect on the duration of illness. There-
the effectiveness of topical corticosteriods in addition to
fore, researchers have suggested adding corticosteroids
antiviral (combined therapy) versus antiviral alone or
to antiviral agents to increase the responsiveness of le-
placebo for the treatment of RHL were eligible for inclu-
sions because herpes infection also triggers im-
sion. Pilot clinical trial studies with similar interventions
mune response. However, some controversy exists about
and outcomes were also included. We excluded observa-
the addition of corticosteroids, as steroidal contents may
tional studies and studies that evaluated the treatment
worsen the infection by reducing the natural defense
for primary herpes labialis, as well as case reports, con-
system against the infection
ference presentations, and editorials. Studies of healthy
Several countries such as US, Germany, and Netherlands
(12–17 years) and
have approved antiviral with topical corticosteroids for the
adults (≥18 years) with a history of RHL were included,
treatment of HSL However, it has not been licensed
irrespective of gender, socioeconomic status, and race.
Arain et al. BMC Infectious Diseases (2015) 15:82
Two types of interventions were considered for the in-
with 95% confidence intervals (CI). P < .05 was con-
clusion: (1) combined therapy versus antiviral therapy
sidered significant. For continuous outcomes, mean
alone; or (2) combined therapy versus placebo. The pri-
differences between the groups along with 95% CIs
mary outcomes of interest were the development of clas-
were measured.
sical lesions and reduction in pain. Secondary outcomes
The meta-analysis of continuous data requires means
were healing time, adverse events, and reduction in size
with variance (or SD) to pool data. Outcomes of con-
of the lesions.
tinuous variables were transformed from medians with
All authors were involved in determining inclusion.
interquartile ranges (IQR) to means with SD, using thefollowing formulas
Data collection and analysisData collection and analysis was performed using Review
1. Conversion of median into mean: χ ≈ a + 2m + b/4
Manager (RevMan Version 5.2, Copenhagen: The Nordic
2. Conversion of range into variance: S = b-a/4 = R/4
Cochrane Centre, the Cochrane Collaboration, 2012).
Data from the studies were extracted using data extrac-
[X = mean, a = smallest value, b = largest value, m = me-
tion form. Risk of bias was assessed using the following
dian, S = variance, R = range].
key domains: randomization, allocation concealment,
A fixed-effects model was used for mild to moderate
sample size, blinding (single, double or triple), and attri-
heterogeneity, whereas random-effects model was used
tion rate ].
for those outcomes when the chi-square test for het-
Odd ratios (ORs) were used for dichotomous out-
erogeneity was significant (P < .10) and I2 was higher
comes to measure the strength of association along
Records identified through
Additional records identified
database searching
through other sources
Records after duplicates removed
Record titles screened
Title not according to
inclusion criteria: not human
subjects, other infections, not
focusing on treatment
Record abstracts screened
-Not an original article-Abstracts were not fulfilling
our inclusion criteria
-Treatment not combined
with hydrocortisone
- Inappropriate study design
Full-text articles assessed
Full-text articles excluded
-Study with children <12
-Study with unhealthy
population(n = 1)
Studies included in
- No comparison condition
quantitative synthesis
-Other interventions (n = 2)
- Unpublished study with
unavailable data (n = 1)
Figure 1 PRISMA flow diagram.
Arain et al. BMC Infectious Diseases (2015) 15:82
studies was 1,892 (range, 29 to 1,443). All four selected
Description of the selected studies
studies appropriately reported the process of random-
A total of 9,450 studies were originally identified, and 4
isation. Most of the randomisation achieved 1:1 treat-
were included in the final review (see Figure for
ment to control ratios whilst Hull et al. (2010)
PRISMA flow diagram). All 4 selected studies
performed 2.7:1 for the treatment and placebo group
were RCTs (see Table for study characteristics). Hull
respectively Allocation concealment was also re-
et al. 2010 was a RCT and topical corticosteroids
ported in all four selected studies. Therefore, a mini-
plus antiviral in the management of RHL was evaluated.
mum risk of selection bias was expected in the selected
A total of 89 patients were recruited. There were cross-
studies as a result of allocation concealment. The par-
overs and there were no losses to follow up. Evans et al.
ticipants as well as personnel involved in providing care
2002 conducted a trial on 380 participants to evalu-
(medication) were blinded in all four selected studies.
ate the effect of ME-609 (topical corticosteroid with
All studies reported the use of identical looking sub-
antiviral) in comparison to placebo. Hull et al. 2009
stances in both group in similar packing and labels.
was a small trial on 39 participants in which valacyclovir
Two studies clearly reported the blinding of personnel
and clobetasol gel were compared with placebo. Spruance
assessing the outcome On the other hand, it
et al. 2000 conducted a trial on 29 participants where
was unclear in two studies ]. The intention to
topical corticosteroid with valacyclovir 2gm was compared
treat analysis was used in all four studies and risk of
with antiviral alone. The antiviral used was acyclovir in 2
bias was minimal in relation to drop outs and loss to
studies [famciclovir and valacyclovir in each of the
follow up patients' data.
other 2 studies ]. The average number of episodes ofthe RHL in the participants ranged from 4.5 to 5.6
Effects of interventions
episodes per year.
The development of the ulcerative lesions
The risk of bias was assessed in the selected studies.
Three studies reported on this outcome -]. There
The total number of participants included in the
was no significant heterogeneity (I2 = 20%) found amongst
Table 1 Characteristics of randomized controlled trials included in the review
Participants (n) Groups (n)
Male, n (%) Frequency of RHL
(mean episodes/year)
Hull et al. 2010 51 sites in the United
States and 4 sites in
1. ME-609 cream (5% Acyclovir, 1%
Canada (July 2006 to
Hydrocortisone) (n = 601)
1. Acyclovir (5% in ME-609 vehicle)
2. Placebo (vehicle) (n = 232)
Evans et al.
4 major university clinics 380
1. ME-609 cream (5% Acyclovir,
1% Hydrocortisone) (n = 190)
1. Placebo (n = 190)
Hull et al. 2009 University of Utah
(August 2004 to March
1. Oral Valacyclovir (2 g 2×/day for 1 day)
plus topical Clobetasol gel (0.05% 2×/dayfor 3 days) (n = 20)
1. Placebo (n = 19)
Spruance et al.
University of Utah
Health Sciences Center,
1. Oral Famciclovir (Famvir, 500 mg 3×/day
for 5 days) plus topical Fluocinonide (0.05%Lidex Gel 3×/day for 5 days) (n = 17)
1. Famciclovir and topical vehicle control
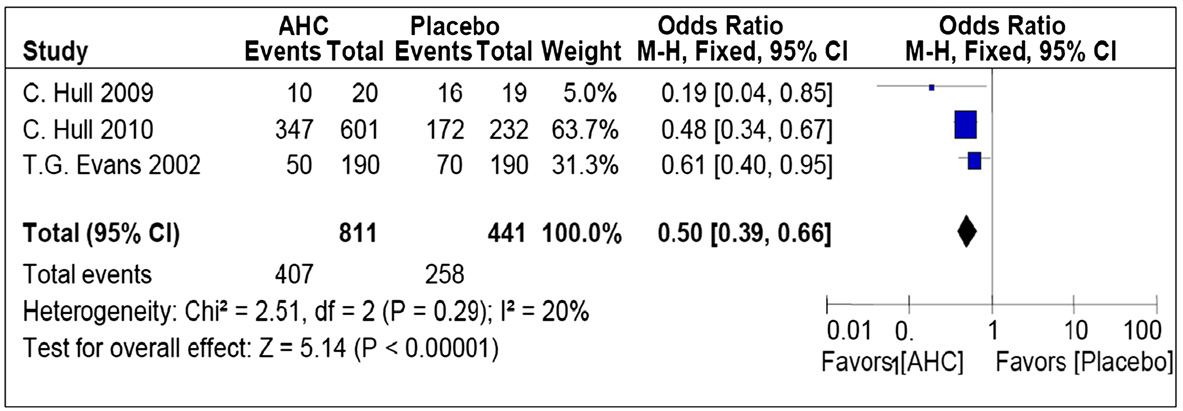
Arain et al. BMC Infectious Diseases (2015) 15:82
trials (Figure . The chi-square test for heterogeneity was
magnitude was observed for reduction in pain and ten-
not significant (P = .29). Meta-analysis showed a statisti-
derness when the combine therapy was compared with
cally significant reduction in the development of ulcerative
antiviral alone. There was a significant heterogeneity
lesions, demonstrating that the odds of the occurrence of
(I2 = 67%) between the 2 studies in this analysis. The
ulcerative lesions were 50% less likely in the intervention
overall effect using the pooled data of 618 participants
group as compared to placebo (OR, 0.50; 95% CI, 0.39-
showed no significant difference in pain between topical
0.66; P < .001).
corticosteroid plus antiviral group compared to antiviral
Similarly, the comparison of combined therapy with
alone (OR, 0.32; 95%CI, 0.03- 3.76; P = .37).
antiviral alone also showed a significant reduction in thedevelopment of ulcerative lesions in the intervention
Adverse reactions
arm as compared to the control (Figure (OR, 0.73;
The most common adverse reactions appeared were dry-
95% CI, 0.58-0.92; P = .007). There was moderate hetero-
ness, irritation, and stinging symptoms, which were re-
geneity among trials (I2 = 56%). Chi-square test for het-
lated to topical application and not specifically due to
erogeneity was insignificant (P = .13).
the corticosteroid component. Studies showed no signifi-cant difference in proportion of adverse reaction ob-
Healing time for ulcerative lesions (complete epithelisation)
served between the intervention and the placebo group.
The meta-analysis of 407 patients showed a significant
There was no heterogeneity between the selected studies
reduction of 1.49 days in the healing time for ulcerative
(I2 = 0%). The overall effect also showed no significant
lesions in the combine therapy group in compared to
difference between the intervention and the placebo
the placebo group (Figure (95% CI, −1.99 to −0.98;
group (OR, 1.09; 95% CI, 0.75-1.59; P = .65).
P < .001). This outcome was observed on the based of
The odds of the occurrence of adverse reactions were
the healing time from the first sign of lesion until
37% more likely in the antiviral alone as compared to
complete epithelisation. All studies showed significant
the combine therapy group; however, the effect was not
reduction in healing time in the intervention group
statistically significant (OR, 1.37; 95% CI, 0.97-1.95,
compared to placebo and there was a moderate hetero-
P = .08). Heterogeneity between the two groups was not
geneity between the studies (I2 = 53%).
statistically significant (I2 = 28%).
No significant difference was found in the healing time
for ulcerative lesions between the combined therapy and
the treatment with antiviral alone (mean difference = −1.68;
To date, this is the first meta-analysis to determine the
95% CI, −4.52 to 1.16) in the pooled data of 357 patients
effect of adding topical corticosteroids in the treatment
(Figure ). There was a highly significant heterogeneity be-
of RHL. The results of this review show that addition of
tween the selected studies (I2 = 93%), so the random effect
topical corticosteroids to antiviral therapy has significant
model was used.
benefit compared to either antiviral therapy alone or pla-cebo. The review was conducted on RCTs only, which
The effect of treatment on pain or tenderness in the lesion
helped in producing rigorous findings about the effect-
In the comparison of topical corticosteroid plus antiviral
iveness of adding topical corticosteroids in the treatment
with placebo, two of the three studies did not show any
significant reduction in pain or tenderness in the inter-
This review indicates that the chance of developing ul-
vention group as compared to the placebo. However, the
cerative lesions is reduced in patients who receive topical
overall effect revealed a significant reduction (OR, 0.59;
corticosteroids in addition to antiviral treatment in com-
95% CI, 0.45-0.77; P < .001). An effect of a smaller
parison to placebo or antiviral treatment alone. Evidence
Figure 2 Forest plot of comparison: topical corticosteroid plus antiviral group versus placebo, outcome: Pooled odds ratio of developmentof ulcerative lesions.
Arain et al. BMC Infectious Diseases (2015) 15:82
Figure 3 Forest plot of comparison: topical corticosteroid plus antiviral group versus antiviral alone, outcome: Pooled odds ratio fordevelopment of ulcerative lesions.
shows that the treatment with antiviral alone decreases
ranging from 0.5 to 2 days ]. Treatment with topical
the duration of ulcerative lesions in herpes labialis ,].
penciclovir alone reduced healing time by 0.5 days in
However, in most cases antiviral treatment alone does not
two clinical trials of 3,057 and 1,573 patients
prevent the development of ulcerative lesion ].
Oral antiviral treatment with famciclovir has shown a re-
Pain is another important determinant of the morbid-
duction of approximately 2 days in healing time
ity in RHL A 24% reduction in the development of
Another trial with valacyclovir 2 grams on 1,524 patients
pain in the lesion was an important outcome observed.
found a median reduction in healing time by 1.5 days
One study reported a 26% faster improvement in pain
which is comparable to the reduction in the healing
among those patients who received topical penciclovir
time with topical corticosteroids plus antiviral demon-
than those who received a placebo However, a re-
strated in this review.
view of 10 antiviral studies showed that none of them
There are a number of limitations of this study. First,
had any significant effect on pain reduction Fur-
the meta-analysis in this study was conducted on a small
thermore, topical anesthetic treatment alone also did not
number of studies, which is likely attributable to the lim-
show any significant impact on pain reduction
ited availability of topical corticosteroid plus antiviral
Photodynamic therapy is effective in relieving pain in
treatment. The statistical tests to identify publication
bias could not be performed due to the small number of
The adverse reactions were lower in corticosteroids
studies in the review. Second, the selected studies used
group than those appeared in the antiviral group alone.
different form and type of antivirals (oral and topical).
A non-randomized clinical trial to determine the safety
The topical antiviral agents that are most commonly rec-
and tolerance of topical corticosteroids plus antiviral in
ommended to treat RHL include acyclovir 5% cream,
adolescents found that the treatment was well tolerated
penciclovir 1% cream and docosanol 10% cream. Most
and safe Studies on animals have also shown that
of these preparations need to be applied every 2 hours
the treatment with acyclovir with hydrocortisone is su-
from the time of prodrome until complete healing
perior to antiviral alone without any significant adverse
Unlike topical agents, systemic medications enable
greater drug exposure, rapid access to site of viral repli-
The healing time was significantly reduced (around
cation, better biocompatibility, less frequent dosing, and
1.5 days) in the treatment group in comparison to pla-
improved compliance. Systemic antiviral agents may be
cebo. A review on topical acyclovir revealed that 9 of 13
administered orally or intravenously. Acyclovir has a
studies did not show any significant effect on healing
short half-life and multiple doses are required to main-
time, while 4 studies demonstrated significant reductions
tain an optimum drug levels in serum. Famciclovir and
Figure 4 The comparison of effect of corticosteroid plus antiviral and placebo on the healing time for ulcerative lesions from the firstsign until complete epithelisation.
Arain et al. BMC Infectious Diseases (2015) 15:82
Figure 5 The comparison of effect of corticosteroid plus antiviral and antiviral alone on the healing time for ulcerative lesions fromthe first sign until complete epithelisation.
valacyclovir have greater bioavailability and are more
Received: 1 September 2014 Accepted: 10 February 2015
convenient for patients [The reduction in healingtime for oral antiviral treatment is generally higher than
topical antivirals; this may have had an impact on the out-
Fatahzadeh M, Schwartz R. Human herpes simplex virus infections:
comes of this study. Finally, 2 studies used ultraviolet-
epidemiology, pathogenesis, symptomatology, diagnosis, and management.
J Am Acad Dermatol. 2007;57(5):737–63. Available at:
induced cold sores, which may lead to more severe
[Accessed: 15th June 2014].
outbreaks. Ultraviolet light is known to be a stimulus for
Skulason S, Peter Holbrook W, Thormar H, Gunnarsson G, Kristmundsdottir T.
the reactivation of herpes simplex virus. Ultraviolet-
A study of the clinical activity of a gel combining monocaprin anddoxycycline: a novel treatment for herpes labialis. J Oral Pathol Med.
induced cold sores may develop very rapidly ]. How-
ever, in this study, we found that the combination therapy
Worrall, G. Herpes labialis. BMJ Clin Evid. 2009 Sep 23;2009. Available at:
was equally effective for the ultraviolet-induced cold sores.
[Accessed: 19th June 2014].
Xu F, Sternberg M, Kottiri B, Mcquillan G, Lee F, Nahmias A, et al. Trends inHerpes Simplex Virus Type 1 and Type 2 Seroprevalence in the United
States. JAMA. 2006;296(8):964–73. Available at: [Accessed: 5th July 2014].
The addition of the steroidal component along with an-
Field H, Vere Hodge R. Recent developments in anti-herpesvirus drugs. Br
tivirals improves treatment of RHL. Adverse reactions
Med Bull. 2013;106:213–49.
are lower in topical corticosteroid plus antivirals treat-
Scoular A, Norrie J, Gillespie G, Mir N, Carman W. Longitudinal study ofgenital infection by herpes simplex virus type 1 in Western Scotland over
ment than treatment with antiviral alone. Thus, the
15 years. Br Med J. 2002;324(7350):1366–13667. Available at:
treatment with topical corticosteroid plus antiviral alone
[Accessed: 20th June 2014].
is safe and more effective in preventing the development
Harmenberg J, Oberg B, Spruance S. Prevention of ulcerative lesions byepisodic treatment of recurrent herpes labialis: A literature review. Acta
of ulcerative lesions than antiviral alone or placebo. The
Derm Venereol. 2010;90(2):122–30. Available at:
healing time may not be further reduced with topical
[Accessed: 5th Aug 2013].
corticosteroid plus antiviral than with antiviral treatment
Arduino P, Porter S. Herpes Simplex Virus Type 1 infection: overview onrelevant clinico-pathological features. J Oral Pathol Med. 2008;37(2):107–21.
Available at: [Accessed:2nd Feb 2013].
Worrall G. Herpes labialis. Clin Evid. 2005;14:2050–7.
Competing interests
Opstelten W, Neven A, Eekhof J. Treatment and prevention of herpes
The authors declare that they have no competing interests.
labialis. Can Fam Physician. 2008;54(12):1683–7. Available at: [Accessed: 9th Apr 2013].
Authors' contributions
Raborn G, Grace M. Recurrent herpes simplex labialis: selected therapeutic
NA and SP planned the overall study. MA participated in data collection and
options. J Can Dental Assoc. 2003;69(8):498–503. Available at:
reviewing papers for the study. NA, MA and SP performed data analysis. All
authors read and approved the final manuscript.
[Accessed: 17th June 2013].
Hull C, Brunton S. The role of topical 5% acyclovir and 1% hydrocortisonecream (Xerese™) in the treatment of recurrent herpessimplex labialis.
Authors' information
Postgrad Med. 2011;122(5):1–6. Available at:
NA (MBBS, MPH) is a Postgraduate student at the University of Sheffield, UK.
[Accessed: 4th Feb 2013].
SP (MBBS, MRCS, PGDip) is a Lecturer at the School of Health and Related
Strand A, Böttiger D, Gever L, Wheeler W. Safety and tolerability of
Research, Section of Health Economics and Decision Science, University of
combination acyclovir 5% and hydrocortisone 1% cream in adolescents
Sheffield, UK. MA (MBBS, MPH, PhD) is a Postdoctoral Fellow at the Faculty of
with recurrent herpes simplex labialis. Pediatr Dermatol. 2012;29(1):105–10.
Nursing, University of Calgary, Canada.
Available at: [Accessed: 9thFeb 2013].
Aciclovir + hydrocortisone. Herpes labialis: a topical antiviral drug perhaps,
We acknowledge Jill Norris, Faculty of Nursing, University of Calgary, for her
but not a steroid. Prescrire Int. 2011 Sep;20(119):205-7. Available at:
support in editing the manuscript.
[Accessed: 15th Feb 2015].
Scheinfeld N. New to the clinic: Therapeutic Review 2009–2010. SKINmed.
2010;8(6):348–9. Available at:
1School of Health and Related Research, The University of Sheffield, Sheffield,
[Accessed: 5th Sep 2013].
UK. 2Faculty of Nursing, University of Calgary, 2500 University Drive NW, T2N
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's
1 N4 Calgary, AB, Canada.
tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928.
Arain et al. BMC Infectious Diseases (2015) 15:82
Antimicrob Agents Chemother. 2003;47(3):1072–80. Available at:
[Accessed: 15th Feb 2015].
[Accessed: 12th Mar 2013].
Hozo S, Djulbegovic B, Hozo I. Estimating the mean and variance from
Stoopler ET, Balasubramaniam R. Topical and systemic therapies for oral and
the median, range, and the size of a sample. BMC Medical Research
perioral herpes simplex virus infections. J Calif Dent Assoc. 2013;41(4):259–62.
Methodology.2005;5:13. Available at:
Balfour Jr HH. Antiviral drugs. N Engl J Med. 1999;340:1255–68.
[Accessed: 6th Aug 2013].
Rooney JF, Bryson Y, Mannix ML, Dillon M, Wohlenberg CR, Banks S, et al.
Hull C, Harmenberg J, Arlander E, Aoki F, Bring J, Darpö B, et al. Early
Prevention of ultraviolet-light-induced herpes labialis by sunscreen. Lancet.
treatment of cold sores with topical ME-609 decreases the frequency of
ulcerative lesions: a randomized, double-blind, placebo-controlled, patient-
Spruance SL. Pathogenesis of herpes simplex labialis: experimental
initiated clinical trial. J Am Acad Dermatol. 2010;64(4):696.e1-1. Available at:
induction of lesions with UV light. J Clin Microbiol. 1985;22(3):366–8.
[Accessed: 05th Mar 2013].
Evans T, Bernstein D, Raborn G, Harmenberg J, Kowalski J, Spruance S, et.al.
Double-blind, randomized, placebocontrolled study of topical 5% acyclovir,1%hydrocortisone cream (ME-609) for treatment of UV radiation-induced herpeslabialis. Antimicrobial agents and chemotherapy. 2002;46(6):1870–4. Availableat: [Accessed: 20th Mar 2013].
Hull C, Mckeough M, Sebastian K, Kriesel J, Spruance S. Valacyclovir andtopical clobetasol gel for the episodic treatment of herpes labialis: apatient-initiated, double-blind, placebo-controlled pilot trial. J Eur AcadDermatol Venereol. 2009;23(3):263–7. Available at: [Accessed: 18th May 2013].
Spruance S, Mckeough M. Combination treatment with famciclovir and atopical corticosteroid gel versus famciclovir alone for experimentalultraviolet radiation-induced herpes simplex labialis: a pilot study. J InfectDis. 2000;181(6):1906–10. Available at: [Accessed: 2nd May 2013].
Spruance S, Nett R, Marbury T, Wolff R, Johnson J, Spaulding T, et.al.
Acyclovir cream for the treatment of herpes simplex labialis: results of tworandomized, double-blind, vehicle-controlled, multicenter clinical trials.
Antimicrob Agents Chemother. 2002;46(7):2238–43. Available at: [Accessed: 6th Aug 2013].
Raborn G, Martel A, Lassonde M, Lewis M, Boon R, Spruance S, et.al.
Effective treatment of herpes simplex labialis with penciclovir cream:combined results of two trials. J Am Dental Assoc. 2002;133(3):303–9.
Available at: [Accessed: 7thAug 2013].
Kaminester L, Pariser R, Pariser D, Weiss J, Shavin J, Landsman L, et al. Adouble-blind, placebo-controlled study of topical tetracaine in thetreatment of herpes labialis. J Am Acad Dermatol. 1999; 41(6):996–1001.
Available at: [Accessed:15th Apr 2013].
Sperandio F, Marotti J, Aranha A, Eduardo Cde P. Photodynamic therapy forthe treatment of recurrent herpes labialis: preliminary results. Gen Dent.
2009;57(4):415–9. Available at: [Accessed: 6th Aug 2013].
Harmenberg J, Awan A, Alenius S, Ståhle L, Erlandsson A, Lekare G, et al.
Augustsson E, Larsson T, Wikström A, Stierna P, Field H, Larsson A. ME-609:a treatment for recurrent herpes simplex virus infections. Antivir ChemChemother. 2003;14(4):205–15. Available at: [Accessed: 4th Aug 2013].
Woo S, Challacombe S. Management of recurrent oral herpes simplexinfections. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103(S12):e1-18. Available at: [Accessed: 4th Aug 2013].
Raborn G, for the Penciclovir Topical Collaborative Study Group. Paperpresented at Thirty-sixth Interscience Conference on Antimicrobial Agentsand Chemotherapy, New Orleans, La. 1996.
Spruance S, Rea T, Thoming C, Tucker R, Saltzman R, Boon R, et.al. Penciclovir
Submit your next manuscript to BioMed Central
cream for the treatment of herpes simplex labialis. A randomized, multicenter,
and take full advantage of:
double-blind, placebo-controlled trial. Topical Penciclovir Collaborative StudyGroup. J Am Med Assoc. 1997;277(17):1374–9. Available at: [Accessed: 2nd Aug 2013].
• Convenient online submission
Spruance S, Bodsworth N, Resnick H, Conant M, Oeuvray C, Gao J, et al.
• Thorough peer review
Single-dose, patient-initiated famciclovir: a randomized, double-blind,
• No space constraints or color figure charges
placebo-controlled trial for episodic treatment of herpes labialis. J Am AcadDermatol. 2006;55(1):47–53.
• Immediate publication on acceptance
[Accessed: 19 Mar 2013].
• Inclusion in PubMed, CAS, Scopus and Google Scholar
Spruance S, Jones T, Blatter M, Vargas-Cortes M, Barber J, et al. High-dose,
• Research which is freely available for redistribution
short-duration, early valacyclovir therapy for episodic treatment of coldsores: results of two randomized, placebo-controlled, multicenter studies.
Submit your manuscript at www.biomedcentral.com/submit
Source: http://bmcinfectdis.biomedcentral.com/track/pdf/10.1186/s12879-015-0824-0?site=bmcinfectdis.biomedcentral.com
P á g i n a 1 McAfee® Multidevice o MultiAccess Guía del Producto La información contenida en este documento se proporciona únicamente con fines informativos y para la conveniencia de los cli entes de McAfee. La información contenida en este documento está sujeta a cambios sin previo aviso y se proporciona "TAL CUAL", sin garantía en cuanto a la exactitud o
PETER UND SABINE ANSARI DIE ANTIDEPRESSIVA-LÜGE mit einem Vorwort von Prof. Dr. med. Bruno Müller-Oerlinghausen Werden im Text Handelsnamen genannt, so handelt es sich in aller Regel um eine subjektive Auswahl ohne Anspruch auf Vollständigkeit. Geschützte Warennamen (Warenzeichen) werden nicht besonders kenntlich gemacht. Aus dem Fehlen eines solchen Hinweises kann
