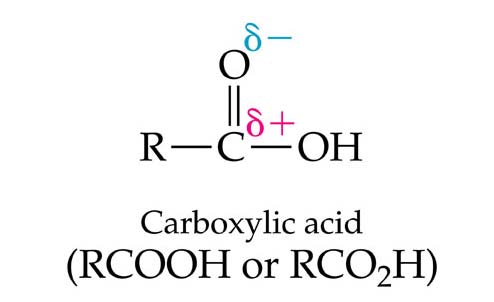Microsoft powerpoint - chm102chapter13a
The Rules for Boiling Points
• The boiling points of compounds depend on how strongly they stick together: The more strongly they stick together, the higher the boilingpoint (the more heat it takes to rip them apart).
• There are two main forces that hold molecules together:
These require both positive hydrogens (from O-H or N-H bonds)
and electron lone pairs (found mainly on
O and
N atoms)
-
London forces (see Chapter 6, pages 169-170)
-Bigger molecules have stronger intermolecular forces, and higherboiling points, other things being equal.
-These forces are very short-range, so this also depends on how
close the molecules can get together. The more branches in an alkane, the harder it is for them to get close. Branched alkanes thus have lower densities and lower boiling points (see page 257).
Copyright Houghton Mifflin Company. All rights reserved.
Recent News
• A recent report asserts that dogs have been trained to detect
cancer by sniffing samples taken from the breaths of healthy
persons and cancer victims.
• Dogs can detect odors at the low parts per billion (ppb) level.
• It is claimed that tumor cells emit different chemicals--
alkanes and benzene derivatives--than healthy cells.
• Reference: The New York Times, January 17, 2006, p. D5.
Copyright Houghton Mifflin Company. All rights reserved.
• It is very important that you begin to
study the material as early as possible.
• Working the practice problems and
answering the practice questions is
absolutely essential.
Copyright Houghton Mifflin Company. All rights reserved.
Functional Groups Containing the
Carbon-Oxygen Double Bond
Copyright Houghton Mifflin Company. All rights reserved.
13.1 The Carbonyl Group
•
A Carbonyl Group consists of a carbon atom
and an oxygen atom joined by a double bond.
This is by definition a carbonyl group (C=O).
» Acetone for example contains a carbonyl group.
Pronounced carbon-EEL
• Carbonyl groups are strongly polarized, with a partial positive charge on carbon and partial negative charge on oxygen.
Copyright Houghton Mifflin Company. All rights reserved.
Because oxygen is more electronegative than carbon,
it pulls the electrons in the double bond toward oxygen
creating a partial negative charge on oxygen and a partial
positive charge on the carbonyl carbon atom. Much of
the reactivity of carbonyl groups results from this
polarization.
Copyright Houghton Mifflin Company. All rights reserved.
The
polarity of the carbonyl group
contributes to its reactivity.
The carbonyl carbon is bonded to oxygen and two other atoms. The bond angles between the three components on carbon are 120o or close to it.
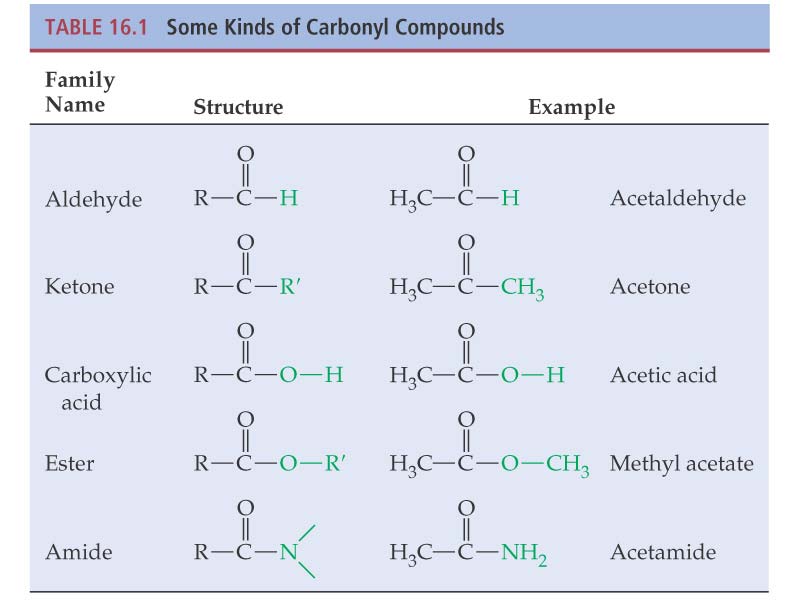
Carbonyl compounds are broadly divided into two groups: (1) aldehydesand ketones are in one group and (2) the second group contains carboxylic acids, esters, and amides.
Copyright Houghton Mifflin Company. All rights reserved.
Copyright Houghton Mifflin Company. All rights reserved.
Structural Characteristics and
Naming of Aldehydes
Copyright Houghton Mifflin Company. All rights reserved.
An aldehyde is a compound that has at least one
hydrogen atom attached to the carbon atom of a
carbonyl group.
The R group in an aldehyde can be
H, alkyl, or aryl.
Copyright Houghton Mifflin Company. All rights reserved.
Some Common Aldehydes
Formaldehyde, H(C=O)H: Toxic but useful. It kills viruses, fungi, and bacteria. It is used in disinfecting and sterilizing equipment.
Acetaldehyde CH (C=O)H: Sweet smelling and
narcotic. Present in ripe fruits, especially in apples. It is less toxic than formaldehyde.
Benzaldehyde Ph(C=O)H: Oil of almonds;
used to make dyes, perfumes, and specific flavors.
Copyright Houghton Mifflin Company. All rights reserved.
Some Common Aldehydes
Some aldehydes are present in nuts and spices.
oil of almonds cinnamaldehyde
Copyright Houghton Mifflin Company. All rights reserved.
The simplest aldehydes are known by their common names, formaldehyde, acetaldehyde, benzaldehyde, and so on. To name aldehydes systematically in the IUPAC system, the final –e of the name of the alkane is replaced by –al.
Copyright Houghton Mifflin Company. All rights reserved.
When a compound contains more than one type of
functional group, the suffix for only one of them can
be used as the ending of the name. The IUPAC rules
define priorities that specify which suffix should be
used:
1. Carboxylic acid
2. Aldehyde
3. Ketone
4. Alcohol
5. Amine
6. Alkene
7. alkyne
Remember that alkoxy (ether), halogen (halide), and alkyl
Groups are treated as substituents and listed in
alphabetical order.
Copyright Houghton Mifflin Company. All rights reserved.
1. To name an aldehyde, select the longest chain which
contains the aldehyde functional group.
2. Then name the parent chain by changing the -e ending
to -al.
3. Number the parent chain starting with 1 for the aldehyde
carbon atom.
4. Determine the identity of the substituents, and add this
information to the front of the parent chain name.
Note that the aldehyde
is the primary functional
pentane Æ pentanal
group and so this isnamed as an aldehyde and
pentanal Æ 3-hydroxypentanal
not as an alcohol.
Copyright Houghton Mifflin Company. All rights reserved.
Properties and Reactions
of Aldehydes
Copyright Houghton Mifflin Company. All rights reserved.
Properties of Aldehydes and Ketones
The polarity of the carbonyl group makes aldehydes and ketones moderately polar compounds. As a result, they have boiling points higher than alkanes and are reactive.
Aldehydes and ketones do not form hydrogen bonds to each other, however, they form hydrogen bonds with water using the lone pairs of electrons on oxygen.
Aldehydes and ketones have boiling points intermediate between alkanes and alcohols of similar size.
Copyright Houghton Mifflin Company. All rights reserved.
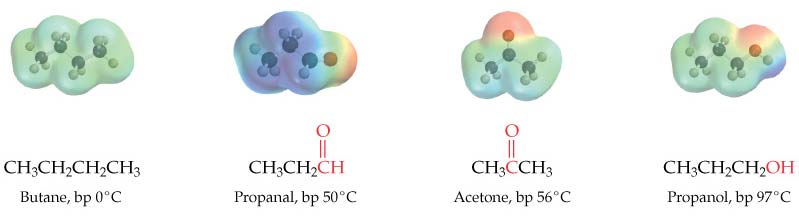
Aldehydes and ketones are soluble in common organic solvents, and those with fewer then five carbons are also soluble in water.
Copyright Houghton Mifflin Company. All rights reserved.
Rules for Solubility
• "Like dissolves like." This means that polar compounds dissolve
best in polar solvents (like water and alcohols) and nonpolar
compounds dissolve best in nonploar solvents (like hexane and
benzene).
• Some compounds, like alcohols, have a polar part (the -OH
group) and a nonpolar part (the hydrocarbon portion). Solubility in
water depends on which part dominates. Alcohols with a large
"organic" (hydrocarbons) portion are not very soluble in water.
• Carbonyl compounds will be soluble in water if their hydrocarbon
portion is not too large. The water can form hydrogen bonds to the
electron lone pairs on the oxygen. But if the organic portion is too
large, solubility is decreased.
• Acetone, CH -(C=O)-CH , is an excellent solvent for both polar and
Copyright Houghton Mifflin Company. All rights reserved.
--Simple ketones are excellent solvents
because they dissolve both polar and
nonpolar compounds; acetone
(CH HC=O) is an example.
--The lower boiling aldehydes and ketones are flammable.
--The simple ketones have low toxicity, however, many simple aldehydes are toxic
because they react with proteins and other
biomolecules; example -formaldehyde.
Copyright Houghton Mifflin Company. All rights reserved.
Oxidation of Aldehydes
• Primary alcohols can be oxidized to
aldehydes and secondary alcohols to ketones.
• Aldehydes can be further oxidized to
carboxylic acids.
• Since ketones cannot be further
oxidized, application with a mild oxidizing agent can be used as a test to distinguish between aldehydes and ketone.
Copyright Houghton Mifflin Company. All rights reserved.
CH CH=O + [O] → CH COOH + H O
[further oxidation
to an organic acid]
CH CH-CH + [O] → CH (C=O)CH + H O
The ketone cannot be further oxidized.
Copyright Houghton Mifflin Company. All rights reserved.



A positive Tollens test for aldehydes involves the formation of a silver mirror. (a) An aqueous solution of ethanal is added to a solution of silver nitrate in aqueous ammonia and stirred. (b) The solution darkens as ethanal is oxidized to ethanoic acid, and Ag+1 ion is reduced to silver. (c) The inside of the beaker becomes coated with metallic silver.
The aldehyde is oxidized and the metal is reduced.
Tollens test: Ag+ (soluble) Æ Ag metal (mirror).
Copyright Houghton Mifflin Company. All rights reserved.
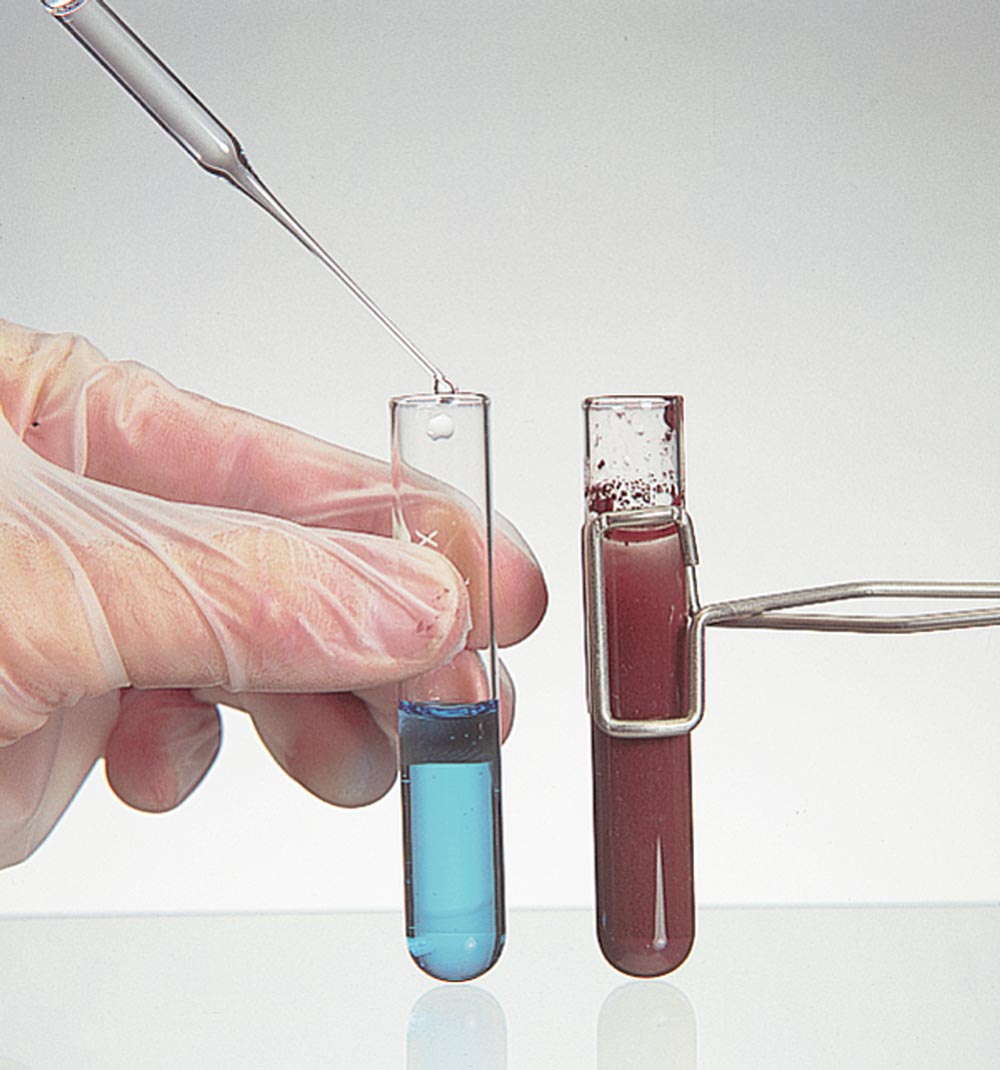
Benedict's solution, which is blue in color, turns brick red when an aldehyde reacts with it.
The aldehyde
is oxidized
and
the metal is
Benedict's test: Cu++ (blue) Æ Cu+ (brick red).
Copyright Houghton Mifflin Company. All rights reserved.
Reduction of Aldehydes and Ketones
Reduction is the reverse of the oxidation reaction. Reduction of a carbonyl group is the addition of hydrogens across the double bond to produce an –OH group.
Aldehydes are reduced to primary alcohols, and ketones are reduced to secondary alcohols.
Reduction of the carbonyl group occurs by formation of a bond to the carbonyl carbon by a hydride, H:-, ion accompanied by bonding of a H+ ion to the carbonyl oxygen atom.
Copyright Houghton Mifflin Company. All rights reserved.
Structural Characteristics and
Naming of Ketones
Copyright Houghton Mifflin Company. All rights reserved.
Acetone, CH (C=O)CH ): It is one of the
most widely used solvents. It can dissolve most organic compounds and is also miscible with water. Acetone is highly volatile and is also highly flammable. Ketones, like aldehydes, occur widely in Nature.
Copyright Houghton Mifflin Company. All rights reserved.
Some ketones are best known by their common names which give the names of the two alkyl groups bonded to the carbonyl group followed by the word ketone. For example, ethyl methyl ketone =
Ketones are named systematically (IUPAC) by replacing the final –e of the corresponding alkane name with –one. The numbering of the alkane chain begins at the end nearest to the carbonyl group. The location of the carbonyl group is indicated by placing the number of the carbonyl carbon in front of the name; e.g. 2-propanone = acetone. The above compound is …
Copyright Houghton Mifflin Company. All rights reserved.
Properties and Reactions
of Ketones
Copyright Houghton Mifflin Company. All rights reserved.
The physical properties of ketones closely parallel
the properties of aldehydes; i.e. their boiling points
are intermediate between alkanes and alcohols.
Ketones are not readily oxidized.
Ketones are readily reduced to secondary alcohols.
Copyright Houghton Mifflin Company. All rights reserved.
Chemistry at a Glance:
Reactions Involving Aldehydes and Ketones
Copyright Houghton Mifflin Company. All rights reserved.
Copyright Houghton Mifflin Company. All rights reserved.
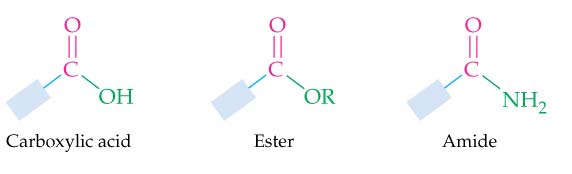
Carboxylic Acids and Their Derivatives:
Properties and Names
Caboxylic acids have an –OH group bonded to a
carbonyl group. In their derivatives, OH is
substituted by other groups. Such as,
Esters have an –OR group bonded to a carbonyl
group.
Amides have an –NH group bonded to a carbonyl
Copyright Houghton Mifflin Company. All rights reserved.
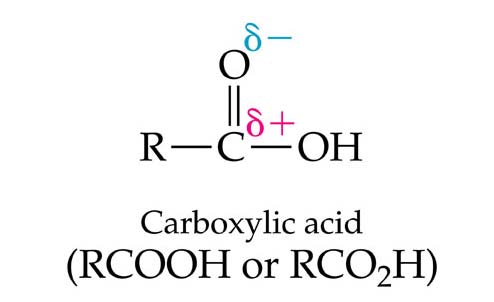
Carboxylic Acids
are acidic
Carboxylic acids donate a proton to bases.
Carboxylic acids hydrogen bond with each other. As a result of hydrogen bonding, they have higher boiling points than similar alkanes.
Copyright Houghton Mifflin Company. All rights reserved.
Learn these names
Copyright Houghton Mifflin Company. All rights reserved.
Two common NSAID's (non-steroidal anti-inflammatory
drugs) are Ibuprofen (a.k.a. Motrin and Advil) and
Naproxen (a.k.a. Naprosyn and Aleve). They are both
carboxylic acids.
Chronic long term used of these OTC drugs has recently
been shown to increase the risk of heart disease.
Copyright Houghton Mifflin Company. All rights reserved.
Advil and Aleve
Copyright Houghton Mifflin Company. All rights reserved.
--Carboxylic acids have a sharp and
strong odor.
--Up to four carbon containing
carboxylic acids are water soluble.
--Carboxylic acids are named
systematically (IUPAC) by replacing
the –e at the end of the alkane name
with – oic acid. If alkyl or other
substituents are present, the chain
is numbered beginning at the
COOH end.
Copyright Houghton Mifflin Company. All rights reserved.
Carboxylic acids have a higher priority when naming.
Therefore, this molecule is not named as a ketone, butinstead is named as a carboxylic acid.
Copyright Houghton Mifflin Company. All rights reserved.
Dicarboxylic acids, which contain two
–COOH groups, include the biochemicals
succinic acid, HOOC-CH -CH -COOH, and
glutaric acid, HOOC-CH -CH -CH -COOH.
Unsaturated acids are named
systematically in the IUPAC system
with the ending –enoic acid.
Acids with larger saturated alkyl
groups are waxy, odorless solids.
Water solubility decreases as the size
of the alkyl group increases.
Copyright Houghton Mifflin Company. All rights reserved.
Chemical Portraits:
Commonly Encountered Carboxylic Acids
Copyright Houghton Mifflin Company. All rights reserved.
Properties and Reactions of
Copyright Houghton Mifflin Company. All rights reserved.
Notes on Some Common Carboxylic Acids
Formic acid, HCOOH: Chemical that is present in the sting of ants.
Acetic acid, CH COOH: dilute (5%) aqueous
acetic acid is known as vinegar.
Butyric acid, CH CH CH COOH: Chemical
responsible for odor of rancid butter.
Amino acids, the building blocks of proteins, have the general formula R-CH(NH )-COOH.
Citric acid: Present in citrus fruits and blood.
Copyright Houghton Mifflin Company. All rights reserved.
Acidity of Carboxylic Acids
Carboxylic acids are weak acids.
Acid strengths of common carboxylic acids are about the same as that for acetic acid.
Carboxylic acids undergo neutralization reactions with bases and produce water and a carboxylic acid salt.
The sodium and potassium salts of carboxylic acids are ionic solids that are more soluble in water than the carboxylic acids themselves.
Copyright Houghton Mifflin Company. All rights reserved.
Carboxylic acids are acids because they donate
a proton to a base; e.g. water.
butyric acid
A carboxylate ion is a negative ion produced when a
carboxylic acid loses a proton (acidic hydrogen atom).
In this case the equilibrium lies far to the left because
carboxylic acids are weak acids and therefore do not
completely ionize.
Copyright Houghton Mifflin Company. All rights reserved.
A given carboxylic acid molecule can form two
hydrogen bonds to another carboxylic acid molecule,
producing a "dimer." Dimers have twice the mass of a
single molecule, and a higher temperature is needed
to boil carboxylic acids than would be needed if no
dimers were present.
Copyright Houghton Mifflin Company. All rights reserved.
The boiling points of monocarboxylic acids compared to those of other types of compounds. All compounds in the comparison have unbranched carbon chains.
Copyright Houghton Mifflin Company. All rights reserved.
The solubility of saturated unbranched-chain carboxylic acids decreases as carbon-chain length increases.
Copyright Houghton Mifflin Company. All rights reserved.
Carboxylic acids react completely with strong bases
such as NaOH. The resulting carboxylate salts are
converted back to the carboxylic acid with strong acids.
Carboxylic acids react with alcohols to produce esters.
Copyright Houghton Mifflin Company. All rights reserved.
Carboxylic acids react with amines to produce amides.
Amides will be discussed in more detail in sections
13.10 and 13.11.
Copyright Houghton Mifflin Company. All rights reserved.
Copyright Houghton Mifflin Company. All rights reserved.
The simple esters are colorless,
volatile liquids with a pleasant smell.
Esters are neither acids nor bases in
aqueous solution.
An ester's name consists of two
words. The name of the alkyl group in
the ester group, -COOR, and the name
of the parent carboxylic acid with the
family name –oic replaced with –ate;
e.g. ethyl acetate: CH (C=O)OCH CH
Copyright Houghton Mifflin Company. All rights reserved.
Properties and Reactions
of Esters
Copyright Houghton Mifflin Company. All rights reserved.
To create an ester the OH group of a carboxylic
acid is replaced by an OR group. Esters cannot
form hydrogen bonds with each other. Therefore,
esters have lower boiling points than the
carboxylic acids from which they are derived.
Copyright Houghton Mifflin Company. All rights reserved.
FORMING ESTERS FROM CARBOXYLIC ACIDS
The reactions of alcohols (R-OH) and amines
(R-NH2) with carboxylic acids follow the same
pattern – both replace the –OH group in the
acid with another group.
Esterification: Esterification is carried out by
warming a mixture of a carboxylic acid and an
alcohol in the presence of a catalytic amount of
a strong acid catalyst.
This is an elimination
reaction. Water is
R-C-OH + HO-R' → R-C-O-R' +H O
split out
Copyright Houghton Mifflin Company. All rights reserved.
Aspirin and Other Over-the-Counter
Carboxylic Acid Derivatives
Aspirin: A member of a group of drugs known as
salicylates. Aspirin is an analgesic (relieves pain),
antipyretic (reduces fever), and anti-inflammatory
(reduces inflammation). Salicylates are esters of
salicyclic acid.
Early people knew that chewing
willow bark could relieve pain. It
was salicylic acid in the bark that
caused the effect. But the acid was
bitter and caused stomach problems.
In 1897 German chemists at Bayer
acetylated salicylic acid and gave
the world Aspirin.
Copyright Houghton Mifflin Company. All rights reserved.
Esterification = forward reaction (remove water)
= reverse reaction = adding
water to break a bond
Copyright Houghton Mifflin Company. All rights reserved.
Selected Esters That Are Used as Flavoring Agents.
Copyright Houghton Mifflin Company. All rights reserved.
Copyright Houghton Mifflin Company. All rights reserved.
• Amides may contain an –NH2 group or one
or both of the hydrogens can be replaced
with alkyl groups.
• Unsubstituted amides, RCONH2, can form
three hydrogen bonds to other amide
molecules and thus have higher melting
and boiling points than the acids from
which they are derived.
Copyright Houghton Mifflin Company. All rights reserved.
• Unsubstituted amides, RCONH2, are
named by replacing the –oic acid by –
• Substituted amides are named by first
specifying the alkyl group and then
identifying the amide name. The alkyl
substituents are preceded by the letter
N to specify that the alkyl groups are
attached to the nitrogen.
Copyright Houghton Mifflin Company. All rights reserved.
Note on Naming These Compounds
CH3- gives "acet-"
HCOOH = formic acid
CH3-COOH = Acetic acid
HCOO- = formate ion
CH3-COO- = acetate ion
H(C=O)H = formaldehyde
CH3(C=O)H = acetaldehyde
H(C=O)NH2 = formamide
CH3(C=O)NH2 = acetamide
CH3(C=O)CH3 = acetone
H(C=O)N(CH3)2 = N,N-dimethylformamideCH3(C=O)NH-CH2CH3 = N-ethylacetamide
Copyright Houghton Mifflin Company. All rights reserved.
NAIL POLISH SOLVENTS
CH3(C=O)CH3 = acetone
CH3(C=O)-O-CH2CH3 = ethylacetate
The Merck Index: "Caution: Inhalation may produce
headache, fatigue, excitement, bronchial irritation,
and, in large amounts, narcosis. Serious poisoning rare."
Copyright Houghton Mifflin Company. All rights reserved.
Chemistry at a Glance:
Summary of Structural Relationships for Hydrocarbon
Derivatives: "H" versus "R"
Copyright Houghton Mifflin Company. All rights reserved.
Urea is a one-carbon amide. Its formation is the
human body's primary method of eliminating
nitrogen "waste". The kidneys remove urea from the blood and
provide a route for its excretion into the urine.
When the kidneys malfunction, urea concentration
builds up to toxic levels, a condition known as
Note that urea is like acetone with the -CH3 groups
replaced by -NH2 groups
Copyright Houghton Mifflin Company. All rights reserved.
Tylenol, a.k.a. acetaminophen, is an amide.
In this case the amine component is an aniline. The
IUPAC name is … N-(4-hydroxyphenyl)-acetamide.
Copyright Houghton Mifflin Company. All rights reserved.
Properties and Reactions of
Copyright Houghton Mifflin Company. All rights reserved.
Amide Formation: Amides are formed by
heating a mixture of a carboxylic acid and
an amine. (The reaction is best
accomplished by treating an acid chloride
with ammonia or an amine.)
Copyright Houghton Mifflin Company. All rights reserved.
Acetaminophen: An amide that also
contains a hydroxyl group. It is best
known as Tylenol. It is an alternative to
aspirin for pain relief, but unlike aspirin it is
not an anti-inflammatory agent.
Copyright Houghton Mifflin Company. All rights reserved.
Hydrolysis of Esters and Amides
Recall: Hydrolysis means the addition of water
to split a bond.
Esters and amides undergo hydrolysis to give
back the carboxylic acid and alcohol or amine.
Ester hydrolysis: Ester hydrolysis reactions can
be catalyzed by either an acid or a base.
Copyright Houghton Mifflin Company. All rights reserved.
• Acid catalyzed ester hydrolysis is simply
the reverse of esterification reaction. In this
reaction, an ester is treated with water in
the presence of a strong acid catalyst such
as sulfuric acid.
• Base catalyzed ester hydrolysis with a
base such as NaOH or KOH is known as
The product of a
saponification reaction is a carboxylate
anion rather then a free carboxylic acid.
Copyright Houghton Mifflin Company. All rights reserved.
Amide hydrolysis: Amides are stable in water, but
undergo hydrolysis when heated in the presence
of a base or an acid. The products of amide
hydrolysis reactions in the presence of a base or
an acid are shown below.
Copyright Houghton Mifflin Company. All rights reserved.
Errors in the Text
Question 13.25(d). The ring part of the structure shown should
be a phenyl (benzene) group., i.e., a hexagon with a circle inside.
On page 353: In the "Chemical Portrait" the structure shown is
actually acetaldehyde, not acetone. (The text is correct for
acetone.)
Acetone is CH -(C=O)-CH
Bonuses: Students who are first to point out any error in the text in the chapters we cover will receive a bonus of 2 points.
Copyright Houghton Mifflin Company. All rights reserved.
Condensation Polymers:
Polyesters and Polyamides
Copyright Houghton Mifflin Company. All rights reserved.
CONDENSATION POLYMERS
These are formed by reacting difunctional monomers to
give a polymer and a small molecule, usually water or
HCl. Note: condensation is the reverse of hydrolysis.
A polyester polymer is a condensation polymer in
which the monomers are joined through ester linkages.
Poly(ethylene terephthalate), a polymer = PET
Copyright Houghton Mifflin Company. All rights reserved.
Polyamides and Polyesters
• Polymer molecules are composed of thousands of
repeating units, known as monomers.
• Both polyamides and polyesters are polymers; they
have many uses.
• Polyamides are formed by reaction between diamines
• Nylons are polyamides.
• Polyesters are formed by reaction between diacids
and dialcohols.
Copyright Houghton Mifflin Company. All rights reserved.
A polyamide is a condensation polymer in which
the monomers are joined through amide linkages.
Copyright Houghton Mifflin Company. All rights reserved.
A white strand of a nylon polymer forms between
two layers of a solution containing a diacid
(bottom layer) and a diamine (top layer).
Copyright Houghton Mifflin Company. All rights reserved.
Polyurethanes are polymers related to polyesters and
polyamides. The backbone of a polyurethane polymer
contains aspects of both ester and amide functional
Foam rubber in furniture upholstery (e.g. on airplanes),
packaging materials, life preservers, elastic fibers, and
many other products such as skin substitute membranes
(Figure 13.13) contain polyurethane polymers.
Copyright Houghton Mifflin Company. All rights reserved.
In the News
The U.S. Environmental Protection Agency (EPA) has recently
questioned the safety of perfluorooctanoic acid (PFOA), a
compound employed in making teflon and a variety of nonstick
Items.
PFOA may be a carcinogen (a cancer-causing agent).
What is the formula of PFOA?
• "Perfluoro" means that all of the hydrogens attached to the carbons
in the compound have been replaced by fluorine atoms.
• "Octanoic acid is an organic acid with 8 carbons, thus
PFOA = CF -(CF ) -COOH
Note that the acid hydrogen is not replaced by a fluorine.
Copyright Houghton Mifflin Company. All rights reserved.
• Carbonyl compounds contain a carbonyl group
• Carbonyl groups are strongly polarized, with a
partial positive charge on carbon and partial
negative charge on oxygen.
• Carbonyl compounds are broadly divided into
two groups: aldehydes and ketones are in one
group and the second group contains carboxylic
acids, esters, and amides.
Copyright Houghton Mifflin Company. All rights reserved.
Chapter Summary (Cont'd)
Systematic or IUPAC names for aldehydes are
derived by replacing –e at the end of the name
of the alkane with –al.
• Ketones are named systematically by replacing
the final –e of the corresponding alkane name
with –one.
• Aldehydes and ketones do not form hydrogen
bonds, as a result they have lower boiling
points than alcohols of similar size.
Copyright Houghton Mifflin Company. All rights reserved.
Chapter Summary (cont'd)
• Aldehydes and ketones are soluble in common organic
solvents, and those with fewer then five carbons are also
soluble in water.
• Simple ketones are excellent solvents because they
dissolve both polar and nonpolar compounds.
• Alcohols can be oxidized to aldehydes and ketones.
• Aldehydes can be further oxidized to carboxylic acids.
• Aldehydes are reduced to primary alcohols, and ketones
are reduced to secondary alcohols.
Copyright Houghton Mifflin Company. All rights reserved.
Chapter Summary (cont'd)
• Most carboxylic acids are weak acids but esters
and amides are neither acids nor bases.
• Carboxylic acids, esters, and amides undergo
carbonyl group substitution reactions.
• Simple acids and esters are liquids; all amides
except formamide are solids.
• In esters, the -OH group in the acid is replaced by
an -OR group of an alcohol.
• In amides, the -OH group in an acid is
replaced by an –NH2 group of ammonia, or
an NHR or –NR2 group of a primary or
secondary amine.
Copyright Houghton Mifflin Company. All rights reserved.
What you absolutely must know from Chapter 13
• You must know the general forms of the following compounds:
• How to name aldehydes, ketones, carboxylic acids, and esters.
• The common names of certain simple compounds: formaldehyde,
acetone, formic acid, benzaldehyde, urea, formamide, acetamide,
• Some important reactions:
--Primary alcohols can be oxidized to form aldehydes.
--Secondary alcohols can be oxidized to form ketones.
--Aldehydes can be further oxidized to form carboxylic acids.
--Ketones resist oxidation
--Esters can be hydrolyzed to form an alcohol and a carboxylic acid
--Amides can be hydrolyzed to form an amine and a carboxylic acid
(Hydrolysis = adding water to split up a compound)
Copyright Houghton Mifflin Company. All rights reserved.
Chapter 13 (continued)
• Polymers are large compound formed by linking smaller units called
monomers.
• Condensation polymers are formed by reacting difunctional
monomers and splitting out small molecules (e.g., H O):
--A polyester is formed by the reaction of a diacid and a dialcohol:
HOOC-R-COOH + HO-R'-OH → HOOC-R-CO-O-R'-OH + H O
--A polyamide (such as nylon) can be formed from the reaction of a
diacid and a diamine:
HOOC-R-COOH + HHN-R'-NH → HOOC-R-CO-NH-R'-OH + H O
Copyright Houghton Mifflin Company. All rights reserved.
To Do List
• Read chapter 13!!
• Do additional problems
• Do practice test T/F
• Do practice test MC
• Review Lecture notes for
Copyright Houghton Mifflin Company. All rights reserved.
Source: http://www.chm.wright.edu/seybold/CHM102Lectures/CHM102chapter13A.pdf
Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial Yung-Jue Bang*, Young-Woo Kim, Han-Kwang Yang, Hyun Cheol Chung, Young-Kyu Park, Kyung Hee Lee, Keun-Wook Lee, Yong Ho Kim, Sang-Ik Noh, Jae Yong Cho, Young Jae Mok, Yeul Hong Kim, Jiafu Ji, Ta-Sen Yeh, Peter Button, Florin Sirzén, Sung Hoon Noh*, for the CLASSIC trial investigators†
Dynamic Energy-Aware Capacity Provisioning for Cloud Mohamed Faten Zhani University of Waterloo University of Waterloo National University of Defense Waterloo, ON, Canada Waterloo, ON, Canada Changsha, Hunan, China Joseph L. Hellerstein University of Illinois at University of Waterloo Google, Inc. Urbana-Champaign, USA Waterloo, ON, Canada Seattle, Washington, USA