Kazansky1.narod.ru
Journal of Immunotoxicology, 2008; 5(4): 369–384
MHC restriction and allogeneic immune responses
Dmitry B. Kazansky
Carcinogenesis Institute, N. N. Blokhin's Cancer Research Center, Moscow, Russia
Discovery of major histocompatability complex (MHC) restriction helped in the understanding of how
T-lymphocytes recognize antigens on bacteria, viruses, and tumor cells. It was initially accepted that MHC
restriction was a consequence of "adaptive differentiation" in the thymus; during differentiation, the form-
ing repertoire of T-lymphocytes "learned" a low affinity for self MHC molecules via positive selection. This
view was later countered by discovery of artifacts in underlying studies and the fact that adaptive differ-
entiation could not explain direct allogeneic and allorestricted recognition phenomena. Data from experi-
ments with TCR transgenic animals, individual MHC/peptide complex expression, and recipients of xeno-
genic thymus glands yielded evidence of an ability to adapt to microenvironment and a low specificity of
positive selection. These facts led to an alternative interpretation of MHC restriction explained, in part, by
specificity of a pool of effector cells activated by primary immunization. Details of this phenomenon were
defined in studies that noted differential primary structures of peptides that bound various allelic forms of
MHC molecules. Here, the T-lymphocyte repertoire formed in the thymus was a result, in part, of random
rearrangement of germinal sequences of TCR gene fragments. Such pre-selected repertoires were inher-
ently capable of reacting with different allelic forms of MHC molecules. In contrast, MHC molecules were
characterized by significant intraspecies polymorphisms; negative and positive selections were aimed at
adaptation of a pre-selected repertoire to a specific microenvironment in an individual. Via elimination
of autoreactive clones and sparing of a broad spectrum of specificity to potential pathogens, selection in
the thymus could be considered a life-long allogeneic reaction of a pre-selected repertoire to self MHC
molecules resulting in tolerance to "self," increased responsiveness to foreign MHC molecules, and cross-
reactivity of the mature T-lymphocyte repertoire to individual foreign peptides plus self MHC.
Keywords: Major histocompatibility complex, MHC restriction, alloreactivity, T lymphocyte, repertoire,
Discovery of MHC restriction
specifically lysed infected targets were found in infected
Dependence of the immune response and immunorec-
immunocompetent mice but not in
nude mice, and that
ognition on MHC Class I molecules was first shown
these cells were critical to disease pathogenesis. Still,
in a lymphocytic choriomeningitis virus (LCMV)
it was critical for them to prove that immune spleno-
infection model (Zinkernagel and Doherty, 1973,
cytes from other strains could kill infected cells with
1974). As later noted, the basis for an MHC restriction
a respective MHC type. Using infected macrophages
hypothesis was development of a test for T-lymphocyte
from varied strains as targets, it was seen that immune
cytotoxicity against LCMV-infected cells. Doherty
T-lymphocytes of H-2b mice lysed only infected cells
(using cerebrospinal liquor) and Zinkernagel (meas-
from H-2b mice but had no effect on cells from hosts of
uring cell cytotoxicity) saw that T-lymphocytes that
a differing haplotype.
The author wishes to thank Professors Garry Abelev and Alexander Chervonsky whose helpful discussions initiated the writing of this manuscript. The author is very grateful to Professor Rolf Zinkernagel for providing some important references, and also wishes to thank Professor Alexander Shtil for being so helpful during the writing process. The work on recognition of MHC molecules by memory cells in the author's laboratory was supported by grants from the Russian Foundation for Basic Research, N-08-04-00563 and N-05-04-49793.
Address correspondence to Dr. Dmitry B. Kazansky, The Laboratory of Regulatory Mechanisms in Immunity, Carcinogenesis Institute, N. N. Blokhin's Cancer Research Center, Moscow, 115478, Russia; e-mail:
[email protected]
(Received 27 February 2008; accepted 7 August 2008)ISSN 1547-691X print/ISSN 1547-6901 online 2008 Informa UK LtdDOI: 10.1080/15476910802476708
370 D. B. Kazansky
Dual specificity to MHC and virus was a pivotal
finding that unveiled the role of MHC and explained T-lymphocyte responses to LCMV and other viruses. Shearer (1974) demonstrated preferential recognition of trinitrophenol (TNP)-labeled syngeneic targets by T-lymphocytes that were immune to TNP. Others proved the reproducibility of data using ectromelia and cowpox vaccine viruses (Blanden et al., 1975; Koszinowski and Ertl, 1975), H-Y antigen (Gordon et al., 1975), and minor histocompatibility antigens (Bevan, 1975); all contrib-uted to a consensus that MHC-restricted recognition was not a casual event but, rather, a general mechanism.
The MHC-restricted manner of CTL-target inter-
actions was subsequently expanded to helper cells, suggesting they too could recognize antigen-induced changes in MHC Class II on B-lymphocytes and mac-
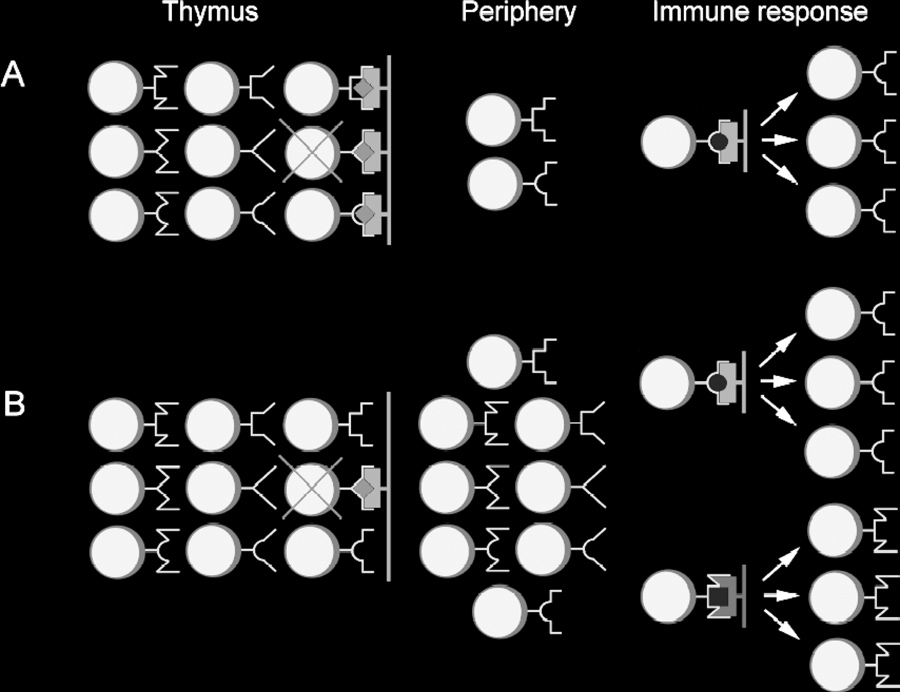
Figure 1. Difference between two concepts explaining origin of
rophages. Most importantly, it became clear why MHC
MHC restriction. (A) According to "adaptive differentiation" hypoth-esis, intrathymic development in contact with self-MHC (purple)
molecules were polymorphic: diversity minimized the
presenting self-peptides (red diamonds) results in skewed diver-
opportunity for non-immunogenic molecule modifi-
sity of T-lymphocytes learned to recognize external antigens (yellow
cation so that immunological tolerance of a population
circles) only in the context of self-MHC molecules. T-Lymphocytes
became improbable. By the mid-1980s, several studies
not capable to interact with self MHC/peptides complexes undergo
noted that transplantation antigens encoded by MHC
extensive "death by neglect." T-Lymphocytes interacting with "self" too well are subject of negative selection. (B) "Intentional priming"
were antigen-presenting molecules and that they were
hypothesis assumes an existence of broad peripheral repertoire
recognized as complexes with antigenic peptides
of T-lymphocytes. According to this hypothesis, MHC restriction is
(Babbitt et al., 1985; Buus et al., 1986; Townsend et al.,
consequence of priming by particular combination of MHC/peptide,
1986; Maryanski et al., 1986).
allowing responses to antigenic peptides (yellow) presented by self- (purple) and foreign (green) MHC molecules.
Origin of MHC restriction: adaptive
(e.g., minor histocompatibility antigens and cow-
differentiation or consequence of priming?
pox vaccine virus). This contradicted the "primary immunization model" wherein lymphocytes recog-
"MHC restriction is an experimental observation of
nized antigen in a complex with MHC molecules of
T-lymphocyte recognition of an antigen in association
any haplotype (Stockinger et al., 1980; Wagner et al.,
with particular MHC-encoded allelic product, but not
1981). Differences between the two models are shown
with the product of another allele." This definition, pro-
in Figure 1.
vided by Schwartz (1984), echoed the intense discus-
The specificity of restriction of a T-lymphocyte
sions of the problem at that time—with the origins of
repertoire in semi-allogeneic chimerae were largely
MHC restriction being the most disputed.
dependent on the time course of appearance of
Defenders of so-called adaptive differentiation
BM-derived antigen-presenting cells (APC) in the
("ontogenetic model of MHC restriction") based
recipient thymus. In the chimerae, donor type (F )
their theory on T-lymphocyte recognition of MHC
APC could be found in the recipient (P ) thymus 2 mo
molecules in the body (Katz, 1977). Here, syngeneic
after BM transplantation. After depletion of periph-
MHC molecules restricted T-lymphocytes, as only the
eral T-lymphocytes, new T-lymphocytes that migrated
former were present during cell maturation. This the-
from the thymus were restricted in MHC of both par-
ory was supported by experiments with bone marrow
ents (Longo and Schwartz, 1980). It was eventually
(BM) chimeras. After transplanting BM of F hybrids
confirmed that positive selection in the thymus was
(after thorough removal of T-lymphocytes) into irradi-
not dependent on the thymic epithelium and could
ated parent (P) recipients (Bevan, 1977; Zinkernagel
be regulated by other cell types (Bix and Raulet, 1992;
et al., 1978; Teh et al., 1982). The resulting chimerae
Hugo et al., 1993). Moreover, selection could proceed
bore hematopoietic cells from the donor (F ), whereas
without MHC molecules and did not require signal-
other cells (including those from thymus) were of
ing via a co-receptor; the only requirement was a low
P1 origin. In these animals, the repertoire of mature
affinity binding of TCR that caused no aggregation
T-lymphocytes was narrower than in donors, and the
(Takahama et al., 1994). Despite some controver-
chimeras recognized mostly antigens in a context
sial results from among the radiation chimerae, the
with recipient MHC molecules. Recipients responded
hypothesis of "adaptive differentiation in the thymus"
mainly to antigens associated with their own MHC
became (and still is) generally accepted.
MHC restriction and allogeneic immune response 371
MHC restriction in nude mice: lessons from
originated from the nude embryo. These mice develop
animals with knocked-out recombinates
mixed chimerism in various tissues; among their
and aggregation chimerae
leukocytes, CD4+, CD8+, and B220+ cells had nude hap-lotypes, whereas two CD11b+ macrophage populations
The problem of MHC restriction in nude mice was thor-
expressed haplotypes of each parent. The thymuses
oughly investigated in RAG knockout and TCR transgenic
of these chimerae bore normal cellular content and a
mice (Zinkernagel and Althage, 1999). In one series of
well-differentiated epithelium with RAG-10/0 haplotype.
studies, nude F (H-2b x H-2d) were sublethally irradiated
To rule out thymic rudiments, double staining for MHC
and then received embryonic H-2b RAG-1 or H-2d SCID
molecules (of RAG-10/0) and cytokeratin was performed.
or H-2d RAG-10/0 thymic transplants. After 12-16 wk, the
This indicated a thymus presence of non-epithelial (i.e.,
mice were immunized with LCMV; at Day 8, CTL restric-
hematopoietic) cells with MHC molecules from a nude
tion was tested and found to be restricted to thymic MHC
parent. None of the chimerae had thymic rudiments with
molecules. These results showed that any "bias" of MHC
mature epithelial cells carrying nude haplotype.
restriction due to transplantation of thymocytes could
Infection of chimerae with LCMV and subsequent CTL
not be associated with specific "suppressor mecha-
responses to H-2b- and H-2d-restricted peptides showed
nisms," as only immuno-deficient mice thymi were used
the T-lymphocyte repertoire of aggregation chimerae
for reconstitution.
was restricted to MHC molecules of both parental haplo-
In a second set of studies, H-2b mice were rescued
types. The response was commensurate with that of wild
with transplanted fully-allogeneic H-2k RAG-10/0 thymi.
type mice and led to complete viral clearance from the
As before, CTL activity was restricted to MHC of the re-
spleen. To better characterize the CD8+ effectors' reper-
cipient but not those of the thymus. The authors were
toire, the cells were stained with MHC tetramers to show
careful to point out that in radiation chimerae, a certain
two distinct populations positive either for LCMV-GP33
amount of hematopoietic cells could survive and influ-
(H-2Db) or for LCMV-NP118 (H-2Ld). Therefore, double
ence positive selection. Along with the transplantation of
restriction of repertoire was associated with real changes
allogeneic H-2k RAG-10/0 thymi, BM cells from the same
in specificity of restriction and not with cross-reactions.
immunodeficient donors were inoculated. Surprisingly,
As noted above, B-lymphocytes of the chimerae
specificity of recipient T-lymphocytes (recipient were chi-
expressed MHC of the nude parent. This meant that in
merae whose hematopoietic cells carried half-recipient
aggregation chimerae, CD4+ helper cells were restricted
half-donor haplotypes) was restricted to MHC molecules
by non-thymic MHC molecules. Apparent contradic-
of the donor and recipient and, in some cases, only to
tions with the results on semi-allogeneic radiation chi-
MHC molecules of the donor. The authors concluded
merae were explained by an incomplete elimination of
that there was an alternative (if not major) way of selec-
TCR-interacting host cells after irradiation; thus, host
tion and maturation of T-lymphocytes that depended on
T-lymphocytes survived regardless of location in the thy-
BM-derived cells.
mus or periphery. As proliferation in the thymus is very
Lastly, in these studies it was seen that MHC
active, these host T-lymphocytes could gain an advan-
molecule expression in transplanted thymi used for H-2b
tage over transplanted donor cells that must first migrate
reconstitution was not necessary for restoration of the
to the thymus. The aggregation chimerae are preferable
T-lymphocyte repertoire. Transplantation of embryonic
because populations developing on thymic and non-
thymi from double Class I and II or 2-microglobulin
thymic MHC are in "equal start" conditions (Martinic
knockouts led to normal CTL responses to LCMV restrict-
et al., 2003). In other words, early data on radiation
ed to a H-2b haplotype. More surprisingly, the complete
chimerae were artifacts, and the ontogenetic model of
restoration of response was achieved after transplanta-
MHC restriction was therefore doubtful. Most likely, this
tion of thymi from xenogenic Lewis rats. Thus, the thymus
model held true only to the dependence of T-lymphocyte
acted mainly as an organ for differentiation, rearrange-
survival on the periphery on MHC molecules. Altogether,
ment, and expression of TCR genes. T-lymphocyte spe-
MHC restriction of the T-lymphocyte repertoire was reg-
cificity reflected expansion of the repertoire and viability
ulated not as much as by the MHC haplotype of thymic
of T-lymphocytes on the periphery, as well as induction
epithelium, but by the haplotype of BM cells (probably,
of effectors by MHC molecules on BM-derived cells.
professional APC).
Martinic et al. (2003) used aggregation allogeneic
chimerae of 8-cell embryos, one of which contained
Adaptive differentiation and positive
a nude homozygote and another a RAG-10/0 or SCID
selection: novel approaches
homozygote. Chimerae SCID H-2d + nude H-2b and RAG-10/0 H-2b + nude H-2d were obtained; thymic
Among findings underlying the ontogenetic model
epithelium of the mice had the haplotype of MHC of
of MHC restriction were results showing that some
SCID or RAG-10/0 embryo, whereas T- and B-lymphocytes
Class II molecules could increase the frequency of
372 D. B. Kazansky
peripheral T-lymphocytes expressing individual V
Still, experiments with transgenic TCR seldom gave
regions (MacDonald et al., 1988; Blackman et al., 1989).
definitive results. Indeed, some TCR were positively
Furthermore, blocking individual allelic MHC products
selected by MHC alleles other than the restricting one,
in F hybrids with allele-specific antibodies inhibited
and positive selection of certain receptors was inefficient
helper and CTL responses restricted to the blocked allele
even by the selecting allele (Bogen et al., 1992). Also, nar-
(Marrack et al., 1988; Marusic-Galesic et al., 1989).
rowing of a repertoire to CD4 or CD8 cells was far from
Importantly, absence of MHC Class I prevented CD8+
absolute (Kirberg et al., 1994; Matechak et al., 1996).
formation, whereas absence of MHC Class II prevented
Logunova gave an interesting example of such receptors
CD4+ cell development (Koller et al., 1990; Zijlstra et al.,
by showing TCR MM14.4 obtained in responses of trans-
1990; Cosgrove et al., 1991; Grusby et al., 1991).
genic mice with a limited peptide repertoire presented to
Mice with transgenic TCR develop a great number of
a syngeneic MHC Class II I-Ab molecule. Transfer of the
T-lymphocytes with certain antigenic and restriction spe-
transgene to wild type C57BL/6 mice led to deletion of
cificity. Initial studies about positive selection of trans-
T-lymphocytes with this receptor. Though the receptor
genic H-Y TCR (specific for sex antigen peptide in the
was initially cloned from T-lymphocyte hybridoma CD4+,
context of H-2Db) (Kisielow et al., 1988; Teh et al., 1988),
predominantly CD8+ cells were positively selected in mice
2C (specific for H-2Ld) (Sha et al., 1988) and AND (specif-
expressing the individual complex Ab with an E chain
ic for pigeon cyt-c fragment in context of I-Ek) (Berg et al.,
AA52-68. In mice totally lacking MHC Class II, CD4+ cells
1989; Kaye et al., 1989) allowed investigators to conclude
were absent; in mice without MHC Class I, CD8+ cells
that a mature T-lymphocyte with a transgenic receptor
were absent. Positive selection of CD4+ T-lymphocytes
could develop in the presence of a restricting MHC allele
with TCR MM14.4 was observed on three alleles of MHC
and absence of specific peptide. This link was clearest in
Class II molecules: in BALB/c (H-2d), A bm12 mutants and
mice with transgenic TCR specific to H-Y antigen. Data
DM knockouts with Ab complexed with CLIP peptide of
demonstrated the link between positive selection and
invariant chain (li). These facts suggested degenerative
MHC restriction of the repertoire, i.e., showing positive
recognition of MHC molecules during positive selection.
selection of CD8+ with this TCR in the thymus of RAG0/0
Other studies of "specificity" of positive selection were
H-2b/d and no selection in the RAG0/0 thymus H-2d/d.
aimed at generating transgenic mice expressing individ-
It eventually became apparent the ontogenetic view-
ual MHC/peptide. The net results were that limitation of
point was idealized. Positive selection of CD8+ cells with
the presenting peptide repertoire lowered the efficiency
transgenic H-Y TCR in H-2b mice appeared associated
of positive selection and presentation of endogenous
with loss of CD4+ cells with the same receptor (Arsov and
superantigens (Golovkina et al., 2001). Nevertheless,
Vukmanovic, 1999). Studies of selection of 2C-TCR in
selection of a diverse repertoire of T-lymphocytes
wild type B6 mice, Kb mutants, and hosts with initially-
occurred in these mice, and T-lymphocytes were
expressed Ld alloantigen revealed at least five phenotypic
capable of reacting on different allelic MHC molecules
patterns of T-lymphocyte selection. These included: (1)
(Ignatowicz et al., 1996, 1997; Chmielowski et al., 1999;
positive selection (Kb and Kbm7); (2) weak positive selection
Lee et al., 1999). Based on all the above, it was con-
(Kbm8); (3) no positive selection (Kbm1 and Kbm10); (4) nega-
cluded that positive selection of a repertoire was due to
tive selection of CD8hi (Kbm3 and Kbm11); and, (5) negative
degenerative recognition of endogenous MHC/peptide
selection of all CD8+ cells (H-2Ld). These results showed
complexes. Though efficacy can depend on variety of
direct interaction of 2C-TCR with various MHC molecules
peptides associated with "self" MHC molecules, positive
during positive and negative selection (Sha et al., 1990).
selection cannot determine the restriction specificity of
In addition, 2C TCR recognized - besides the
the forming T-lymphocyte repertoire.
immunizing complex of Ld plus peptide p2Ca (LSPFPFDL) - Kbm3 plus peptide dEV8 (EQYKFYSV) and positively-
Allorestricted recognition as evidence in
selecting Kb molecule associated with peptide SIYR-8
support of "priming" hypothesis
(SIYRYYGL) (Tallquist et al., 1996; Udaka et al., 1992, 1996). Recognition of peptide antigens by this receptor
The most critical conclusion of the "adaptive differentia-
was specific in the context of the allogeneic Ld molecule,
tion" hypothesis—after experiments with semi-allogeneic
whereas in the context of positively-selecting H-2Kb mol-
chimerae—was that MHC restriction was a process
ecule, all three peptides showed degenerative recogni-
adopted by T-lymphocytes during antigen-independent
tion (Tallquist et al., 1998). Moreover, positive selection
differentiation in a thymus. This "narrowed" a lymphocyte
of TCR could be observed in bm3 TAP0/0 mice, i.e., on
repertoire to the extent that only clones specific to anti-
"empty" heavy chains of Kbm3 (Kuhns et al., 2000). It was
gen associated with self-MHC molecules proliferated
also established that positive selection of T-lymphocyte
in response to antigen. Therefore, this model presumed
receptor AND could proceed on different MHC alleles
that non-immune T-lymphocytes did not recognize the
(Kaye et al., 1992).
antigen associated with allogeneic MHC molecules. This
MHC restriction and allogeneic immune response 373
contrasted with the "priming" hypothesis that surmised
tumor-associated antigens. These clones were specific
that T-lymphocyte clones recognized antigen associ-
to a complex of H-2Kb plus mdm-2-derived peptide; it
ated with allogeneic MHC molecules. The question
is noteworthy that mdm-2 is often over-expressed in
arising from this collision of hypotheses was whether
tumor cells. In culture, these clones selectively reacted
allorestricted recognition exists.
with, and killed, melanoma and lymphoma cells - but
Using the method of limiting dilutions, the frequency
not normal H-2Kb-expressing dendritic cells. In vivo,
of auto- and allorestricted CTL in this depleted
allorestricted clones caused retardation of the growth
population was determined. The frequency of precur-
of melanoma and lymphoma cells in syngeneic (H-2b)
sors with syngeneic restriction was ≈6 times higher than
recipients (Stanislawski et al., 2001). The authors also
that of precursors with allogeneic restriction. This dif-
attempted to obtain allorestricted clones specific to a
ference fluctuated from 2–10-fold depending on inbred
cyclin D1 peptide in the context of human HLA-A2. The
strain combination (Stockinger et al., 1980; Wagner et
clones lysed cyclin D1-over-expressing breast carcinoma
al., 1981). Similar results were observed for precursors of
cells, but not Epstein-Barr-transformed lymphoblast-
thymus CTL devoid of alloreactive cells and recognizing
oid cells (Sadovnikova et al., 1998). Thus, allorestricted
TNP derivatives in the context of syngeneic and alloge-
recognition became an efficient means of breaking toler-
neic MHC molecules (Stockinger et al., 1981).
ance to tumor-associated antigens and to get responses
Wagner et al. (1981) were correct to suggest that a
to leukemia-associated markers like WT1, CD68 and
preference of the T-lymphocyte repertoire for antigen
CD45 (Gao et al., 2000; Sadovnikova et al., 2002; Amrolia
recognition in the context of "self" MHC could be a con-
et al., 2003).
sequence of an experimental procedure and breaking
An additional significant impact on the theory of allor-
normal repertoire during the course of chimerism for-
estriction was made in studies that identified MHC bind-
mation or depletion of alloreactive cells. This was veri-
ing motifs in peptides that interact with allele-specific
fied in subsequent studies that determined frequencies
forms of MHC molecules. Obst et al. (1998) stimulated
of allorestricted clones in the repertoire of normal allo-
a repertoire of T-lymphocytes H-2d with a mixture of
geneic mice. Using combinations of allogeneic strains, it
synthetic peptides from combinatorial libraries of pep-
was seen that stimulation of CTL precursors with TNF-
tides that had an MHC binding motif for interaction with
modified allogeneic cells caused unusually high frequen-
H-2Kb, and cells lacking TAP (to present the synthetic
cies of clones that react with these CTL (1/30–1/300);
peptides on APC). Incubation of the cells with peptides
CTL reacting clones did not react with non-modified
whose structures were optimal for binding with MHC
allogeneic targets (Reimann et al., 1985a). The responses
resulted in successful formation of an MHC molecule/ -
of combinations of B6 and bm1 stimulators and respond-
microglobulin/peptide complex and subsequent trans-
ers to herpes simplex virus and TNP derivatives showed
port onto the plasma membrane. Allorestricted, as well
that ≈30% of reacting clones recognized their targets in
as autorestricted, CTL lines obtained in response to
an allorestricted manner, i.e., they did not react with
such cells widely varied in their peptide specificity and
non-infected or non-modified targets (Reimann et al.,
avidity. The authors concluded that positive selection in
1985b). Kabelitz et al. (1987) proved the existence of
the context of certain MHC molecule was not required
allorestricted T-lymphocytes in humans that responded
for generating high avidity TCR restricted to the same
to parotitis virus.
molecule, but increased the frequency of these CTL. The
Restriction of allorestricted T-lymphocyte responses
authors also analyzed precursors of allorestricted CTL in
to MHC molecules absent in the thymus was used to
peripheral blood of HLA-A2- and HLA-A3- donors. It was
obtain high avidity clones capable of recognizing tumor-
noted that TAP- targets that expressed these HLA (after
associated antigens in patients. Indeed, normally nega-
incubation with combinatorial peptide libraries bear-
tive selection ablates high avidity lymphocyte clones that
ing proper MHC binding motifs) induced responses.
can react with self antigens of an organism (P1) in the
CTL specific to these peptide libraries in the context of
context of self MHC molecules (H-2x-P1). But the clones
allogeneic MHC molecules comprised a major part of
specific to H-2x-P1 could be presented in an allogeneic
the repertoire. However, the frequency of allo-restricted
P2 because negative selection deleted the clones specific
CTL was two times lower than that of CTL restricted to
to tumor-associated antigens in the context of "another
self-MHC molecules.
self" MHC (H-2y-P2). Thus, allorestricted recognition
Any links between self-MHC expression and
could supposedly provide a basis to obtain clones of P2
alloreactive -restricted repertoires was subsequently
specific to a combination of MHC molecule with H-2x-P1
studied. The approach used was as above, plus testing
peptide (for adoptive immunotherapy).
allorestricted responses to viral and self peptides. It was
noted that the closer the structures of allogeneic MHC
allorestricted CTL clones of H-2d mice, thereby demon-
molecule and T-lymphocyte MHC molecule were, the
strating successful use of allorestricted recognition of
greater the ratio of allorestricted CTL that recognize
374 D. B. Kazansky
antigen peptides:CTL recognizing the allogeneic mol-
but different allele-specific motifs (Falk et al., 1990,
ecule independently of the peptide. As expected, the
1991a; Rotzschke et al., 1990). These studies showed that
highest ratio of peptide-specific clones was found in a
one could identify allele-specific sequences from among
response to H-2b stimulators of mutant bm13 and bm14
the huge variety of peptides derived from one antigenic
with the mutations in the H-2Db antigen-binding groove.
protein (Falk et al., 1991a, 1991b).
This link could be associated with effects on the allore-
These motifs formed by "anchoring" AA residues nec-
active repertoire of positive selection in the thymus and
essary for high affinity binding of the peptide to respec-
lymphocyte survival in periphery. However, this did not
tive MHC also indicated that APC expressing different
prohibit recognition of peptides in an allogeneic con-
MHC haplotypes would present various peptides of the
text (Obst et al., 2000). Using MHC tetramer technology,
same antigen. For example, influenza virus nucleopro-
allorestricted T-lymphocytes that specifically recognize
tein contains an epitope that binds H-2Kd in positions
antigenic peptides were later isolated (Moris et al., 2001).
AA147-155 (TYQRTRALV) and H-2Db in positions AA366-
It was thus concluded that T-lymphocytes recognize
374 (MTEMNENSA). For human MHC, the epitope for
antigens in the context of MHC molecules absent during
binding HLA-A2 is within AA85-94 (KLGEFYNQM),
thymocyte differentiation. The existence of lymphocytes
and HLA-B27 in AA383-391 (SRYWAIRTR) (underlined
capable of recognizing antigens in the context of alloge-
= anchoring [motif-forming] residues). Mechanisms of
neic MHC molecules was by itself a solid argument against
peptide/MHC molecule association allowed for pre-
an ontogenetic origin of MHC restriction. Evidently,
dicting the structure of lymphocyte peptide epitopes,
positive selection in the thymus, and T-lymphocyte sur-
including tumor antigens (Rotzschke et al., 1991; Wallny
vival on the periphery, have low impact on formation
et al., 1992; Rammensee et al., 1993). Further, these
of MHC-restriction. This makes the concept of adaptive
studies established a molecular basis for an association
differentiation inappropriate to explain the early experi-
between autoimmune diseases and certain MHC haplo-
mental results of Zinkernagel and Doherty.
types (Vartdal et al., 1996; Kalbus et al., 2001; Munz et al., 2002). Finally, the ability of individual allelic products of
The molecular basis of MHC restriction:
MHC molecules to bind particular peptides of the patho-
MHC binding motifs
gen directly linked MHC with genetically determined immune responses to pathogens.
Can primary priming explain difficulties and contro-
The Rammensee group also discovered the molecular
versies in data that adaptive differentiation cannot?
mechanism of MHC restriction. In initial experiments,
Dependence of repertoire restriction on the replace-
immunization of CBA(H-2k) mice with LCMV induced
ment of host APC with donor cells in radiation chimerae
CTL that recognized viral peptides with MHC H-2k bind-
(Longo and Schwartz, 1980) and the simultaneous trans-
ing motifs. Clearly, these specific CTL lysed infected
plantation of the thymus and BM from RAG knockouts
L929 cells/macrophages (H-2k) that presented the same
into nude mice (Zinkernagel and Althage, 1999) led to an
viral peptides. These CTL did not kill infected macro-
assumption that MHC restriction was controlled at the
phages of H-2d haplotype that presented totally different
level of antigen presentation. Indeed, measures of spe-
peptides of the virus. This was shown in analyses of im-
cific functions of effector cells always required antigen
munogenicity of three LCMV epitopes restricted by the
priming of the naive T-lymphocytes. The "non-thymic
H-2Db molecule, i.e., GP33-41 (KAVYNFATC), GP276-286
cells from BM" that partook in immune responses were
(SGVENPGGYCL), NP396-404 (FQPQNGQFI). Efficacy
professsional APC, i.e., dendritic cells, B-lymphocytes,
of presentation of these peptides correlated with inten-
macrophages. Differing life spans of these cells after
sity of the antiviral CTL response. The NP396-404 pep-
lethal irradiation and different roles in immune respons-
tide (bearing two anchoring residues for H-2Db binding)
es might have been a source of experimental artifact
showed the highest protective effect, regardless of its
and misinterpretation. Conversely, it was evident that
relatively low levels on APC (Gallimore et al., 1998).
primary responses (i.e., allogeneic response, reaction to bacterial superantigens) were MHC-unrestricted.
Origins of allogeneic response: direct and
Rammensee's group uncovered how APC determined
restriction of effector lymphocytes. As noted in a review on MHC binding motifs in antigens (Rammensee, 1995),
All transplantation antigens fall into two categories: ma-
Rotzschke and Falk made this discovery in studies that
jor (i.e., classical H-2-encoded MHC molecules) and mi-
examined the structures of peptides that interact with
nor histocompatibility antigens, i.e., other polymorphic
Class I MHC molecules. The isolated peptides revealed
transplantation antigens. The allogeneic MHC molecules
invariant amino acid (AA) residues near the C- and
are most important for rejection of a transplant. It was
N- termini. Importantly, peptides bound by different
seen early on that a transplant bearing foreign MHC was
allelic forms of MHC Class I molecules had similar lengths
rejected within 8–10 d, whereas one with alien minor
MHC restriction and allogeneic immune response 375
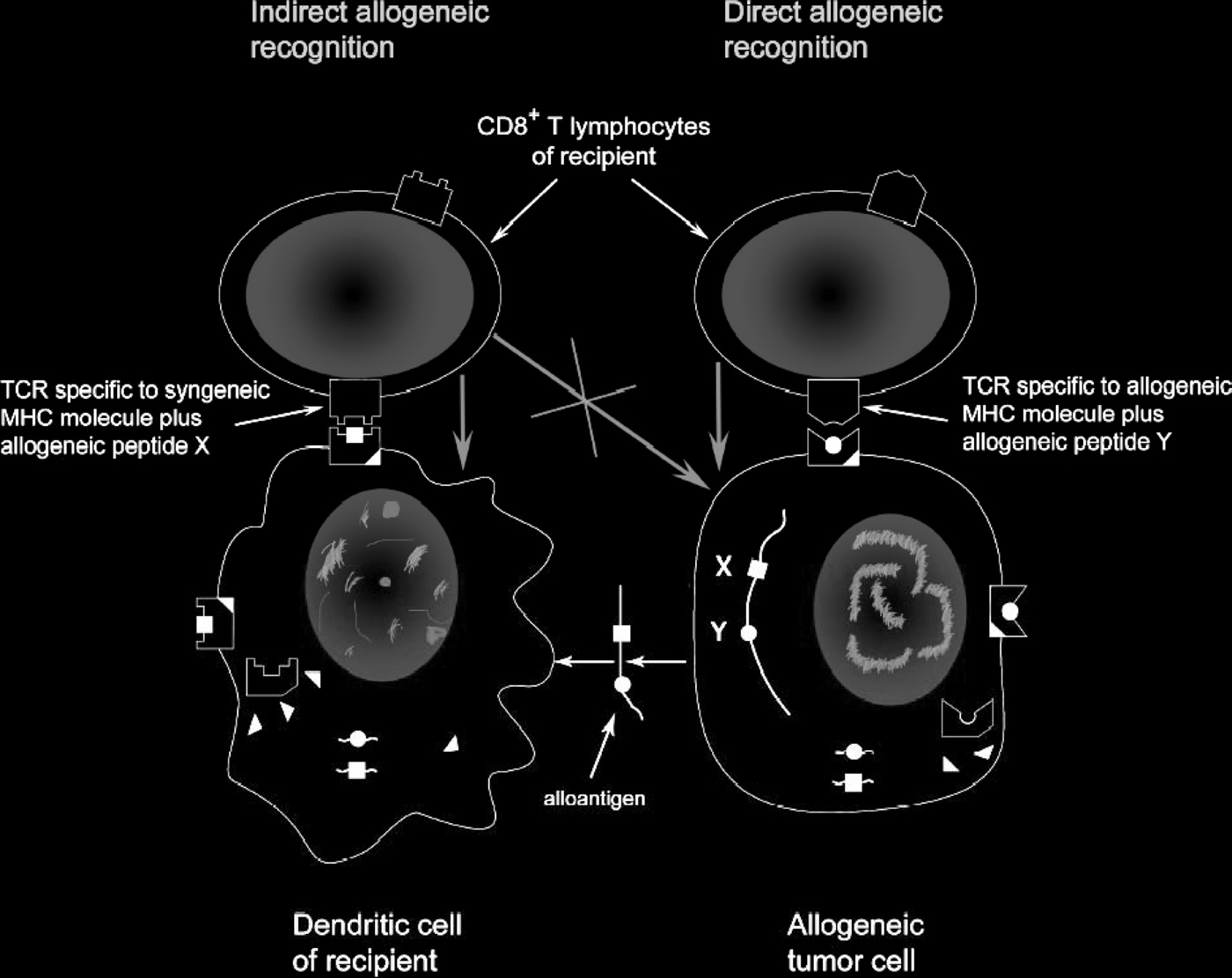
Figure 2. Direct and indirect recognition of allogeneic tumor cells. Rejection of allogeneic tumors represents a basic phenomenon of transplan-
tational immunology and immunogenetics, which cannot be fully explained in frames of current immunological paradigm. To destroy allogeneic
tumor cells, CTL should react to allogeneic MHC Class I molecules, not expressed on recipient's professional APC, whereas cross-priming by
self-APC should result in induction of CTL destroying self-APC presenting foreign peptides (black squares) but sparing engrafted tumor cells
expressing foreign MHC alleles and presenting unrelated peptides (black circles). In reality, in response to allogeneic tumor cells we obviously
see selective expansion of CTL directly reacting with allogeneic MHC molecules on tumor cells. Blue arrows show the direction of killing.
antigens remained viable at least 3 wk. MHC alloantigens
graft proteins could be processed for presentation in
induced very strong T-lymphocyte responses in culture
the context of recipient MHC Class II or transferred
(i.e., 1° responses), while responses to conventional
into endoplasmic reticulum to be associated with
antigens (i.e., ovalbumin) required pre-immunization.
Class I molecules for further cross-priming of recipi-
Further, alloreactive precursors were more frequent
ent T-lymphocytes (Bevan, 1976; Benichou et al., 1999;
than cells specific to antigens presented with self-MHC
Gould and Auchincloss, 1999).
molecules. The frequency of alloreactive lymphocytes
Hypothesis of adaptive differentiation was in agree-
was seen to be as high as 2–5% of the total T-lymphocyte
ment only with the second model, whereas primary
population, whereas cells that react to soluble/viral anti-
priming was in concert with both models. According to
gens were normally 1:10,000 (Fischer et al., 1977).
adaptive differentiation, allogeneic recognition must be
Two models explain T-lymphocyte alloantigen recog-
a consequence of recognition of allogeneic peptides in
nition. Direct allogeneic recognition presumed interac-
the context of self-MHC of the responder. Direct inter-
tion of T-lymphocyte receptor with an allogeneic MHC
action with allogeneic MHC molecules can occur only
molecule bound to the peptide from allogeneic APC.
as a random cross-reaction of T-lymphocyte receptors
After grafting, this response was mediated by migration
"instructed" to react with self-MHC.
of donor APC to the recipient's lymphoid tissue. This
To demonstrate indirect recognition, donor APC
explained how the allogeneic response was not MHC-
lysates were added to a MLR (i.e., a setting wherein direct
restricted and was in concert with a dominant genetic
presentation of antigen was impossible). Lysed APC of
control of inducibility. Indirect allogeneic recogni-
MHC-incompatible donors induced T-lymphocyte pro-
tion implied the allogeneic peptide was recognized by
liferation in the mixed lymphocyte cultures. However,
T-lymphocytes bound to an MHC of the recipient and
this effect was detectable only after preliminary immu-
functioned in responses to minor histocompatibility
nization of the recipients and not confirmed by: inhibi-
antigens, and was MHC-restricted (inheritance being
tion with antibodies against presenting MHC allele; use
co-dominant). This mode of recognition was a result of
of APC from MHC-deficient mice; or, APC from recom-
presentation of allogeneic peptides derived from the
binant mice that express other presenting alleles, factors
graft by the recipient's dendritic cells. After engulfment,
that would bolster an indirect mechanism. Moreover, no
376 D. B. Kazansky
phenotyping of proliferating cells was performed due to
gene products could react with MHC molecules the more
a certitude that the a priori proliferating cells were CD4+
specific was thymic selection.
(Gould and Auchincloss, 1999).
Jerne's hypothesis was supported by some studies,
To study responses to allogeneic Class I MHC, we
even though the efficacy of thymic selection was low
have used a similar system in which C57BL/10 (H-2b)
(Zerrahn et al., 1997; Sebzda et al., 1999). Alloreactive
mice were immunized with P815 mastocytoma (H-2d)
T-lymphocytes could recognize determinants independ-
cells (Kazansky et al., 1998, 1999). Re-stimulation dur-
ent of the bound peptide (Mullbacher et al., 1991; Smith
ing in vitro MLR was performed 2 mo later using heat-
et al., 1997). Nevertheless, the bulk of data indicated that
shocked stimulator splenocytes of C57BL/10 (H-2b),
peptide-independent recognition was rare, and alloreac-
B10.D2 or BALB/c (H-2d), and C3H (H-2k) mice. Primary
tive cells recognized allogeneic MHC in association with
proliferative response to dead allogeneic APC should
the peptides (Rotzschke et al., 1990; Heath et al., 1991;
have been absent. Dead allogeneic APC triggered
Alexander-Miller et al., 1993). Dependence of alloreac-
T-lymphocyte proliferation of pre-immunized recipients;
tive memory CD8+ cells on MHC-bound peptides was
however, in response to immunizing antigen, only CD8+
seen in our studies also. Memory CD8+ cells from B10.
cells of immune animals proliferated. Similar results
D2(R101) (KdIdDb) (obtained in response to EL4 thymoma
were obtained in a system with B10.D2 (R101) (KdIdDb)
(KbDb)) proliferated in MLR in response to heat-shocked
mice immunized with EL4 (H-2b) cells, followed by
allogeneic stimulators from C57BL/6 (KbDb) wild-type
re-stimulation during in vitro MLR with heated spleno-
mice. Proliferation was abrogated if TAP knockout stimu-
cytes from B10.D2 (R101), C57BL/6 (H-2b), and C3H
lators were used. Thus, direct recognition allogeneic
(H-2k) mice. It was noted that the ability to proliferate in
cell depended on the peptides bound to this molecule
response to dead allogeneic APC was not a consequence
(Pobezinskaya et al., 2004).
of indirect recognition of the alloantigen but, rather, a
Interesting and convincing data on the role of pep-
specific feature of CD8+ memory cells primed by the an-
tides in alloreactivity were obtained in a "single MHC/
tigen and trans-co-stimulated.
peptide" system (Kovalik et al., 2000) One study, using
The tumor cells used were not professional APC, so pri-
transgenic pEa mice in which all Class II MHC were rep-
marily indirect recognition of the alloantigen by CD4+ cells
resented by the individual complex of Ab with AA52-68
could be expected. However, only CD8+ cells proliferated.
peptide of E, and DM-KO mice in which Class II mol-
Primed CD8+ cells recognized the antigen directly because
ecules were bound with individual CLIP peptide of the Ii
proliferation of R101 mouse memory cells was blocked by
invariant chain, demonstrated important mechanisms of
antibody to H-2Kb and because the response was absent
allogeneic and allorestricted recognition. First, both lig-
if stimulation in MLR was performed with cells from TAP
ands seemed "poor" stimulators of allogeneic responses,
and -microglobulin knockouts on a C57BL/6 (H-2b)
supporting the "frequency of determinants" hypoth-
background (Kazansky et al., 1999; Pobezinskaya et al.,
esis and identifying presentation of a diverse peptide
2004). Thus, even if indirect recognition of allogeneic Class
repertoire for induction of intense responses. Second,
I MHC was favored (immunization with non-professional
T-lymphocyte hybridomas obtained in the responses to
tumor APC), CD8+ cells directly interacting with foreign
these "allorestricting complexes" were more sensitive to
MHC Class I were a major component of the response.
stimulation with antigenic peptide; these hybridomas recognized peptide in a degenerated manner unlike hybridomas from syngeneic "autorestricted" responders.
Direct allogeneic recognition: peptides or
Third, testing >500 alloreactive hybridomas showed that
side chains?
the majority of alloreactive T-lymphocytes depended
"Initial priming" explained alloreactivity as a conse-
on the peptide (only 17% recognized this peptide spe-
quence of an innate preference of T-lymphocyte recep-
cifically). The authors suggested that peptides influenced
tors to recognize MHC molecules of a species/a higher
the allogeneic response by inducing weak conforma-
density or frequency of allogeneic determinants present-
tional changes in the MHC molecule -helix, and these
ed by allogeneic MHC. Jerne (1971) was first to provide
changes were recognized by alloreactive lymphocyte
a hypothesis of evolutionary preference of TCR genes
receptors. Degenerated recognition of the peptide, and
according to their products' ability to interact with MHC
high sensitivity to the peptide ligand, were key features of
molecules of the same species. This suggested that after
intrathymic elimination of self-reactive T-lymphocytes,
MHC also interact with TCR; this interaction is impor-
a repertoire was left that comprised of a high frequency
tant for alloreactivity. CTL lyse TAP- allogeneic targets that
of cells specific to all other MHC antigens. As selection
express only low amounts of the "empty" heavy chains of
led to enrichment of T-lymphocytes able to react with
Class I MHC molecules. Moreover, in bm3 TAP knockout
MHC, the TCR gene pool would be too abundant and
mice, CD8+ cells formed in the thymus and accumulated
"senseless" precursors produced. Thus, the more TCR
in peripheral lymphoid organs, indicating that positive
MHC restriction and allogeneic immune response 377
selection could occur in an absence of bound peptide
peptide complex and are available for interaction with
(Kuhns et al., 2000). Schneck et al. (1989a, 1989b) showed
the TCR. This is why the most variable regions of the TCR
that allogeneic recognition of H-2Kb by CTL was blocked
CDR3 and CDR3 chains have optimal access to the
by the peptide AA163-174 of the same molecule, meaning
most variable component of the ligand, i.e., the peptide
there was a region for binding the MHC heavy chain with
(Garcia et al., 1996; Davis et al., 1998).
TCR. In our own work, peptides from C-terminal regions of
A similar principle of TCR/MHC interaction was
-helices devoid of MHC binding motifs for B10.D2(R101)
found for allospecific TCR Bm3.3 that bind Class I H-2Kb
recipients—when injected—still induced cell-mediated
complexed with a naturally-processed octapeptide
suppression of allogeneic responses and extended the life
(pBM1:INFDFNTI). In this complex, TCR and MHC-
span of allogeneic skin grafts in recipients (Brondz et al.,
bound peptide were linked via a CDR3 region whereas
1995). Further evidence of the interaction of TCR with
in another TCR, the - and -chains had equal impact
fragments of MHC molecules was demonstrated with mu-
on interactions. Accordingly, only a few C-terminus
tant MHC. Some individual point mutations in the MHC
residues were involved in interactions. Another peculi-
side -helices had no effect on the spectrum of bound
arity of the complex was the very small TCR and MHC
peptides, but caused intense immune responses (Falk
interface; this was surprising, as the affinity of interac-
et al., 1992; Grandea and Bevan, 1993). Noun et al. (1998)
tion was very high. The CDR3 region of this TCR was
showed that mutations in positions 62, 65, 69, 72, 152,
large and was shifted from the peptide-binding groove.
163, and 166 in -helices away from the binding groove
This region interacted only with Gln65 of the -helix of
could be antigenic. The repertoire of peptides that bound
the domain of the MHC, whereas CDR3 consisted
individual mutants did not correlate with the ability of the
of nine residues that all interacted with the peptide. The
mutants to evoke primary immune responses.
CDR1 and CDR2 were shifted to the N-terminus of
Still, it remained unclear which AA residues were
the -helix of the -domain of the MHC molecule, i.e.,
critical for recognition of Class I MHC by alloreactive/
away from the peptide binding area, which abrogated
autorestricted CTL. Using large panels of alloreactive and
their interaction with the -helices. Thus, the position of
autorestricted clones and targets that expressed mutated
the ligand-bound TCR was oblique and mediated main-
AA residues, it was shown that recognition by alloreactive
ly by interactions with the V chain of the receptor with
and autorestricted clones depended on the same residues
the MHC side-chain and the peptide C-terminal part.
in the heavy chains that formed common clusters of rec-
These spatial considerations were relevant to the degen-
ognition (Sun et al., 1995; Hornell et al., 1999). Ala muta-
erative mode of peptide recognition in the allogeneic
genesis-based mapping of relative energies of interaction
response. For a particular TCR/MHC/peptide combina-
of TCR with the MHC heavy chain revealed that ≈67% of
tion, the predicted number of peptides interacting with
the surface and energy in the interface between TCR and
Bm3.3 TCR could increase 400-fold (Reiser et al., 2000).
MHC molecules belonged to interaction of the receptors
Nevertheless, evidence supports a general degen-
with the Class I heavy chain (Manning et al., 1998). These
erative recognition of MHC/peptide complexes by all
data indicated that alloreactivity not be explained solely
TCR (Eisen, 2001). Usually, a detailed analysis of cross-
by differences in the peptide repertoire presented by
reactivity of individual TCR reveals additional MHC or
various MHC. Side -helices also play an important role
peptide ligands capable of interacting with receptors of
being capable of direct interaction with the TCR.
interest. Cross-reactivity was found in our studies of an
MHC molecules present "self" and "alien" peptides to
alloreactive MCC-1 clone of CD8+ memory cells obtained
T-lymphocytes. The groove MHC-peptide is directed to
in a response to an allogeneic H-2Kb molecule. This clone
the extracellular milieu and is a plane formed by -helices
could be activated by the immunizing antigen as well
and the peptide. Topologically, interactions within each
as in a response to H-2Dd(Ld) and H-2Dq(Lq). However,
TCR/MHC/peptide complex are similar to those in all
lengths of the CDR3 and - chains of its TCR were
others, i.e., TCR is oriented diagonally to external sur-
similar (Pobezinskaya et al., 2004). Most likely, a degen-
face of MHC/peptide complex. Spatially, the CDR1 and
erative manner of recognition is an important trait of the
CDR2 of the TCR -chains are localized near the peptide
immune response that allows T-lymphocytes to recog-
N-terminus, whereas similar parts of the -chain are
nize an enormously wide variety of MHC-bound pep-
positioned near the C terminus. CDR1 and CDR2 chains
tides; the specificity is sufficient to discriminate between
encoded by the V region interact mainly with MHC AA
"self" and "foreign".
residues. The third region in the TCR is most variable and
It can thus be concluded that there are no critical
determines the complementary interaction CDR3-MHC.
differences in recognition of a peptide in the context of
The regions of TCR CDR3 and CDR3 are oriented to
syngeneic or allogeneic MHC. Both types of interaction
the center of the TCR/MHC/peptide and interact mainly
can induce highly-specific immune responses to indi-
with the central portion of the peptide. Several residues
vidual MHC/peptide complexes and are efficient in rec-
in the peptide form the external surface of the MHC/
ognizing other combinations. Nevertheless, responses to
378 D. B. Kazansky
allogeneic MHC/peptide complexes are characterized
and most HLA-DR-peptide conjugates might be sterically
by a large number of degeneratively recognizing clones
optimized coordination sites for Ni. In the complexes, Ni
that cross-react with other MHC by interacting with their
may effectively bridge the TCR-chain to His81 in most
-helices. These clones supposedly appear since nega-
DR. Thus, analogous to super-antigens, Ni may link TCR
tive selection does not eliminate them.
to MHC in a peptide-independent manner. However, unlike super-antigens, Ni requires idiotypic (i.e., CDR3-
Role of MHC in immunotoxicology:
determined) TCR AAs. This novel TCR-MHC linkage might
recognition of xenobiotics
explain the high frequency of Ni-reactive T-lymphocytes in humans (Gamerdinger et al., 2003).
In light of the increased understanding of the MHC and its
CBD, a granulomatous lung disorder caused by work-
role(s) in normal host immune responses, several studies
site beryllium (Be), is characterized by accumulation of
have sought to determine the bases for T-lymphocyte-
Be-specific CD4+ T-lymphocytes. Depending on genetic
mediated phenomena like contact hypersensitivity
susceptibility and the nature of exposure, CBD occurs in
(CHS) and allergic contact dermatitis (ACD) in response
up to 20% of exposed workers. Susceptibility has been
to small chemical compounds, chromium and nickel
associated with particular HLA-DP alleles, especially
agents, as well as chronic lung beryllium disease (CBD)
those possessing a negatively-charged Glu residue at
in response to beryllium ions.
AA69 of the -chain. The basis for this association lies
Low-molecular chemicals with an intrinsic potential to
in the ability of these HLA-DP to bind and present Be
covalently modify proteins are classified as haptens. Many
to pathogenic CD4+ cells. Large numbers of effector
are strong inducers of T-lymphocyte-mediated CHS, and
memory, Be-specific CD4+ lymphocytes are recruited to
hapten-specific lymphocytes are known to interact with
the lung and secrete T 1-type cytokines upon Be recog-
hapten-modified MHC-associated peptides. In contrast
nition. A presence of circulating Be-specific CD4+ cells
to these classical haptens, nickel (Ni) ions do not form
directly correlates with severity of lymphocytic alveolitis.
covalent bonds to proteins, but become caught in revers-
As such, CBD serves as an important model of immune-
ible coordination complexes. Some T-lymphocytes may
mediated organ destruction. The findings related to CBD
react to such Ni complexes on the MHC/peptide-surface,
have implications for studies of autoimmune diseases,
akin to what happens with common haptens. In other
in particular, those with unknown inciting antigens and
cases, Ni ions may activate lymphocytes by cross-linking
inaccessible target organs (Amicosante and Fontenot,
their receptors to MHC independent of the nature of the
2006; Newman, 2007).
peptide (Thierse et al., 2004, 2005). The MHC restriction
Drug-induced hypersensitivity reactions have been
element in Ni-reactive T-lymphocytes (ANi-2.3) was des-
explained by the hapten concept, i.e., a compound is
ignated DR52c. A series of experiments established that
too small to be recognized by the immune system. After
the functional ligand for these lymphocytes was a pre-
the drug is covalently bound to an endogenous protein,
formed complex of Ni bound to a combination of DR52c
the hapten-carrier complex (larger modified protein) is
and a specific peptide that generated in B-lymphocytes
immunogenic to B- and T-lymphocytes. Consequently,
(but not in fibroblasts or any other antigen processing-
an immune response (to the drug) with very heteroge-
deficient cells). In addition, ANi-2.3 recognition of this
neous clinical manifestations develops. In recent years,
complex was dependent on His81 of the MHC -chain,
evidence has shown that not all drugs need covalently
suggesting a role for this AA in Ni binding to MHC. Lu
bind to the MHC-peptide complex in order to trigger
et al. (2003) proposed that a general model for Ni recogni-
an immune response. Rather, some may directly and
tion was one in which His81 and two AA from the NH -
reversibly bind to immune receptors like the MHC or
terminal part of the MHC bound peptide-coordinated
TCR, thereby stimulating the cells similar to a pharma-
Ni which, in turn, interacts with some portion of the V
cological activation of other receptors. This concept has
CDR1 or CDR2 region.
been termed "pharmacological interaction with immune
In another study using the T-lymphocyte clone SE9, po-
receptors," the (p-i) concept.
tential Ni contact sites in the TCR and the restricting histo-
While the exact mechanism is still a matter of debate,
compatibility leukocyte antigen (HLA)-DR structure were
non-covalent drug presentation clearly leads to activa-
identified. The specificity of this HLA-DR-favoring V22/
tion of drug-specific T-lymphocytes by various agents
V17+ TCR was primarily due to its -chain. Ni reactivity
(e.g., lidocaine, sulfamethoxazole, lamotrigine, car-
was neither dependent on APC protein processing nor
bamazepine, p-phenylendiamine, etc.). In some patients
affected by the nature of HLA-DR-associated peptides.
with drug hypersensitivity, such a response may occur
However, SE9 activation by Ni did depend on Tyr29 in
within hours upon first exposure to the drug. Thus, the
CDR1, an N-nucleotide-encoded Tyr94 in CDR3, and
reaction may not be due to a classic primary response but,
a conserved His81 in the HLA-DR -chain. This indicated
rather, be mediated by stimulating existing pre-activated,
that labile non-activating complexes between SE9 TCR
peptide-specific lymphocytes cross-specific for the drug.
MHC restriction and allogeneic immune response 379
By this, certain drugs may circumvent checkpoints in
repertoire; this frequency was similar to that after
immune activation imposed by antigen processing and
selection. This indicated the innate predisposition of
presentation mechanisms; this may explain the peculiar
TCR to interact with MHC. Genes coding for TCR were
nature of many drug hypersensitivity reactions (Pichler
evolutionary designed in a way such that their products
et al., 2006).
bind predominantly with MHC side -helices; this is par-
As with indirect and direct responses to grafted tissues,
ticularly true for V-segment CDR1 and CDR2 regions.
the role of chemically-modified peptides (in the context
These results were surprising. Indeed: (1) preferential
of MHC molecules) as well as direct influence on TCR/
interaction of TCR with the MHC is not a consequence
MHC interactions in these reactions can be clarified. In
of positive selection in a thymus; (2) the T-lymphocyte
a structure of Class I domains recognized by TCR, AA
repertoire is primarily specific to all variants of classic
residues have been identified that are invariant even in
MHC molecules, presuming a coordinated evolution of
evolutionary—very distant species. Their location on
three independent genetic loci (/, , and MHC); and,
3D-structure models indicates that in structure-forming
(3) alloreactivity and MHC restriction can be a sequelae
function, as a rule, they are located in contact sites
of this innate specificity. Yang et al. (2002) estimated
between -helices and -sheets. Studies of combinatorial
there were four combinations of peptides with the H-2Kb
homology between Class I and II molecules have identi-
that induced auto- and allorestricted responses in three
fied a fragment located in a "kink"-region of both Class I
mouse strains.
(AA154-164) and -chains of Class II molecules (AA63-73,
Responders were primed in vitro with stimulators
A-molecules; AA 69-79, E molecules) at which a spectra
loaded with peptides, followed by evaluation of frequen-
of "allowed" AA substitutions significantly coincide. Thus,
cies of peptide specific CTL. Three out of four peptides
this region could play a role as "anchor" for interactions
induced responses restricted by self-MHC better than
with TCR, providing the capability for the latter to distin-
by alien MHC, but differences were only 3–5-fold. The
guish MHC from other molecules (Kazanskii et al., 2004).
fourth peptide induced auto- and allorestricted CTL
Importantly, point mutations here result in well-known
with equal efficacy. Titration of peptides showed that
Kbm1 and A bm12 molecules capable of inducing vigorous
high avidity CTL were present among the auto- and
allogeneic responses in wild-type C57BL/6 mice.
allorestricted CTL. The authors concluded that narrow-
Although knowledge of the details of interaction
ing the repertoire to a preferential recognition of antigens
between small xenobiotics and MHC molecules is im-
in the context of self-MHC (which can be expected from
perfect, it can be anticipated that chemical modification
positive selection in thymus) was minor. Further analy-
or acquiring reversible coordination complexes in/near
sis of lectin-stimulated maturation of thymocytes from
a kink-region will result in highly immunogenic forms
mice deficient in MHC showed that the number of Kb-
of these molecules and increased risk for development
restricted CTL among these lymphocytes was similar to
of autoimmune and allergic diseases. The involvement of
the number of allorestricted CTL. Thus, MHC-restricted
His81 and Glu69 in Class II -chains into recognition of
recognition of peptides was innate and imminent for a
Ni and Be ions may suggest this idea (Gamerdinger et al.,
T-lymphocyte repertoire, and this recognition did not
2003; Lu et al., 2003; Newman, 2007).
require thymic selection on MHC molecules.
As early as 1971, Jerne postulated the genetic predis-
Back to Jerne's hypothesis
position of the repertoire for MHC recognition, i.e., "anti-body specificity is determined by structural V-genes that
Is it reasonable that the ability of T-receptor repertoires
code for AA sequences of variable regions on antibody
to interact with MHC are genetically determined? Two
polypeptide chains." The present hypothesis proposes
groups have seen that non-selected lymphocyte receptor
that a host's germ-cells carry a set of V-genes determin-
repertoires had an innate capability to recognize MHC in
ing combining sites of antibodies directed against a com-
the thymus (Merkenschlager et al., 1997; Zerrahn et al.,
plete set of a given class of histocompatibility antigens of
1997). One demonstrated that 20% of thymocytes rec-
the host species. Though this hypothesis is 37 years old,
ognize MHC prior to positive and negative selection, as
its importance remains great.
CD69 activation marker was expressed upon recognizing cells. The other used T-lymphocytes from mice that did
What is really going on?
not express MHC, i.e., DP lymphocytes (immature) with a receptor repertoire not positively or negatively selected
Much data appeared to not fit the hypothesis of
for MHC. Using monoclonal antibodies against TCR/
ontogenetic origin of MHC restriction. Among the prob-
and CD4, maturation of the lymphocytes was induced
lems encountered has been: experiments on which the
in fetal thymic organ cultures. Analysis of TCR spe-
hypothesis was founded have been criticized; results in
cificity revealed a high frequency of clones that reacted
transgenic TCR hosts have been largely refuted; and, spe-
with allogeneic MHC in a pre-selected T-lymphocyte
cificity of restriction by T-lymphocytes was associated
380 D. B. Kazansky
not with the thymus, but with cells originating in the BM
i.e., antigen presentation. However, the reason could be
(minor impact from microenvironment and periphery
deduced based on a hypothesis that pinpointed MHC
survival). As a result, an "initial priming" hypothesis
molecule alloantigenic traits. Indeed, changes in alloan-
emerged that based itself on the critical role of a primary
tigenic "image" of the molecule would change the char-
immune response to a given MHC/peptide combination.
acter of negative selection of the repertoire; this would
Due to this priming, a portion of the lymphocyte reper-
provide an opportunity to rescue individual clones that
toire arose that was specific to recognition of particular
respond to the pathogen.
MHC/peptide complexes, i.e., specificity was dictated
The proposed hypothesis seemed to both reconcile
by antigen-presenting cell. Limiting this specificity by
transplantation immunology with MHC restriction and
thymic "bringing-up" to given MHC/peptide complexes
explain alloreactivity, and provided a foundation for the
resulted in a variable repertoire of T-lymphocytes capa-
phenomena, i.e., interaction of repertoire with histo-
ble of reacting with allogeneic MHC molecules.
compatibility molecules. Adaptive differentiation failed
Identification of MHC-binding motifs in antigenic
to explain alloreactivity, and even genetic control of
peptides provided a structural basis for explanation of
allogeneic reactions and MHC-restricted recognition was
the origin of MHC restriction in terms of "initial prim-
considered differentially. The ability to induce a specific
ing." Combinatory peptide libraries proved the existence
allogeneic response was inherited as a dominant trait,
of a broad repertoire of allorestricted T-lymphocytes
whereas an ability to induce MHC-restricted responses
and revealed an insignificant role for positive selection
was co-dominant. To researchers of MHC-restricted
in narrowing repertoires down to autorestricted clones.
recognition, allogeneic effects were distracting; geneti-
Taking into account that adaptive differentiation could
cally defined hosts were needed to avoid these. It is thus
not provide explanations to phenomena of allorestricted
understandable that allogeneic phenomena were put
responses and direct allogeneic recognition, it was time
aside; this situation changed after discovery of allor-
to admit that an ontogenic origin of MHC restriction,
though still predominant in immunology textbooks, was
Jerne's hypothesis solved the enigma of dominant
misleading. The hypothesis also tried to explain allore-
inheritance of alloantigenicity by linking V gene struc-
activity as cross-reactivity with "self." But for what reason
tures and an innate predisposition of a repertoire to rec-
were "cross-reactive" alloreactive clones not deleted
ognize transplantation antigens. A precise provision of
during thymic negative selection? Were cross-reactions
Jerne was confirmed by discovery of MHC peptide pres-
more pronounced than specific ones? The most obvious
entation in studies that showed that allelic forms of the
answer was no. Logic suggested that "the reaction of the
molecules presented different peptides. Other studies
repertoire to foreign transplantation antigens was spe-
proved the critical role of a broad peptide repertoire in the
cific, whereas recognition of peptides in the context of
intensity of allogeneic responses. Overall, this hypothesis
self MHC was a "cross-reaction."
stated that "the high intensity of an allogeneic response
Loss of some specificities potentially useful for
was explainable by two non-exclusive peculiarities of
responses to pathogens was inevitable and created an
T-lymphocyte recognition: (1) a genetically,determined
Achilles' heel in the organism being attacked. Because
ability of a repertoire to interact with the entire spectrum
MHCs were highly polymorphic and their allelic forms
of a species' MHC; and, (2) specificity of negative selec-
presented different peptides from the same protein,
tion that maintained the ability of a repertoire to respond
this vulnerability was individual within a species. This
to peptides in the context of allogeneic MHC molecules."
was why selection of virus variants for ability to escape
Apparently, the major problem for the immune
immunological attack in one organism would not rescue
system was to avoid transplantation conflict in a host.
a variant from the immune system of a different host.
Indeed, receptors of adaptive immunity "see" self-
Thus, MHC polymorphism created a safety net that al-
antigens more frequently than pathogens. In any case,
lowed slowly-evolving vertebrata to survive even in the
existence of a potentially dangerous system in the host
presence of quickly evolving pathogenic organisms.
could be no less a factor in the evolution of its immune
The traits of the immune system used to be consid-
system than the pathogen. Thus, MHC-restricted recog-
ered in the context of co-evolution with pathogenic
nition could develop not as much in the struggle with
microorganisms. By this, polymorphism among MHCs
pathogenic microorganisms, but in inhibiting reactions
would mean the variability of only those AA residues
with "self." By this, restriction of immune reactions by
responsible for peptide presentations. However, the
several types of recognized molecules would be helpful.
majority of variable residues localized in TCR binding
MHC have allowed the immune system to redirect these
regions and had no impact on peptide binding specifi-
reactions to short peptides containing AA substitutions
city; nevertheless, these variable residues were necessary
not presented in the responding organism. On the other
for MHC alloantigenicity. The reason for polymorphism
hand, they have allowed the responding organism to
was unclear, considering the traditional role of MHC,
form specific central tolerance to self, not involving in
MHC restriction and allogeneic immune response 381
this process a huge diversity of conformations created by
Benichou, G., Valujskikh, A., and Heeger, P. S. 1999. Contributions of
other proteins. As such, alloreactivity can be, not only a
direct and indirect T-cell alloreactivity during allograft rejection in mice. J. Immunol. 162:352–358.
backstop for efficiency during induction of tolerance, but
Berg, L. J., Pullen, A. M., Fazekas de St. Groth, B., Mathis, D., Benoist, C.,
a general feature of T-lymphocyte repertoires.
and Davis, M. M. 1989. Antigen/MHC-specific T-cells are preferen-
One argument of adepts in differentiation was the
tially exported from the thymus in the presence of their MHC ligand. Cell 58:1035–1046.
artificial, purely laboratory character of alloreactivity,
Bevan, M. J. 1975. The major histocompatibility complex determines sus-
i.e., it is a phenomenon that does not exist naturally.
ceptibility to cytotoxic T-cells directed against minor histocompat-
Nevertheless, the reaction of thymocytes to self-MHC
ibility antigens. J. Exp. Med. 142:1349–1364.
Bevan, M. J. 1976. Cross-priming for a secondary cytotoxic response to
during intra-thymic selection had much in common with
minor H antigens with H-2 congenic cells which do not cross-react
the reaction of a mature repertoire to transplantation anti-
in the cytotoxic assay. J. Exp. Med. 143:1283–1288.
gens. Both depended on the same co-stimulatory ligands
Bevan, M. J. 1977. In a radiation chimera, host H-2 antigens determine
immune responsiveness of donor cytotoxic cells. Nature 269:417–418.
and co-receptors (Punt et al., 1994, 1997), and avidity of
Bix, M., and Raulet, D. 1992. Inefficient positive selection of T-cells
T-lymphocyte-APC interactions - although the threshold
directed by hematopoietic cells. Nature 359:330–333.
of activation for thymocytes seemed to be lower than that
Blackman, M. A., Marrack, P., and Kappler, J. 1989. Influence of the major
histocompatibility complex on positive thymic selection of V17+
of mature peripheral lymphocytes (Ashton-Rickardt et al.,
T-cells. Science 244:214–217.
1994; Sebzda et al., 1994). Clearly, positive selection could
Blanden, R. V., Doherty, P. C., Dunlop, M. B., Gardner, I. D.,
be an analog of peripheral interactions of T-lymphocytes
Zinkernagel, R. M., and David, C. S. 1975. Genes required for cyto-toxicity against virus-infected target cells in K and D regions of H-2
with self-MHC necessary for survival (Viret and Janeway,
complex. Nature 254:269–270.
1999). Intra-thymic negative selection was evidently
Bogen, B., Gleditsch, L., Weiss, S., and Dembic, Z. 1992. Weak posi-
similar to deletion of peripheral T-lymphocytes upon
tive selection of transgenic T-cell receptor-bearing thymocytes: Importance of major histocompatibility complex Class II, T-cell
binding of endogenous superantigens (Chervonsky
receptor and CD4 surface molecule densities. Eur. J. Immunol.
et al., 1995). A significant difference was that thymocyte
interactions with self-MHC led to deletion of the former,
Brondz, B. D., Kazansky, D. B., Chernysheva, A. D., and Ivanov, V. S. 1995.
Peptides of a major histocompatibility complex Class I (Kb) molecule
whereas mature lymphocytes gained effector functions
cause prolongation of skin graft survival and induce specific down-
after reaction to alloantigen (Pircher et al., 1991). Thus,
regulatory T-cells demonstrable in the mixed lymphocyte reaction.
thymic selection could be considered first and last as a
Buus, S., Colon, S., Smith, C., Freed, J. H., Miles, C., and Grey, H. M. 1986.
lifelong allogeneic reaction of pre-selected repertoire to
Interaction between a "processed" ovalbumin peptide and Ia mol-
ecules. Proc. Natl. Acad. Sci. USA 83:3968–3971.
The goal of this review was to illustrate conflicts
Chervonsky, A. V., Golovkina, T. V., Ross, S. R., and Janeway, C. A., Jr. 1995.
Differences in the avidity of TCR interactions with a superantigenic
among the modern theories of MHC restriction and
ligand affect negative selection but do not allow positive selection. J.
allogeneic recognition, and to present an alterna-
tive concept that reconciled these views. The ideas
Chmielowski, B., Muranski, P., and Ignatowicz, L. 1999. In the normal reper-
toire of CD4+ T-cells, a single Class II MHC/peptide complex positively
expressed here were initiated by those of Jerne (1971)
selects TCR with various antigen specificities. J. Immunol. 162:95–105.
and the findings of Zerrahn and Raulet (1997), as well
Cosgrove, D., Gray, D., Dierich, A., Kaufman, J., Lemeur, M., Benoist, C.,
as of those that subsequently evolved (Janeway et al.,
and Mathis, D. 1991. Mice lacking MHC Class II molecules. Cell 66:1051–1066.
1997; Viret and Janeway, 1999; Huseby et al., 2003,
Davis, M. M., Boniface, J. J., Reich, Z., Lyons, D., Hampl, J., Arden, B., and
2004; Whitelegg and Barber, 2004).
Chien, Y. 1998. Ligand recognition by T-cell receptors. Annu. Rev. Immunol. 16:523–544.
Eisen, H. N. 2001. Specificity and degeneracy in antigen recognition: Yin
and yang in the immune system. Annu. Rev. Immunol. 19:1–21.
Falk, K., Rotzschke, O., and Rammensee, H. G. 1990. Cellular peptide
Alexander-Miller, M. A., Burke, K., Koszinowski, U. H., Hansen, T. H., and
composition governed by major histocompatibility complex Class I
Connolly, J. M. 1993. Alloreactive cytotoxic T-lymphocytes generat-
molecules. Nature 348:248–251.
ed in the presence of viral-derived peptides show exquisite peptide
Falk, K., Rotzschke, O., and Rammensee, H. G. 1992. A self peptide natu-
and MHC specificity. J. Immunol. 151:1–10.
rally presented by both H-2Kb and H-2Kbm1 molecules demon-
Amicosante M., and Fontenot, A. P. 2006. T-Cell recognition in chronic
strates MHC restriction of self tolerance at the molecular level. Int.
beryllium disease. Clin. Immunol. 121:134–143.
Amrolia, P. J., Reid, S. D., Gao, L., Schultheis, B., Dotti, G., Brenner, M.
Falk, K., Rotzschke, O., Deres, K., Metzger, J., Jung, G., and Rammensee, H. G.
K., Melo, J. V., Goldman, J. M., and Stauss, H. J. 2003. Allorestricted
1991a. Identification of naturally processed viral nonapeptides allows
cytotoxic T-cells specific for human CD45 show potent anti-leukemic
their quantification in infected cells and suggests an allele-specific
activity. Blood 101:1007–1014.
T-cell epitope forecast. J. Exp. Med. 174:425–434.
Arsov, I., and Vukmanovic, S. 1999. Dual MHC Class I and Class II restric-
Falk, K., Rotzschke, O., Stevanovic, S., Jung, G., and Rammensee, H. G.
tion of a single T-cell receptor: Distinct modes of tolerance induc-
1991b. Allele-specific motifs revealed by sequencing of self-peptides
tion by two classes of autoantigens. J. Immunol. 162:2008–2015.
eluted from MHC molecules. Nature 351:290–296.
Ashton-Rickardt, P. G., Bandeira, A., Delaney, J. R., Van Kaer, L.,
Fischer Lindahl, K., and Wilson, D. B. 1977. Histocompatibility antigen-
Pircher, H. P., Zinkernagel, R. M., and Tonegawa, S. 1994. Evidence
activated cytotoxic T lymphocytes II. Estimates of frequency and
for a differential avidity model of T-cell selection in the thymus. Cell
specificity of precursors. J. Exp. Med. 145:508–522.
76: 651–663.
Gallimore, A., Dumrese, T., Hengartner, H., Zinkernagel, R. M., and
Babbitt, B. P., Allen, P. M., Matsueda, G., Haber, E., and Unanue, E. R.
Rammensee, H. G. 1998. Protective immunity does not correlate
1985. Binding of immunogenic peptides to Ia histocompatibility
with the hierarchy of virus-specific cytotoxic T-cell responses to nat-
molecules. Nature 317:359–361.
urally processed peptides. J. Exp. Med. 187:1647–1657.
382 D. B. Kazansky
Gamerdinger, K., Moulon, C., Karp, D. R., Van Bergen, J., Koning, F.,
Kazanskii, D. B., Pobezinskii, L. A., and Tereshchenko, T. S. 2004. Motifs in
Wild, D., Pflugfelder, U., and Weltzien, H. U. 2003. A new type of
the primary structure of MHC Class I molecules and their use for the
metal recognition by human T-cells: Contact residues for peptide-
design of synthetic T-cell receptor ligands. Vestn. Ross. Akad. Med.
independent bridging of T-cell receptor and major histocompatibil-
ity complex by nickel. J. Exp. Med. 197:1345–1353.
Kazansky, D. B., Chernysheva, A. D., Sernova, N. V., Petrishchev, V. N.,
Gao, L., Bellantuono, I., Elsasser, A., Marley, S. B., Gordon, M. Y.,
Pobezinskii, L. A., Agafonova, E. L., and Chervonskii, A. V. 1998. The
Goldman, J. M., and Stauss, H. J. 2000. Selective elimination of
nature of epitopes, recognized by T-lymphocytes in the allogeneic
leukemic CD34+ progenitor cells by cytotoxic T-lymphocytes specific
immune response. Mol. Biol. (Mosk). 32:692–702.
for WT1. Blood 95:2198–2203.
Kazansky, D. B., Petrishchev, V. N., Shtil', A. A., Chernysheva, A. D.,
Garcia, K. C., Degano, M., Stanfield, R. L., Brunmark, A., Jackson, M. R.,
Sernova, N. V., Abronina, I. F., Pobezinskii, L. A., and Agafonova, E. L.
Peterson, P. A., Teyton, L., and Wilson, A. 1996. An T-cell recep-
1999. Use of heat shock of antigen-presenting cells for functional
tor structure at 2.5 A° and its orientation in the TCR-MHC complex.
testing of allospecific memory T-cells. Bioorg. Khim. 25:117–128.
Kirberg, J., Baron, A., Jakob, S., Rolink, A., Karjalainen, K., and von
Golovkina, T., Agafonova, Y., Kazansky, D., and Chervonsky, A.
Boehmer, H. 1994. Thymic selection of CD8+ single positive cells
2001. Diverse repertoire of the MHC Class II-peptide complexes
with a Class II major histocompatibility complex-restricted receptor.
is required for presentation of viral superantigens. J. Immunol.
J. Exp. Med. 180:25–34.
Kisielow, P., Teh, H. S., Bluthmann, H., and von Boehmer, H. 1988. Positive
Gordon, R. D., Simpson, E., and Samelson, L. E. 1975. In vitro cell-mediated
selection of antigen-specific T-cells in thymus by restricting MHC
immune responses to the male specific (H-Y) antigen in mice. J. Exp.
molecules. Nature 335:730–733.
Koller, B. H., Marrack, P., Kappler, J. W., and Smithies, O. 1990. Normal
Gould, D. S., and Auchincloss, H., Jr. 1999. Direct and indirect recogni-
development of mice deficient in M, MHC Class I proteins, and
tion: The role of MHC antigens in graft rejection. Immunol. Today
CD8+ T-cells. Science 248:1227–1229.
Koszinowski, U., and Ertl, H. 1975. Lysis mediated by T-cells and restrict-
Grandea, A. G., and Bevan, M. J. 1993. A conservative mutation in a
ed by H-2 antigen of target cells infected with vaccinia virus. Nature
Class I MHC molecule outside the peptide binding groove stimulates
responses to self peptides. J. Immunol. 151:3981–3987.
Kovalik, J. P., Singh, N., Mendiratta, S. K., Martin, W. D., Ignatowicz, L.,
Grusby, M. J., Johnson, R. S., Papaioannou, V. E., and Glimcher, L. H.
and Van Kaer, L. 2000. The alloreactive and self-restricted CD4+
1991. Depletion of CD4+ T-cells in major histocompatibility complex
T-cell response directed against a single MHC Class II/peptide com-
Class II-deficient mice. Science 253:1417–1420.
bination. J. Immunol. 165:1285–1293.
Heath, W. R., Kane, K. P., Mescher, M. F., and Sherman, L. A. 1991.
Kuhns, S. T., Tallquist, M. D., Johnson, A. J., Mendez-Fernandez, Y.,
Alloreactive T-cells discriminate among a diverse set of endogenous
and Pease, L. R. 2000. T-Cell receptor interactions with Class I
peptides. Proc. Natl. Acad. Sci. USA 88:5101–5105.
heavy-chain influence T-cell selection. Proc. Natl. Acad. Sci. USA
Hornell, T. M., Solheim, J. C., Myers, N. B., Gillanders, W. E.,
Balendiran, G. K., Hansen, T. H., and Connolly, J. M. 1999. Alloreactive
Lee, D. S., Ahn, C., Ernst, B., Sprent, J., and Surh, C. D. 1999. Thymic selec-
and syngeneic CTL are comparably dependent on interaction with
tion by a single MHC/peptide ligand: Autoreactive T-cells are low-
MHC Class I -helical residues. J. Immunol. 163:3217–3225.
affinity cells. Immunity 10:83–92.
Hugo, P., Kappler, J. W., McCormack, J. E., and Marrack, P. 1993. Fibroblasts
Logunova, N. N., Viret, C., Pobezinsky, L. A., Miller, S. A., Kazansky, D. B.,
can induce thymocyte positive selection in vivo. Proc. Natl. Acad. Sci.
Sundberg, J. P., and Chervonsky, A. V. 2005. Restricted MHC-peptide
repertoire predisposes to autoimmunity. J. Exp. Med. 202:73–84.
Huseby, E. S., Crawford, F., White, J., Kappler, J., and Marrack, P. 2003.
Longo, D. L., and Schwartz, R. H. 1980. T-cell specificity for H-2 and Ir
Negative selection imparts peptide specificity to the mature T-cell
gene phenotype correlates with the phenotype of thymic antigen-
repertoire. Proc. Natl. Acad. Sci. USA 100:11565–11570.
presenting cells. Nature 287:44–46.
Huseby, E., Kappler, J., and Marrack, P. 2004. TCR-MHC/peptide inter-
Lu, L., Vollmer, J., Moulon, C., Weltzien, H. U., Marrack, P., and Kappler,
actions: Kissing-cousins or a shotgun wedding? Eur. J. Immunol.
J. 2003. Components of the ligand for a Ni2+ reactive human T-cell
clone. J. Exp. Med. 197:567–574.
Ignatowicz, L., Kappler, J., and Marrack, P. 1996. The repertoire of T-cells
MacDonald, H. R., Lees, R. K., Schneider, R., Zinkernagel, R. M., and
shaped by a single MHC/peptide ligand. Cell 84:521–529.
Hengartner, H. 1988. Positive selection of CD4+ thymocytes control-
Ignatowicz, L., Rees, W., Pacholczyk, R., Ignatowicz, H., Kushnir, E.,
led by MHC Class II gene products. Nature 336:471–473.
Kappler, J., and Marrack, P. 1997. T-Cells can be activated by peptides
Manning, T. C., Schlueter, C. J., Brodnicki, T. C., Parke, E. A., Speir, J. A.,
that are unrelated in sequence to their selecting peptide. Immunity
Garcia, K. C., Teyton, L., Wilson, I. A., and Kranz, D. M. 1998. Alanine
scanning mutagenesis of an T-cell receptor: Mapping the energy
Janeway, C. A., Chervonsky, A. V., and Sant'Angelo, D. 1997. T-cell
of antigen recognition. Immunity 8:413–425.
receptors: Is the repertoire inherently MHC-specific? Curr. Biol.
Marrack, P., Kushnir, E., Born, W., McDuffie, M., and Kappler, J. 1988.
The development of helper T-cell precursors in mouse thymus. J.
Jerne, N. K. 1971. The somatic generation of immune recognition. Eur. J.
Martinic, M. M., Rulicke, T., Althage, A., Odermatt, B., Hochli, M.,
Kabelitz, D., Herzog, W. R., Heeg, K., Wagner, H., and Reimann, J. 1987.
Lamarre, A., Dumrese, T., Speiser, D. E., Kyburz, D., Hengartner, H.,
Human cytotoxic T-lymphocytes. III. Large numbers of peripheral
and Zinkernagel, R. M. 2003. Efficient T-cell repertoire selection in
blood T-cells clonally develop into allo-restricted anti-viral cytotoxic
tetraparental chimeric mice independent of thymic epithelial MHC.
T-cell populations in vitro. J. Mol. Cell. Immunol. 3:49–60.
Proc. Natl. Acad. Sci. USA 100:1861–1866.
Kalbus, M., Fleckenstein, B. T., Offenhausser, M., Bluggel, M., Melms, A.,
Marusic-Galesic, S., Longo, D. L., and Kruisbeek, A. M. 1989. Preferential
Meyer, H. E., Rammensee, H. G., Martin, R., Jung, G., and Sommer, N.
differentiation of T-cell receptor specificities based on the MHC
2001. Ligand motif of the autoimmune disease-associated mouse
glycoproteins encountered during development. Evidence for posi-
MHC Class II molecule H2-A(s). Eur. J. Immunol. 31:551–562.
tive selection. J. Exp. Med. 169:1619–1630.
Katz, D. H. 1977. The role of histocompatibility complex in lymphocyte
Maryanski, J. L., Pala, P., Corradin, G., Jordan, B. R., and Cerottini, J. C.
differentiation. Cold Spring Harbor Symp. Quant. Biol. 41:611–624.
1986. H-2-restricted cytolytic T-cells specific for HLA can recognize
Kaye, J., Hsu, M. L., Sauron, M. E., Jameson, S. C., Gascoigne, N. R., and
a synthetic HLA peptide. Nature 324:578–579.
Hedrick, S. M. 1989. Selective development of CD4+ T-cells in trans-
Matechak, E. O., Killeen, N., Hedrick, S. M., and Fowlkes, B. J. 1996. MHC
genic mice expressing a Class II MHC-restricted antigen receptor.
Class II-specific T-cells can develop in the CD8 lineage when CD4 is
absent. Immunity 4:337–347.
Kaye, J., Vasquez, N. J., and Hedrick, S. M. 1992. Involvement of the same
Merkenschlager, M., Graf, D., Lovatt, M., Bommhardt, U., Zamoyska, R.,
region of the T-cell antigen receptor in thymic selection and foreign
and Fisher, A.G 1997. How many thymocytes audition for selection?
peptide recognition. J. Immunol. 148:3342–3353.
J. Exp. Med. 186:1449–1158.
MHC restriction and allogeneic immune response 383
Moris, A., Teichgraber, V., Gauthier, L., Buhring, H. J., and Rammensee, H. G.
complex Class I molecules: Reagents for tumor immunotherapy.
2001. Cutting edge: Characterization of allorestricted and pep-
Proc. Natl. Acad. Sci. USA 93:13114–13118.
tide-selective alloreactive T-cells using HLA-tetramer selection. J.
Sadovnikova, E., Jopling, L. A., Soo, K. S., and Stauss, H. J. 1998. Generation
of human tumor-reactive cytotoxic T-cells against peptides present-
Mullbacher, A., Hill, A. B., Blanden, R. V., Cowden, W. B., King, N. J., and
ed by non-self HLA Class I molecules. Eur. J. Immunol. 28:193–200.
Hla, R. T. 1991. Alloreactive cytotoxic T-cells recognize MHC Class I
Sadovnikova, E., Parovichnikova, E. N., Savchenko, V. G., Zabotina, T., and
antigen without peptide specificity. J. Immunol. 147:1765–1772.
Stauss, H. J. 2002. The CD68 protein as a potential target for leukae-
Munz, C., Hofmann, M., Yoshida, K., Moustakas, A. K., Kikutani, H.,
mia-reactive CTL. Leukemia 16:2019–2026.
Stevanovic, S., Papadopoulos, G. K., and Rammensee, H. G.
Schneck, J., Maloy, W. L., Coligan, J. E., and Margulies, D. H. 1989a.
2002. Peptide analysis, stability studies, and structural modeling
Inhibition of an allospecific T-cell hybridoma by soluble Class I pro-
explain contradictory peptide motifs and unique properties of
teins and peptides: Estimation of the affinity of a T-cell receptor for
the NOD mouse MHC Class II molecule H2-Ag7. Eur. J. Immunol.
MHC. Cell 56:47–55.
Schneck, J., Munitz, T., Coligan, J. E., Maloy, W. L., Margulies, D. H.,
Newman, L. S. 2007. Immunotoxicology of beryllium lung disease.
and Singer, A. 1989b. Inhibition of allorecognition by an H-2Kb-
Environ. Health Prevent. Med. 12:161–164.
derived peptide is evidence for a T-cell binding region on a major
Noun, G., Reboul, M., Abastado, J. P., Kourilsky, P., Sigaux, F., and Pla, M.
histocompatibility complex molecule. Proc. Natl. Acad. Sci. USA
1998. Strong alloantigenicity of the -helices residues of the MHC
Class I molecule. J. Immunol. 161:148–153.
Schwartz, R. H. 1984. The role of gene products of the major histocom-
Obst, R., Munz, C., Stevanovic, S., and Rammensee, H.G. 1998. Allo- and
patibility complex in T-cell activation and cellular interactions. In:
self-restricted cytotoxic T-lymphocytes against a peptide library:
Fundamental Immunology (Paul, W. E., Ed.), New York: Raven Press,
Evidence for a functionally diverse allorestricted T-cell repertoire.
pp. 385–392.
Eur. J. Immunol. 28:2432–2443.
Sebzda, E., Mariathasan, S., Ohteki, T., Jones, R., Bachmann, M. F., and
Obst, R., Netuschil, N., Klopfer, K., Stevanovic, S., and Rammensee, H. G.
Ohashi, P. S. 1999. Selection of the T-cell repertoire. Annu. Rev.
2000. The role of peptides in T-cell alloreactivity is determined by
self-major histocompatibility complex molecules. J. Exp. Med.
Sebzda, E., Wallace, V. A., Mayer, J., Yeung, R. S., Mak, T. W., and Ohashi, P. S.
1994. Positive and negative thymocyte selection induced by different
Pichler, W. J., Beeler, A., Keller, M., Lerch, M., Posadas, S., Schmid, D.,
concentrations of a single peptide. Science 263:1615–1618.
Spanou, Z., Zawodniak, A., and Gerber, B. 2006. Pharmacological in-
Sha, W. C., Nelson, C. A., Newberry, R. D., Kranz, D. M., Russell, J. H., and
teraction of drugs with immune receptors: The p-i concept. Allergol.
Loh, D. Y. 1988. Positive and negative selection of an antigen recep-
tor on T-cells in transgenic mice. Nature 336:73–76.
Pircher, H., Rohrer, U. H., Moskophidis, D., Zinkernagel, R. M., and
Sha, W. C., Nelson, C. A., Newberry, R. D., Pullen, J. K., Pease, L. R.,
Hengartner, H. 1991. Lower receptor avidity required for thymic
Russell, J. H., and Loh, D. Y. 1990. Positive selection of transgenic
clonal deletion than for effector T-cell function. Nature 351:482–485.
receptor-bearing thymocytes by Kb antigen is altered by Kb mutations
Pobezinskaya, E. L., Pobezinskii, L. A., Silaeva, Y. Y., Anfalova, T.
that involve peptide binding. Proc. Natl. Acad. Sci. USA 87:6186–6190.
V., Khromykh, L. M., Tereshchenko, T. S., Zvezdova, E. S., and
Shearer, G. M. 1974. Cell-mediated cytotoxicity to trinitrophenyl- modified
Kazanskii,D. B. 2004. Cross-reactivity of T-cell receptor on memory
syngeneic lymphocytes. Eur. J. Immunol. 4:527–533.
CD8+ cells isolated after immunization with allogeneic tumor cells.
Smith, P. A., Brunmark, A., Jackson, M. R., and Potter, T. A. 1997. Peptide-
Bull. Exp. Biol. Med. 137:493–498.
independent recognition by alloreactive cytotoxic T lymphocytes
Punt, J. A., Havran, W., Abe, R., Sarin, A., and Singer, A. 1997. T-cell re-
(CTL). J. Exp. Med. 185:1023–1033.
ceptor (TCR)-induced death of immature CD4+CD8+ thymocytes by
Stanislawski, T., Voss, R. H., Lotz, C., Sadovnikova, E., Willemsen, R.
two distinct mechanisms differing in their requirement for CD28 co-
A., Kuball, J., Ruppert, T., Bolhuis, R. L., Melief, C. J., Huber, C.,
stimulation: Implications for negative selection in the thymus. J. Exp.
Stauss, H. J., and Theobald, M. 2001. Circumventing tolerance to a
human MDM2-derived tumor antigen by TCR gene transfer. Nat.
Punt, J. A., Osborne, B. A., Takahama, Y., Sharrow, S. O., and Singer, A.
1994. Negative selection of CD4+CD8+ thymocytes by T-cell receptor-
Stockinger, H., Bartlett, R., Pfizenmaier, K., Rollinghoff, M., and Wagner, H.
induced apoptosis requires a co-stimulatory signal that can be pro-
1981. H-2 Restriction as a consequence of intentional priming:
vided by CD28. J. Exp. Med. 179:709–713.
Frequency analysis of alloantigen-restricted trinitrophenyl-specific
Rammensee, H. G. 1995. Chemistry of peptides associated with MHC
cytotoxic T-lymphocyte precursors within thymocytes of normal
Class I and Class II molecules. Curr. Opin. Immunol. 7:85–96.
mice. J. Exp. Med. 153:1629–1639.
Rammensee, H. G., Falk, K., and Rotzschke, O. 1993. Peptides natu-
Stockinger, H., Pfizenmaier, K., Hardt, C., Rodt, H., Rollinghoff, M., and
rally presented by MHC Class I molecules. Annu. Rev. Immunol.
Wagner, H. 1980. H-2 restriction as a consequence of intentional
priming: T-Cells of fully allogeneic chimeric mice as well as of nor-
Reimann, J., Heeg, K., Miller, R. G., and Wagner, H. 1985a. Alloreactive
mal mice respond to foreign antigens in the context of H-2 determi-
cytotoxic T-cells. I. Alloreactive and allorestricted cytotoxic T-cells.
nants not encountered on thymic epithelial cells. Proc. Natl. Acad.
Eur. J. Immunol. 15:387–393.
Sci. USA 77:7390–7394.
Reimann, J., Kabelitz, D., Heeg, K., and Wagner, H. 1985b. Allorestricted
Sun, R., Shepherd, S. E., Geier, S. S., Thomson, C. T., Sheil, J. M., and
cytotoxic T-cells. Large numbers of allo-H-2Kb-restricted antihap-
Nathenson, S. G. 1995. Evidence that the antigen receptors of cyto-
ten and antiviral cytotoxic T-cell populations clonally develop in
toxic T-lymphocytes interact with a common recognition pattern on
vitro from murine splenic precursor T-cells. J. Exp. Med.
the H-2Kb molecule. Immunity 3:573–582.
Takahama, Y., Suzuki, H., Katz, K. S., Grusby, M. J., and Singer, A. 1994.
Reiser, J. B., Darnault, C., Guimezanes, A., Gregoire, C., Mosser, T., Schmitt-
Positive selection of CD4+ T-cells by TCR ligation without aggrega-
Verhulst, A. M., Fontecilla-Camps, J. C., Malissen, B., Housset, D.,
tion even in the absence of MHC. Nature 371:67–70.
and Mazza, G. 2000. Crystal structure of a T-cell receptor bound to an
Tallquist, M. D., Weaver, A. J., and Pease, L. R. 1998. Degenerate recog-
allogeneic MHC molecule. Nat. Immunol. 1:291–297.
nition of alloantigenic peptides on a positive-selecting Class I mol-
Rotzschke, O., Falk, K., Deres, K., Schild, H., Norda, M., Metzger, J.,
ecule. J. Immunol. 160:802–809.
Jung, G., and Rammensee, H. G. 1990. Isolation and analysis of
Tallquist, M. D., Yun, T. J., and Pease, L. R. 1996. A single T-cell receptor
naturally processed viral peptides as recognized by cytotoxic T-cells.
recognizes structurally distinct MHC/peptide complexes with high
specificity. J. Exp. Med. 184:1017–1026.
Rotzschke, O., Falk, K., Stevanovic, S., Jung, G., Walden, P., and
Teh, H. S., Bennink, J., and Von Boehmer, H. 1982. Selection of the
Rammensee, H.G. 1991. Exact prediction of a natural T-cell epitope.
T-cell repertoire during ontogeny: Limiting dilution analysis. Eur. J.
Eur. J. Immunol. 21:2891–2894.
Sadovnikova, E., and Stauss, H. J. 1996. Peptide-specific cytotoxic
Teh, H. S., Kisielow, P., Scott, B., Kishi, H., Uematsu, Y., Bluthmann, H.,
T-lymphocytes restricted by nonself major histocompatibility
and von Boehmer, H. 1988. Thymic major histocompatibility
384 D. B. Kazansky
complex antigens and the T-cell receptor determine the CD4/
complex- and allo-major histocompatiblilty complex-restricted
CD8 phenotype of T-cells. Nature 335:229–233.
virus-specific T-cells. J. Exp. Med. 153:1517–1532.
Thierse, H. J., Gamerdinger, K., Junkes, C., Guerreiro, N., and
Wallny, H. J., Deres, K., Faath, S., Jung, G., Van Pel, A., Boon, T., and
Weltzien, H. U. 2005. T-Cell receptor (TCR) interaction with haptens:
Rammensee, H. G. 1992. Identification and quantification of a naturally
Metal ions as non-classical haptens. Toxicology 209:101–107.
presented peptide as recognized by cytotoxic T-lymphocytes specific
Thierse, H. J., Moulon, C., Allespach, Y., Zimmermann, B., Doetze, A.,
for an immunogenic tumor variant. Int. Immunol. 4:1085–1090.
Kuppig, S., Wild, D., Herberg, F., and Weltzien, H. U. 2004. Metal-
Whitelegg, A., and Barber, L. D. 2004. The structural basis of T-cell allo-
protein complex-mediated transport and delivery of Ni2+ to TCR/
recognition. Tissue Antigens 63:101–108.
MHC contact sites in nickel-specific human T-cell activation.
Yang, T. H., Lovatt, M., Merkenschlager, M., and Stauss, H. J. 2002.
J. Immunol. 172:1926–1934.
Comparison of the frequency of peptide-specific cytotoxic
Townsend, A. R., Rothbard, J., Gotch, F. M., Bahadur, G., Wraith, D., and
T-lymphocytes restricted by self- and allo-MHC following in vitro
McMichael, A. J. 1986. The epitopes of influenza nucleoprotein rec-
T-cell priming. Int. Immunol. 14:1283–1290.
ognized by cytotoxic T-lymphocytes can be defined with short syn-
Zerrahn, J., Held, W., and Raulet, D. H. 1997. The MHC reactivity of
thetic peptides. Cell 44:959–968.
the T-cell repertoire prior to positive and negative selection. Cell
Udaka, K., Tsomides, T. J., and Eisen, H. N. 1992. A naturally occurring
peptide recognized by alloreactive CD8+ cytotoxic lymphocytes in
Zijlstra, M., Bix, M., Simister, N. E., Loring, J. M., Raulet, D. H., and
association with a Class I MHC protein. Cell 69:989–998.
Jaenisch, R. 1990. -Microglobulin-deficient mice lack CD4-8+
Udaka, K., Wiesmuller, K. H., Kienle, S., Jung, G., and Walden, P. 1996.
cytolytic T-cells. Nature 344:742–746.
Self-MHC-restricted peptides recognized by an alloreactive
Zinkernagel, R. M., and Althage, A. 1999. On the role of thymic epithelium
T-lymphocyte clone. J. Immunol. 157:670–678.
vs. bone marrow-derived cells in repertoire selection of T-cells. Proc.
Vartdal, F., Johansen, B. H., Friede, T., Thorpe, C. J., Stevanovic, S.,
Natl. Acad. Sci. USA 96:8092–8097.
Eriksen, J. E., Sletten, K., Thorsby, E., Rammensee, H. G., and
Zinkernagel, R. M., and Doherty, P. C. 1973. Cytotoxic thymus-derived
Sollid, L. M. 1996. The peptide binding motif of the disease
lymphocytes in cerebrospinal fluid of mice with lymphocytic chori-
associated HLA-DQ (1*0501, 1*0201) molecule. Eur. J. Immunol.
omeningitis. J. Exp. Med. 138:1266–1269.
Zinkernagel, R. M., and Doherty, P. C. 1974. Restriction of in vitro T-cell-
Viret, C., and Janeway, C. A., Jr. 1999. MHC and T-cell development. Rev.
mediated cytotoxicity in lymphocytic choriomeningitis within a syn-
geneic or semi-allogeneic system. Nature 248:701–702.
Wagner, H., Hardt, C., Bartlett, R., Stockinger, H., Rollinghoff, M., Rodt, H.,
Zinkernagel, R. M., Klein, P. A., and Klein, J. 1978. Host-determined T-cell
and Pfizenmaier, K. 1981. Frequency analysis of cytotoxic T lym-
fine specificity for self H-2 in radiation bone-marrow chimera of
phocyte precursors in chimeric mice. Evidence for intra-thymic
C57BL/6 (H-2b), mutant Hz1 (H-2ba), and F mice. Immunogenetics
maturation of clonally distinct self-major histocompatibility
Source: http://kazansky1.narod.ru/works/369.pdf
RA Journal of Applied Research Volume 1 Issue 1 Pages-18-43 November -2014 ISSN (e): Appli Importance of Physicochemical Properties In Drug Discovery. (Review Article) Kapadia Akshay Bhupendra Department of Pharmaceutical Sciences and Technology, Institute of Chemical Technology, Nathalal Parekh Marg, Matunga (East), Mumbai.
Koffer 1. Carle, Eric: Die kleine Raupe Nimmersatt, Gerstenberg, Bilderbuch, 2014.25 Exemplare Auch kleine Raupen können großen Hunger haben. Deshalb macht sich die Raupe Nimmersatt auf die Suche nach etwas zu essen - und wird fündig. Sie frisst sich von Montag bis Sonntag Seite für Seite durch einen Berg von Leckereien, bis sie endlich satt ist. Nun ist die Zeit gekommen, sich einen Kokon zu bauen, und nach zwei Wochen des Wartens schlüpft aus ihm ein wunderschöner Schmetterling. Die Kleinsten spielen mit der Kleinen Raupe Nimmersatt und sind fasziniert von den gestanzten Raupenfresslöchern. Etwas größere Kinder entdecken mit ihr die Wochentage, Früchte, Zahlen und die Metamorphose in der Natur.

