Carbohydrate.ru
MOLECULAR AND CELLULAR BIOLOGY, Aug. 2007, p. 5699–5710
0270-7306/07/$08.00⫹0 doi:10.1128/MCB.00383-07Copyright 2007, American Society for Microbiology. All Rights Reserved.
Distinct Endocytic Mechanisms of CD22 (Siglec-2) and Siglec-F
Reflect Roles in Cell Signaling and Innate Immunity䌤
Hiroaki Tateno,1 Hongyi Li,1 Melissa J. Schur,3 Nicolai Bovin,2 Paul R. Crocker,4
Warren W. Wakarchuk,3 and James C. Paulson1*
Departments of Molecular Biology and Molecular and Experimental Medicine, The Scripps Research Institute, San Diego,
California 920371
; Shemyakin and Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences,
16/10 ul. Miklukho-Maklaya, 117997 Moscow, Russia2
; Institute for Biological Sciences, National Research Council,
Ottawa, Ontario K1A OR6, Canada3
; and Division of Cell Biology and Immunology,
The Wellcome Trust Biocentre, School of Life Sciences, University of Dundee,
Dow Street, Dundee DD1 5EH, United Kingdom4
Received 2 March 2007/Returned for modification 15 April 2007/Accepted 1 June 2007
Sialic acid-binding immunoglobulin-like lectins (siglecs) are predominately expressed on immune cells. They
are best known as regulators of cell signaling mediated by cytoplasmic tyrosine motifs and are increasingly
recognized as receptors for pathogens that bear sialic acid-containing glycans. Most siglec proteins undergo
endocytosis, an activity tied to their roles in cell signaling and innate immunity. Here, we investigate the
endocytic pathways of two siglec proteins, CD22 (Siglec-2), a regulator of B-cell signaling, and mouse eosin-
ophil Siglec-F, a member of the rapidly evolving CD33-related siglec subfamily that are expressed on cells of
the innate immune system. CD22 exhibits hallmarks of clathrin-mediated endocytosis and traffics to recycling
compartments, consistent with previous reports demonstrating its localization to clathrin domains. Like CD22,
Siglec-F mediates endocytosis of anti-Siglec-F and sialoside ligands, a function requiring intact tyrosine-
based motifs. In contrast, however, we find that Siglec-F endocytosis is clathrin and dynamin independent,
requires ADP ribosylation factor 6, and traffics to lysosomes. The results suggest that these two siglec
proteins have evolved distinct endocytic mechanisms consistent with roles in cell signaling and innate
immunity.
The siglec (sialic-acid binding immunoglobulin [Ig]-like lec-
tein tyrosine phosphatase SHP-1, which dephosphorylates the
tins) proteins are subset of the Ig superfamily of cell recogni-
BCR and dampens the B-cell response, setting a threshold for
tion molecules that bind to sialic acid-containing glycans of cell
B-cell activation (27, 73).
surface glycoconjugates as ligands (27, 78). Of the 13 human
CD22 is also known to undergo endocytosis following liga-
and 9 murine siglec proteins, 4 are highly conserved in mam-
tion by anti-CD22 antibody (38, 64) or high-affinity multiva-
malian species: sialoadhesin (Sn)/Siglec-1, CD22/Siglec-2, my-
lent-sialoside ligands (22). The tyrosine-based ITIMs of CD22
elin-associated glycoprotein (MAG)/Siglec-4, and Siglec-15.
also fit the sorting signal YXXØ (where Ø is a hydrophobic
The others comprise a rapidly evolving subfamily homologous
residue) for association with the adaptor complex 2 (AP2),
to CD33/Siglec-4, known as the CD33-related siglec proteins.
which directs recruitment of receptors into clathrin-coated pits
With two exceptions (MAG and Siglec-6) the siglec proteins
(13). John et al. reported that CD22 associates with the AP50
are predominantly expressed in various white blood cells of the
subunit of AP2 through these tyrosine-based motifs and that
immune system (25, 43, 78, 85). All contain a N-terminal V-set
they are required for endocytosis (37). Consistent with this
sugar-binding Ig domain, a variable number of additional C-set
observation, CD22 is predominantly localized in clathrin-rich
Ig domains, a transmembrane domain, and a cytoplasmic do-
domains (23, 34). Antigen ligation of the BCR results in mo-
main that typically contains tyrosine-based motifs implicated in
bilization of the BCR to "activation rafts," which subsequently
regulation of cell signaling and endocytosis.
fuse with clathrin domains prior to endocytosis (69, 70). Since
Many of the siglec proteins contain one or more immunore-
CD22 is specifically excluded from activation rafts (54), the
ceptor tyrosine-based inhibitory motifs (ITIMs), (I/L/V)XYXX
negative regulatory effect of CD22 on BCR signaling has been
(L/V), suggesting that they play important roles as inhibitory
proposed to occur following its movement to clathrin domains
receptors of cell signaling (25, 78), as exemplified by CD22,
(23), linking the endocytic activity of CD22 to its role in reg-
which is well documented as a regulator of B-cell receptor
ulation of BCR signaling.
(BCR) signaling. Upon antigen binding to the BCR, the ITIMs
Sn and most CD33-related siglec proteins are expressed on
of CD22 are quickly tyrosine phosphorylated and recruit pro-
cells of the innate immune system, including monocytes, mac-rophages, neutrophils, eosinophils, and dendritic cells (25, 43,50, 85), focusing investigations into their precise functions on
* Corresponding author. Mailing address: The Scripps Research
the known activities of these cells. Like CD22, ligation of
Institute, 10550 N. Torrey Pines Rd. MEML71, La Jolla, CA 92037.
CD33-related siglec proteins (CD33 and Siglec-5, -7, and -9)
Phone: (858) 784-9633. Fax: (858) 784-9690. E-mail: jpaulson@scripps
also induces recruitment of SHP-1 via phosphorylated ITIMs
䌤 Published ahead of print on 11 June 2007.
(6, 7, 53, 72). Antibody ligation of the same siglec proteins
TATENO ET AL.
MOL. CELL. BIOL.
initiates their endocytosis, suggesting that endocytic activity is
conjugates were synthesized by coupling spacered oligosaccharides and spacered
also a general property of this subfamily (9, 38, 50, 81, 84).
biotin to poly (
N-oxysuccinimide acrylate) as described previously (67); the1,000-kDa lactose conjugate was obtained from The Consortium for Functional
Over 20 pathogenic microorganisms express sialic acid-con-
taining glycans on their surface (25). Recent demonstration of
Mice. The IL5 transgenic (IL5-Tg) mouse line NJ.1638 was the generous gift
the binding or uptake of several sialylated pathogens, including
of James J. Lee (Mayo Clinic, Scottsdale, AZ) (41). C57BL/6 mice were obtained
Neisseria meningitidis,
Trypanosoma cruzi,
Campylobacter je-
from the Scripps Research Institute. Protocols were conducted in accordance
juni, and group B
Streptococcus, by Sn and Siglec-5, -7, and -9
with the National Institutes of Health and the Scripps Research Institute.
Plasmids. The full-length cDNA fragment of Siglec-F and human CD22 was
has suggested various roles of these siglec proteins in the im-
amplified by PCR and cloned into pcDNA5/FRT/V5-His vector (Invitrogen)
mune responses to these organisms (8, 18, 25, 30, 38, 45). Since
(71). Site-directed mutagenesis was performed using a Genetailor site-directed
mechanisms of pathogen entry and engulfment are increasingly
mutagenesis system (Invitrogen). The primers used for construction of Siglec-F
recognized to involve the host cells' endocytic machinery (12),
mutants were as follows: for T535F, 5⬘-GGATGAGCCTGAACTCCACTTTG
the endocytic functions of the siglec proteins are also relevant
CCTCCCTC-3⬘ (sense) and 5⬘-GGATGAGCCTGAACTCCACTTTGCCTCCCTC-3⬘ (antisense); for T568F, 5⬘-GGAGGCTATGAAATCTGTATTCACAG
to their roles in pathogen recognition and uptake.
ACATC-3⬘ (sense) and 5⬘-TACAGATTTCATAGCCTCCGTGTTCTGAG-3⬘
In this report, we contrast the mechanisms of endocytosis of
(antisense); and for the cytoplasmic tail deletion, dC, 5⬘-TTTTCACAGTGAA
CD22 and a CD33-related siglec, murine Siglec-F, predomi-
GGTCCTCTGATAGAAATCAGCCC-3⬘ (sense) and 5⬘-GAGGACCTTCACT
nately expressed on eosinophils (85). Eosinophils are best
GTGAAAAAGATGAGGCA-3⬘ (antisense). Dynamin-1 cDNA constructs (wildtype [WT] and K44A) were obtained from Sandra L. Schmid (The Scripps
known for their role in allergic diseases (62). In this regard, a
Research Institute, San Diego, CA). ADP ribosylation factor 6 (ARF6)-en-
recent report analyzing Siglec-F null mice revealed enhanced
hanced green fluorescent protein (EGFP) cDNA constructs (WT, T27N, and
eosinophil infiltration and blood eosinophilia in response to
Q67L) were obtained from Julie G. Donaldson (National Institutes of Health,
allergen challenge, suggesting that Siglec-F is a negative reg-
Bethesda, MD). Cells were grown to 70 to 80% confluence on coverslips and
ulator of eosinophil response to allergens (86). Eosinophils
transiently transfected with plasmid using Lipofectamine 2000 (Invitrogen).
Cell culture. CHO cells were cultured in Ham's F-12 medium, respectively,
also contribute to an immune response against foreign patho-
supplemented with 10% fetal calf serum, penicillin (100 U/ml), and streptomycin
gens, including binding, engulfment, and killing of microbes (1,
(100 g/ml). Stable clones of CHO cells expressing Siglec-F, Siglec-F mutants,
15, 19, 32, 36, 83). While investigating the endocytic activity of
and human CD22 were generated by Lipofectamine 2000 (Invitrogen) and se-
Siglec-F, we observed efficient endocytosis of anti-Siglec anti-
lection in 0.5 mg/ml hygromycin B (Roche Molecular Biochemicals).
body, high-affinity sialoside ligand probes, and
Neisseria men-
Agent treatments. Cells were preincubated for 30 min at 37°C in Ham's
F-12–10% fetal calf serum medium containing 10 mM methyl--cyclodextrin
ingitidis bearing sialylated glycans. Like CD22, endocytosis
(CD) (Sigma-Aldrich), 5 M latrunculin A (Sigma-Aldrich), 1 M jasplakinolide
was dependent on its cytoplasmic ITIM and ITIM-like mo-
(EMD Biosciences, CA), 100 M genistein (Sigma-Aldrich), 100 M PP2 (EMD
tifs. Surprisingly, however, Siglec-F exhibited no colocaliza-
Biosciences), 100 M sodium pervanadate, 1 M nocodazole (Sigma-Aldrich),
tion with clathrin, raising doubt that it was endocytosed by
10 M amiloride (Sigma-Aldrich), 50 M nystatin (Sigma-Aldrich), or 10 Mbrefeldin A (Sigma-Aldrich). Cells were then incubated with PE–anti-Siglec-F in
a clathrin-mediated mechanism, as proposed for CD22.
the continued presence of the agents at 37°C for 1 h. Agent treatment did not
More detailed investigations showed that while endocytosis
affect cell viability.
of CD22 is indeed mediated by a clathrin-dependent mech-
Flow cytometry. Cells (5 ⫻ 105) suspended in PBS/BSA (10 mM phosphate-
anism and is sorted to early endosome and recycling com-
buffered saline [pH 7.0] containing 10 mg/ml bovine serum albumin) were incu-
partments, Siglec-F endocytosis is directed to lysosomal
bated with 10 g/ml of primary antibody or sialoside-PAA probe for 30 min onice. After washing with PBS/BSA, cells were incubated with 10 g/ml of FITC-
compartments and is mediated by a mechanism that is in-
secondary antibody or streptavidin-Cy5-PE for 30 min on ice. For sialidase
dependent of clathrin and dynamin. The results suggest that
pretreatment, cells in PBS/BSA were incubated with 25 mU of
A. ureafaciens
siglec proteins have evolved different mechanisms of endo-
sialidase (Roche Molecular Biochemicals) for 30 min at 37°C. Flow cytometry
cytosis consistent with roles in regulation of cell signaling
data were acquired on a FACS Calibur flow cytometer (BD Biosciences, SanJose, CA) and analyzed using the CellQuest software.
and innate immunity.
Immunofluorescence microscopy. For the internalization assay, CHO cells
cultured on coverslips for 2 days were labeled with fluorescence-conjugatedantibody (10 g/ml) in PBS/BSA for 30 min on ice. Cells were then incubated at
MATERIALS AND METHODS
37°C for various times, and internalization was stopped by transferring the cells
Antibodies and reagents. Purified rat anti-Siglec-F (E50-2440), phycoerythrin
to ice. After washing, cells were fixed with 4% paraformaldehyde (PFA; Electron
(PE)-conjugated anti-Siglec-F (E50-2440), fluorescein isothiocyanate (FITC)-
Microscopy Services) for 15 min at room temperature. For colocalization exper-
conjugated anti-human CD22 (HIB22), PE–Cy5-streptavidin, anti-Syntaxin-8
iments, cells were further permeabilized with 0.1% saponin in PBS for 20 min at
(48), and anti-EEA1 (14 polyclonal antibodies) were purchased from BD Bio-
room temperature and then incubated with the desired antibody for 1 h at room
sciences-Pharmingen (San Diego, CA). Anti-hamster LAMP2 (UH3) was pur-
temperature or 4°C overnight.
chased from the Developmental Studies Hybridoma Bank. Anti-clathrin heavy
For eosinophil staining, eosinophils from IL5-Tg mice at 107/ml in PBS/BSA
chain (x22) was purchased from Abcam. Affinity-purified sheep anti-Siglec-F IgG
were fixed with 2% PFA for 10 min at 4°C before staining with the desired
was prepared as described previously (85). Alexa Fluor 488 (AF488)- and
antibodies for 30 min at 4°C. After cell surface staining, cells were cytospun onto
AF555–goat anti-mouse IgG, AF555–anti-mouse IgG1, and AF555–anti-mouse
glass slides and fixed with 4% PFA for 20 min at room temperature. Cells were
IgG2a, AF555-conjugated goat anti-rat IgG, AF555–goat anti-rabbit IgG, Quan-
further permeabilized with 0.1% saponin in PBS for 20 min at room temperature
tumdot 655 (Qdot655)-streptavidin, and AF488-streptavidin were purchased
and then incubated with anticlathrin antibody (clone x22). Cells were mounted
from Invitrogen. FITC-donkey anti-sheep IgG and FITC-donkey anti-rabbit IgG
with Prolong gold antifade reagent (Invitrogen) and examined using a Zeiss
were purchased from Jackson Immunoresearch (West Grove, PA). Anti-CD45
Axiovert S100TV microscope equipped with a Bio-Rad MRC 1024 confocal laser
and anti-CD90 antibody-conjugated magnetic beads were purchased from Milte-
scanner. Images were collected with Bio-Rad LaserSharp 2000 software and
nyi Biotech (Auburn, CA).
analyzed with Image J (v. 1.33), Zeiss LSM image, and Adobe Photoshop.
Synthesis of high-molecular-mass sialoside-PAA probe. Biotinylated low-mo-
Bacteria. Cells of
Neisseria meningitidis MC58 (NRCC 6200 Cap⫹ and NRCC
lecular-mass (30 kDa) sialoside-polyacrylamide (PAA) conjugates bearing
4728 Cap⫺) were grown on chocolate agar in the presence of 20 g/ml CMP-
NeuAc to increase the level of sialylation of the lipooligosaccharide (LOS). The
4]Gal1-4[Fuc␣1-3]GlcNAc (6⬘-sulfo-sLeX) or 9-biphenylcar-
bonyl (BPC)-Neu5Ac␣2-6Gal1-4GlcNAc were synthesized by coupling spac-
cells were killed by heat treatment at 68°C for 1 h. The killed cells were labeled
ered oligosaccharides and spacered biotin to poly (4-nitrophenyl acrylate) as
with FITC by resuspending them first in PBS and then mixing them with 1 mg/ml
described previously (16) Biotinylated high-molecular-mass (1,000 kDa) PAA
FITC in sodium bicarbonate buffer at pH 9. Cells were reacted with the dye for
ENDOCYTIC MECHANISMS OF SIGLEC PROTEINS
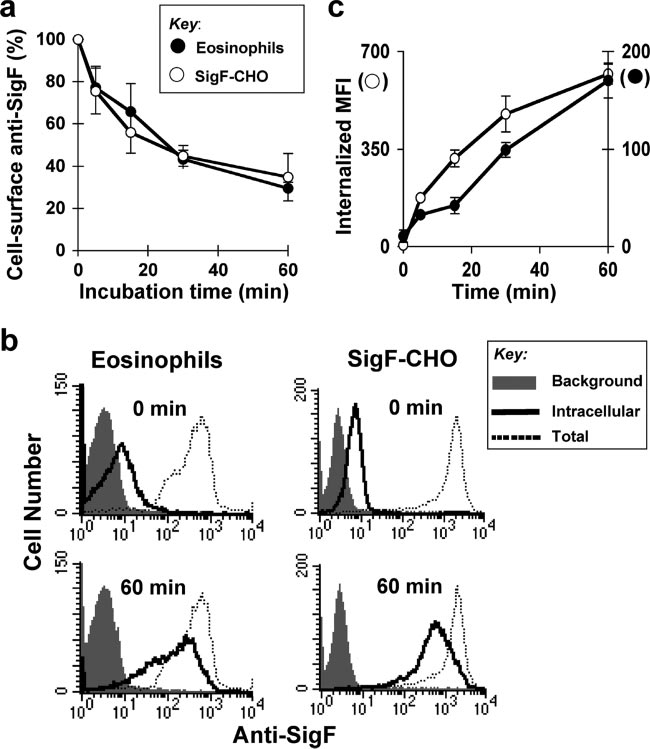
FIG. 1. Siglec-F mediates endocytosis following ligation by antibody. (a) SigF-CHO cells and mouse eosinophils were incubated with sheep
anti-Siglec-F pAb on ice for 30 min and allowed to internalize at 37°C for the indicated time. Cell-surface anti-Siglec-F pAb was detected byFITC–anti-sheep IgG. Data are the average ⫾ standard deviation of three independent experiments. (b) SigF-CHO cells and mouse eosinophilswere incubated with PE–anti-Siglec-F (dotted line and solid line) or isotype control (shaded) for 30 min on ice. After washing, the cells wereallowed to internalize at 37°C for the indicated time. Following the incubation, cells were washed with 0.2 M glycine buffer pH 2.0 (solid line,intracellular) to remove surface-bound antibody or PBS/BSA (shaded area and dotted line, total probe) as a control. Bound or internalizedantibody was measured by flow cytometry. Representative results are shown. (c) Data are expressed as internalized mean fluorescence intensity(MFI) of PE–anti-Siglec-F measured as in panel b. Data are the average ⫾ standard deviation of triplicate determinants.
15 min and then washed extensively. Cells were observed by phase-contrast
ternalized at a rate similar to that observed for eosinophils
microscopy to ensure they were intact and to estimate the cell number.
(Fig. 1). Anti-CD22 monoclonal antibody (MAb) was similarlyinternalized from the cell surface of CD22-CHO cells, with
50% internalization observed within 30 min (data not shown).
The endocytosis of Siglec-F was also assessed by monitoring
Endocytosis of Siglec-F following ligation by antibody.
the internalization of fluorescently labeled anti-Siglec-F MAb.
Endocytosis of Siglec-F was assessed by monitoring the de-
Eosinophils or SigF-CHO cells were stained with PE–anti-
crease of anti-Siglec-F from the surface of eosinophils using
Siglec-F MAb at 4°C and warmed to 37°C for up to 60 min.
flow cytometry. Siglec-F was labeled with sheep anti-Si-glec-F on ice for 30 min. The temperature was raised to
Cell-associated antibody was monitored by flow cytometry be-
37°C to initiate endocytosis, and at various times, cells were
fore (total) or after removing residual cell surface antibody by
transferred to 4°C. Residual cell-surface anti-Siglec-F was then
a brief low-pH wash. After incubation at 37°C for 60 min, the
detected by incubation with FITC–anti-sheep IgG. As shown in
level of intracellular PE–anti-Siglec-F MAb increased gradu-
Fig. 1a, anti-Siglec-F was internalized over time, reaching ⬃60%
ally over time (Fig. 1b and c). The combined results demon-
internalization relative to time zero after 30 min.
strate that antibody ligation initiates efficient endocytosis of
CHO cells are often used to study the mechanism of endo-
Siglec-F on both mouse eosinophils and SigF-CHO cells.
cytosis of immunoreceptors (14, 39, 68). Anti-Siglec-F bound
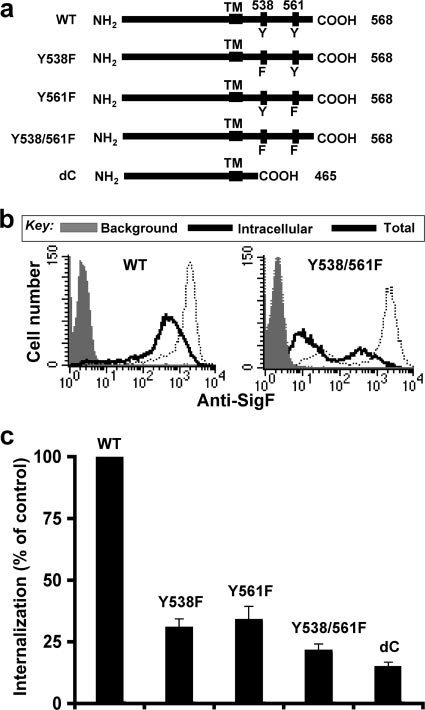
Mutation of tyrosines 538 and 561 abrogates endocytosis of
to CHO cells stably expressing Siglec-F (SigF-CHO) was in-
Siglec-F. Siglec-F carries two potential cytoplasmic tyrosine-
TATENO ET AL.
MOL. CELL. BIOL.
FIG. 3. Siglec-F is not colocalized with clathrin. SigF-CHO cells or
mouse eosinophils were fixed with 2% PFA before staining with mouseanti-Siglec-F MAb and AF555–anti-rat IgG. After permeabilization,cells were stained with mouse anti-clathrin MAb and AF488–anti-mouse IgG and examined by confocal microscopy. Representative
images are shown. Bars, 10 m.
mutant is similar to that of the double-tyrosine mutant Y538/
561F (85% inhibition), suggesting that the tyrosine-based mo-tifs are dominant sorting signals. Since mutation of eitherTyr538 or Tyr561 also inhibited (66 to ⬃69%) endocytosis ofSiglec-F, we conclude that both tyrosine-based motifs are re-quired for optimal endocytosis of Siglec-F. In contrast to themutations in the cytoplasmic domain, an Arg-to-Ala mutationof the conserved arginine residue required for sialoside bind-ing (3) showed no effect on internalization of Siglec-F, sug-
FIG. 2. Tyrosine-based motifs are required for endocytosis of
gesting that ligand-binding activity is not directly involved in
Siglec-F. (a) Schematic representation of WT and mutant forms of
the endocytosis of Siglec-F (data not shown), as reported pre-
Siglec-F. The sequence at the C-terminal end is 431 SETSRGTVLGAIWGAGMALLAVCLCLIFFTVKVLRKKSALKVAATKGNHLA
viously for endocytosis of CD22 (87).
Siglec-F is not colocalized with clathrin domains. Since the
Siglec-F tyrosine-based motifs (YxxI/L) conform to the binding
569, where underlined letters are the transmembrane domain and
sites for clathrin-associated adapter complex 2 (AP2) and
tyrosines 538 and 561 are in boldface. (b) Rates of internalization ofSiglec-F for CHO cells stably expressing WT and mutant forms of
CD22 had previously been demonstrated to localize predomi-
Siglec-F were compared as in Fig. 1b. Representative flow cytometry
nantly in clathrin domains (23), we investigated the localization
data of the WT and the double-tyrosine mutant (Y538/561F) of
of clathrin and Siglec-F on mouse eosinophils and SigF-CHO
Siglec-F are shown. (c) Percent internalization is calculated as (intra-
cells. Fixed cells were first stained with anti-Siglec-F MAb and
cellular mean fluorescence intensity [MFI]/total MFI) ⫻ 100 and ex-
AF555-labeled (60) anti-rat IgG. Cells were then permeabil-
pressed as a percentage of internalization in CHO cells expressingwild-type Siglec-F. Data are the average ⫾ standard deviation of trip-
ized and stained with anti-clathrin MAb and AF488-labeled
(green) anti-mouse IgG. As shown in Fig. 3, on both mouseeosinophils and SigF-CHO cells anti-Siglec-F staining ap-peared punctate and did not overlap with anticlathrin staining.
based sorting motifs (YxxL/I) at amino acids Tyr 538 and 561.
Staining of mouse eosinophils or SigF-CHO cells with anti-
To assess whether the intact motifs are required for internal-
Siglec-F and secondary antibody prior to fixation and perme-
ization, we designed mutant constructs substituting phenylala-
abilization also showed no association with clathrin (data not
nine for tyrosine 538 and 561, either individually or in combi-
nation, and a cytoplasmic deletion mutant (Fig. 2a). The
Endocytosis of Siglec-F and CD22 results in sorting to dif-
internalization of the mutant Siglec-F constructs was com-
ferent intracellular compartments. To further assess the ab-
pared to that of WT Siglec-F by flow cytometry. Although
sence of Siglec-F and clathrin localization, the internalization
PE–anti-Siglec-F MAb was efficiently internalized in CHO
of Siglec-F was followed by confocal fluorescence microscopy
cells expressing WT Siglec-F, the level of internalized antibody
and compared to that of CD22 and transferrin (Tf) receptor,
was significantly decreased in CHO cells expressing the dou-
which is known to be mediated by a clathrin-mediated mech-
ble-tyrosine mutant Y538/561F (Fig. 2b). Mutation of both
anism (46). SigF-CHO, CD22-CHO, and parental CHO cells
tyrosines to phenylalanine reduced internalization by nearly
were grown on coverslips and were labeled with PE–anti-
80% (Fig. 2c). The inhibitory effect of the cytoplasmic deletion
Siglec-F MAb, FITC–anti-CD22 MAb, and AF488-Tf, respec-
ENDOCYTIC MECHANISMS OF SIGLEC PROTEINS
FIG. 4. Endocytosis of Siglec-F and CD22 results in sorting to different intracellular compartments. (a) CD22-CHO and SigF-CHO cells grown
on coverslips were labeled with FITC–anti-CD22 and PE–anti-Siglec-F on ice for 30 min, respectively, and allowed to internalize after shifting to37°C for the indicated times. As a control, internalization of Tf is shown. (b) Cells were incubated with antibody (FITC–anti-CD22 orPE–anti-Siglec-F) and Tf (AF555-Tf or AF488-Tf) on ice for 30 min and allowed to internalize after shifting to 37°C for 30 min. Cells were alsostained with a lysosome marker, anti-Syntaxin-8. Representative images are shown. Bars, 10 m.
tively, and allowed to internalize at 37°C for various times over
recycling compartments, with negligible colocalization with
a 60-min period. Slides were then fixed for microscopy. As
the lysosomal marker Syntaxin-8 (Fig. 4b). The results are
shown in Fig. 4a, all three proteins were diffusely localized at
consistent with endocytosis occurring by a clathrin-mediated
time zero (4°C). Within 15 min of warming to 37°C, Tf was
mechanism. In contrast, anti-Siglec-F was predominantly colo-
clearly observed in early endosomes and the tubules of the
calized with anti-Syntaxin-8 and another lysosomal marker,
recycling compartment, visible as a bright spot near the nucleus
anti-LAMP2 (not shown), and exhibited minimal colocaliza-
as reported previously (46). CD22 exhibited visually identical
tion with Tf. Endocytosed anti-Siglec-F also partially colocal-
localization as the Tf receptor with slightly delayed kinetics. In
ized with the early endosomal marker anti-EEA-1 but not with
contrast, anti-Siglec-F endocytosed at a much reduced rate and
anti-clathrin or anti-AP2 (data not shown). The results indicate
accumulated in intracellular compartments with a lysosomal
that Siglec-F and CD22 are sorted to different intracellular
appearance visually distinct from Tf and anti-CD22 staining.
compartments, with Siglec-F being predominantly sorted to
The labeling at 60 min was a result of intracellular staining for
all three proteins since there was no change in appearance if
The effect of tyrosine-based motifs on endocytosis of Siglec-F
cells were subjected to a low-pH wash (0.2 M glycine, pH 2.0)
was confirmed by confocal fluorescence microscopy. While
prior to fixation.
anti-Siglec-F showed predominantly intracellular punctate
To better characterize the intracellular localization of endo-
staining in CHO cells expressing WT Siglec-F, cells expressing
cytosed CD22 and Siglec-F, we investigated their localization
the double-tyrosine mutant (Y538/561F) showed only diffuse
in relationship to Tf, a marker for the early endosome and
surface staining that was removed with a brief low-pH wash
recycling compartments, and anti-Syntaxin-8, a marker for late
(not shown).
endosomes and lysosomes (5, 55, 76). As expected, CD22 was
A high-affinity sialoside probe competes with cis ligands and
predominantly colocalized with Tf in early endosomes and
binds to Siglec-F on native cells. Recently, Collins et al. showed
TATENO ET AL.
MOL. CELL. BIOL.
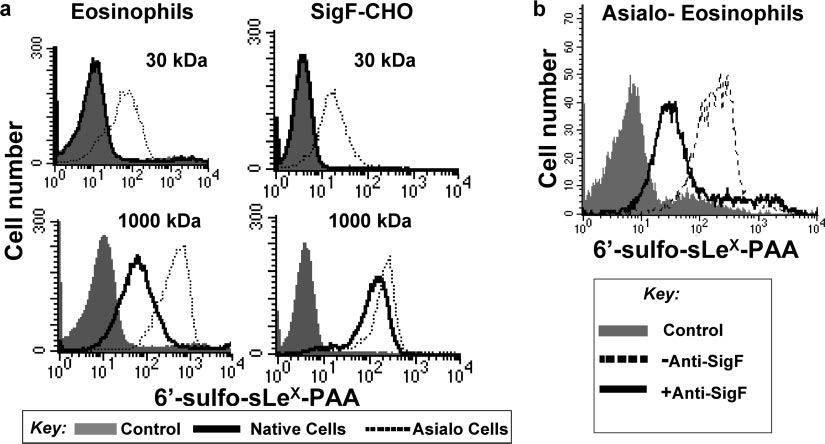
FIG. 5. High-molecular-mass sialoside probe binds to Siglec-F on native cells. (a) Mouse eosinophils prepared from IL5-Tg mice or SigF-CHO
cells pretreated with or without A. ureafaciens sialidase were incubated with biotinylated 1,000-kDa lactose-PAA (negative control) or 1,000-kDa6⬘-sulfo-sLeX-PAA probe on ice for 60 min. After washing, bound probe was detected with PE-Cy5-streptavidin and analyzed by flow cytometry.
(b) Sialidase-treated mouse eosinophils were preincubated with or without anti-Siglec-F MAb and then were incubated with the biotinylated
sialoside-PAA probes on ice for 60 min. After washing, bound probe was detected with PE-Cy5-streptavidin and analyzed by flow cytometry.
that high-affinity sialoside ligands could compete with cis ligands,
nonspecific interaction (71). No binding of a control 1,000-kDa
bind to CD22 on native B cells, and serve as a ligand-based
lactose-PAA probe was observed.
marker of CD22 endocytosis (22). The ligand was comprised of a
Siglec-F and CD22 mediate endocytosis of glycan ligands to
high-affinity synthetic ligand of CD22, 9-biphenylcarboxyl-
different subcellular compartments. Using the high-affinity
NeuAc␣2-6Gal1-4GlcNAc (BPC-NeuAc-LN), attached to a
sialoside probes that can bind to Siglec-F and CD22 on native
1,000-kDa PAA (74) backbone. To determine if CD22 and
cells, we compared their abilities to internalize and traffic their
Siglec-F carry sialoside ligands to the same compartments as
respective sialoside probes to intracellular compartments (Fig.
their respective anti-siglec antibodies, we sought to prepare an
6). Cells were incubated with the appropriate 1,000-kDa bio-
analogous sialoside ligand for Siglec-F. We previously reported
tinylated sialoside-PAA probe on ice for 60 min followed by
that the preferred glycan ligand for Siglec-F is 6⬘-sulfo-sLeX
incubation with PE-Cy5-streptavidin on ice for an additional
(Neu5Ac␣2-3[6-SO ]Gal1-4[Fuc␣1-3]GlcNAc) (71). How-
30 min. The cells were then shifted to 37°C, and the degree of
ever, Siglec-F appears to be masked to synthetic 30-kDa PAA
probe internalization assessed before and after low-pH wash
probes containing this sequence unless the cells are treated
was measured by flow cytometry at various times. As shown in
with sialidase to destroy cis ligands, a general property of the
Fig. 6a, CD22-CHO cells efficiently internalized the BPC-
siglec family (25, 47, 58, 59, 78). To create high-avidity probes
NeuAc-LN-PAA probe. Similarly, the 6⬘-sulfo-sLeX-PAA
for Siglec-F, 6⬘-sulfo-sLeX was attached to the 1,000-kDa-mo-
probe was internalized by Siglec-F on SigF-CHO cells with
lecular-mass PAA along with 5% biotin to allow detection with
somewhat slower kinetics, as seen for the anti-Siglec-F anti-
streptavidin (74). A standard enzyme-linked immunosorbent
body (Fig. 3). No binding or internalization was observed with
assay using immobilized Siglec-F-Fc chimera showed that the
the control 1,000-kDa lactose-PAA by either CD22-CHO or
resulting 1,000-kDa 6⬘-sulfo-sLeX-PAA probe bound with
Siglec-F-CHO cells (data not shown). No binding or internal-
much higher binding avidity than did the standard 30-kDa
ization was observed for either the 1,000-kDa BPC-NeuAc-
6⬘-sulfo-sLeX-PAA probe (data not shown), indicating that the
LN-PAA or 6⬘-sulfo-sLeX-PAA probe with untransfected
increased multivalency did increase binding avidity for
CHO cells (data not shown).
Siglec-F. The 6⬘-sulfo-sLeX-PAA probes (30 kDa and 1,000
CD22 and Siglec-F sort glycan ligands to the same intracel-
kDa) were then compared for their abilities to compete with cis
lular compartments as their respective antibodies. Endocyto-
ligands and bind to native eosinophils and SigF-CHO cells, as
sis of sialoside probes was investigated by confocal fluores-
illustrated in Fig. 5a. As found earlier, the 30-kDa probe failed
cence microscopy (Fig. 6b). Cells grown on coverslips were
to bind to native cells but bound only to the sialidase-treated
incubated with the biotinylated 1,000-kDa sialoside-PAA
(asialo) cells (71). In contrast, the 1,000-kDa probe bound to
probe on ice for 60 min. After washing, cells were incubated
native cells as well, demonstrating that the 1,000-kDa 6⬘-sulfo-
with Qdot655-streptavidin on ice for 30 min and allowed to
sLeX-PAA probe can effectively compete with cis ligands on
internalize at 37°C. After 60 min at 37°C, CD22 had carried the
both mouse eosinophils and SigF-CHO cells. Blocking with
1,000-kDa BPC-NeuAc-LN-PAA probe (CD22-L) to the early
anti-Siglec-F MAb inhibited binding of the sialoside probe to
endosome/recycling compartments marked by Tf. In contrast,
the cells (Fig. 5b), indicating that the binding is not due to a
Siglec-F carried the 1,000-kDa 6⬘-sulfo-sLeX-PAA probe
ENDOCYTIC MECHANISMS OF SIGLEC PROTEINS
FIG. 6. Siglec-F and CD22 mediate endocytosis of glycan ligands.
(a) SigF-CHO cells or CD22-CHO cells were incubated with the 1,000-kDa 6⬘-sulfo-sLeX-PAA probe and the 1,000-kDa BPC-NeuAc-LN-PAA probe on ice for 60 min followed by incubation with PE-Cy5-streptavidin on ice for 30 min. After washing, the cells were thenshifted to 37°C for the indicated times. Following the incubation, cellswere washed with 0.2 M glycine buffer, pH 2.0, to remove surface-bound probe or PBS/BSA as a control. Bound or internalized probe
FIG. 7. Endocytosis of Siglec-F is Dyn1 independent. (a) SigF-
was measured by flow cytometry. The 1,000-kDa lactose-PAA probe
CHO cells were transiently transfected with Dyn1-WT or Dyn1-K44A,
was used as a negative control. Data are expressed as internalized
both tagged with hemagglutinin (HA). Cells were incubated with Tf or
mean fluorescence intensity (MFI) of the probes and the average ⫾
PE–anti-Siglec-F for 60 min, fixed, and stained with anti-HA to detect
standard deviation of triplicates. (b) Endocytosis of sialoside probes
transfected cells. Representative images are shown. Bar, 10 m. (b)
was investigated by confocal fluorescence microscopy. CD22 carried
SigF-CHO and CD22-CHO cells were transfected with Dyn1-WT or
the 1,000-kDa BPC-NeuAc-LN-PAA probe (CD22-L) to the early
Dyn1-K44A and analyzed for internalization of Siglec-F and Tf recep-
endosome/recycling compartments marked by Tf, whereas Siglec-F
tor as for panel a and for CD22 with FITC–anti-CD22. Data are
carried the 1,000-kDa 6⬘-sulfo-sLeX-PAA probe (SigF-L) to lysosomes
expressed as the percentage of transfected cells with Dyn1-K44A rel-
marked with anti-LAMP2. Representative images are shown. Bar, 10 m.
ative to Dyn1-WT that contained internalized Tf, anti-CD22, or anti-Siglec-F. More than 50 transfected cells were counted. Data are theaverage ⫾ standard deviation of three independent transfections. Ex-pression of Dyn1-K44A inhibited endocytosis of Tf and anti-CD22 but
(SigF-L) to lysosomes labeled with anti-LAMP2. These results
not endocytosis of Siglec-F.
indicate that the glycan ligand is sorted to the same intracel-lular compartment as is antibody.
Endocytosis of Siglec-F is dynamin-1 independent. To fur-
ther investigate the mechanism of endocytosis, we evaluated
and anti-CD22 was virtually inhibited by Dyn1-K44A, but not
the dependence on dynamin-1. Dynamin-1 is a large GTPase
by WT dynamin-1 (Dyn1-WT), whereas that of anti-Siglec-F
that regulates vesiculation events at the plasma membrane for
was not inhibited by the expression of either Dyn1-K44A or
several clathrin-dependent and clathrin-independent endocytic
Dyn1-WT (Fig. 7). These results indicate that endocytosis of
pathways (24). Expression of a dominant-negative dynamin-1
CD22 is dynamin dependent, characteristic of a clathrin-me-
mutant (Dyn1-K44A) inhibits both clathrin- and caveolin-me-
diated pathway, whereas endocytosis of Siglec-F is dynamin
diated endocytic pathways (28, 35, 40, 51). Internalization of Tf
TATENO ET AL.
MOL. CELL. BIOL.
untreated cells (Fig. 9b), suggesting that cholesterol is requiredfor internalization of Siglec-F. However, the cholesterol-se-questering agent nystatin, which inhibits endocytic pathwaysinvolving lipid rafts and caveolin, caused no inhibition at aconcentration of 50 g/ml (61). Inhibitors of actin cytoskeletonpolymerization (latrunculin A and jasplakinolide) and micro-tuble polymerization (nocodazole) showed partial (40 to 50%)inhibition. Although genistein (general tyrosine kinase inhibi-tor) strongly inhibited internalization (87% inhibition), little orno effect was observed by PP2 (Src family kinase inhibitor).
Amiloride, a macropinocytosis inhibitor, showed no inhibitoryeffect, rather increased the internalization of Siglec-F. In CHOcells, clathrin-mediated endocytosis is known to be insensitiveto treatments with CD and genistein, so inhibition of Siglec-F
endocytosis by these agents is further evidence that the processis clathrin independent (21). Similarly, caveolin-mediated en-docytosis is sensitive to the treatment with PP2 (21), so lack ofinhibition of Siglec-F endocytosis by PP2 supports a caveolin-independent mechanism.
Endocytic pathway of Siglec-F can mediate uptake of
sialylated bacteria. Having shown that Siglec-F mediates inter-
FIG. 8. Endocytosis of Siglec-F is ARF6 dependent. SigF-CHO
nalization of antibody and synthetic sialoside probe, we inves-
cells were transfected by cDNAs coding for ARF6-WT, ARF6-T27N,
tigated whether the endocytic machinery of Siglec-F expressed
or ARF6-Q67L, all tagged with EGFP. Cells were allowed to internal-
in nonphagoctic CHO cells can mediate uptake of Neisseria
ize PE–anti-Siglec-F at 37°C for 60 min. Expression of ARF6-Q67Lresulted in accumulation of anti-Siglec-F in vacuole-like structures.
meningitidis MC58 with (MC58cap⫹) or without (MC58cap⫺)
Representative images are shown. Bars, 10 m.
capsule. MC58cap⫺ expresses LOS (31) with a terminalNeuAc␣2-3Gal1-4GlcNAc sequence, whereas MC58cap⫹ ex-presses, in addition to the LOS, a polysialic acid capsule
Endocytosis of Siglec-F is ARF6 dependent. ARF6 is a small
[(NeuAc␣2-8NeuAc) ] (80). The former sequence is recog-
GTPase that regulates the movement of endosomes between
nized as a weak ligand by Siglec-F, while the latter is not
the plasma membrane and a non-clathrin-derived endosomal
recognized (3, 71). SigF-CHO cells pretreated with or without
compartment (56). ARF6 regulates clathrin- and caveolin-in-
sialidase were incubated with fixed FITC-labeled bacteria on
dependent endocytosis (2, 4, 17, 48, 49, 56). Trafficking of these
ice for 30 min and allowed to internalize at 37°C for 60 min.
molecules is blocked by a constitutively active ARF6 mutant
Noninternalized bacteria were removed with PBS/BSA wash
(ARF6-Q67L) and results in accumulation in vacuole-like
and were confirmed to be internalized by resistance to ethidium
structures (17). Although expression of WT and a constitu-
bromide quenching of fluorescence (not shown). While neither
tively inactive ARF6 mutant (ARF6-T27N) had no effect on
the MC58cap⫺ or MC58cap⫹ bacteria were internalized in un-
the internalization of anti-Siglec-F, expression of ARF6-Q67L
treated SigF-CHO cells (Fig. 10), MC58cap⫺ was selectively in-
inhibited trafficking of anti-Siglec-F to the lysosomal compart-
ternalized by cells treated with sialidase treatment to destroy cis
ment (Fig. 8). Instead, anti-Siglec-F appears to be associated
interactions of Siglec-F with the NeuAc␣2-3Gal linkages known
with vacuole-like structure, indicating that endocytosis of
to be expressed by CHO cells. No internalization of N. meningi-
Siglec-F is affected by ARF6. Recently, flotillin-1 was reported
tidis was observed in control CHO cells. These results indicate
to define a clathrin-independent endocytic pathway (33). How-
that endocytic machinery of Siglec-F is capable of internalizing
ever, no colocalization or internalized anti-Siglec-F with anti-
bacteria containing a terminal sialoside sequence on a cell surface
flotillin-1 was observed (data not shown).
glycolipid that is recognized as a ligand.
Cholesterol, tyrosine kinases, and intact actin cytoskelton
are required for endocytosis of Siglec-F. To better understand
the mechanism of endocytosis of Siglec-F, we investigated theeffects of various agents known to affect endocytic processes
While the siglec proteins are best known as a family of sialic
(Fig. 9). SigF-CHO cells were pretreated with the agent at
acid-specific lectins that regulate cell-signaling receptors and
37°C for 30 min. PE–anti-Siglec-F was then allowed to inter-
mediate cell-cell interactions (25, 26, 78), they are increasingly
nalize at 37°C for 1 h in the continued presence of the agent.
recognized for endocytic activity that can link to their cell
After residual cell surface antibody was removed by a brief
surface functions (9, 22, 50, 57, 64, 75, 77, 81, 84, 87). Results
low-pH wash (0.2 M glycine buffer, pH 2.0), the level of inter-
presented in this report confirm and extend the suggestion that
nalized PE–anti-Siglec-F was compared to the level in un-
endocytosis of CD22 occurs by a clathrin-dependent mecha-
treated cells, as shown in Fig. 9a for the cholesterol depletion
nism mediated by tyrosine-based motifs (22, 34, 37) and show
agent CD. For each agent, the degree of internalization rela-
that CD22 is transported to early endosomes and para-Golgi
tive to no agent (using mean channel fluorescence) is summa-
recycling compartments. In contrast, the CD33-related siglec,
rized in Fig. 9b and c. The CD treatment showed 92% inhibi-
Siglec-F, undergoes endocytosis dependent on intact cytoplas-
tion of internalization relative to the internalization in
mic tyrosine residues, but by a clathrin-independent mecha-
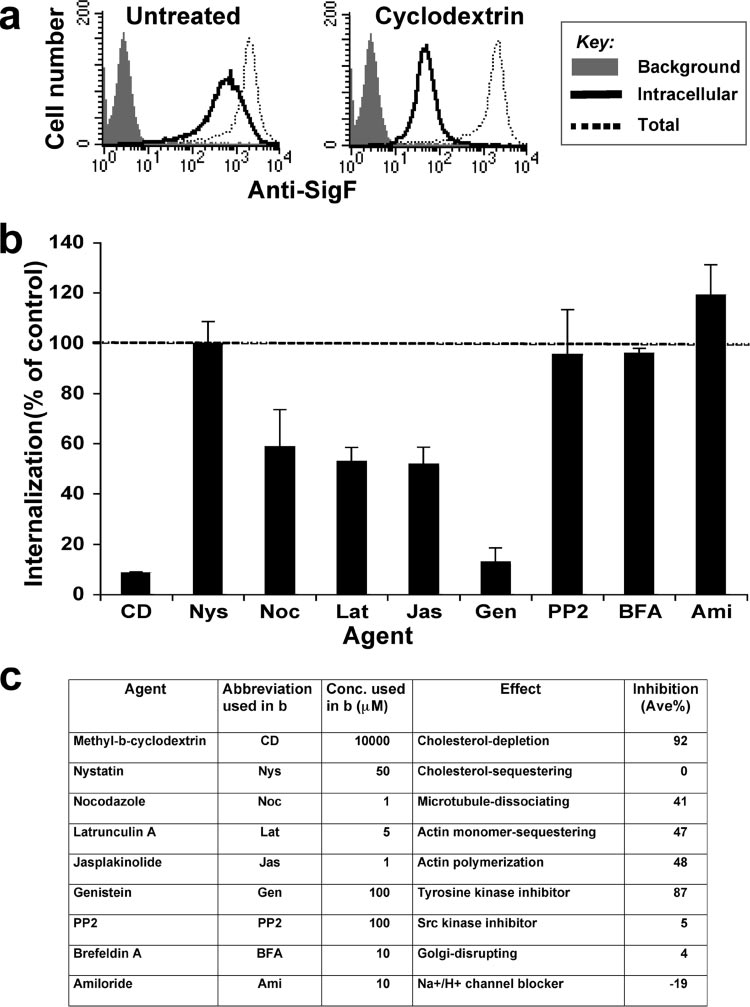
ENDOCYTIC MECHANISMS OF SIGLEC PROTEINS
FIG. 9. Endocytosis of Siglec-F requires cholesterol, tyrosine kinases, and actin cytoskeleton. SigF-CHO cells were left untreated (control) or
pretreated with the indicated agents at 37°C for 30 min, and internalization of PE–anti-Siglec-F was measured by flow cytometry as in Fig. 1b andc. Representative flow cytometry data are shown in panel a. (b) Percent internalization is calculated as (intracellular mean fluorescence intensity[MFI]/total MFI) ⫻ 100 and expressed as a percentage of internalization in control cells. Data are the average ⫾ standard deviation of triplicatedeterminants. (c) Summary of the effects of the agent on endocytosis of Siglec-F.
nism, and is sorted to lysosomes. The different endocytic mech-
endocytosis of CD22 is consistent with its activity as a regulator
anisms of these two siglec proteins reflect the divergence in
of BCR signaling since the endocytosis of the BCR is also
their functions in B cells and eosinophils, respectively.
predominantly clathrin dependent and required for dampening
Anti-CD22 antibody and sialoside ligands endocytosed by
of BCR signaling (70). It is of interest that the CD22 ITIMs
CD22 colocalize to early endosomes and recycling compart-
involved in recruitment of SHP-1 are the same tyrosine motifs
ments with the Tf receptor, well documented to be an inter-
that mediate binding of the clathrin adaptor protein AP2 (37).
nalized clathrin-dependent mechanism (46). Moreover, endo-
However, while tyrosine phosphorylation is required for SHP-1
cytosis is dependent on the dynamin-1 GTPase required for
binding, phosphorylation blocks binding of AP2, suggesting an
clathrin-mediated vesicle formation (24). Clathrin-dependent
inverse regulation of these two activities (37).
TATENO ET AL.
MOL. CELL. BIOL.
pathway utilized by Siglec-F is also suggestive of receptorswhose function is to deliver cargo to lysosomes for degradationand thus may also function as an innate immune receptorrelated to bacterial clearance or antigen presentation, as pro-posed for Siglec-H, another CD33-related siglec expressed onplasmacytoid dendritic cell precursors (84). Clinical and exper-imental investigations have shown that eosinophils can func-tion as antigen-presenting cells that can process microbial,viral, and parasitic antigens and migrate to lymph nodes topresent antigens to CD4⫹ T cells, thereby promoting T-cellproliferation and polarization (29, 44, 65, 66). It was reportedrecently that Siglec-F is highly expressed on CD11c⫹ lungmacrophages (52). We also found that Siglec-F is expressed onalveolar macrophages, bone marrow-derived dendritic cells,
and bone marrow-derived macrophages (data not shown).
FIG. 10. The endocytic machinery of Siglec-F can mediate uptake
of sialylated bacteria. SigF-CHO cells grown on coverslips were pre-
Recent reports on other members of the siglec family also
treated with or without Arthrobacter ureafaciens sialidase and were
suggest that they may function as receptors for clearance of
incubated with fixed, FITC-labeled Neisseria meningitidis MC58cap⫺
pathogens as part of the innate immune response. For exam-
containing the NeuAc␣2-3Gal sequence on ice for 30 min and allowed
ple, Sn and Siglec-5 bind to and internalize the invasive human
to internalize at 37°C for 60 min. Noninternalized bacteria were re-
moved with a PBS/BSA wash. Cells were examined by confocal mi-
pathogen Neisseria meningitidis, expressing sialylated lipopoly-
croscopy. Representative images are shown. Bar, 10 m.
saccharides containing the NeuAc␣2-3Gal linkage in a sialicacid-dependent manner even in the presence of cis ligands(38). As shown in this report, Siglec-F can also mediate uptake
Rapid mobilization of CD22 to the cell surface following
of Neisseria meningitidis expressing sialylated lipooligosaccha-
BCR ligation is consistent with redistribution of an intracellu-
rides containing the NeuAc␣2-3Gal linkage recognized as a
lar pool of CD22 localized in the para-Golgi recycling com-
ligand by Siglec-F (3, 71). Sn was also found to mediate asso-
partment shared by other recycling receptors (82). Conversely,
ciation of macrophages with Trypanosoma cruzi, a parasite
CD22 antibody induces rapid endocytosis of CD22 to the same
expressing cell surface sialic acids (45), and porcine Sn was
compartment, reaching a plateau of 60 to 70% internalized.
shown to mediate the entry of porcine reproductive and respi-
This redistribution is achieved without increased degradation
ratory viruses into porcine alveolar macrophages (30, 77).
(64, 87) and has been suggested to be consistent with redistri-
Thus, bacteria, parasites, and viruses expressing sialic acids on
bution of CD22 to an intracellular recycling pool (73). Elegant
their cell surfaces are likely to be natural trans ligands of siglec
studies by Shan and Press (64) earlier suggested that the con-
proteins (20, 79). The diversity of interactions of siglec proteins
stitutive endocytosis of CD22 was terminal, leading to degra-
with sialylated pathogens suggests that they may play multiple
dation with a half-life of ⬃8 h without recycling to the cell
roles in their interactions with immune cells (8, 18, 30, 38, 45).
surface. However, given the slower kinetics of CD22 endocy-
The roles emerging for the rapidly evolving siglec family
tosis relative to the Tf receptor (Fig. 4a), recycling of CD22
reflect diverse functions resulting from their varied specificity
from the intracellular pool might also be slower, and it is
for sialylated ligands by the N-terminal Ig domain and regula-
possible that recycling was not observed over the time course
tory motifs in their cytoplasmic domains that regulate cell
sufficient to demonstrate recycling of the Tf receptor.
signaling receptors and endocytosis. As documented in this
In contrast to CD22, Siglec-F endocytosis traffics to lysoso-
report, two siglec proteins of unrelated function, CD22 and
mal compartments by a mechanism that is clathrin, caveolin,
Siglec-F, exhibit clathrin-dependent and clathrin-independent
and dynamin-1 independent. The endocytic pathway utilized
mechanisms of endocytosis, both of which require intact cyto-
by Siglec-F appears to be similar to that utilized by major
plasmic tyrosine-based motifs.
histocompatibility complex class 1 (MHC-1), and several other
Since Siglec-F is a typical CD33-related siglec that shares
leukocyte receptors including interleukin-2 receptor ␣ subunit
highly conserved cytoplasmic tyrosine-based motifs, we predict
(Tac), E-cadherin, integrins, and CD59 (2, 4, 17, 48, 49, 56). In
that other CD33-related siglec proteins show similar endocytic
this pathway, receptors are first sorted to ARF6-positive en-
properties to Siglec-F. While there is no clear human ortholog
dosomes, which then fuse with early endosomes derived from
of Siglec-F, human Siglec-8 is considered a functionally con-
clathrin-cargo, before being sorted to late endosomes and ly-
vergent paralog due to its restricted expression on eosinophils
sosomes. As shown for Siglec-F (Fig. 6), expression of the
and unique specificity for the sialoside ligand 6⬘-sulfo-sLeX
ARF6-Q67L mutant, which blocks fusion of ARF6-positive
(11, 71). Thus, it will be of particular interest to see if the
endosomes with early endosomes, results in the accumulation
functional convergence of Siglec-F and Siglec-8 extends to
of receptors in vacuole-like structures (49). The requirement
their mechanisms of endocytosis. However, it is premature to
for tyrosine-dependent endocytosis of Siglec-F has also been
generalize the mechanisms of endocytosis for the siglec family.
demonstrated for MHC-1 (42, 63), although the exact role of
For example, some CD33-related siglec proteins such as
this sequence in the endocytic mechanism remains to be de-
Siglec-H and Siglec-14 lack cytoplasmic tyrosine-based motifs,
but they associate with the DAP12 coreceptor that contains a
As recently shown by Zhang et al. (86), Siglec-F negatively
cytoplasmic immunoreceptor tyrosine-based activation motif
regulates eosinophil responses to allergens. The endocytic
(ITAM) (10, 84). Thus, siglec proteins may undergo endocy-
ENDOCYTIC MECHANISMS OF SIGLEC PROTEINS
tosis by yet other mechanisms than those of CD22 and Siglec-F
Phosphatidylinositol 4,5-bisphosphate and Arf6-regulated membrane traffic.
described here. It is likely that a better understanding of the
J. Cell Biol. 154:1007–1017.
18. Carlin, A. F., A. L. Lewis, A. Varki, and V. Nizet. 2007. Group B strepto-
endocytic mechanisms of the siglec proteins will provide in-
coccal capsular sialic acids interact with Siglecs (immunoglobulin-like lec-
sights into their biological roles.
tins) on human leukocytes. J. Bacteriol. 189:1231–1237.
19. Castro, A. G., N. Esaguy, P. M. Macedo, A. P. Aguas, and M. T. Silva. 1991.
Live but not heat-killed mycobacteria cause rapid chemotaxis of large num-
bers of eosinophils in vivo and are ingested by the attracted granulocytes.
Infect. Immun. 59:3009–3014.
We thank Jill Ferguson, Mary O'Reilly, and Anna Tran-Crie for
20. Chava, A. K., S. Bandyopadhyay, M. Chatterjee, and C. Mandal. 2004.
help in preparing this article. We are grateful to Sandra L. Schmid for
Sialoglycans in protozoal diseases: their detection, modes of acquisition and
dynamin-1 cDNA constructs (WT and the K44A mutant), discussions,
emerging biological roles. Glycoconj. J. 20:199–206.
and suggestions; Julie G. Donaldson for ARF6-EGFP cDNA con-
21. Cheng, Z. J., R. D. Singh, D. K. Sharma, E. L. Holicky, K. Hanada, D. L.
structs (WT and the T27N and Q67L mutants); James J. Lee for
Marks, and R. E. Pagano. 2006. Distinct mechanisms of clathrin-indepen-
dent endocytosis have unique sphingolipid requirements. Mol. Biol. Cell
IL5-Tg mice; and The Consortium for Functional Glycomics for the
1,000-kDa lactose-PAA probe.
22. Collins, B. E., O. Blixt, S. Han, B. Duong, H. Li, J. K. Nathan, N. Bovin, and
This work was supported by grants GM60938 and AI050143 from
J. C. Paulson. 2006. High-affinity ligand probes of CD22 overcome the
the National Institutes of Health, by a Wellcome Trust Senior Fellow-
threshold set by cis ligands to allow for binding, endocytosis, and killing of B
ship awarded to P.R.C., by Presidium RAS "Molecular and Cell Biol-
cells. J. Immunol. 177:2994–3003.
ogy" to N.V.B., and by a JSPS Postdoctoral Fellowship for Research
23. Collins, B. E., B. A. Smith, P. Bengtson, and J. C. Paulson. 2006. Ablation
Abroad awarded to H.T.
of CD22 in ligand-deficient mice restores B cell receptor signaling. Nat.
Immunol. 7:199–206.
24. Conner, S. D., and S. L. Schmid. 2003. Regulated portals of entry into the
cell. Nature 422:37–44.
1. Ahluwalia, J., A. Tinker, L. H. Clapp, M. R. Duchen, A. Y. Abramov, S. Pope,
25. Crocker, P. R. 2005. Siglecs in innate immunity. Curr. Opin. Pharmacol.
M. Nobles, and A. W. Segal. 2004. The large-conductance Ca2⫹-activated
K⫹ channel is essential for innate immunity. Nature 427:853–858.
26. Crocker, P. R. 2002. Siglecs: sialic-acid-binding immunoglobulin-like lectins
2. Aikawa, Y., and T. F. Martin. 2003. ARF6 regulates a plasma membrane
in cell-cell interactions and signalling. Curr. Opin. Struct. Biol. 12:609–615.
pool of phosphatidylinositol(4,5)bisphosphate required for regulated exocy-
27. Crocker, P. R., J. C. Paulson, and A. Varki. 2007. Siglecs and their roles in
tosis. J. Cell Biol. 162:647–659.
the immune system. Nat. Rev. Immunol. 7:255–266.
3. Angata, T., R. Hingorani, N. M. Varki, and A. Varki. 2001. Cloning and
28. Damke, H., D. D. Binns, H. Ueda, S. L. Schmid, and T. Baba. 2001. Dynamin
characterization of a novel mouse Siglec, mSiglec-F: differential evolution of
GTPase domain mutants block endocytic vesicle formation at morphologi-
the mouse and human (CD33) Siglec-3-related gene clusters. J. Biol. Chem.
cally distinct stages. Mol. Biol. Cell 12:2578–2589.
29. Del Pozo, V., B. De Andres, E. Martin, B. Cardaba, J. C. Fernandez, S.
4. Arnaoutova, I., C. L. Jackson, O. S. Al-Awar, J. G. Donaldson, and Y. P. Loh.
Gallardo, P. Tramon, F. Leyva-Cobian, P. Palomino, and C. Lahoz. 1992.
2003. Recycling of Raft-associated prohormone sorting receptor car-
Eosinophil as antigen-presenting cell: activation of T cell clones and T cell
boxypeptidase E requires interaction with ARF6. Mol. Biol. Cell 14:4448–
hybridoma by eosinophils after antigen processing. Eur. J. Immunol. 22:
5. Augustin, R., J. Riley, and K. H. Moley. 2005. GLUT8 contains a [DE]XXX-
30. Delputte, P. L., and H. J. Nauwynck. 2004. Porcine arterivirus infection of
L[LI] sorting motif and localizes to a late endosomal/lysosomal compart-
alveolar macrophages is mediated by sialic acid on the virus. J. Virol. 78:
ment. Traffic 6:1196–1212.
6. Avril, T., H. Floyd, F. Lopez, E. Vivier, and P. R. Crocker. 2004. The
31. Dobrina, A., R. Menegazzi, T. M. Carlos, E. Nardon, R. Cramer, T. Zacchi,
membrane-proximal immunoreceptor tyrosine-based inhibitory motif is crit-
J. M. Harlan, and P. Patriarca. 1991. Mechanisms of eosinophil adherence
ical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related
to cultured vascular endothelial cells. Eosinophils bind to the cytokine-
Siglecs expressed on human monocytes and NK cells. J. Immunol. 173:6841–
induced ligand vascular cell adhesion molecule-1 via the very late activation
antigen-4 integrin receptor. J. Clin. Investig. 88:20–26.
7. Avril, T., S. D. Freeman, H. Attrill, R. G. Clarke, and P. R. Crocker. 2005.
32. Galioto, A. M., J. A. Hess, T. J. Nolan, G. A. Schad, J. J. Lee, and D.
Siglec-5 (CD170) can mediate inhibitory signaling in the absence of immu-
Abraham. 2006. Role of eosinophils and neutrophils in innate and adaptive
noreceptor tyrosine-based inhibitory motif phosphorylation. J. Biol. Chem.
protective immunity to larval Strongyloides stercoralis in mice. Infect. Immun.
8. Avril, T., E. R. Wagner, H. J. Willison, and P. R. Crocker. 2006. Sialic
33. Glebov, O. O., N. A. Bright, and B. J. Nichols. 2006. Flotillin-1 defines a
acid-binding immunoglobulin-like lectin 7 mediates selective recognition of
clathrin-independent endocytic pathway in mammalian cells. Nat. Cell Biol.
sialylated glycans expressed on Campylobacter jejuni lipooligosaccharides.
Infect. Immun. 74:4133–4141.
34. Grewal, P. K., M. Boton, K. Ramirez, B. E. Collins, A. Saito, R. S. Green, K.
9. Biedermann, B., D. Gil, D. T. Bowen, and P. R. Crocker. 2007. Analysis of the
Ohtsubo, D. Chui, and J. D. Marth. 2006. ST6Gal-I restrains CD22-depen-
CD33-related siglec family reveals that Siglec-9 is an endocytic receptor
dent antigen receptor endocytosis and Shp-1 recruitment in normal and
expressed on subsets of acute myeloid leukemia cells and absent from nor-
pathogenic immune signaling. Mol. Cell. Biol. 26:4970–4981.
mal hematopoietic progenitors. Leuk. Res. 31:211–220.
35. Henley, J. R., E. W. Krueger, B. J. Oswald, and M. A. McNiven. 1998.
10. Blasius, A. L., M. Cella, J. Maldonado, T. Takai, and M. Colonna. 2006.
Dynamin-mediated internalization of caveolae. J. Cell Biol. 141:85–99.
Siglec-H is an IPC-specific receptor that modulates type I IFN secretion
36. Inoue, Y., Y. Matsuwaki, S. H. Shin, J. U. Ponikau, and H. Kita. 2005.
through DAP12. Blood 107:2474–2476.
Nonpathogenic, environmental fungi induce activation and degranulation of
11. Bochner, B. S., R. A. Alvarez, P. Mehta, N. V. Bovin, O. Blixt, J. R. White,
human eosinophils. J. Immunol. 175:5439–5447.
and R. L. Schnaar. 2005. Glycan array screening reveals a candidate ligand
37. John, B., B. R. Herrin, C. Raman, Y. N. Wang, K. R. Bobbitt, B. A. Brody,
for Siglec-8. J. Biol. Chem. 280:4307–4312.
and L. B. Justement. 2003. The B cell coreceptor CD22 associates with
12. Bonazzi, M., and P. Cossart. 2006. Bacterial entry into cells: a role for the
AP50, a clathrin-coated pit adapter protein, via tyrosine-dependent interac-
endocytic machinery. FEBS Lett. 580:2962–2967.
tion. J. Immunol. 170:3534–3543.
13. Bonifacino, J. S., and L. M. Traub. 2003. Signals for sorting of transmem-
38. Jones, C., M. Virji, and P. R. Crocker. 2003. Recognition of sialylated
brane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395–
meningococcal lipopolysaccharide by siglecs expressed on myeloid cells leads
to enhanced bacterial uptake. Mol. Microbiol. 49:1213–1225.
14. Booth, J. W., M. K. Kim, A. Jankowski, A. D. Schreiber, and S. Grinstein.
39. Keller, G. A., M. W. Siegel, and I. W. Caras. 1992. Endocytosis of glyco-
2002. Contrasting requirements for ubiquitylation during Fc receptor-medi-
phospholipid-anchored and transmembrane forms of CD4 by different en-
ated endocytosis and phagocytosis. EMBO J. 21:251–258.
docytic pathways. EMBO J. 11:863–874.
15. Borelli, V., F. Vita, S. Shankar, M. R. Soranzo, E. Banfi, G. Scialino, C.
40. Le, P. U., G. Guay, Y. Altschuler, and I. R. Nabi. 2002. Caveolin-1 is a
Brochetta, and G. Zabucchi. 2003. Human eosinophil peroxidase induces
negative regulator of caveolae-mediated endocytosis to the endoplasmic
surface alteration, killing, and lysis of Mycobacterium tuberculosis. Infect.
reticulum. J. Biol. Chem. 277:3371–3379.
41. Lee, N. A., M. P. McGarry, K. A. Larson, M. A. Horton, A. B. Kristensen,
16. Bovin, N. V., E. Korchagina, T. V. Zemlyanukhina, N. E. Byramova, O. E.
and J. J. Lee. 1997. Expression of IL-5 in thymocytes/T cells leads to the
Galanina, A. E. Zemlyakov, A. E. Ivanov, V. P. Zubov, and L. V. Mochalova.
development of a massive eosinophilia, extramedullary eosinophilopoiesis,
1993. Synthesis of polymeric neoglycoconjugates based on N-substituted
and unique histopathologies. J. Immunol. 158:1332–1344.
polyacrylamides. Glycoconj. J. 10:142–151.
42. Lizee, G., G. Basha, J. Tiong, J. P. Julien, M. Tian, K. E. Biron, and W. A.
17. Brown, F. D., A. L. Rozelle, H. L. Yin, T. Balla, and J. G. Donaldson. 2001.
Jefferies. 2003. Control of dendritic cell cross-presentation by the major
TATENO ET AL.
MOL. CELL. BIOL.
histocompatibility complex class I cytoplasmic domain. Nat. Immunol.
67. Shilova, N. V., O. E. Galanina, T. V. Pochechueva, A. A. Chinarev, V. A.
Kadykov, A. B. Tuzikov, and N. V. Bovin. 2005. High molecular weight
43. Lock, K., J. Zhang, J. Lu, S. H. Lee, and P. R. Crocker. 2004. Expression of
neoglycoconjugates for solid phase assays. Glycoconj. J. 22:43–51.
CD33-related siglecs on human mononuclear phagocytes, monocyte-derived
68. Sigismund, S., T. Woelk, C. Puri, E. Maspero, C. Tacchetti, P. Transidico,
dendritic cells and plasmacytoid dendritic cells. Immunobiology 209:199–
P. P. Di Fiore, and S. Polo. 2005. Clathrin-independent endocytosis of
ubiquitinated cargos. Proc. Natl. Acad. Sci. USA 102:2760–2765.
44. Mawhorter, S. D., J. W. Kazura, and W. H. Boom. 1994. Human eosinophils
69. Stoddart, A., M. L. Dykstra, B. K. Brown, W. Song, S. K. Pierce, and F. M.
as antigen-presenting cells: relative efficiency for superantigen- and antigen-
Brodsky. 2002. Lipid rafts unite signaling cascades with clathrin to regulate
induced CD4⫹ T-cell proliferation. Immunology 81:584–591.
BCR internalization. Immunity 17:451–462.
45. Monteiro, V. G., C. S. Lobato, A. R. Silva, D. V. Medina, M. A. de Oliveira,
70. Stoddart, A., A. P. Jackson, and F. M. Brodsky. 2005. Plasticity of B cell
S. H. Seabra, W. de Souza, and R. A. DaMatta. 2005. Increased association
receptor internalization upon conditional depletion of clathrin. Mol. Biol.
of Trypanosoma cruzi with sialoadhesin positive mice macrophages. Parasi-
tol. Res. 97:380–385.
71. Tateno, H., P. R. Crocker, and J. C. Paulson. 2005. Mouse Siglec-F and
46. Mukherjee, S., R. N. Ghosh, and F. R. Maxfield. 1997. Endocytosis. Physiol.
human Siglec-8 are functionally convergent paralogs that are selectively
expressed on eosinophils and recognize 6⬘-sulfo-sialyl Lewis X as a preferred
47. Nakamura, K., T. Yamaji, P. R. Crocker, A. Suzuki, and Y. Hashimoto. 2002.
glycan ligand. Glycobiology 15:1125–1135.
Lymph node macrophages, but not spleen macrophages, express high levels
72. Taylor, V. C., C. D. Buckley, M. Douglas, A. J. Cody, D. L. Simmons, and
of unmasked sialoadhesin: implication for the adhesive properties of mac-
S. D. Freeman. 1999. The myeloid-specific sialic acid-binding receptor,
rophages in vivo. Glycobiology 12:209–216.
CD33, associates with the protein-tyrosine phosphatases, SHP-1 and SHP-2.
48. Naslavsky, N., R. Weigert, and J. G. Donaldson. 2004. Characterization of a
J. Biol. Chem. 274:11505–11512.
nonclathrin endocytic pathway: membrane cargo and lipid requirements.
73. Tedder, T. F., J. C. Poe, and K. M. Haas. 2005. CD22: a multifunctional
Mol. Biol. Cell 15:3542–3552.
receptor that regulates B lymphocyte survival and signal transduction. Adv.
49. Naslavsky, N., R. Weigert, and J. G. Donaldson. 2003. Convergence of
non-clathrin- and clathrin-derived endosomes involves Arf6 inactivation and
74. Toppila, S., T. Paavonen, A. Laitinen, L. A. Laitinen, and R. Renkonen. 2000.
changes in phosphoinositides. Mol. Biol. Cell 14:417–431.
Endothelial sulfated sialyl Lewis x glycans, putative L-selectin ligands, are
50. Nguyen, D. H., E. D. Ball, and A. Varki. 2006. Myeloid precursors and acute
preferentially expressed in bronchial asthma but not in other chronic inflam-
myeloid leukemia cells express multiple CD33-related Siglecs. Exp. Hema-
matory lung diseases. Am. J. Respir. Cell Mol. Biol.
75. Tuscano, J. M., R. T. O'Donnell, L. A. Miers, L. A. Kroger, D. L. Kukis, K. R.
51. Oh, P., D. P. McIntosh, and J. E. Schnitzer. 1998. Dynamin at the neck of
Lamborn, T. F. Tedder, and G. L. DeNardo. 2003. Anti-CD22 ligand-block-
caveolae mediates their budding to form transport vesicles by GTP-driven
ing antibody HB22.7 has independent lymphomacidal properties and aug-
fission from the plasma membrane of endothelium. J. Cell Biol. 141:101–114.
ments the efficacy of 90Y-DOTA-peptide-Lym-1 in lymphoma xenografts.
52. Padigel, U. M., J. J. Lee, T. J. Nolan, G. A. Schad, and D. Abraham. 2006.
Eosinophils can function as antigen-presenting cells to induce primary and
76. Uthayakumar, S., and B. L. Granger. 1995. Cell surface accumulation of
secondary immune responses to Strongyloides stercoralis. Infect. Immun. 74:
overexpressed hamster lysosomal membrane glycoproteins. Cell Mol. Biol.
53. Paul, S. P., L. S. Taylor, E. K. Stansbury, and D. W. McVicar. 2000. Myeloid
77. Vanderheijden, N., P. L. Delputte, H. W. Favoreel, J. Vandekerckhove, J.
specific human CD33 is an inhibitory receptor with differential ITIM func-
Van Damme, P. A. van Woensel, and H. J. Nauwynck. 2003. Involvement of
tion in recruiting the phosphatases SHP-1 and SHP-2. Blood 96:483–490.
sialoadhesin in entry of porcine reproductive and respiratory syndrome virus
54. Pierce, S. K. 2002. Lipid rafts and B-cell activation. Nat. Rev. Immunol.
into porcine alveolar macrophages. J. Virol. 77:8207–8215.
78. Varki, A., and T. Angata. 2006. Siglecs—the major subfamily of I-type lec-
55. Prekeris, R., B. Yang, V. Oorschot, J. Klumperman, and R. H. Scheller.
tins. Glycobiology 16:1R–27R.
1999. Differential roles of syntaxin 7 and syntaxin 8 in endosomal trafficking.
79. Vimr, E. R., K. A. Kalivoda, E. L. Deszo, and S. M. Steenbergen. 2004.
Mol. Biol. Cell 10:3891–3908.
Diversity of microbial sialic acid metabolism. Microbiol. Mol. Biol. Rev.
56. Radhakrishna, H., and J. G. Donaldson. 1997. ADP-ribosylation factor 6 reg-
ulates a novel plasma membrane recycling pathway. J. Cell Biol. 139:49–61.
80. Wakarchuk, W., A. Martin, M. P. Jennings, E. R. Moxon, and J. C. Richards.
57. Rapoport, E. M., Y. B. Sapot'ko, G. V. Pazynina, V. K. Bojenko, and N. V.
1996. Functional relationships of the genetic locus encoding the glycosyl-
Bovin. 2005. Sialoside-binding macrophage lectins in phagocytosis of apop-
transferase enzymes involved in expression of the lacto-N-neotetraose ter-
totic bodies. Biochemistry (Moscow) 70:330–338.
minal lipopolysaccharide structure in Neisseria meningitidis. J. Biol. Chem.
58. Razi, N., and A. Varki. 1999. Cryptic sialic acid binding lectins on human
blood leukocytes can be unmasked by sialidase treatment or cellular activa-
81. Walter, R. B., B. W. Raden, D. M. Kamikura, J. A. Cooper, and I. D.
tion. Glycobiology 9:1225–1234.
Bernstein. 2005. Influence of CD33 expression levels and ITIM-dependent
59. Razi, N., and A. Varki. 1998. Masking and unmasking of the sialic acid-
internalization on gemtuzumab ozogamicin-induced cytotoxicity. Blood 105:
binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc. Natl.
Acad. Sci. USA 95:7469–7474.
82. Yamashiro, D. J., B. Tycko, S. R. Fluss, and F. R. Maxfield. 1984. Segrega-
60. Rodal, S. K., G. Skretting, O. Garred, F. Vilhardt, B. van Deurs, and K.
tion of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the
Sandvig. 1999. Extraction of cholesterol with methyl-beta-cyclodextrin per-
recycling pathway. Cell 37:789–800.
turbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 10:961–
83. Yazdanbakhsh, M., C. M. Eckmann, A. A. M. Bot, and D. Roos. 1986.
Bactericidal action of eosinophils from normal human blood. Infect. Immun.
61. Rothberg, K. G., J. E. Heuser, W. C. Donzell, Y. S. Ying, J. R. Glenney, and
R. G. Anderson. 1992. Caveolin, a protein component of caveolae membrane
84. Zhang, J., A. Raper, N. Sugita, R. Hingorani, M. Salio, M. J. Palmowski, V.
coats. Cell 68:673–682.
Cerundolo, and P. R. Crocker. 2006. Characterization of Siglec-H as a novel
62. Rothenberg, M. E., and S. P. Hogan. 2006. The eosinophil. Annu. Rev.
endocytic receptor expressed on murine plasmacytoid dendritic cell precur-
sors. Blood 107:3600–3608.
63. Santos, S. G., A. N. Antoniou, P. Sampaio, S. J. Powis, and F. A. Arosa. 2006.
85. Zhang, J. Q., B. Biedermann, L. Nitschke, and P. R. Crocker. 2004. The
Lack of tyrosine 320 impairs spontaneous endocytosis and enhances release
murine inhibitory receptor mSiglec-E is expressed broadly on cells of the
of HLA-B27 molecules. J. Immunol. 176:2942–2949.
innate immune system whereas mSiglec-F is restricted to eosinophils. Eur.
64. Shan, D., and O. W. Press. 1995. Constitutive endocytosis and degradation
J. Immunol. 34:1175–1184.
of CD22 by human B cells. J. Immunol. 154:4466–4475.
86. Zhang, M., T. Angata, J. Y. Cho, M. Miller, D. H. Broide, and A. Varki. 2007.
65. Shi, H. Z. 2004. Eosinophils function as antigen-presenting cells. J. Leukoc.
Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on
mouse eosinophils. Blood 109:4280–4287.
66. Shi, H. Z., A. Humbles, C. Gerard, Z. Jin, and P. F. Weller. 2000. Lymph
87. Zhang, M., and A. Varki. 2004. Cell surface sialic acids do not affect primary
node trafficking and antigen presentation by endobronchial eosinophils.
CD22 interactions with CD45 and surface IgM nor the rate of constitutive
J. Clin. Investig. 105:945–953.
CD22 endocytosis. Glycobiology 14:939–949.
Source: http://carbohydrate.ru/pdf/cv280.pdf
Federal Register / Vol. 75, No. 169 / Wednesday, September 1, 2010 / Notices (8) Narrow woven ribbons comprised Countervailing Duty Order merchandise in an amount equal to the at least 85 percent by weight of threads According to section 706(b)(2) of the net countervailable subsidy rates noted having a denier of 225 or higher; Act, duties shall be assessed on subject
GUIDELINES FOR THE MANAGEMENT OF THE INFANT WITH NEONATAL ABSTINENCE SYNDROME Background Neonatal Abstinence Syndrome (NAS) is a syndrome of drug withdrawal observed in infants of mothers physically dependent on drugs. Also known as neonatal withdrawal syndrome or passive addiction, NAS is a condition resulting from exposure in utero or postnatal exposure to opioids and other illicit drugs. It is more common in infants born to opioid-dependent women than in infants born to women dependent on other drugs or alcohol.1



