Ctn.uoregon.edu
5990 • The Journal of Neuroscience, April 15, 2015 • 35(15):5990 –5997
Striatal D1- and D2-type Dopamine Receptors Are Linked to
Motor Response Inhibition in Human Subjects
Chelsea L. Robertson,1,5
Kenji Ishibashi,3,4
Mark A. Mandelkern,5,6
Amira K. Brown,3
Dara G. Ghahremani,3
Fred Sabb,3
Robert Bilder,3
Tyrone Cannon,2
Jacqueline Borg,2
and Edythe D. London1,3,4,5
Departments of 1Molecular and Medical Pharmacology, and 2Psychology, 3Department of Psychiatry and Biobehavioral Sciences, The Semel Institute for
Neuroscience and Human Behavior at UCLA, and 4Brain Research Institute, University of California, Los Angeles, Los Angeles, California 90024, 5Veterans
Administration Greater Los Angeles Healthcare System, Los Angeles, California 90073, and 6Department of Physics, University of California, Irvine, Irvine,
California 92697
Motor response inhibition is mediated by neural circuits involving dopaminergic transmission; however, the relative contributions of
dopaminergic signaling via D1- and D2-type receptors are unclear. Although evidence supports dissociable contributions of D1- and
D2-type receptors to response inhibition in rats and associations of D2-type receptors to response inhibition in humans, the relationship
between D1-type receptors and response inhibition has not been evaluated in humans. Here, we tested whether individual differences in
striatal D1- and D2-type receptors are related to response inhibition in human subjects, possibly in opposing ways. Thirty-one volunteers
participated. Response inhibition was indexed by stop-signal reaction time on the stop-signal task and commission errors on the
continuous performance task, and tested for association with striatal D1- and D2-type receptor availability [binding potential referred to
nondisplaceable uptake (BPND)], measured using positron emission tomography with [11C]NNC-112 and [18F]fallypride, respectively.
Stop-signal reaction time was negatively correlated with D1- and D2-type BPND in whole striatum, with significant relationships involving
the dorsal striatum, but not the ventral striatum, and no significant correlations involving the continuous performance task. The results
indicate that dopamine D1- and D2-type receptors are associated with response inhibition, and identify the dorsal striatum as an impor-
tant locus of dopaminergic control in stopping. Moreover, the similar contribution of both receptor subtypes suggests the importance of
a relative balance between phasic and tonic dopaminergic activity subserved by D1- and D2-type receptors, respectively, in support of
response inhibition. The results also suggest that the stop-signal task and the continuous performance task use different neurochemical
mechanisms subserving motor response inhibition.
Key words: dopamine; impulsivity; PET imaging
2013). Impaired inhibitory control can disrupt goal-directed be-
Impulsive actions are premature, poorly conceived, or difficult to
with negative consequences that contribute to psycholog-
suppress (Dalley et al., 2008), and lack of inhibitory control over
ical distress associated with these disorders. Clarifying the
of attention deficit hyperactivity dis-
mechanisms that mediate inhibitory control, therefore, ulti-
order (ADHD) and substance use disorders (Bari and Robbins,
mately may help to guide treatment for disorders characterizedby an impulsive phenotype.
Research findings have indicated a role for dopamine in im-
Received Nov. 24, 2014; revised Feb. 10, 2015; accepted Feb. 24, 2015.
pulsive behavior. Syndromes such as ADHD (Vaidya et al., 1998;
Author contributions: F.S., R.B., T.C., J.B., and E.D.L. designed research; C.L.R., K.I., and A.K.B. performed re-
search; C.L.R., K.I., M.A.M., and D.G.G. analyzed data; C.L.R., M.A.M., and E.D.L. wrote the paper.
Bedard et al., 2003; Senderecka et al., 2012
This work was supported by the Consortium for Neuropsychiatric Phenomics (National Institutes of Health Road-
map for Medical Research Grants UL1-DE019580, RL1MH083269, RL1DA024853, and PL1MH083271), The UCLA
Training Program in Translational Neuroscience of Drug Abuse (Grant T32DA024635), and Endowments from the
dysfunction. In addition, studies of genetic poly-
Thomas P. and Katherine P. Pike Chair in Addiction Studies and the Marjorie Greene Trust.
The authors declare no competing financial interests.
morphisms (Colzato et al., 2010, 2013) and pharmacological manip-
Correspondence should be addressed to Edythe D. London, Semel Institute, University of California, Los Angeles,
ulations (; Eagle and Baunez, 2010) have
760 Westwood Plaza, C8-831, Los Angeles, CA 90024. E-mail:
[email protected].
K. Ishibashi's present address: Tokyo Metropolitan 173-0022, Japan.
bition, an index of inhibitory control (Chamberlain et al., 2006;
A.K. Brown's present address: Life Sciences Institute, Charles R. Drew University of Medicine and Science, Los
Angeles, CA 90059.
Eagle and Baunez, 2010). For or
F. Sabb's present address: Research Team for Neuroimaging, Lewis Center for Neuroimaging, University of Ore-
improves response inhibition in
gon, Eugene, OR 97403.
ADHD patients and healthy subjects, respectively (Tannock et al.,
R. Borg's present address: Department of Clinical Neuroscience, Karolinska Institute, 171 77 Stockholm, Sweden.
1989; de Wit et al., 2000; Aron et al., 2003), of
T. Cannon's present address: Departments of Psychology and Psychiatry, Yale University, New Haven, CT 06520.
response inhibition
Copyright 2015 the authors 0270-6474/15/355990-08$15.00/0
(Colzato et al., 2014; Ramdani et al., 2014).
Robertson et al. • Dopamine Receptors and Motor Response Inhibition
J. Neurosci., April 15, 2015 • 35(15):5990 –5997
• 5991
Despite an evident role for dopamine, the relative contribu-
They were instructed to respond quickly and accurately, and that stop-
tions of dopamine signaling via dopamine D
ping and going were equally important. The SSD was adjusted on a
1- and D2-like recep-
tor subtypes are unclear. In rats, systemic administration of
trial-by-trial basis according to performance; values were drawn from
dopaminergic antagonists does not affect response inhibition
two interleaved ladders to ensure equal performance levels across partic-
(Eagle et al., 2007, 2008; Bari and Robbins, 2013). However, di-
ipants, producing successful inhibition on !50% of stop trials. Partici-
pants received task training before task initiation, consisting of eight
trials (three of which were stop trials).
dorsal-medial striatum improves response inhibition, whereas
During the CPT, participants viewed a series of go stimuli (alphabet
infusion of the D2 receptor antagonist sulpiride has the opposite
letters, go trials) and were instructed to respond with a key press. On
effect. Similar infusions into the ventral striatum have no effect
some trials (no-go trials, 10%), a no-go stimulus was presented (the letter
(Eagle et al., 2011). Thus, the effects of dopamine receptor sub-
"X") in lieu of the go stimulus, and participants were instructed to with-
response inhibition appear to be regionally spe-
hold responding. The task comprised 18 blocks presented at random,
cific and possibly opposing. In addition, the administration of the
each containing 20 trials at a fixed intertrial interval (ITI): 1000, 2000, or
4000 ms. Participants received task training before initiation, consisting
2-specific agonist cabergoline improves response inhibition
(Nandam et al., 2013), and striatal D
of 10 trials from the 2000 ms ITI type.
2-type receptor availability is
capacity for response inhibition and corre-
Analysis of neurobehavioral data. SST data were analyzed using the
sponding neural activation during inhibition in humans (Ghah-
same methods as in a prior study of a separate sample (Ghahremani et al.,2012). The median and SD of reaction time on go
remani et al., 2012). Nonetheless, human
all-correct go trials [go trial reaction times (GoRTs)]. The average
in response inhibition and direct com-
SSD was calculated using all-successful stop trials. The stop-signal reac-
parisons of D1- vs D2-type receptor contributions to motor re-
tion time (SSRT) was estimated by subtracting each participant's average
sponse inhibition have not been performed.
SSD from his/her median GoRT (Band et al., 2003). The percentage of
We used positron emission tomography (PET) with
inhibition on stop trials was of successful stop trials
[11C]NNC-112 and [18F]fallypride as radioligands for dopamine
to all stop trials presented. As recommended (Congdon et al., 2012),
participant data meeting the following
1- and D2-type receptors, respectively (Mukherjee et al., 1995;
Ekelund et al., 2007), to examine the
ysis: (1) "25% or #75% inhibition on stop trials (
n $ 3); (2) "60%
receptor availability [binding potential re-
correct responding on go trials (
n $ 0); (3) #10% direction errors on go
ferred to nondisplaceable uptake (BP
trials (
n $ 0); and (4) SSRT estimate that was negative or "50 ms (
n $ 1,
ND)] with measures from
computer failure). SST data from 27 participants were subject to analysis,
prototypical assessments of motor response inhibition—the
as follows: 22 participants with D
stop-signal task (SST) and continuous performance task (CPT)
1-type dopamine receptor availability
(Logan et al., 1984; Tannock et al., 1989; Aron et al., 2014). We
1-type BPND); 24 participants with D2-type BPND; and 19 participants
with both. Performance data from the CPT were used to calculate the mean
and SD of the GoRT on all go trials. The commission error (CE) was calcu-
not the ventral striatum, would be linked to response inhibition
lated as the number of failed no-go trials (response to a no-go stimulus).
task performance, and that D1- and D2-type receptor contribu-
PET scanning. D1-type BPND was assayed using [11C]NNC-112, a
tions would be dissociable in this region, reflecting opposing
high-affinity ligand for D1-type receptors (Andersen et al., 1992; Ekelund
et al., 2007) in 26 subjects (14
a different day using [ 18F]fallypride, a high-affinity radioli-gand for D
Materials and Methods
2-type receptors (Mukherjee et al., 1995) in 27 subjects (14
females). PET scanning Gemini Tru Flight
Research participants. All study procedures were approved by the Univer-
PET/CT scanner in 3D mode (FWHM $ 5.0 mm % 4.8 mm; 90 slices;
sity of California, Los Angeles (UCLA) Institutional Review Board.
voxel size, 2 mm 3). A CT transmission scan was performed to obtain data
Thirty-one healthy volunteers (16 females; mean age, 30.68 years; SD, 8.3
for measured attenuation correction. After a bolus injection of
years), who were participating in the UCLA Consortium for Neuropsy-
[ 11C]NNC-112 (!15 mCi &5%; specific activity, !1 Ci/"mol), dy-
chiatric Phenomics (CNP; www.phenomics.ucla.edu), completed exten-
namic emission data were acquired for 90 min. For [18F]fallypride (!5
sive response inhibition
mCi &5%, specific activity ! 1 Ci/"mol), data were acquired in two
and MRI scanning (Bilder et al., 2009). CNP participants who expressed
scanning blocks of 80 min each, with a short break between blocks. Data
interest in being studies were offered flyers or
were reconstructed using the 3D row action maximum likelihood algo-
were called via telephone, and were invited to participate in this study
rithm. Scatter and random corrections were applied.
involving PET scanning. On average, PET scanning occurred !17
PET image processing. Reconstructed [ 11C]NNC-112 PET data (1
months after participation in the CNP study. Participants received a
min % 90 frames) were averaged into 23 frames, consisting of 4 1 min
complete description of this study and provided written informed con-
frames, 3 2 min frames, and 16 5 min frames. Reconstructed [ 18F]fally-
sent. Health screening was performed using the Structured Clinical In-
pride PET data (2 blocks; 1 min % 80 frames) were combined into 16
terview for the
Diagnostic and Statistical Manual of Mental Disorders, 4th
frames, each consisting of an average of 10 min. PET images were motion
edition, and a physical examination. Participants were excluded if they
corrected (Jenkinson et al., 2002) then coregistered to the corresponding
met the following criteria: current axis I psychiatric diagnoses other than
MRI (). Volume of interest (VOI)-based time
nicotine dependence; use of psychotropic medications or substances,
kinetic modeling using PMOD version
except marijuana or alcohol; CNS, cardiovascular, or systematic disease;
3.1. Time–activity curves were fit using the simplified reference tissue
or HIV-seropositive status, hepatic disease, or pregnancy. On all test
model (SRTM; Lammertsma and Hume, 1996). The cerebellum was se-
days, negative urine samples for recent drug use and pregnancy (women)
lected as the ; Abi-Dargham et al., 2000;
were required.
Ishibashi et al., 2013). A
Neurobehavioral tasks. The SST (Logan et al., 1984) and CPT (Tannock
the reference region tissue), estimated from high-
et al., 1989) were administered
activity regions (caudate and putamen), was computed. Time–activity
Software Tools). During the SST, participants viewed a series of
curves were then refit using SRTM2 (Wu and Carson, 2002), applying the
go stimuli (left/right arrows) and were instructed to respond with corre-
computed k2' values to all VOIs. subtracting 1.0
sponding left or right key presses, respectively (go trials). On some trials
from the product of R1 (ratio of radiotracer delivery in the target region
(stop trials, 25%), an audible tone (stop-signal) was presented after a
tissue relative to that of the reference region tissue) and k2'/k2a.
short delay [stop-signal delay (SSD)] following the go stimulus. Partici-
MRI scanning and volumes of interest. MRI scanning was performed on
pants were instructed to withhold their responses upon hearing the tone.
a Siemens Trio scanner (MPRAGE: repetition time, 1.9 s; echo time, 2.26
5992 • J. Neurosci., April 15, 2015 • 35(15):5990 –5997
Robertson et al. • Dopamine Receptors and Motor Response Inhibition
ms; voxel size, 1 mm 3; 176 slices), and processed using the FMRIB Soft-
Table 1. Performance variables for the stop-signal task and the continuous
ware Library (FSL; http://www.fmrib.ox.ac.uk/fsl/index.html; Oxford
Selected VOIs included the whole striatum and functional striatal sub-
divisions: limbic striatum, associative striatum, and sensory–motor stria-
Stop-signal task (n $ 27)
tum. A VOI for the whole striatum was created by combining
anatomically defined VOIs for the caudate, putamen, and nucleus ac-
cumbens using the FSL software package (Patenaude et al., 2011). Func-
tional subdivisions of the striatum () and the
Correct go responding (%)
midbrain region (Zald et al., 2010) previously.
Inhibition on stop-trials (%)
The manually in standard space and trans-
Continuous performance task (n $ 31)
formed to each subject's MRI.
Data analysis and statistical analysis. Striatal VOIs were selected a pri-
ori on the basis of evidence that dopaminergic transmission in these
regions is important for inhibitory control (Lee et al., 2009; Buckholtz et
Commission errors
al., 2010; Ghahremani et al., 2012).
subdivisions were tested
post hoc if a sig-
Table 2. D
nificant relationship was found using the whole-striatum VOI. Relation-
1-type and D2-type BPND in the striatum and within-region correlations
Region of interest
ND, SST, and CPT were conducted analyzed using SPSS
version 22 (IBM Corp.). Analyses reported here were conducted using
measurements from bilateral VOIs. These correlations were nearly iden-
tical to those examined using measurements from left and right VOIs
Associative striatum
separately. Exploratory investigations of D1- and D2-type BPND with SST
Sensory motor striatum
and CPT performance included a voxelwise analysis of correlations be-
Data are reported as the mean (SD).
tween cortical BPND and SSRT, GoRT, or CE, and VOI-based analysis
*p $ 0.028: n $ 26, 14 females; n $ 27, 14 females; n $ 22, 12 females.
using measurements of midbrain BPND.
Relationships of regional D1-type and D2-type BPND. Within-region
correlations of D1- and D2-type BPND were performed for all striatal
samples (Boehler et al., 2010; Ghahremani et al., 2012). On the
VOIs by Pearson correlation analysis. Of the 31 participants included inthe study, 22 (12 female) underwent PET scans for the determination of
13 CEs (of 36 no-go trials). Mean GoRT values were similar to
1- and D2-type BPND, and their data were used for this analysis.
Dopamine receptor BP
those reported previously (Steele et al., 2013).
ND, and performance on the SST and CPT. Rela-
tionships of striatal BPND with SSRT were tested using partial correlation
analysis controlling for age and sex. Similar analyses were performed
Dopamine receptor BPND
Overall, BPND values for both receptor subtypes were higher in
The Hotelling–Williams test (Van Sickle, 2003) was used to test for
the dorsal than in ventral regions of the striatum. D1- and D2-type
equality of the correlations dorsal striatum BPND
versus SSRT and ventral striatum BP
ND values were approximately equal in the associative and sen-
ND. For this test, BPND values of the
sory motor striatum, while D
associative and sensory motor regions were combined to create a BP
2-type BPND was higher in the sen-
sory motor than the associative striatum (Table 2; see Fig. 4).
value for the dorsal striatum, and compared with the BPND value of the
ventral striatum.
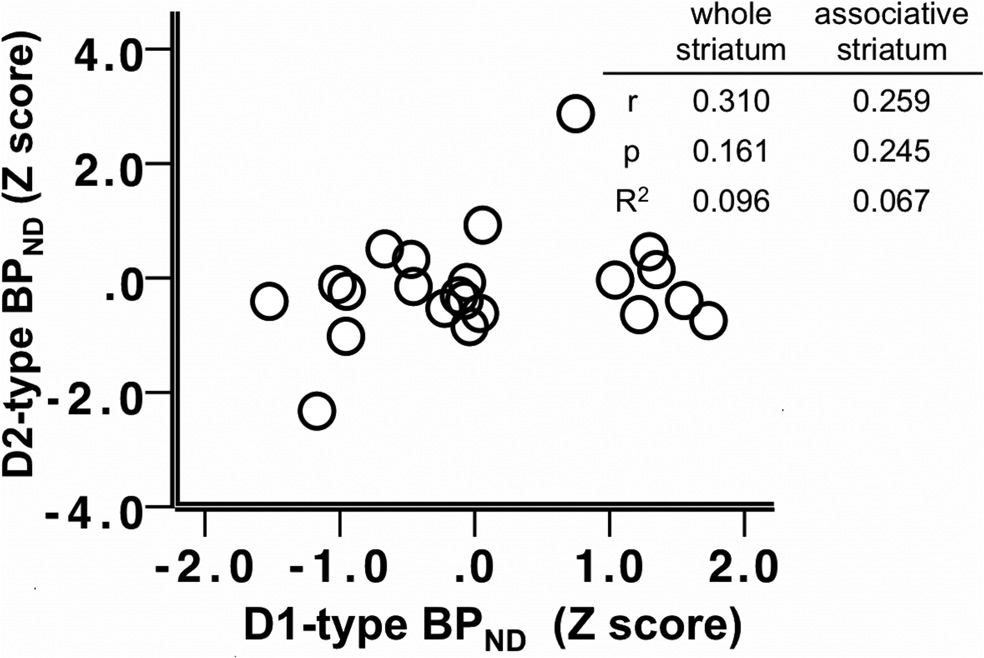
D1-type receptor BPND and D2-type BPno
To examine the contributions of both receptor subtypes (BP
correlation in the associative or limbic striatal subdivisions, but
SSRT, a stepwise regression analysis was used. To determine the effect of
were significantly positively correlated in the sensory motor stria-
tum (
r $ 0.469,
p $ 0.028).
2-type BPND to a model with D1-type BPND, the variables en-
tered into the first step of the regression were age, sex, and D1-type BPND;
D2-type BPND was included in the second step. Next, the reverse relation-
Dopamine receptor BPND and response inhibition on the SST
ship was tested to determine the effect of adding D1-type BPND to a
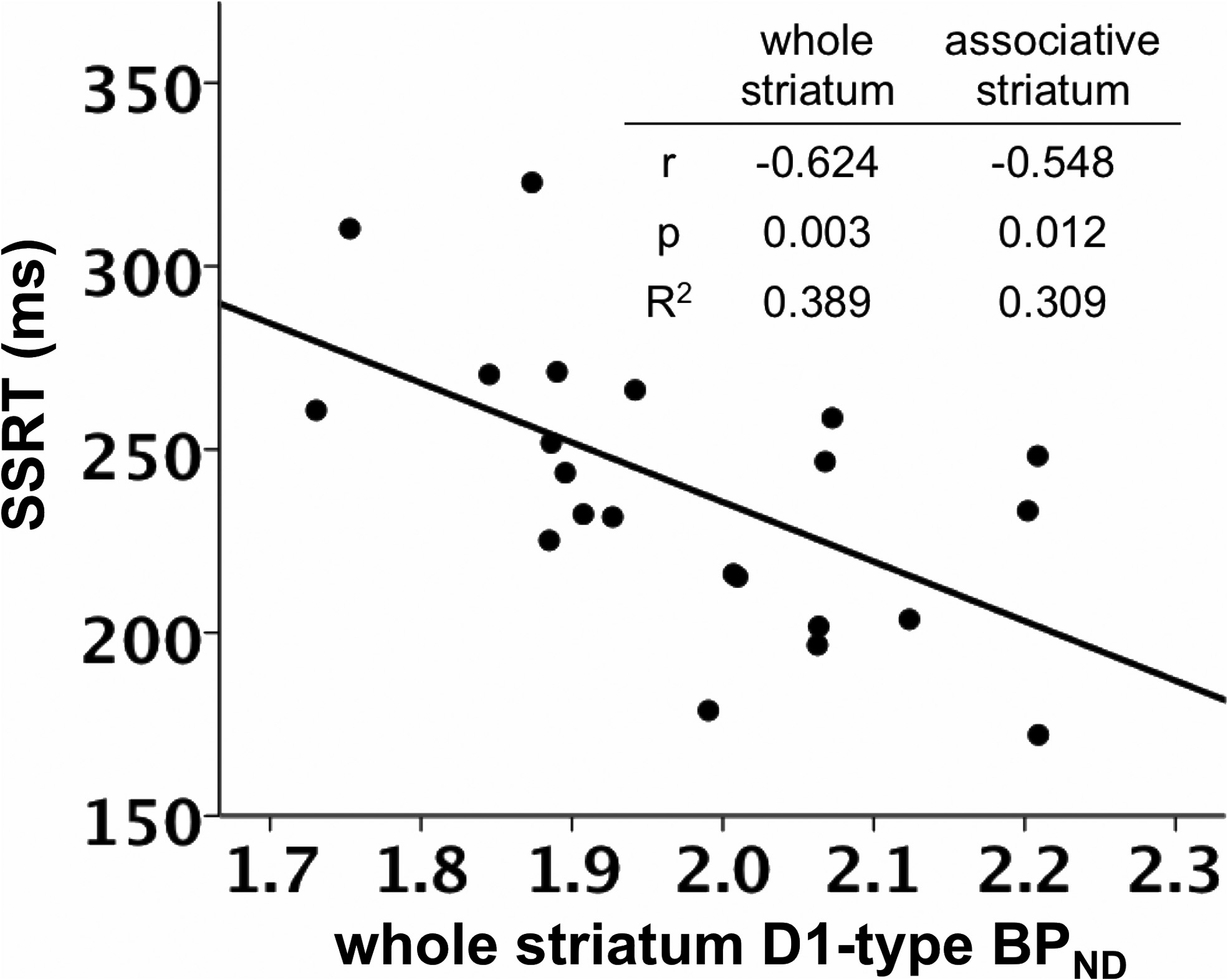
SSRT was negatively correlated with D1-type BPND in the whole
model using D2-type BPND, with D1-type BPND included in the second
striatum, controlling for the effects of age and sex (
r $ (0.624,
step instead.
p $ 0.003; (Figs. 1, 3; Table 3).
Post hoc evaluations of data from
The relationships of receptor BPND with CEs, the outcome variable
striatum revealed significant rela-
reflecting response inhibition in the CPT, and GoRTs were assessed usingpartial correlation analysis, controlling for sex and age.
tionships in the dorsal regions (associative striatum:
r $ (0.548,
To estimate effects of time lapse between neuroimaging and neurobe-
p $ 0.012; sensory motor striatum:
r $ (0.527,
p $ 0.017), but
havioral procedures (average elapsed time, 17 months), the stability of
not in the ventral region (limbic striatum:
r $ (0.342,
p $
neurobehavioral task performance over time was evaluated. For this
0.139). A difference in correlations between SSRT and D1-type
analysis, a subset of participants (
n $ 10) was invited to return for retest-
BPND in the dorsal versus ventral region of striatum was detected
ing of SST and CPT performance after an average elapsed time of 40
using the Hotelling–Williams test at a trend level (
p $ 0.083). To
months. Reliability assessments were assessed using the intraclass corre-
determine the specificity of the association to the stopping pro-
lation coefficient (ICC).
cess, correlations between GoRT and D1-type BPND were exam-
1-type BPND in the whole striatum showed a trend toward
a negative correlation with GoRT (
r $ (0.425,
p $ 0.062). We
therefore conducted a
post hoc analysis of the functional sub-
On the stop-signal task, participants performed at a level of 99%
divisions and found that the correlation involving D1-type
correct on go trials and inhibited their responses on approxi-
BPND in the ventral striatum reached a trend level (
p $ 0.082),
mately half of the stop trials [mean (SD), 52% (0.056)], indicat-
but the Hotelling–Williams test indicated no difference in
ing that the adaptive staircase procedure for equating stop-trial
dorsal versus ventral correlations.
performance across participants was successful (Table 1). SSRT
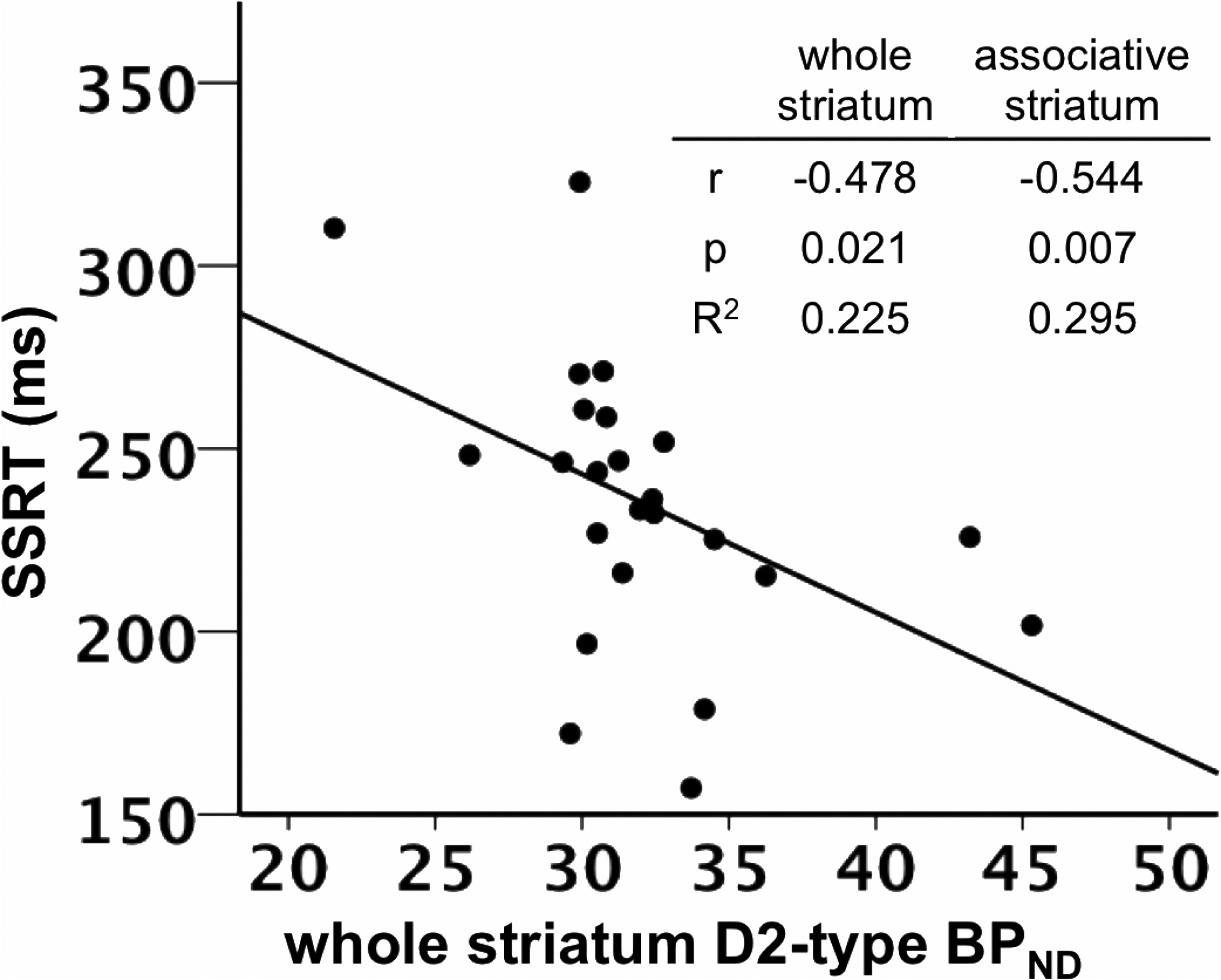
SSRT was negatively correlated with D2-type BPND in the
values were similar to those observed in prior separate
whole striatum, controlling for the effects of age and sex (
r $
Robertson et al. • Dopamine Receptors and Motor Response Inhibition
J. Neurosci., April 15, 2015 • 35(15):5990 –5997 • 5993
striatum. Table insert displays partial correlation coefficients, p values, and R 2 values for therelationship between whole striatum and associative striatum D1-type BPND and SSRT, control-
ling for age and sex.
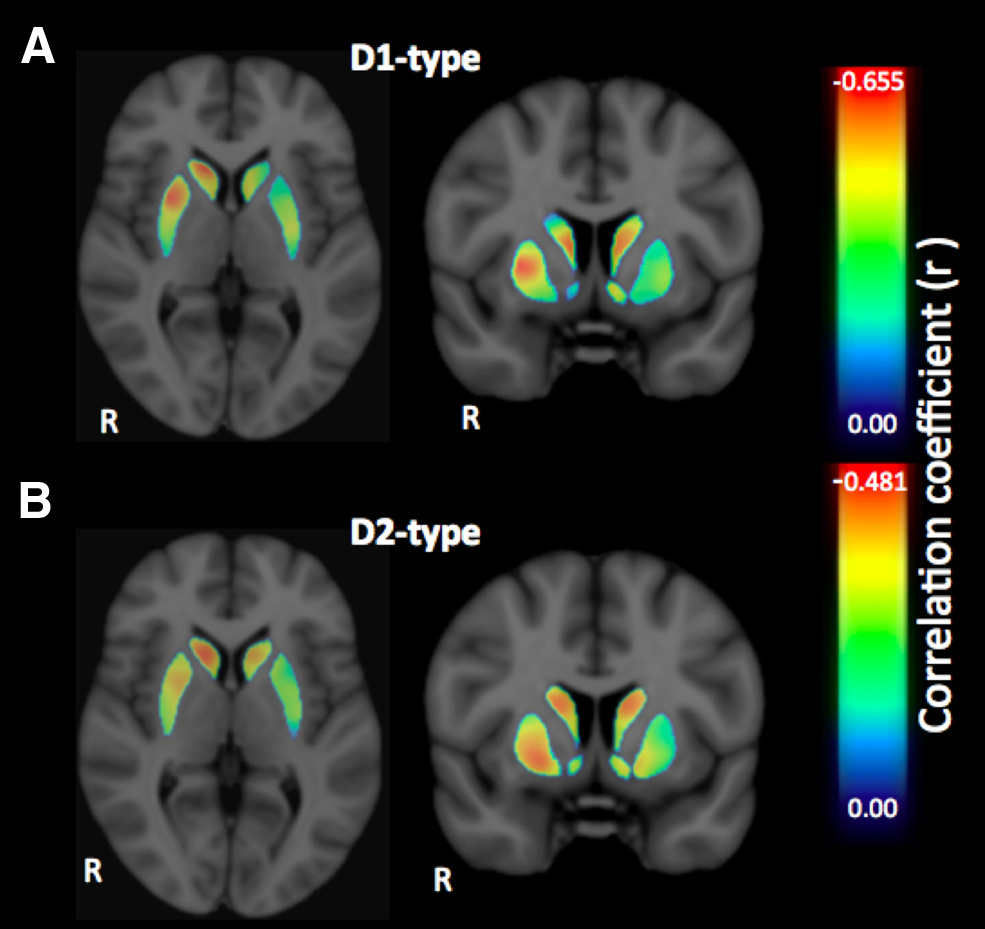
Figure 3. A, B, Voxelwise effect size maps depicting the partial correlation coefficient (r)
between individual SSRT and D1-type (A) and D2-type (B) receptor BPND in the striatum, con-
trolling for the effects of age and sex.
Figure 4. Scatter plot depicting the relationship between D2-type BPND and D1-type
BPND in the whole striatum; z-scores of BPND were used for presentation purposes. Table
striatum. Table insert displays partial correlation coefficients, p values, and R 2 values for the
insert displays correlation coefficients, p values, and R 2 values for the correlations in the
relationship between whole striatum and associative striatum D2-type BPND and SSRT, control-
whole and associative striatum.
ling for age and sex.
associative striatum was present at trend level when controlling
(0.478, p $ 0.021; Figs. 2, 3; Table 3). Post hoc tests involving
for D2-type BPND (r $ (0.473, p $ 0.064). SSRT was negatively
functional showed significant nega-
correlated with D2-type BPND in associative striatum when con-
tive correlations in the associative striatum (r $ (0.544, p $
trolling for D1-type BPND (r $ (0.599, p $ 0.014).
0.007) and sensory motor striatum (r $ (0.419, p $ 0.046), but
To examine the effect of both receptor BPND measures on
not in the limbic striatum (r $ (0.308, p $ 0.153; Table 3). The
SSRT, a stepwise regression was used to determine the effect of
Hotelling–Williams test of equality of that
adding additional BPND measures to a model of SST performance
the relationships of SSRT and D2-type BPND in the dorsal versus
using only one BPND measure. A model using age, sex and D1-
ventral regions of striatum differed significantly from one an-
type BPND to predict SST performance was improved by adding
other (p $ 0.039), suggesting that the correlation of D2-type
D2-type BPND to the model (D1: F(3,18) $ 2.937, p $ 0.067; D1
BPND with SSRT was specific to the dorsal striatum.
) D2: F(4,18) $ 3.776, p $ 0.028). In the reverse analysis, adding
Since BPND for each receptor subtype in the dorsal striatum
D1-type BPND to a model of SST performance using D2-type
was negatively correlated with SSRT, we tested the correlations
BPND also improved the model (D2: F(3,18) $ 2.176, p $ 0.133; D1
between BPND for each receptor subtype and SSRT, controlling
) D2: F(4,18) $ 3.776, p $ 0.028). The model including both
for the effects of age, sex, and BPND for the other receptor sub-
receptors showed the effects of both D1- and D2-type BPND (D1:
type. A negative correlation of SSRT with D1-type BPND in the
t $ (2.506, p $ 0.025; D2: t $ (2.082, p $ 0.056).
5994 • J. Neurosci., April 15, 2015 • 35(15):5990 –5997
Robertson et al. • Dopamine Receptors and Motor Response Inhibition
Table 3. Relationships of dopamine receptor binding potential (BPND) and stop-
receptors are activated at high dopamine concentrations during
signal task performance variables
phasic increases in extracellular dopamine (Dreyer et al., 2010).
D1- and D2-type receptor signaling can as
shown by the observation that coadministration of D
Region of interest
type dopamine receptor agonists, at doses that are behaviorally
inactive when administered alone, increases locomotor behavior
in rats (Vermeulen et al., 1994). Such an interaction between
Associative striatum
pathways may govern the perfor-
Sensory motor striatum
mance on the SST.
The results obtained here align with a model of striatal motor
control of response inhibition in which D1- and D2-type recep-
tors support competing processes via the modulation of stria-
tonigral and striatopallidal pathways (Logan et al., 1984; Mink,
Associative striatum
1996; Frank, 2005; Frank et al.,
Sensory motor striatum
facilitate the "go" process
Partial correlation coefficients for the relationships between dopamine receptor availability (BP
2-expressing striatopallidal neurons facilitate the "stop"
ND ) and stop-signal
task performance variables, controlling for the effects of sex and age. Significant relationships are highlighted in
process (Alexander and Crutcher, 1990; Surmeier et al., 2007;
ND and SSRT is consistent with this
Dopamine receptor BP
model and corroborates findings from other human studies
ND and response inhibition assessed by
showing that the administration of the D2-type receptor agonist,
Tests of the correlations between dopamine receptor subtype
cabergoline, enhances stopping ability (Nandam et al., 2013), and
findings from neuroimaging results
ND and CE or GoRT on the CPT showed no statistically signif-
icant relationships. Furthermore, CE was not correlated with re-
BPND is correlated with SSRT and inhibition-related striatal neu-
sponse inhibition capacity (SSRT) on the SST. Although GoRT
ral activity (Ghahremani et al., 2012).
on the SST and GoRT on the CPT showed a significant associa-
contributions of D1-
tion (r $ 0.419, p $ 0.024), GoRT on the CPT did not show any
and D2-mediated dopamine signaling to cognitive function and
significant relationship with either D
behavior. For example, individual differences in the ability to
1- or D2-type BPND in any
region tested.
learn from positive and negative feedback are related to D1- and
1- nor D2-type BPND in the cortex showed a signif-
2-type BPND values, respectively (Cox et al., 2015). A theory of
icant correlation with SSRT, GoRT, or CE in a voxelwise analysis,
prefrontal dopamine function between D1-
using a liberal threshold (p " 0.05, uncorrected). Analysis of D
2-type receptor-mediated signaling in modulating fronto-
striatal function (Durstewitz and Seamans, 2008). Moreover, a
2-type BPND in the midbrain showed no significant correla-
tions with SSRT, GoRT, or CE.
new model of posit thatD1 receptor activation prepares a set of possible responses, then
Repeated measures of neurobehaviorial task performance
D2 receptor activation functions in selecting the final response
Task performance variables showed a high degree of test–retest
(Keeler et al., 2014). The present findings are consistent with such
reliability over an average elapsed time of 40 months. The average
suggesting that there is cooperative sig-
percentage change in CE and SSRT was small (8% and 7%, re-
naling between D1- and D2-type receptor-mediated pathways
spectively), and intraclass correlations were moderately high (CE:
during stopping.
ICC $ 0.913, p $ 0.001, n $ 10; SSRT: ICC $ 0.738, p $ 0.029,
The effect of D2-type BPND on SSRT appears to be specific to
n $ 10). Adding the time interval between neuroimaging and
stopping a motor response, as indicated by the lack of correlation
neurocognitive tests as a covariate in statistical analyses did not
with GoRT. In contrast, the relationship between D1-type BPND
change the results.
and SSRT may reflect a general motor effect. This view is sup-ported by the trend-level correlation found with GoRT on the
SST, and by literature showing consistently that the activation
This study extends evidence for a contribution of striatal dopa-
of D1 receptors enhances motor activity (Kreitzer and Berke,
minergic function to motor response inhibition in humans
2011). D1-type BPND, however, was not T
(Ghahremani et al., 2012; Bari and Robbins, 2013; Nandam et al.,
The anatomical specificity of the correlations between SSRT
receptors in the dorsal striatum. D1 and D2 receptors
and BPND corroborate findings from rodent studies (Eagle and
are localized to striatonigral and striatopallidal neurons, respec-
Robbins, 2003a; Eagle et al., 2011). These studies
tively, with minimal colocalization (Hersch et al., 1995). Dopa-
dorsal striatum, but not the
mine regulates striatal activation 1 receptor
ventral striatum, is necessary for SST performance. Specifically,
activation, which enhances the function of striatonigral neurons,
neither excitotoxic lesions nor direct antagonist infusions into
and via D2-receptor activation, which suppresses the function of
the nucleus accumbens affected SST performance in rats (Eagle
striatopallidal neurons (Creese et al., 1983; Surmeier et al., 2007;
and Robbins, 2003a,b; Eagle et al., 2011). Moreover, this
performance was also ob-
activation of either subtype de-
served in humans in whom D2-type BPND and fMRI activation
pends on the intrasynaptic dopamine concentration and the
during stopping was found in dorsal striatum, but not in ventral
respective affinities of the receptors for the neurotransmitter. D2-
striatum (Ghahremani et al., 2012). Last, although D2-type BPND
type receptors, which have higher affinity than D1-type receptors
in the with self-reports of impul-
for dopamine, mediate tonic dopaminergic signaling. D1-type
sivity and novelty seeking (Zald et al., 2008; Buckholtz et al.,
Robertson et al. • Dopamine Receptors and Motor Response Inhibition
J. Neurosci., April 15, 2015 • 35(15):5990 –5997 • 5995
2010), there were no significant relationships between behavioral
in D1- and D2-type BPND with aging, a decrease of only !8% with
measures and D2-type BPND in the midbrain. This
every decade of life. In addition, the test–retest reliability of the
difference between findings may reflect differences between what
SST and CPT performance variables is well established, showing
is measured by self-reports of impulsivity compared with neuro-
high reliability over several weeks (Soreni et al., 2009; Weafer et
cognitive tasks (Reynolds et al., 2006, 2008; Fields et al., 2009).
al., 2013; but also see Wo¨
ciated with dopamine receptor availability suggests that the tasks
average elapsed time of 40 months between assessments. Adding
tap into different neurochemical mechanisms subserving motor
the time interval between neuroimaging and neurocognitive tests
response inhibition. Whereas the SST measures the ability to
as a covariate in statistical analyses did not change the results,
cancel a motor response that has been initiated, the CPT mea-
suggesting that the time-related influences on the relationships
sures action restraint (i.e., not going). Brain-imaging studies have
between dopamine receptor BPND and task performance re-
shown that these tasks engage overlapping, but distinct, neural
ported here are likely to be minimal.
circuits (Rubia et al., 2001; Zheng et al., 2008; Swick et al., 2011,;
In summary, we present direct evidence for associations of
striatal D1- and D2-type receptor availability with capacity for
they would be governed by the same neu-
response inhibition on the SST in humans. These relationships
rotransmitter systems and would show comparable relationships
were specific to the dorsal striatum, identifying this region as an
with neurochemical markers (Jentsch et al., 2014). Our findings,
important locus for differential dopaminergic control of motor
however, support a stopping
response inhibition. The results support the notion that the bal-
(SST) and not going (CPT) as separate constructs (Robinson et
ance between D1- and D2-type receptor-mediated signaling is
al., 2009; Swick et al., 2011) that are subserved
important for motor response inhibition. The findings represent
et al., 2008; Robinson et al., 2009).
an important advance as the understanding of dopaminergic sig-
naling in the human brain has implications for the development
process (SSRT) is likely influenced by dopaminergic signaling,
of specific agents, possibly D1 targeted, to treat patients with neu-
the ability to withhold a response (CPT) is not (Eagle and Baunez,
ropsychiatric disorders that are characterized by an impulsive
2010). Different cognitive requirements,
phenotype, such as observed in ADHD and addictive disorders.
or working memory, may influence overall task perfor-mance and links to dopamine markers. Such differences may also
explain the lack of correlation between scores on the CPT and
Abi-Dargham A, Martinez D, Mawlawi O, Simpson N, Hwang DR, Slifstein
M, Anjilvel S, Pidcock J, Guo NN, Lombardo I, Mann JJ, Van Heertum R,
SST in both rodents and human subjects (Broos et al., 2012).
Foged C, Halldin C, Laruelle M (2000) Measurement of striatal and ex-
Finally, while dopamine receptors were
trastriatal dopamine D1 receptor binding potential with [11C]NNC 112
study, contributions of other neurotransmitter systems cannot be
in humans: validation and reproducibility. J Cereb Blood Flow Metab
overlooked, as there is substantial evidence for a role of norad-
20:225–243. CrossRef Medline
renergic and other transmitter systems in the striatal control of
Alexander Functional architecture of basal ganglia
response inhibition (Zheng et al., 1999; Eagle et al., 2011; Bari and
circuits: neural substrates of parallel processing. Trends Neurosci 13:266 –271. CrossRef Medline
Robbins, 2013).
FC, Hohlweg R, Hansen LB, Guddal E, Braestrup C,
has limitations. Among them are its correlative
Nielsen EB (1992) NNC-112, NNC-687 and NNC-756, new selective
design, which cannot inform on causal relationships between
and highly potent dopamine D1 receptor antagonists. Eur J Pharmacol
dopamine receptor subtype signaling and motor response inhi-
219:45–52. CrossRef Medline
bition, and the relatively small sample size. Another is the imper-
Aron AR, BJ, Robbins TW (2003) Methylphenidate
fect selectivity of the radioligands used. [11C]NNC-112 has an
improves response inhibition in adults with attention-deficit/hyperactiv-
!10-fold higher in vivo affinity for D
ity disorder. Biol Psychiatry 54:1465–1468. CrossRef Medline
1-type over 5HT2A recep-
Aron AR, Robbins TW, Poldrack RA right infe-
tors (Slifstein et al., 2007), and pharmacological blocking studies
rior frontal cortex: one decade on. Trends Cogn Sci 18:177–185. CrossRef
(that !5% of the [11C]NNC-112 sig-
5HT2A binding. Although contam-
van der Molen MW, Logan GD (2003) Horse-race model simu-
lations of the stop-signal procedure. Acta Psychol (Amst) 112:105–142.
1 receptor signal with 5HT2A binding is minor in
the striatum, it should be acknowledged. [18F]Fallypride has
nearly equal affinity for D
2 and D3 dopamine receptors in vivo
pulsivity, attention and monitoring behaviour in rats performing the stop-signal
(Slifstein et al., 2004) and cannot distinguish between them; how-
task: possible relevance to ADHD. Psychopharmacology (Berl) 230:89–111.
in the dorsal striatum are almost exclu-
sively D2 receptors with very low D3 expression (Murray et al.,
A, Logan GD, Hogg-Johnson S, Schachar R, Tannock R
(2003) Selective inhibition in children with attention-deficit hyperactiv-
ND measurements in the dorsal -
ity disorder off and on stimulant medication. J Abnorm Child Psychol
2 receptor availability, and those in the ventral stria-
tum are likely a combination of signals from D
31:315–327. CrossRef Medline
2 and D3 receptors.
Bilder RM, London ED, Jentsch JD, Parker DS, Poldrack
[18F]Fallypride also binds to both isoforms of the D2 receptor
RA, Evans C, Freimer NB (2009) Phenomics: the systematic study of phe-
(D2S and D2L); therefore, BPND measurements using [18F]fally-
notypes on a genome-wide scale. Neuroscience 164:30–42. CrossRef
pride do not distinguish between presynaptic and postsynaptic
D2 receptors.
Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG (2010)
Another limitation is the time interval between the behavioral
Pinning down response inhibition in the brain— conjunction analyses of
and PET assessments, which was 17 months on average. Of rele-
the stop-signal task. Neuroimage 52:1621–1632. CrossRef Medline
vance is the low test–retest variation in BP
Broos N, Schmaal L, Wiskerke J, Kostelijk L, L,
Ham J, de Geus EJ, Schoffelmeer AN, van den Brink W, Veltman DJ, de
made using [11C]NNC-112 or [18F]fallypride, which has been
Vries TJ, Pattij T, Goudriaan AE (2012) The relationship between im-
determined in previous studies to be 5–10% (Abi-Dargham et al.,
pulsive choice and impulsive action: a cross-species translational study.
2000; Fujita et al., 2006; Dunn et al., 2013
PLoS One 7:e36781. CrossRef Medline
5996 • J. Neurosci., April 15, 2015 • 35(15):5990 –5997
Robertson et al. • Dopamine Receptors and Motor Response Inhibition
Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS,
Fields S, Collins C, Leraas K, Reynolds B (2009) Dimensions of impulsive
Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald
behavior in adolescent smokers and nonsmokers. Exp Clin Psychophar-
DH (2010) Dopaminergic network differences in human impulsivity.
macol 17:302–311. CrossRef Medline
Science 329:532. CrossRef Medline
Fillmore MT, Rush inhibitory control of behavior in
Chamberlain SR, AD, Clark L, Robbins TW, Sahakian BJ
chronic cocaine users. Drug Alcohol Depend 66:265–273. CrossRef
(2006) Neurochemical modulation of response inhibition and probabi-
listic learning in humans. Science 311:861– 863. CrossRef Medline
(2005) Dynamic dopamine modulation in the basal ganglia: a
Colzato LS, van den Wildenberg WP, Van der (2010)
neurocomputational account of cognitive deficits in medicated and non-
Genetic markers of striatal dopamine predict individual differences in
medicated Parkinsonism. J Cogn Neurosci 17:51–72. CrossRef Medline
dysfunctional, but not functional impulsivity. Neuroscience 170:782–
Frank MJ, Santamaria A, O'Reilly RC, Willcutt E
788. CrossRef Medline
tational models of dopamine and noradrenaline dysfunction in atten-
WP, Hommel B (2013) The genetic impact
tion deficit/hyperactivity disorder. Neuropsychopharmacology 32:
(C957T-DRD2) on inhibitory control is magnified by aging. Neuropsy-
1583–1599. CrossRef Medline
chologia 51:1377–1381. CrossRef Medline
Fujita M, Brown A, Sangare J, Ryu Y, Sprague K,
Colzato LS, Jongkees Wildenberg WP, Hommel B
Berman K, Pike V, Innis R (2006) Test retest reproducibility and influ-
(2014) Eating to stop: tyrosine supplementation enhances inhibitory
ence of dopamine levels on [18F]fallypride PET quantification. J Nucl
control but not response execution. Neuropsychologia 62:398 – 402.
Med 47 [Suppl 1]:282P.
Gerfen CR, Surmeier DJ (2011) Modulation of striatal projection systems
JA, Cohen JR, Galvan A, Canli T, Poldrack RA (2012)
by dopamine. Annu Rev Neurosci 34:441– 466. CrossRef Medline
Measurement and reliability of response inhibition. Front Psychol 3:37.
Ghahremani DG, Lee B, Robertson CL, Shetler
N, Brown AK, Monterosso JR, Aron AR, Mandelkern MA, Poldrack RA,
Larcher K, Fellows LK, Clark CA, Leyton M, Dagher A
London ED (2012) Striatal dopamine D2/D3 receptors mediate response
(2015) Striatal D1 and D2 signaling differentially predict learning from
inhibition and related activity in frontostriatal neural circuitry in humans.
positive and negative outcomes. Neuroimage 109:95–101. CrossRef
J Neurosci 32:7316 –7324. CrossRef Medline
Hall H, Sedvall G, C, Farde L (1994) Distri-
DR, Hamblin MW, Leff SE (1983) The classification of do-
bution of D1- and D2-dopamine receptors, and dopamine and its metab-
pamine receptors: relationship to radioligand binding. Annu Rev Neuro-
olites in the human brain. Neuropsychopharmacology 11:245–256.
sci 6:43–71. CrossRef Medline
Dalley JW, D, Robbins TW (2008) Neurobehavioral
Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam
mechanisms of impulsivity: fronto-striatal systems and functional neuro-
JP, Ince E, Yi H, Levey AI (1995) Electron microscopic analysis of D1 and
chemistry. Pharmacol Biochem Behav 90:250 –260. CrossRef Medline
D2 dopamine receptor proteins in the dorsal striatum and their synaptic
de Wit H, Crean J, Richards JB (2000) Effects of etha-
relationships with motor corticostriatal afferents. J Neurosci 15:5222–
nol on a measure of behavioral inhibition in humans. Behav Neurosci
114:830 – 837. CrossRef Medline
CL, Mandelkern MA, Morgan AT, London ED
Dreyer JK, JD (2010) Influence of phasic
(2013) The simplified reference tissue model with 18F-fallypride posi-
and tonic dopamine release on receptor activation. J Neurosci 30:14273–
tron emission tomography: choice of reference region. Mol Imaging 12:
14283. CrossRef Medline
1536 – 0121. CrossRef Medline
Dunn C, Marsden P, Baker S, Cleij M, Kapur S, Kessler R,
Jenkinson global optimisation method for robust af-
Howard R, Reeves SJ (2013) Establishing test-retest reliability of an
fine registration of brain images. Med Image Anal 5:143–156. CrossRef
adapted [(18)F]fallypride imaging protocol in older people. J Cereb Blood
Flow Metab 33:1098 –1103. CrossRef Medline
M, Bannister P, Brady M, Smith S (2002) Improved optimization
Durstewitz D, Seamans JK theory of prefrontal cortex
for the robust and accurate linear registration and motion correction of
dopamine function with relevance to catechol-o-methyltransferase geno-
brain images. Neuroimage 17:825– 841. CrossRef Medline
types and schizophrenia. Biol Psychiatry 64:739 –749. CrossRef Medline
Jentsch JD, Ashenhurst JR, Cervantes AS, Penning-
Eagle DM, Baunez C (2010) Is there an
ton ZT (2014) Dissecting impulsivity and its relationships to drug ad-
in the rat? Evidence from anatomical and pharmacological studies of
dictions. Ann N Y Acad Sci 1327:1–26. CrossRef Medline
behavioral inhibition. Neurosci Biobehav Rev 34:50 –72. CrossRef
Keeler JF, Pretsell DO, Robbins TW of do-
pamine D1 vs. D2 receptors: a "prepare and select" model of the striatal
Robbins TW (2003a) Lesions of the medial prefrontal cortex or
direct vs. indirect pathways. Neuroscience 282C:156 –175. CrossRef
nucleus accumbens core do not impair inhibitory control in rats perform-
ing a stop-signal reaction time task. Behav Brain Res 146:131–144.
Berke JD (2011) Investigating striatal function through cell-
type-specific manipulations. Neuroscience 198:19 –26. CrossRef Medline
TW (2003b) Inhibitory control in rats performing a
Lammertsma AA, Hume SP (1996) Simplified
stop-signal reaction-time task: effects of lesions of the medial striatum
PET receptor studies. Neuroimage 4:153–158. CrossRef Medline
and d-amphetamine. Behav Neurosci 117:1302–1317. CrossRef Medline
Lane SD, Moeller FG, Steinberg JL, Buzby Perfor-
Eagle DM, Tufft MR, Goodchild HL, Robbins TW
mance of cocaine dependent individuals and controls on a response inhi-
fects of modafinil and methylphenidate on stop-signal reaction time task
bition task with varying levels of difficulty. Am J Drug Alcohol Abuse
performance in the rat, and interactions with the dopamine receptor
33:717–726. CrossRef Medline
antagonist cis-flupenthixol. Psychopharmacology (Berl) 192:193–206.
Lee B, Farahi J, Nacca A, Monterosso JR, Mumford
JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Man-
Robbins TW (2008) The neuropsychopharmacology of
delkern MA (2009) Striatal dopamine D2/D3 receptor availability is re-
action inhibition: cross-species translation of the stop-signal and go/
duced in methamphetamine dependence and is linked to impulsivity.
no-go tasks. Psychopharmacology (Berl) 199:439 – 456. CrossRef Medline
J Neurosci 29:14734 –14740. CrossRef Medline
Eagle DM, Wong JC, Allan ME, Mar AC, Theobald DE,
Logan GD, Cowan WB, Davis ability to inhibit simple and
Contrasting roles for dopamine D1 and D2 receptor subtypes in the dor-
choice reaction time responses: a model and a method. J Exp Psychol
somedial striatum but not the nucleus accumbens core during behavioral
Hum Percept Perform 10:276 –291. CrossRef Medline
inhibition in the stop-signal task in rats. J Neurosci 31:7349 –7356.
Mawlawi O, Martinez D, Slifstein R, Hwang DR,
Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M (2001) Im-
Narendran R, Guillin O, Belani H, Guo NN, Hwang Y,
aging human mesolimbic dopamine transmission with positron emission
Hwang DR, Abi-Dargham A, Laruelle M (2007) In vivo DA D(1) recep-
tomography: I. Accuracy and precision of D(2) receptor parameter mea-
tor selectivity of NNC 112 and SCH 23390. Mol Imaging Biol 9:117–125.
surements in ventral striatum. J Cereb Blood Flow Metab 21:1034 –1057.
Robertson et al. • Dopamine Receptors and Motor Response Inhibition
J. Neurosci., April 15, 2015 • 35(15):5990 –5997 • 5997
Mink JW (1996) The basal ganglia: focused selection and inhibition of com-
continuous performance tasks: test–retest reliability of two inhibition
peting motor programs. Prog Neurobiol 50:381– 425. CrossRef Medline
measures in ADHD children. J Atten Disord 13:137–143. CrossRef
Monterosso JR, Aron AR, Cordova X, Xu J, London in
response inhibition associated with chronic methamphetamine abuse.
Aharoni E, Munro GE, Calhoun VD, Nyalakanti P, Stevens MC,
Drug Alcohol Depend 79:273–277. CrossRef Medline
Pearlson G, Kiehl KA (2013) A large scale (N $ 102) functional neuro-
imaging study of response inhibition in a Go/NoGo task. Behav Brain Res
256:529 –536. CrossRef Medline
fluoropropyl)-2, 3-dimethoxybenzamide as an improved dopamine D-2 recep-
Surmeier DJ, Z, Shen W (2007) D1 and D2 dopamine-
tor tracer. Nucl Med Biol 22:283–296. CrossRef Medline
receptor modulation of striatal glutamatergic signaling in striatal medium
Murray AM, Ryoo HL, Gurevich Localization of dopa-
spiny neurons. Trends Neurosci 30:228 –235. CrossRef Medline
mine D3 receptors to mesolimbic and D2 receptors to mesostriatal re-
Swick D, Ashley V, Turken U (2011) Are stopping
gions of human forebrain. Proc Natl Acad Sci U S A 91:11271–11275.
and not going identical? Quantitative meta-analysis of two response inhi-
bition tasks. Neuroimage 56:1655–1665. CrossRef Medline
R, Wagner J, Dean AJ, Messer C, Honeysett A, Nathan PJ,
Tannock R, Schachar RJ, Carr RP, Effects of
Bellgrove MA (2013) Dopamine D(2) receptor modulation of human
methylphenidate on inhibitory control in hyperactive children. J Abnorm
response inhibition and error awareness. J Cogn Neurosci 25:649 – 656.
Child Psychol 17:473– 491. CrossRef Medline
Vaidya CJ, Austin G, Desmond JE, Glover GH,
SM, Kennedy DN, Jenkinson M (2011) A Bayesian
Gabrieli JD (1998) Selective effects of methylphenidate in attention def-
model of shape and appearance for subcortical brain segmentation. Neu-
icit hyperactivity disorder: a functional magnetic resonance study. Proc
roimage 56:907–922. CrossRef Medline
Natl Acad Sci U S A 95:14494 –14499. CrossRef Medline
Ramdani C, C, Dagher A, Hasbroucq T
Van Sickle J (2003) Analyzing and watershed
(2014) Dopamine precursors depletion impairs impulse control in
attributes. JAWRA 39:717–726. CrossRef
healthy volunteers. Psychopharmacology (Berl) 232:477– 487. CrossRef
Vermeulen RJ, Drukarch B, Goosen C, Wolters EC, Stoof JC
(1994) The dopamine D1 agonist SKF 81297 and the dopamine D2 ago-
B, Ortengren A, Richards JB, de Wit H (2006) Dimensions of
nist LY 171555 act synergistically to stimulate motor behavior of 1-methyl-
impulsive behavior: personality and behavioral measures. Pers Individ
Dif 40:305–315. CrossRef
Mov Disord 9:664–672. CrossRef Medline
Reynolds B, M (2008) Dimensions of impulsive behavior
Weafer J, Baggott MJ, Test-retest reliability of behavioral
in adolescents: laboratory behavioral assessments. Exp Clin Psychophar-
measures of impulsive choice, impulsive action, and inattention. Exp Clin
macol 16:124 –131. CrossRef Medline
Psychopharmacol 21:475– 481. CrossRef Medline
Robinson ES, Eagle Theobald DE, Mar AC, Murphy ER,
Wo¨stmann NM, Aichert DS, HJ, Ettinger U (2013)
Robbins TW, Dalley JW (2009) Behavioural characterisation of high
Reliability and plasticity of response inhibition and interference control.
impulsivity on the 5-choice serial reaction time task: specific deficits in
Brain Cogn 81:82–94. CrossRef Medline
"waiting" versus "stopping." Behav Brain Res 196:310 –316. CrossRef
Wu Y, Carson RE in the simplified reference tissue
model for neuroreceptor functional imaging. J Cereb Blood Flow Metab
Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T,
22:1440 –1452. CrossRef Medline
Simmons A, Williams SC, Giampietro V, Andrew CM, Taylor E (2001)
Zald DH, RM, Ansari MS, Li R, Shelby ES,
Mapping motor inhibition: conjunctive brain activations across different
Smith CE, McHugo M, Kessler RM (2008) Midbrain dopamine receptor
versions of go/no-go and stop tasks. Neuroimage 13:250 –261. CrossRef
availability is inversely associated with novelty-seeking traits in humans.
J Neurosci 28:14372–14378. CrossRef Medline
M, Grabowska A, Szewczyk J, Gerc K, Chmylak R (2012) Re-
Zald DH, Woodward ND, P, Ansari MS, Baldwin RM,
sponse inhibition of children with ADHD in the stop-signal task: an
Cowan RL, Smith CE, Hakyemez H, Li R, Kessler RM (2010) The inter-
event-related potential study. Int J Psychophysiol 85:93–105. CrossRef
relationship of dopamine D2-like receptor availability in striatal and ex-
trastriatal brain regions in healthy humans: a principal component
Hwang DR, Huang Y, Guo N, Sudo Y, Narendran R, Talbot P,
analysis of [18F]fallypride binding. Neuroimage 51:53– 62. CrossRef
Laruelle M (2004) In vivo affinity of [18F]fallypride for striatal and ex-
trastriatal dopamine D2 receptors in nonhuman primates. Psychophar-
Oka T, Bokura H, Yamaguchi S (2008) The key locus of common
macology (Berl) 175:274 –286. CrossRef Medline
response inhibition network for no-go and stop signals. J Cogn Neurosci
Slifstein M, Kegeles LS, Xu X, Laruelle M, Abi-
20:1434 –1442. CrossRef Medline
Dargham A (2007) [11C]NNC 112 selectivity for dopamine D1 and se-
Zheng P, WX (1999) Opposite modulation of
rotonin 5-HT(2A) receptors: a PET study in healthy human subjects.
cortical N-methyl-D-aspartate receptor-mediated responses by low and
J Cereb Blood Flow Metab 27:1733–1741. CrossRef Medline
high concentrations of dopamine. Neuroscience 91:527–535. CrossRef
Soreni N, Crosbie J, Ickowicz A, Schachar R and Conners'
Source: http://ctn.uoregon.edu/wp-content/uploads/2015/12/Robertson-2015-Striatal-D1-and-D2-type-Dopamine-Receptors-Are-Linked-to-Motor-Response-Inhibition-in-Human-Subjects.pdf
Package ‘openintro' February 20, 2015 Title OpenIntro data sets and supplemental functions Author David M Diez, Christopher D Barr, and Mine Cetinkaya-Rundel Maintainer David M Diez <[email protected]> Description This package is a supplement to OpenIntro Statistics, which is a free textbook available at openintro.org (at costpaperbacks are also available for under $10 on Amazon). Thepackage contains data sets used in the textbook along withcustom plotting functions for reproducing book figures. Notethat many functions and examples include color transparency.Some plotting elements may not show up properly (or at all) insome Windows versions.
Fernando de Souza Costa and Ricardo Vieira Preliminary Analysis of Hybrid Fernando de Souza Costa Rockets for Launching Nanosats into [email protected] 12630-000 Cachoeira Paulista, SP, Brazil This work determines the preliminary mass distribution of hybrid rockets using 98% H2O2 and solid paraffin mixed with aluminum as propellants. An iterative process is used to



