Dmpkd.com
Eur J Clin Pharmacol (2011) 67:701–707DOI 10.1007/s00228-011-0994-7
Effects of SLCO1B1 polymorphisms on the pharmacokineticsand pharmacodynamics of repaglinide in healthy Chinesevolunteers
Jiake He & Zhixia Qiu & Ning Li & Yang Yu & Yang Lu & Deen Han & Tingting Li &Di Zhao & Wei Sun & Fang Fang & Jianheng Zheng & Hongwei Fan & Xijing Chen
Received: 12 August 2010 / Accepted: 7 January 2011 / Published online: 17 February 2011
# Springer-Verlag 2011
tively (P=0.028, 0.032). The clearance in the former two
Purpose Repaglinide is commonly used in the treatment of
genotype groups was significantly attenuated (by 27.39 and
patients with type 2 diabetes mellitus to reduce postprandial
28.55%, respectively) compared with individuals with
hyperglycemia. The objective of this research was to study
SLCO1B1*1B/*1B genotype (P=0.015, 0.019). No signif-
the effects of SLCO1B1 polymorphisms on the pharmaco-
icant differences in blood glucose-lowering effect were
kinetics and pharmacodynamics of repaglinide in healthy
observed among three genotype groups.
Chinese volunteers.
Conclusions SLCO1B1*1B/*1B genotype is associated
Methods A total of 22 healthy young male participants were
with reduced pharmacokinetic exposure after a single dose
recruited from a pool of pharmacogenetically characterized
oral administration of 2 mg repaglinide, including de-
participants genotyped for SLCO1B1, CYP3A4, and CYP2C8
creased AUC0-∞ and increased clearance of repaglinide.
SNPs by polymerase chain reaction-restriction fragment
Moreover, this polymorphism of SLCO1B1 has significant
length polymorphism (PCR-RFLP). Volunteers with
influence on the pharmacokinetics of repaglinide, but no
CYP2C8*3 and CYP3A4*4 alleles were excluded from the
effects on its pharmacodynamics.
clinical study. Then selected volunteers took part in theclinical pharmacokinetic study, receiving 2 mg repaglinide.
Keywords Repaglinide . SLCO1B1 polymorphisms .
Results Healthy participants with SLCO1B1*1A/*1B or
OATP1B1 . Pharmacogenetics
*1A/*1A genotype and SLCO1B1 *15/*1A or *5/*1Agenotype had significantly higher AUC0-∞ than participants
with SLCO1B1*1B/*1B genotype, with the former showing
The encoding gene of the human organic
an increase over the latter of 39.81 and 42.09%, respec-
anion-transporting polypeptides OATP1B1
Cytochrome P-450 2C8 gene
Cytochrome P-450 3A4 gene
F. Fang J. Zheng X. Chen (*)Center for Drug Metabolism and Pharmacokinetics,
Single nucleotide polymorphisms
China Pharmaceutical University,
Peak plasma concentration
Mailbox 210, #24 Tongjiaxiang,
Area under plasma concentration-time curve
Nanjing, Jiangsu 210009, People's Republic of China
Area under plasma concentration-time curve
from 0 to infinity
Bioanalysis Service, WuXi AppTec Co. Ltd,
Shanghai 200131, People's Republic of China
Elimination half-life
Clinical Pharmacology Laboratory, Nanjing First Hospital
Area above blood glucose level-time curve
Affiliated to Nanjing Medical University,
Nanjing, Jiangsu 210006, People's Republic of Chinae-mail:
[email protected]
Eur J Clin Pharmacol (2011) 67:701–707
addition to the polymorphisms of SLCO1B1, theCYP2C8*3 variant allele could also be associated with
Repaglinide is commonly used in the treatment of type 2
reduced plasma concentrations of repaglinide Fur-
diabetes (T2D) to lower postprandial hyperglycemia As
thermore, the Cmax and AUC0-∞ values of pitavastatin
a short-acting insulin secretagogue, it reduces blood
were about 71 and 85% higher in Chinese volunteers with
glucose concentrations by enhancing glucose-stimulated
the SLCO1B1 c.388GA and c.388GG genotypes than
insulin release in pancreatic beta cells. Repaglinide is a
those in volunteers with the SLCO1B1 c.388AA genotype
drug of high plasma protein binding ratio (>98%) []. It is
rapidly absorbed after oral administration with a bioavail-
The available repaglinide pharmacogenetic studies
ability about 60% During elimination, repaglinide is
reported to date were almost exclusively performed in
first taken up from the blood to hepatocytes by human
Finnish populations. It still remains unknown whether the
organic anion-transporting polypeptides 1B1 (OATP1B1)
SLCO1B1 genotypes influence the pharmacokinetics and
expressed on the basolateral membrane of hepatocytes and
pharmacodynamics of repaglinide in the Chinese popula-
then transformed into inactive metabolites via cytochrome
tion. Due to the common interracial variability, we carried
P-450 (CYP) 2C8 and 3A4 in liver [, The pharmaco-
out a prospective and comprehensive pharmacogenetic and
kinetics of repaglinide shows large interindividual variabil-
pharmacodynamic study into repaglinide in healthy Chinese
ity not only in healthy volunteers but also in T2D patients,
mainly due to the genetic polymorphisms of drug trans-porter or metabolizing enzymes [].
Several single nucleotide polymorphisms (SNPs) have
been identified in the SLCO1B1 gene encoding forOATP1B1. One common SNP of the SLCO1B1 gene,
c.388A > G (p.Asn130Asp), has been associated withincreased OATP1B1 transport activity in vitro [, ], and
All of the pharmacokinetic data were obtained from a
the other common SNP c.521 T>C (p.Val174Ala) has been
clinical pharmacokinetic study approved by the Human
associated with reduced OATP1B1 transport activity in
Ethics Committee of Nanjing First Hospital Affiliated to
vitro and increased plasma concentrations of several of its
Nanjing Medical University (Nanjing, China). Informed
substrate drugs in the human body ]. Four haplotypes
written consent was obtained from each of the subjects. A
can be formed through these two SNPs, including
total of 22 healthy young male participants were recruited
SLCO1B1*1A (c.388A–c.521 T), *1B (c.388 G–c.521 T),
from a pool of pharmacogenetically characterized partic-
*5 (c.388A–c.521 C), and *15 (c.388 G–c.521 C) [].
ipants genotyped for SLCO1B1, CYP2C8, and CYP3A4
In East Asians, the frequencies of the c.388A>G and the
SNPs. Volunteers with CYP2C8*3 and CYP3A4*4 alleles
c.521 T>C variants are about 55–89% and 0–22% ],
were excluded from the clinical study , ]. In all
respectively. In Chinese, SLCO1B1*1B and *15 haplotype
enrolled volunteers, four were genotyped with
frequencies are 59.9 and 14%, respectively ].
SLCO1B1*1B/*1B (group 1), six with SLCO1B1 *1A/
Genetic polymorphism in SLCO1B1 is a major
*1A, five with SLCO1B1 *1A/*1B, three with SLCO1B1
determinant of interindividual variability in the pharma-
*15/*1A, and four with SLCO1B1 *5/*1A (Table
cokinetics of repaglinide []. Subjects with the c.521CC
Participants with SLCO1B1 *1A/*1B or *1A/*1A genotype
genotype (including SLCO1B1*5 or SLCO1B1*15 homo-
were combined into group 2, and participants with
zygous haplotype) have 59 or 72% greater repaglinide
SLCO1B1 *15/*1A or *5/*1A genotype were combined
AUC0-∞ and higher mean plasma concentration compared
into group 3. Each of the volunteers was healthy as
with participants with c.521TC or c.521TT genotypes
demonstrated by medical history, physical examination,
At low administration dose of 0.5 mg repaglinide, the
routine blood tests, and electrocardiography. All subjects
SLCO1B1*1B/*1B haplotype has been associated with
were nonsmokers, abstained from drugs for at least 2 weeks
increased OATP1B1 transport activity and SLCO1B1*1A/
before entry into the study, and did not take any coffee or
*1A with impaired activity in healthy Finnish []. In
alcohol for 1 week before the study.
Table 1 Clinical characteristics
of selected healthy participants
SLCO1B1 *1A/*1B or *1A/*1A
SLCO1B1 *15/*1A or *5/*1A
Eur J Clin Pharmacol (2011) 67:701–707
Genotyping of SLCO1B1, CYP2C8*3, CYP3A4*4
validated within the linear range of 0.1–50 ng/mL. The
interday precision (expressed as the coefficient of variation)was 9.5% at 0.2 ng/mL, 9.5% at 2.0 ng/mL, and 1.7% at
DNA was extracted from peripheral whole blood of each
20 ng/mL of repaglinide (n=5), while the intraday precision
subject using a Tiangen DNA extraction kit (Biotech,
was 10.8% at 0.2 ng/mL, 8.0% at 2.0 ng/mL, and 4.7% at
Beijing, China). The genotypes of SLCO1B1 [c.521 T>C
20 ng/mL of repaglinide, respectively (n=15).
(rs4149056), c.388A> G (rs2306283)], CYP2C8*3, andCYP3A4*4 were identified by polymerase chain reaction-
Pharmacokinetics and pharmacodynamics
restriction fragment length polymorphism (PCR-RFLP) aspreviously described with little modification , ].
Pharmacokinetic parameters of repaglinide, such as Cmax
PCR products were then digested with the respective
and Tmax, were directly obtained from the measured data.
restriction enzymes (New England Biolabs, Beverly, MA,
Other important pharmacokinetic parameters (T1/2, AUC0-8,
USA or MBI Fermentas, Vilnius, Lithuania), and separated
AUC0-∞, CL) were calculated using WinNonlin 5.0.1
with agarose gel of proper concentration (Basingstoke,
(Pharsight, Mountain View, CA). Blood glucose decremen-
Hampshire, England). Positive controls with definite spe-
tal AAC0-3h, maximum glucose decrease, minimum glucose
cific genotype templates and negative controls without
concentration, and decremental extent were used to char-
DNA templates were run with every assay to assess the
acterize the blood glucose response to repaglinide.
Statistical analysis
Statistical analysis was performed using SPSS v. 12.0
Twenty-two healthy male Chinese volunteers (mean age
(SPSS, Chicago, IL). Results were expressed as mean±SD
24.2±2.0 years, mean body mass index 21.9±1.3 kg/m2)
in the text and tables and as mean±SEM in the figures for
were entered into the trial. After an overnight fast, each
clarity. Cmax and AUC data were logarithmically trans-
of the volunteers ingested a single dose of 2 mg
formed to fit a normal distribution. Analysis of variance
repaglinide (NovoNorm, 1 mg tablet; NovoNordisk
(one-way ANOVA) with Dunnett for post-hoc analysis was
Bagsvaerd, Denmark) with 250 ml water at 07:30 AM
used to compare the pharmacokinetic and pharmacodynam-
and was not allowed to take in water until 2 h later.
ic parameters among different genotypes. Tmax data were
Volunteers remained seated during the first 2 h and
analyzed by the Kruskal-Wallis test. Pearson correlation
received a standardized warm meal 3.5 h after repagli-
coefficient was used to evaluate the relationship between
nide administration. All the volunteers were directly
the pharmacokinetic and pharmacodynamic parameters of
under clinical supervision and avoided strenuous exer-
repaglinide. A probability (P) of less than 0.05 was
cises throughout the whole experiment. If obvious
considered to be statistically significant.
symptoms of hypoglycemia occurred, 28 g glucosepowder dissolved in 200 ml water was given to thevolunteers. Timed blood samples (3 ml each) were drawn
before and 0.167, 0.33, 0.5, 0.75, 1.0, 1.5, 2.0, 2.5, 3.0,4.0, 5.0, 6.0, 7.0, and 8.0 h after the administration of
Pharmacokinetic parameters of repaglinide
repaglinide. Plasma was then immediately separated andstored at −70°C for further analysis. Blood glucose
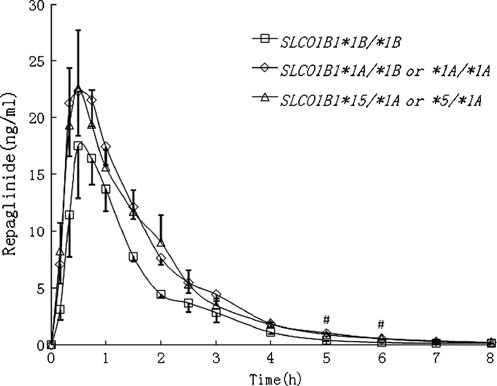
After repaglinide administration, healthy participants in the
concentrations were measured immediately after each
SLCO1B1*1A/*1B or *1A/*1A genotype group (n=11) and
blood sampling by the glucose oxidase method with
the SLCO1B1 *15/*1A or *5/*1A genotype group (n=7)
Glucose Assay Kit (Rongsheng Biotech, Shanghai,
showed numerically but not significantly higher mean
China). The interday coefficient of variation (CV) was
plasma concentrations than the SLCO1B1*1B/*1B geno-
4.8% at 2.5 mmol/L, 3.6% at 12.5 mmol/L, and 4.5% at
type group at most time points (P=0.348 and 0.389 at Cmax,
25 mmol/L (n=18).
respectively; P=0.008 and 0.022 at 5 h, respectively; P=0.018 and 0.010 at 6 h, respectively) (Fig. Table ). The
Determination of plasma repaglinide concentrations
AUC0-8 and AUC0-∞ in those two genotype groups werehigher by 39.8 and 39.8% (P=0.028 and 0.028, respective-
Plasma concentrations of repaglinide were quantified by a
ly), and 41.8 and 42.1% (P=0.033 and 0.032, respectively)
(Fig. ), which was in good agreement with the character-
mass spectrometry (LC-ESI-MS/MS) system [The
istics of their transport activities. Moreover the CLs in
limit of quantification was 0.1 ng/mL. The method was
participants with the SLCO1B1*1A/*1B or *1A/*1A geno-
Eur J Clin Pharmacol (2011) 67:701–707
decremental AAC0–3h (r=−0.035, P=0.883) in bloodglucose. After repaglinide administration, two volunteersgenotyped as SLCO1B1*5/*1A in the SLCO1B1 *15/*1Aor *5/*1A genotype group showed obvious symptoms ofhypoglycemia, and each received 28 g of glucose powder.
One volunteer genotyped as SLCO1B1*1A/*1B in theSLCO1B1*1A/*1B or *1A/*1A genotype group received28 g of glucose powder at 1 h and an additional 28 g at 2 hafter repaglinide intake. The blood glucose parameters ofthese three subjects were excluded from the final pharma-codynamic statistics analysis.
In this study, we investigated the influence of SLCO1B1
Fig. 1 Mean±SEM plasma concentration-time curve after a singleoral dose of 2 mg repaglinide in healthy participants with the
genotypes on the pharmacokinetics and pharmacodynamics
SLCO1B1*1B/*1B (n=4, open squares), SLCO1B1*1A/*1B or *1A/
of repaglinide in healthy Chinese. Healthy participants with
*1A (n =11, open diamonds), and SLCO1B1*15/*1A or *5/*1A
SLCO1B1*1B/*1B genotype had significantly smaller
genotypes (n=7, open triangles). All the participants experienced an
overnight fast and received a standardized warm meal 3.5 h after
0-∞ and larger CL than participants in the other two
repaglinide administration. #P<0.05 vs. SLCO1B1*1B/*1B group
genotype groups. The present findings on repaglinidepharmacokinetics were consistent with an enhanced hepaticuptake of repaglinide by OATP1B1 in the SLCO1B1*1B/
types and the SLCO1B1 *15/*1A or *5/*1A genotypes were
*1B genotype , ]. Moreover, this paper first reported
significantly lower (by 27.4 and 28.6%, respectively) than
significant alterations in CL, but not in T1/2, after single
those participants with the SLCO1B1*1B/*1B genotype (P=
oral administration of 2 mg repaglinide. Such a phenome-
0.015 and 0.019, respectively).
non might be neglected in previous studies due to nostatistical differences in T1/2. The polymorphisms of OATP
Pharmacodynamic parameters of repaglinide
could have a profound influence on CL.
Pasanen et al. depicted the positions of c.388A > G (p.
The baseline blood glucose concentrations (P=0.770 and
Asn130Asp) in the predicted second extracellular loop of
0.995, respectively), minimum concentrations (P=0.334
OATP1B1 and c.521 T > C (p.Val174Ala) in the predicted
and 0.940, respectively), maximum decrease (P=0.854 and
transmembrane domains []. The combined effect of amino
0.894, respectively), decremental AAC0–3h (P=0.944 and
acid exchange and impaired protein sorting to the cell
0.955, respectively) as well as decremental extent (P=0.529
membrane will result in functional changes of OATP1B1,
and 0.890, respectively) of lowering blood glucose showed
making substrate specificity possible [–It is
no significant differences among the three genotype
reasonable to assume that the level of functional changes in
groups after a single oral dose of 2 mg repaglinide,
OATP1B1 may be intricate in individuals homozygous or
while a tendency toward an effect of attenuated blood
compound heteroygous for SLCO1B1 depending on repagli-
glucose lowering presented in SLCO1B1*1B/*1B geno-
nide dose, which may be a partial reason why the
type (Table ). The AUC0-∞ in healthy participants with
pharmacokinetic exposures in the SLCO1B1*1A/*1B or
different genotypes did not significantly correlate with
*1A/*1A genotype group and the SLCO1B1 *15/*1A or
either the maximum decrease (r = 0.052, P= 0.828) or the
*5/*1A genotype group were similar. However, it is also
Table 2 Pharmacokinetic parameters of a single oral dose of 2 mg repaglinide for different SLCO1B1 genotypes
AUC0-8 (ng·h/ml)
AUC0-∞ (ng·h/ml)
*1A/*1B or *1A/*1A
*15/*1A or *5/*1A
Data are mean±SD# P<0.05 vs. SLCO1B1*1B/*1B group
Eur J Clin Pharmacol (2011) 67:701–707
dose from 0.5 to 2 mg , there was no significant differencein blood glucose variables between different genotypes]. The following reasons could be possible explanations.
The adjustment of blood glucose is multifaceted andcomplicated. Blood glucose can be regulated by self-adjustment to a certain extent in healthy participants whena strong blood glucose-lowering effect is present at highconcentration, which could offset the blood glucose-lowering effect of repaglinide, resulting in no apparentassociation between pharmacodynamics and pharmacoki-netics. Repaglinide can stimulate insulin secretion frompancreatic ß-cells to reduce the blood glucose concentra-tion. However, the polymorphisms in insulin secretion andeffects sites, producing diversified ion channels andreceptors, would affect the direct efficiency of insulin]. Thus, it is suggested that even in healthy subjects, the
Fig. 2 Individual AUC0-∞ values in healthy participants with threedifferent SLCO1B1 genotypes. For SLCO1B1*1B/*1B genotype
same amount of insulin may be of different potencies,
group, n=4; for SLCO1B1*1A/*1B or *1A/*1A genotype group, n=11;
resulting in various blood glucose-lowering effects. The
for SLCO1B1*15/*1A or *5/*1A genotype group, n=7. Some values
detection of plasma insulin level after repaglinide adminis-
were so close that they overlapped. #1: P=0.028 SLCO1B1*1A/*1B or
tration could be a better indicator of the influence of
*1A/*1A genotype vs. SLCO1B1*1B/*1B genotype. #2: P=0.032SLCO1B1 *15/*1A or *5/*1A genotype vs. SLCO1B1*1B/*1B genotype
The frequencies of the c.388A> G and c.521 T>C
variants are about 55–89% and 0–22%, respectively, in
possible that some inevitable impact factors could affect the
East Asians [, ]. And the widespread polymorphisms of
pharmacokinetics of repaglinide, e.g., differences in gastric
SLCO1B1 make them nonnegligible factors causing the
emptying and intestinal absorption.
interindividual and interracial variability in the clinical
Pharmacodynamic findings were quite interesting. At a
pharmacokinetics of repaglinide. In the Finnish population,
dose of 2 mg, the AUC0-∞ of repaglinide did not
genetic polymorphism in SLCO1B1 is a major determinant
significantly correlate with either the maximum decrease
of interindividual variability in the pharmacokinetics of
or the decremental AAC0–3h in blood glucose level. It also
repaglinide, and the effect of SLCO1B1 c.521 T > C
seemed that participants with SLCO1B1*1B/*1B genotype
polymorphism persists throughout the clinically relevant
were not as sensitive to the blood glucose-lowering effect
dose range [Thus, keeping a normal and comfort-
as individuals in the other genotype groups (NS). Similarly,
able pharmacokinetic exposure to repaglinide could be of
in a study carried out by Kalliokoski et al. that escalated the
Table 3 Blood glucose variables before and after a single oral dose of 2 mg repaglinide in healthy participants with different genotypes. Bloodglucose data were collected from 0 to 3 h after repaglinide administration because participants received a standardized warm meal at 3.5 h
Baseline concentration
Minimum concentration
Decremental AAC0–3h
*1A/*1B or *1A/*1A
*15/*1A or *5/*1A
Data are mean±SD. After repaglinide administration, one volunteer genotyped as SLCO1B1*1A/*1B in SLCO1B1*1A/*1B or *1A/*1A genotypegroup and two volunteers genotyped as SLCO1B1*5/*1A in SLCO1B1 *15/*1A or *5/*1A genotype group showed obvious symptoms ofhypoglycemia, and each received 28 g of glucose powder. The blood glucose parameters of these subjects were excluded from the final statisticsanalysisa P=0.770 vs. SLCO1B1*1A/*1B or *1A/*1A genotype and P=0.995 vs. SLCO1B1 *15/*1A or *5/*1A genotypeb P=0.334 vs. SLCO1B1*1A/*1B or *1A/*1A genotype and P=0.940 vs. SLCO1B1 *15/*1A or *5/*1A genotype.
c P=0.854 vs. SLCO1B1*1A/*1B or *1A/*1A genotype and P=0.894 vs. SLCO1B1 *15/*1A or *5/*1A genotyped P=0.529 vs. SLCO1B1*1A/*1B or *1A/*1A genotype and P=0.890 vs. SLCO1B1 *15/*1A or *5/*1A genotypee P=0.944 vs. SLCO1B1*1A/*1B or *1A/*1A genotype and P=0.955 vs. SLCO1B1 *15/*1A or *5/*1A genotype
Eur J Clin Pharmacol (2011) 67:701–707
During the clinical research, two volunteers genotyped as
impaired membrane localization of the hepatocyte uptake trans-porter. J Biol Chem 277:43058–43063
SLCO1B1*5/*1A and one volunteer genotyped as
7. Kameyama Y, Yamashita K, Kobayashi K, Hosokawa M, Chiba K
SLCO1B1*1A/*1B showed obvious symptoms of hypogly-
(2005) Functional characterization of SLCO1B1 (OATP-C)
cemia. These side effects seem to be in accordance with high
variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15 +
pharmacokinetic exposure, but it is still necessary to
C1007G, by using transient expression systems of HeLa andHEK293 cells. Pharmacogenet Genomics 15:513–522
investigate the genotype-adverse effect relationship and
8. Niemi M (2007) Role of OATP transporters in the disposition of
elucidate molecular mechanisms. Beginning with an opti-
drugs. Pharmacogenomics 8:787–802
mum dose and adjusting the dosage according to the
9. Kalliokoski A, Neuvonen PJ, Niemi M (2010) SLCO1B1
genotype if significant genotype-therapeutic effects are
polymorphism and oral antidiabetic drugs. Basic Clin PharmacolToxicol 107:775–781
revealed and keeping proper maintenance dose will not only
10. Tirona RG, Leake BF, Merino G, Kim RB (2001) Polymorphisms
help develop a more secure and effective clinical use of
in OATP-C: identification of multiple allelic variants associated
repaglinide, reducing the risk of unwanted side effects such
with altered transport activity among European- and African-
as hypoglycemia, but also could relieve patients' burden.
Americans. J Biol Chem 276:35669–35675
11. Nozawa T, Nakajima M, Tamai I, Noda K, Nezu J, Sai Y, Tsuji A,
Due to ethical problems, we cannot conduct pharmacokinetic
Yokoi T (2002) Genetic polymorphisms of human organic anion
research in T2D patients at this time. But it remains to be
transporters OATP-C (SLC21A6) and OATP-B (SLC21A9): allele
investigated whether the analogous response could appear in
frequencies in the Japanese population and functional analysis. J
Chinese T2D patients with the same genotype through more
Pharmacol Exp Ther 302:804–813
12. Niemi M, Schaeffeler E, Lang T, Fromm MF, Neuvonen M,
ingenious and secure experiments. Further investigations
Kyrklund C, Backman JT, Kerb R, Schwab M, Neuvonen PJ,
centering on the separate and combined effects of SLCO1B1
Eichelbaum M, Kivistö KT (2004) High plasma pravastatin
polymorphisms on repaglinide disposition as well as a
concentrations are associated with single nucleotide poly-
potential risk evaluation during combination treatment in
morphisms and haplotypes of organic anion transportingpolypeptide-C (OATP-C, SLCO1B1). Pharmacogenetics
T2D patients would be of great importance.
In conclusion, after a single oral administration of 2 mg
13. Kalliokoski A, Backman JT, Neuvonen PJ, Niemi M (2008)
of repaglinide, SLCO1B1*1B/*1B genotype is associated
Effects of the SLCO1B1*1B haplotype on the pharmacokinetics
with reduced pharmacokinetic exposure such as decreased
and pharmacodynamics of repaglinide and nateglinide. Pharma-cogenet Genomics 18:937–942
AUC0-∞ and enhanced clearance of repaglinide. Moreover,
14. Pasanen MK, Neuvonen PJ, Niemi M (2008) Global analysis of
the polymorphism of SLCO1B1 has significant influence on
genetic variation in SLCO1B1. Pharmacogenomics 9:19–33
the pharmacokinetics of repaglinide but no effects on its
15. Xu LY, He YJ, Zhang W, Deng S, Li Q, Zhang WX, Liu ZQ,
Wang D, Huang YF, Zhou HH, Sun ZQ (2007) Organic aniontransporting polypeptide-1B1 haplotypes in Chinese patients. ActaPharmacol Sin 28:1693–1697
The study was supported by the 863 Hi-tech
16. Niemi M, Backman JT, Kajosaari LI, Leathart JB, Neuvonen M,
Program of China (No. 2007AA02Z171).
Daly AK, Eichelbaum M, Kivistö KT, Neuvonen PJ (2005)Polymorphic organic anion transporting polypeptide 1B1 is amajor determinant of repaglinide pharmacokinetics. Clin Pharma-
col Ther 77:468–478
17. Kalliokoski A, Neuvonen M, Neuvonen PJ, Niemi M (2008)
Different effects of SLCO1B1 polymorphism on the pharmacoki-
1. Hatorp V (2002) Clinical pharmacokinetics and pharmacodynam-
netics and pharmacodynamics of repaglinide and nateglinide. J
ics of repaglinide. Clin Pharmacokinet 41:471–483
Clin Pharmacol 48:311–321
2. Plum A, Müller LK, Jansen JA (2000) The effects of selected
18. Niemi M, Leathart JB, Neuvonen M, Backman JT, Daly AK,
drugs on the in vitro protein binding of repaglinide in human
Neuvonen PJ (2003) Polymorphism in CYP2C8 is associated with
plasma. Methods Find Exp Clin Pharmacol 22:139–143
reduced plasma concentrations of repaglinide. Clin Pharmacol
3. Hatorp V, Oliver S, Su CA (1998) Bioavailability of repaglinide, a
Ther 74:380–387
novel antidiabetic agent, administered orally in tablet or solution
19. Wen J, Xiong Y (2010) OATP1B1 388A>G polymorphism and
form or intravenously in healthy male volunteers. Int J Clin
pharmacokinetics of pitavastatin in Chinese healthy volunteers. J
Pharmacol Ther 36:636–641
Clin Pharm Ther 35:99–104
4. König J, Cui Y, Nies AT, Keppler D (2000) A novel human organic
20. Ruzilawati AB, Suhaimi AW, Gan SH (2007) Genetic poly-
anion transporting polypeptide localized to the basolateral hepatocyte
morphisms of CYP3A4:CYP3A4*18 allele is found in five
membrane. Am J Physiol Gastrointest Liver Physiol 278:156–164
healthy Malaysian subjects. Clin Chim Acta 383:158–162
5. Bidstrup TB, Bjørnsdottir I, Sidelmann UG, Thomsen MS,
21. Yu M, Xu XJ, Yin JY, Wu J, Chen X, Gong ZC, Ren HY, Huang
Hansen KT (2003) CYP2C8 and CYP3A4 are the principal
Q, Sheng FF, Zhou HH, Liu ZQ (2010) KCNJ11 Lys23Glu and
enzymes involved in the human in vitro biotransformation of
TCF7L2 rs290487(C/T) polymorphisms affect therapeutic effica-
the insulin secretagogue repaglinide. Br J Clin Pharmacol
cy of repaglinide in Chinese patients with type 2 diabetes. Clin
Pharmacol Ther 87:330–335
6. Michalski C, Cui Y, Nies AT, Nuessler AK, Neuhaus P, Zanger
22. He J, Zhou W, Wang LC, He TT, Wang D, Fan L, Zhang W, Zhou HH
UM, Klein K, Eichelbaum M, Keppler D, Konig J (2002) A
(2009) Genetic polymorphisms of CYP2C8 and CYP2C9 in Chinese
naturally occurring mutation in the SLC21A6 gene causing
type 2 diabetes mellitus. Chin J Clin Pharmacol Ther 14:300–306
Eur J Clin Pharmacol (2011) 67:701–707
23. Niemi M, Backman JT, Neuvonen M, Neuvonen PJ, Kivistö KT
25. Iwai M, Suzuki H, Ieiri I, Otsubo K, Sugiyama Y (2004)
(2000) Rifampin decreases the plasma concentrations and effects
Functional analysis of single nucleotide polymorphisms of hepatic
of repaglinide. Clin Pharmacol Ther 68:495–500
organic anion transporter OATP1B1 (OATP-C). Pharmacogenetics
24. Wang M, Miksa IR (2007) Multi-component plasma quantitation
of anti-hyperglycemic pharmaceutical compounds using liquid
26. Kalliokoski A, Neuvonen M, Neuvonen PJ, Niemi M (2008) The
chromatography-tandem mass spectrometry. J Chromatogr B
effect of SLCO1B1 polymorphism on repaglinide pharmacokinetics
Analyt Technol Biomed Life Sci 856:318–327
persists over a wide dose range. Br J Clin Pharmacol 66:818–825
Source: http://dmpkd.com/doc/2011paper02.pdf
A Macroeconomic Analysis of Selected Economic Development Indicators in the Local Government Units in Specific Regions in the Philippines Ivy Benito-Lim The development concerns affected by economic indicators and being addressed are not new; they are in fact common to most Local Government Units (LGUs). The practices they employ, likewise, do not involve
s of Exploring Relief and A CME/CE Supplement to PWJ—PAINWeek Journal RELEASE DATE: December 1, 2014 EXPIRATION DATE: December 31, 2015 Sponsored by Global Education Group. Med Learning Group is the education partner. This activity is supported by an educational grant from AstraZeneca.
