A critical role of follicle-stimulating hormone (fsh) in mediating the effect of clotrimazole on testicular steroidogenesis in adult zebrafish


Contents lists available at
A critical role of follicle-stimulating hormone (Fsh) in mediating the effect of
clotrimazole on testicular steroidogenesis in adult zebrafish
Damien Baudiffier , Nathalie Hinfray , Mélanie Vosges , Nicolas Creusot , Edith Chadili ,
Jean-Marc Porcher , Rüdiger W. Schulz , Franc¸ois Brion
a Institut National de l'environnement industriel et des risques (INERIS), Direction des Risques Chroniques, Unité d'écotoxicologie in vitro et in vivo, BP 2, 60550 Verneuil-en-Halatte,
Franceb University of Utrecht, Science Faculty, Department Biology, Division Developmental Biology, Reproductive Biology Group, Kruyt Building room W-606, Padualaan 8, NL-3584 CH
Utrecht, The Netherlands
Clotrimazole is a pharmaceutical fungicide known to inhibit several cytochrome P450 enzyme activi-
Received 22 February 2012
ties, including several steroidogenic enzymes. This study aimed to assess short-term in vivo effects of
Received in revised form 3 April 2012
clotrimazole exposure on blood 11-ketotestosterone (11-KT) levels and on the transcriptional activity of
Accepted 21 April 2012
genes in pituitary and testis tissue that are functionally relevant for androgen production with the view
Available online 28 April 2012
to further characterize the mode of action of clotrimazole on the hypothalamus-pituitary-gonad axis in
zebrafish, a model vertebrate in toxicology. Adult male zebrafish were exposed to measured concentra-
tions in water of 71, 159 and 258 g/L of clotrimazole for 7 days. Expression of pituitary gonadotropins
 subunit (lhb, fshb), testicular gonadotropins receptors (lhcgr, fshr) and testicular steroidogenesis-
Pituitary-testis axis
related genes (e.g., star, cyp17a1, cyp11c1) were assessed. Blood concentrations of 11-KT were measured.
Short-term exposure to clotrimazole induced a concentration-dependent increase of star, cyp17a1, and
cyp11c1 gene expression and Cyp17a1 and Cy11c1 protein synthesis in Leydig cells, but androgen lev-
els in blood remained unchanged. fshb, but not lhb mRNA levels in the pituitary tended to increase in
clotrimazole-exposed zebrafish. Testicular expression of the Fsh receptor gene was significantly up-
regulated following exposure, when expression of this receptor was significantly correlated to the
expression of steroidogenesis-related genes. Moreover, the Fsh-regulated insulin-like growth factor 3
(igf3) gene, a fish-specific Igf peptide expressed in Sertoli cells, was induced in testes. By using a network
of genes functioning in pituitary and testis tissue, our study demonstrated that clotrimazole induced a
cascade of molecular and cellular events which are in agreement with a role for Fsh (1) in stimulating
Leydig cell steroidogenesis to compensate the inhibitory action of clotrimazole on 11-KT synthesis and
(2) in inducing the expression of Fsh-regulated igf3 in Sertoli cells.
2012 Elsevier Ireland Ltd. All rights reserved.
Gonadotropins control steroidogenesis and gametogenesis through
interaction with their gonadal G protein-coupled receptors, FSH
Steroidogenesis is a multi-step process consisting of a series
receptor (FSHR) and LH receptor (LHR). Androgen production in
of enzymatic reactions that convert cholesterol to biologically
the testis takes place in the steroidogenic Leydig cells that are situ-
active steroid hormones (
ated between the seminiferous tubules close to blood vessels in
that control a large array of important physiological functions
the interstitial tissue of the testis. The regulation of Leydig cell
In vertebrates, gonadal steroidogenesis is under
androgen production is a typical domain of LH in all vertebrates.
the control of the hypothalamus-pituitary-gonad (HPG) axis. The
However, in fish, Leydig cells are also expressing the fshr gene
hypothalamus secretes gonadotropin-releasing hormones (GnRHs)
and piscine Fshs have been shown to be potent steroidogenic hor-
that interact with their pituitary receptors (GnRH-Rs) to control
mones in several species
synthesis and release of gonadotropins, luteinizing hormone (LH)
Hence, in fish Fsh is involved in regulat-
and follicle-stimulating hormone (FSH)
ing both, spermato- and steroidogenesis via the Fshr-expressing
Sertoli and Leydig cells, respectively (
Steroid hormones, in
turn, exert positive or negative feedback on neuroendocrine circuits
∗ Corresponding author. Tel.: +33 3 44 55 65 12; fax: +33 3 44 55 66 05.
(Dopamine, GABA and GnRH neurons) that control the synthesis
E-mail address: (F. Brion).
and release of gonadatropins (
0300-483X/$ – see front matter
2012 Elsevier Ireland Ltd. All rights reserved.
D. Baudiffier et al. / Toxicology 298 (2012) 30–39
Several chemical compounds belonging to the azole family alter
family 17, subfamily A polypeptide 1 (cyp17a1); cytochrome P450,
gonadal steroidogenesis in fish leading to reproductive impair-
family 11, subfamily C, polypeptide 1 showing a 11-hydroxylase
activity (cyp11c1; previously referred to as cyp11b2); hydroxys-
These compounds were designed to inhibit a cytochrome
teroid (11-) dehydrogenase 3a (hsd11b3a); cytochrome P450,
P450 (CYP) enzyme involved in ergosterol synthesis of fungi
family 19, subfamily A, polypeptide 1a (cyp19a1a); luteinizing
However, they can also inhibit other
hormone/choriogonadotropin receptor (lhcgr); follicle stimulat-
CYP enzyme activities, including steroidogenic cytochrome P450
ing hormone receptor (fshr); were quantified. The Sertoli cell
17␣-hydroxylase/17,20-lyase (CYP17) and aromatase (CYP19) in
markers insulin-like growth factor 3 (igf3) and anti-müllerian
mammals and fish
hormone (amh) as well as the spermatogonial marker piwi-like
1 (Drosophila) (piwil1, previously referred to as ziwi) were also
In addition, azole compounds have been shown to interact
with different cytoplasmic or nuclear receptors such as aryl hydro-
carbon receptor (pregnane X receptor (
2. Methods
or androgen receptor (
2.1. Animals and treatments
and therefore can interfere with a
The ethical committee of the National Institute of Industrial Environment and
broad range of physiological processes.
Risks (INERIS) approved all experiments. Mature wild type male zebrafish (Danio
rerio, AB strain) were obtained from our breeding unit. Fish were raised under
In vivo exposure of fish to prochloraz, ketoconazole or clotrima-
controlled photoperiod (14 h light/10 h dark cycle) in a recirculated water system
zole increased expression of steroidogenic genes in gonads
(Techniplast, France) at 25 ± 1 ◦C. They were fed with TetraMin Pro® twice a day and
live brine shrimp (Artemia spp.; Ocean Nutrition). Clotrimazole (CLO) was purchased
we have reported that in adult zebrafish clotrimazole affected tes-
from Sigma–Aldrich (St Quentin Fallavier, France) and all the stock solutions were
ticular steroidogenesis differently in vivo as compared to an ex vivo
prepared in dimethylsulfoxide (DMSO; Sigma–Aldrich).
Adult male zebrafish were exposed to three concentrations of clotrimazole or
testis tissue explant culture system (The data
solvent alone (DMSO, 0.004% v:v) for 7 days under semi-static conditions with a total
suggested that clotrimazole-induced 17␣-hydroxylase/17,20 lyase
renewal of the water every 24 h. Each group contained 20 fish equally distributed
(cyp17a1) expression was not due to a direct action on the testes
in two replicate 4 L-glass tanks. Water samples were collected from each condition
to regulate cyp17a1 transcription but could involve indirect actions
at day 5 at t = 0 h and t = 24 h before renewal of water.
at the pituitary level that may then alter the regulation of testic-
2.2. Fish sampling
ular steroidogenesis. Azoles are known to cause, directly and/or
indirectly, endocrine disruption thereby affecting gene expression
At the end of the exposure, fish were sacrificed in ice-cold water, measured and
along the hypothalamic-pituitary-gonadal (HPG) axis (e.g.,
weighed. Blood (2.5 or 5 L) was collected by cardiac puncture for each fish with
However, the precise
a heparinized syringe (1000 U of heparin per ml) and transferred into Eppendorf
tubes (held on ice) containing enzyme immunoassay (EIA) buffer supplemented
mechanism responsible for stimulating gonadal steroidogenesis
with proteinase inhibitor phenylmethanesulfonylfluoride (PMSF, 1 mM) and hep-
has not been fully established yet.
arin (1000 U/ml). Blood was diluted 1:10 (v:v) in this solution. Then, blood was
In the present study we used zebrafish as a relevant model
vortexed, centrifuged (3000 g, 15 min, 4 ◦C) and stored at −20 ◦C until analysis. Liver
vertebrate for toxicology (to
and pituitary were removed and preserved in RNA laterTM (Sigma-Aldrich, France)
investigate clotrimazole mode of action. Indeed, in addition to
at −20 ◦C until analysis. Testes were removed and preserved in RNA later TM (Sigma-
Aldrich, France) at −20 ◦C until analysis or fixed in Bouin's fluid. Testis weight was
practicalities (small size, low cost, high fecundity, etc.), zebrafish
determined to calculate the gonado-somatic index (GSI, gonad wet weight/total
physiology, genetics, and development under "normal conditions"
body wet weight × 100).
have been studied in great detail and the species has also been
used to investigate several types of toxicity from vascular toxicity to
2.3. Analytical chemistry for clotrimazole actual concentrations determination
endocrine disruption For toxicology, advances in
Clotrimazole concentrations in exposure tanks were determined using solid
zebrafish genetics and genomics provide useful tools to investigate
phase extraction (SPE) followed by high-pressure liquid chromatography (HPLC)
molecular mechanisms of toxicity of chemicals. Furthermore, con-
coupled to UV–Vis detection (� = 194 nm). The extraction protocol was adapted from
served cell signaling pathways and development process between
(Briefly, water samples were collected from tanks (250 mL
zebrafish and mammals, and global concordance between zebrafish
per condition) and adjusted to pH of 2. Then, samples were filtered under vac-
uum through SPE cartridges (Waters® OASIS-HLB, 6cc, 200 mg). Prior to extraction,
and mammalian toxicity studies showed that the zebrafish model
the cartridges were conditioned with 6 mL of n-heptane, 6 mL of methanol, and
could be representative also for higher vertebrates (
5 mL of ultrapure water (pH 2). After drying under vacuum for 90 min, clotrimazole
was eluted with 3 × 1.5 mL of acetone. Finally, acetone extracts were evaporated to
The aim of this work was therefore to assess the in vivo effect of
dryness under nitrogen gas and residues were redissolved in 1 mL of acetonitrile.
short-term exposure to clotrimazole on androgen levels in blood
Samples were analyzed using a Varian® HPLC system (Prostar 230 ternary pump,
Prostar 420 autosampler, Prostar 9050 UV-Vis detector) based on a C18 column
and on the transcriptional activity of genes in pituitary and testes
(Poursuit C18, 5 M, 250 × 4.6 d.i., Varian®). Actual clotrimazole concentrations in
that are functionally relevant for androgen production (i) to char-
exposure tanks have been determinated using external calibration based on four
acterize the mode of action of clotrimazole on the HPG axis and
points calibration curve (0 to 100 g/L). The limit of detection (LOD), obtained
(ii) to investigate a potential role of gonadotropins in mediat-
with signal/noise ratios equal to 3, was 1.5 g/L. For each experiment, a 20 g/L
clotrimazole-spiked has been processed as quality control of the extraction proce-
ing the effect of clotrimazole on testicular steroidogenesis. For
dure. The recovery of clotrimazole was higher than 90% in all the experiment.
that purpose, male fish were exposed for 7 days to clotrima-
zole (71–258 g/L). The rational for choosing these concentrations
2.4. Gene expression analysis
was based on previous experiments that showed up-regulation
of cyp17a1 gene expression in zebrafish testis after 7 days of
Total RNA was extracted from testis or pituitary using Trizol Reagent (Life Tech-
nologies Inc., Gaithersburg, MD) following manufacturer's instructions by using a
exposure to clotrimazole These are higher
fast tissue homogenizer (Precellys 24,). The mRNA quality was verified with a 1%
concentrations than those recently reported in waste water treat-
agarose gel electrophoresis stained with Sybr Safe (Invitrogen). For each sample,
ment plants (e.g.,
the total RNA concentration was quantified using a Nanodrop ND-8000 spectropho-
In the pituitary, changes in lhb and fshb transcript lev-
tometer (Nanodrop Technologies, Wilmington, DE) and the samples were diluted to
0.5 g/L. DNA for the real-time PCR reactions was generated using a combination of
els were measured. In testis tissue, expression of steroidogenic
random hexamers, M-MLV reverse transcriptase, 2.5 mM dNTP and RNAsin to avoid
acute regulatory protein (star); hydroxyl-�-5-steroid dehydroge-
RNA degradation. The expression of target genes was analyzed using an Eppendorff
nase, 3- and steroid �-isomerase 1 (hsd3b1); cytochrome P450,
realplex4 Mastercycler epgradient S (Eppendorf, France). Standard curves from 10−4
D. Baudiffier et al. / Toxicology 298 (2012) 30–39
to 10−11 g/L were created for each gene to determine the PCR efficiency and to
obtain concentrations in g/L for each sample. Dilution of standards, mRNA and
Clotrimazole concentrations measured in tank at t = 0 h and t = 24 h.
cDNA samples were prepared in RNAse-free water (Sigma–Aldrich). Every 25-L
cDNA amplification reaction contained 5 L of diluted sample or standard, 15 L of
QuantiTect Sybr Green Mix (Qiagen, France) and 2 L of each 400 nM forward and
Measured (g/L) t = 0 h
400 nM reverse primers. Primer sequences are presented in the real-
Measured (g/L) t = 24 h
time PCR program included an enzyme activation step at 95 ◦C (15 min) and 40 cycles
SC: solvent control, LOD: limit of detection.
of 95 ◦C (5 s), 60 ◦C (30 s) and 72 ◦C (30 s). Determination of transcript abundance of
genes was conducted in duplicate.
Normalization to total RNA in association with calibration to a gene-specific
mRNA standard curve was employed in testes and pituitaries rather than normal-
ization to "housekeeping" genes. Indeed, when we assayed "housekeeping" genes
3. Results
(gapdh and elf1˛), we observed significant changes between treatments (data not
shown). We assume that both normalization to "housekeeping" gene and to total
3.1. Water chemistry
RNA have limitations when changes in tissue composition occur in response to an
exposure ("house-
keeping" genes often simply rely on normalization to total RNA (
Water concentrations of clotrimazole measured at T0 (day 5) in
the clotrimazole-treated tanks were 71, 159 and 258 g/L for the
low, medium, and high contaminations, respectively (After
2.5. Fluorescent immunohistochemistry
24 h, concentrations declined by 22%, 6% and 11% of the concentra-
tions measured at T0 respectively. Control water samples were all
The antiserum raised against zebrafish Cyp17a1 has been described previously
(The antiserum against Cyp11c1 was a generous gift of Yann
below the limit of detection; i.e. 1.5 g/L.
Guiguen (INRA-Scribe, Rennes, France). The antiserum was produced in rats by co-
injection of two synthetic peptides of the rainbow trout Cyp11c1 sequence (EMBL
accession number: Q918S6). The first peptide (PWATHRETRQHSKGV) showed 93%
3.2. Biometrical parameters
identity with the zebrafish Cyp11c1 protein sequence (EMBL accession number:
Q0P493) while no sequence identity was found between the second rainbow trout
peptide (EKDGGKEERGHSLTI) and the zebrafish Cyp11c1 amino-acids sequence.
There was no clotrimazole-induced mortality or any obser-
At the end of exposure, testes were fixed in Bouin's fluid for 48 h at 4 ◦C. After
vation of abnormal behavior during the study. Furthermore,
fixation, samples were dehydrated in ethanol, cleared in toluene and embedded in
no significant differences were observed between clotrimazole-
paraffin, according to conventional procedures. Samples were sectioned at 5 m
contaminated fishes and controls in body weight, length or GSI
(longitudinal sections) and sections were mounted on gelatin-coated slides.
Then, Cyp17a1 and Cyp11c1 labeling on zebrafish testes were performed by flu-
orescent immunochemistry as described below. Sections from four or five fish per
condition were used in the present study. Briefly, sections were dewaxed and rehy-
drated, and antigens were unmasked for 3 h at 80 ◦C in ethylenediaminetetraacetic
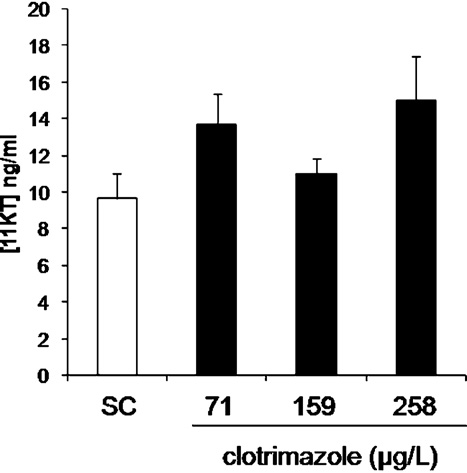
3.3. 11-KT concentrations in blood
acid buffer (pH 8.5). Tissue sections were then incubated for 1 h in a saturation PBS
solution containing 0.2% Triton X-100 and 1% milk powder. Incubations with the
anti-zebrafish Cyp17a1 antibody or the anti-rainbow trout Cyp11c1 antibody were
Circulating concentrations of 11-KT were quantified in control
performed overnight (1:300 with 0.5% milk powder in PBS) at room temperature.
and exposed-fish. In control fish, the mean measured concentration
After rinsing, sections were incubated for 90 min with a goat anti-rabbit antibody
was 9.6 ± 5.5 ng/mL, which agrees with previously reported data in
(for Cyp17a1) or a goat anti-rat antibody (for Cyp11c1) coupled to Alexafluor 594
adult male zebrafish (In
(1:200 with 0.5% milk powder in PBS). The specificity of the staining was controlled
by processing adjacent sections without primary antibody, with the pre immune
controls, 11-KT concentrations ranged between 6.4 and 21.8 ng/mL.
serum or with the antibody pre-absorbed with the synthetic peptides.
Although there was no significant difference between control
and clotrimazole-exposed fish (we noted an increased
2.6. 11-KT blood level determination
inter-individual variation in the group exposed to the highest con-
centration of clotrimazole: 2 of 20 fish had concentrations of 0.36
11-KT was quantified in blood samples by means of a competitive ELISA, fol-
lowing manufacturer's instructions (11-KT EIA Kit, Cayman Chemical Company, Ann
and 1 ng/mL and 5 of 20 fish had elevated concentrations of 11-KT
Harbor, U.S.A.). The mean EC50 ± standard deviation was 8.6 ± 0.7 pg/mL (n = 6 inde-
between 25.1 and 33.7 ng/mL.
pendent experiments). The calculated coefficient of variation between assays was
8%, and the detection limit was 2.8 pg/mL ± 0.3 pg/mL. For the analysis, blood sam-
ples were assayed in triplicates with differential dilutions: 1:300; 1:900; 1:2700
in EIA buffer. To sum up the principle, acetylcholinesterase (AChE)-labeled 11-KT
(secondary antibody) was added to the pre-coated wells with primary antibody
anti-11-KT. A competition was established between 11-KT-AChE and natural 11-KT
supplied by samples. The plate was then incubated overnight (18 h, 4 ◦C), washed
5 times with wash buffer and Ellman's reagent was added to the wells. Finally, the
plates were developed on an orbital shaker for 75 min, before reading at 420 nm
using a microtiter plate reader (EL340, Bio-Tek Instrument).
2.7. Data analysis
One-way analysis of variance (ANOVA) was performed to test for differences
between treatments. Then, differences among treatments were determined using a
post hoc test (Tukey Honestly Significant Difference). Normality of the data was pre-
viously assessed using a Shapiro test and homogeneity of variance was also verified
using the Bartlett test. Non-normally distributed data were log-transformed prior to
analysis and a non-parametric Kruskal–Wallis test, followed by a multiple compar-
ison test, was used when data did not meet parametric assumptions. Furthermore,
non-parametric Spearman rank correlation tests were applied to test correlations
among expression levels of different testicular genes for each condition.
Levels of gene expressions were expressed as fold changes relative to the aver-
age value of the control. Statistical analyses were conducted using RTM (R 2.13.1,
software, R development Core Team). All data are presented as mean ± standard
Fig. 1. In vivo effect of clotrimazole on circulating 11-KT concentration following
error to the mean (SEM) except for biometrical parameters that are presented as
a 7 days exposure. Data are represented as mean ± SEM and expressed in pg/ml
mean ± standard deviation (SD). Significance level (p) was fixed at 0.05 (p < 0.05*;
(N = 16–20 fish per condition). Each blood sample was analyzed in triplicate. 11-
p < 0.01**; p < 0.001***).
KT = 11-ketotestosterone.
D. Baudiffier et al. / Toxicology 298 (2012) 30–39
Fish biometrical parameters after the 7 days exposure.
Gonad weight (mg)
Clotrimazole medium
Clotrimazole high
GSI = Gonado somatic index.
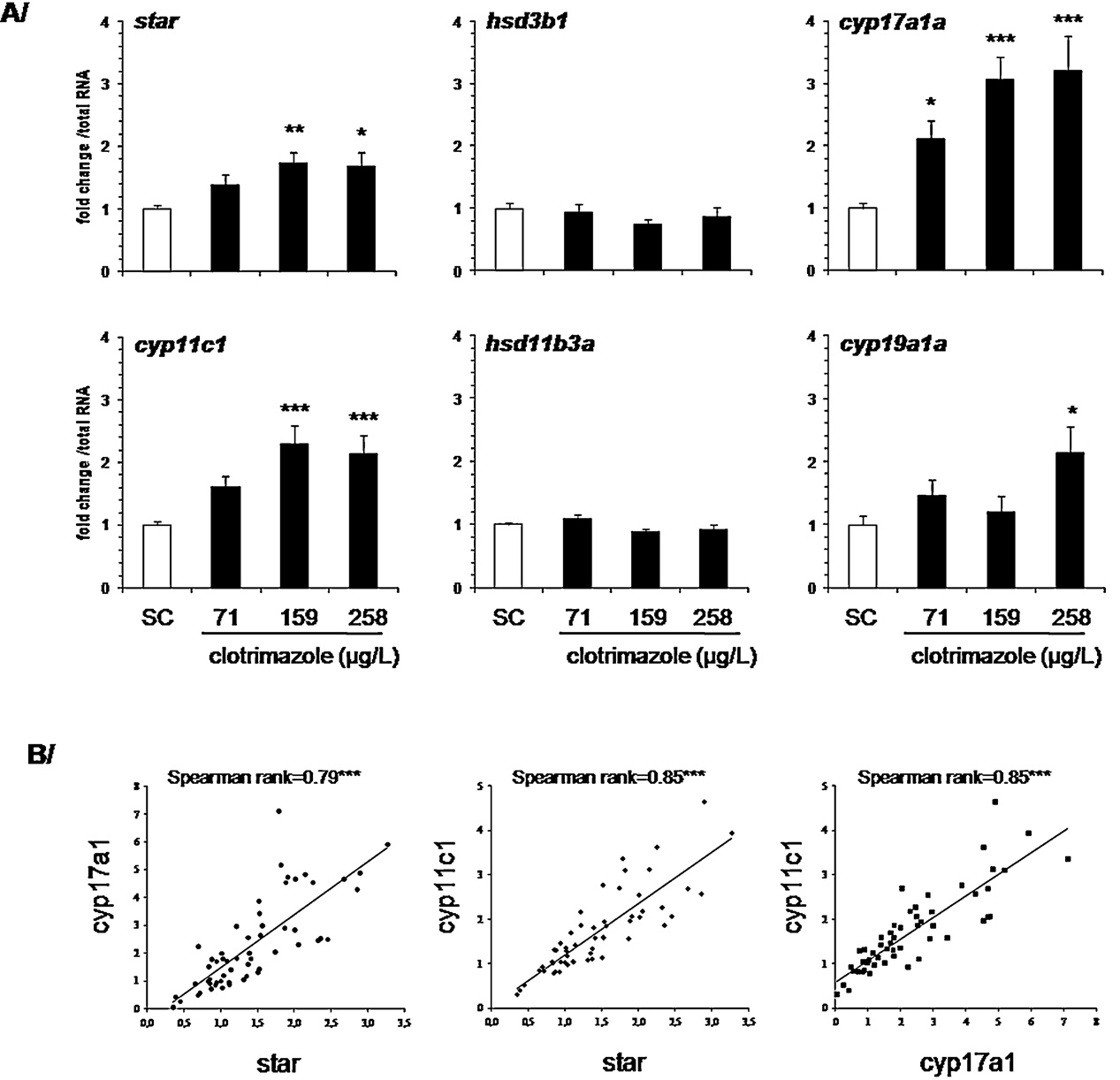
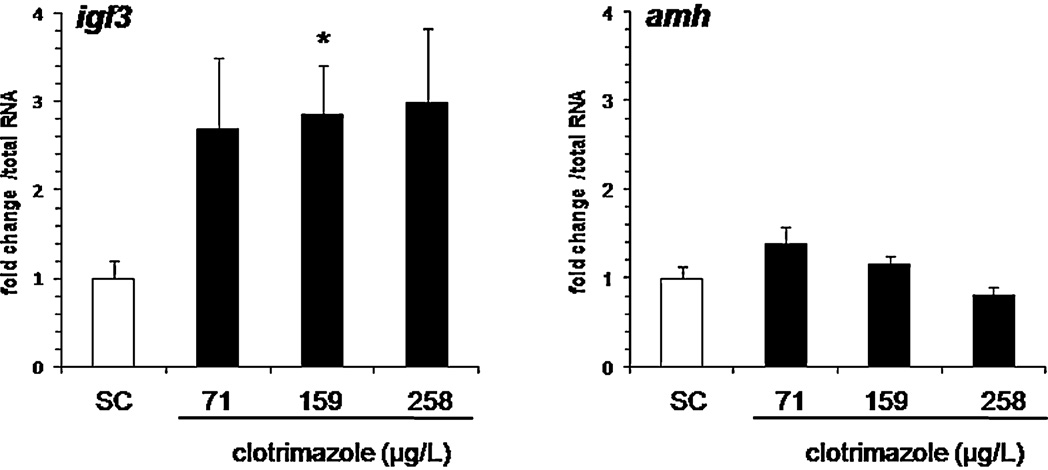
3.4. Expression of steroidogenesis-related genes
amh and igf3 mRNA levels. While the exposure had no effect on amh
expression, significantly increased igf3 mRNA levels (3-fold induc-
Transcriptional levels of genes involved in gonadal steroido-
tion) were found at the intermediate clotrimazole concentration of
genesis were determined by Q-PCR and compared in control and
159 g/L (p < 0.05,
4 of the 6 steroidogenic genes measured were up-regulated in
a concentration-dependent manner (A 1.7-fold induction
was observed for star transcript levels at 159 g/L (p < 0.01) and
In this study, we investigated the effects of clotrimazole on
258 g/L (p < 0.05). The 3 other over-expressed genes belonged
the expression and function of genes operating at crucial sites
to the CYP family. Among them, cyp17a1 was induced most
within the pituitary-gonad axis in adult male zebrafish. Our data
clearly, with a significant up-regulation at the lowest concen-
demonstrate that clotrimazole induced the expression of steroido-
tration (p < 0.001) and a 3-fold induction at 159 (p < 0.001) and
genic enzymes in Leydig cells (mRNA and protein level) that
258 g/L (p < 0.001). For cyp11c1, a significant 2-fold increase was
are required for the production of the 11-oxygenated androgens
measured at 159 g/L and 258 g/L (p < 0.001, p < 0.001 respec-
typically found in fish (like 11-KT), while circulating androgen
tively). Star, cyp17a1 and cyp11c1 showed similar patterns of
concentrations were not altered. Measurement of transcript lev-
expression and their expression was significantly correlated in
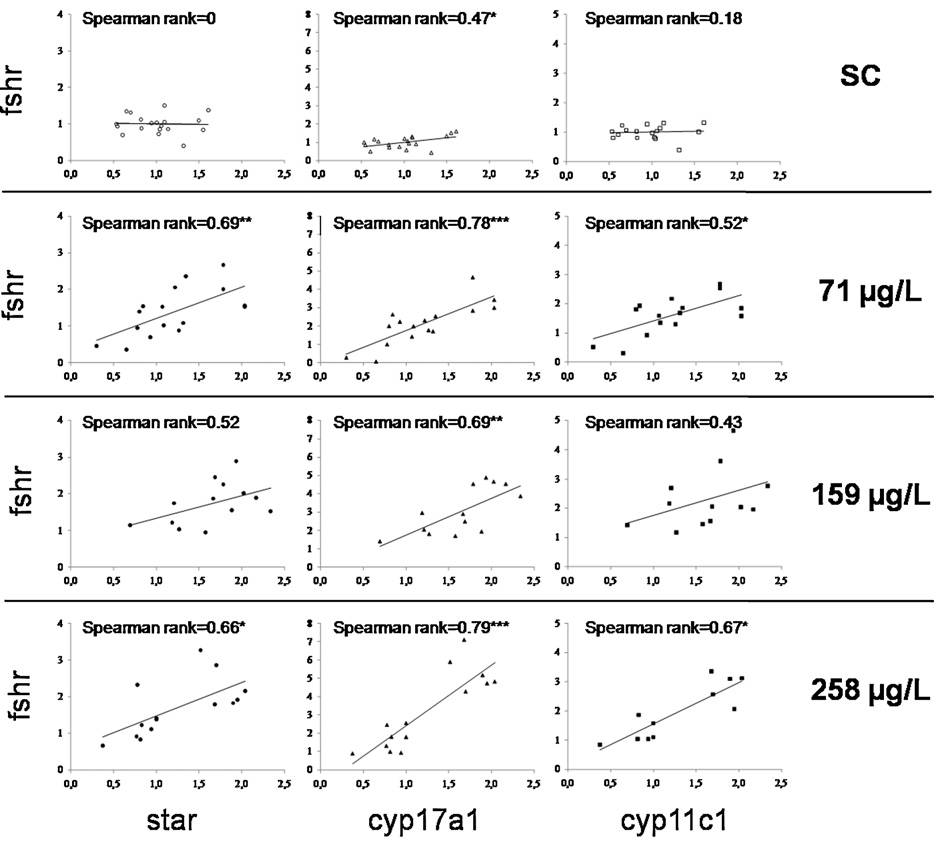
els of pituitary fshb, testicular fshr and the significant correlations
control and clotrimazole-exposed groups (Interestingly,
between fshr and expression of steroidogenesis-related genes sug-
the correlation between steroidogenic genes was stronger in all
gest that gonadotropins, in particular Fsh, are involved in the
clotrimazole-exposed groups than in the control group (
clotrimazole-induced changes in testicular physiology. Additional
Cyp19a1a was also significantly (2-fold) up-regulated (p < 0.05) but
evidence for an increased signaling via Fsh/Fshr is provided by the
only at the highest concentration of clotrimazole.
induction of igf3, a gene expressed by Sertoli cells and up-regulated
by Fsh (Nobrega, Morais, de Waal, Bogerd and Schulz; unpublished
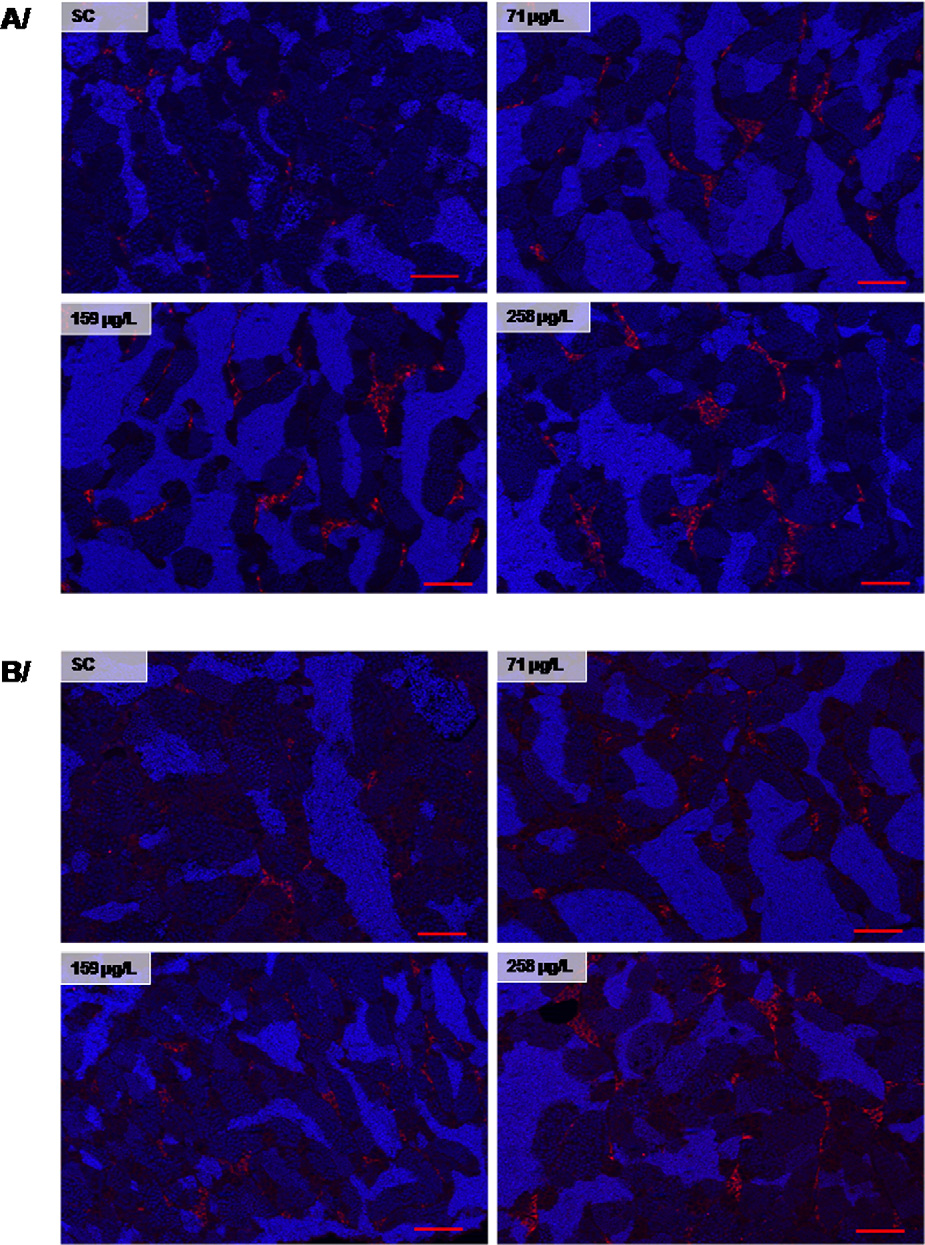
3.5. Cyp17a1 and Cyp11c1 immunostaining in testes
data). This data set highlights the importance of studying expres-
sion and functioning of key genes of the pituitary-gonad axis to start
The effect of clotrimazole on Cyp17a1 and Cyp11c1 protein
elucidating the mode of action of clotrimazole on the endocrine
expression was examined by fluorescent immunohistochemistry
system of fish.
in testis sections using specific polyclonal antibodies (We
observed a strong increase of Cyp17a1 and Cyp11c1 protein expres-
4.1. Clotrimazole-induced expression of testicular genes related
sions that both were localized in Leydig cells. Pictures presented
to steroidogenesis
in representative of all individuals for each condition.
Cyp17a1 immunostaining was induced from 71 g/L to 258 g/L
In the present study, short-term in vivo exposure of zebrafish
and Cyp11c1 was clearly induced at the highest concen-
to clotrimazole led to over-expression in zebrafish testis of star
tration of clotrimazole (
and steroidogenic enzymes crucial for fish androgen production,
i.e. cyp17a1 and cyp11c1. Interestingly, expression of these genes
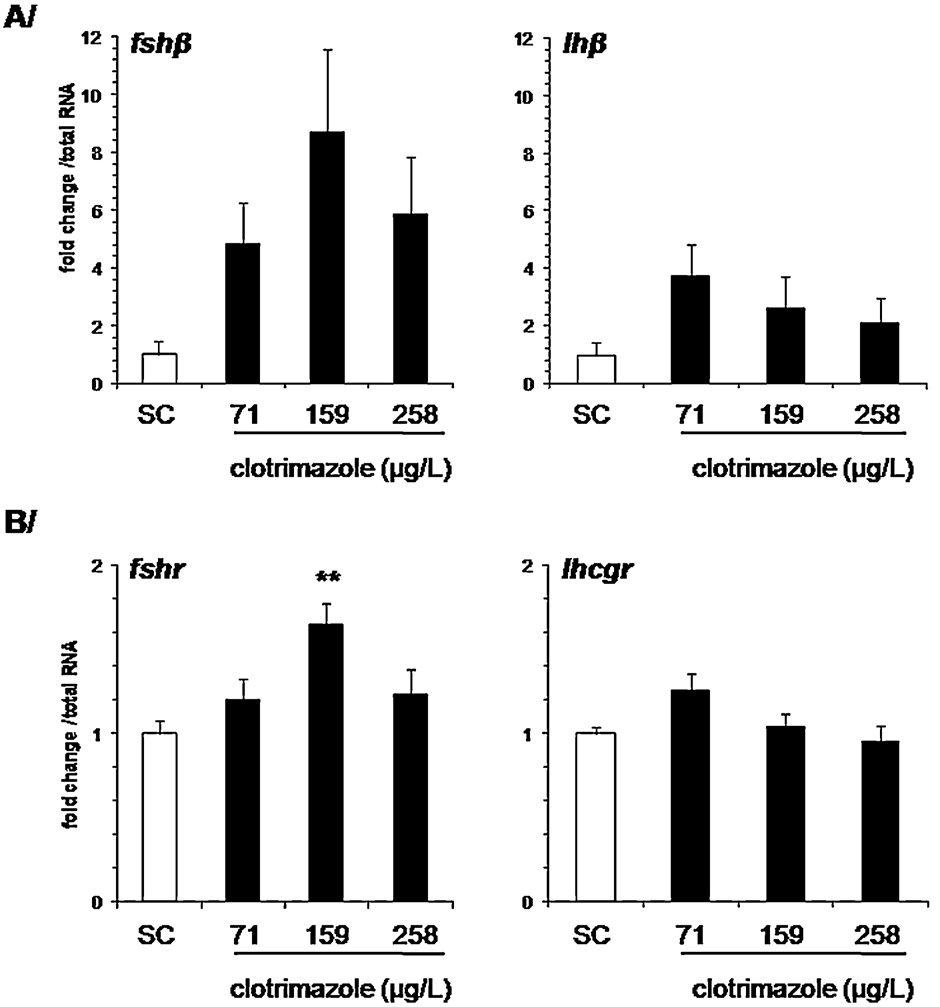
3.6. Expression of pituitary gonadotropins and their gonadal
was strongly correlated in clotrimazole-treated groups, suggest-
ing an orchestrated response to clotrimazole By
means of immunohistochemistry, we further showed an increased
An 8- and 6-fold up-regulation of pituitary fshb was observed
of Cyp17a1 and Cyp11c1 protein levels in Leydig cells, suggest-
at 159 g/L and 258 g/L, respectively (Still, statistical sig-
ing de novo synthesis of these enzymes. This is in agreement
nificance was not reached, probably due to the high variability of
with previously published data on the effect of clotrimazole
transcript levels, in particular in the clotrimazole-exposed groups.
on cyp17a1 expression in zebrafish (and
In testes, fshr was up-regulated 1.65-fold in the 159 g/L
extends the findings to another steroidogenic enzyme. Even though
clotrimazole-exposed group (p < 0.01), whereas no effect was
clotrimazole concentrations used to trigger in vivo changes in
observed for lhcgr transcripts levels Interestingly, signif-
testicular steroidogenesis-related genes are higher than concen-
icant and strong correlations were found between fshr expression
trations found in the aquatic environment
and either star, cyp17a1 or cyp11c1 in clotrimazole-exposed groups,
it should be stressed that clotri-
whereas such correlations were not found in the control group
mazole can potentially bioaccumulated in fish given its lipophilic
Induction of testicular steroidogenic gene expression following
3.7. Expression of insulin-like growth factor 3 (igf3) and
exposure to other azole fungicides (ketoconazole and prochlo-
anti-müllerian hormone (amh)
raz) has been reported in fathead minnow and medaka
Interestingly, in fathead minnow
The data presented above showed that Leydig cell genes related
exposed to flutamide, an androgen receptor antagonist, cyp17a1
to steroidogenesis were affected by clotrimazole. To determine
and hsd11b3a were induced in testes (Induction
whether genes expressed in Sertoli cells and known to be regu-
of steroidogenic genes in testes of flutamide-exposed fathead min-
lated by Fsh respond to clotrimazole exposure in vivo, we quantified
now has been interpreted as an inhibitory action on endogenous
D. Baudiffier et al. / Toxicology 298 (2012) 30–39
Fig. 2. (A) In vivo effect of clotrimazole on key testicular gene expressions following a 7 days exposure. Data are represented as mean ± SEM and expressed as fold change in
mRNA expression from the control. Relative mRNA expression was determined as the ratio of target gene mRNA/2 g total RNA (N = 12–19 fish per condition). Each sample
was analyzed in duplicate. (B) Correlation between star, cyp17a1 and cyp11c1 expressions in control group and clotrimazole-exposed groups. Spearman rank number is
indicated. Asterisks indicated a significant difference compared to control group (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
androgen negative feedback pathways (While
at least partially, the inhibition of 11-KT production that we mea-
prochloraz and ketoconazole behaved also as androgen recep-
sured previously in the culture medium of zebrafish testicular
tor agonists (clotrimazole did
explants after a 6-days in vitro exposure to 1 M (equivalent to
not bind to AR in mammals (
344.8 g/L) clotrimazole These data, demon-
information as regards the possible binding to piscine ARs is not
strating the in vitro inhibitory action of clotrimazole on 11-KT
available. Therefore, it is unlikely that induction of steroidogenic
production, seemingly contrast with in vivo results showing that
genes in zebrafish exposed to clotrimazole involve an inhibitory
11-KT blood concentrations were not affected in males after a
action of endogenous androgens. On the contrary, the primary
comparable time (7 days) and concentration (258 g/L) of expo-
mode of action of clotrimazole on testicular steroidogenesis is
sure to clotrimazole, as reported herein and in our previous work
through the inhibition of cytochrome P-450 dependent steroido-
(The in vivo over-expression of steroidogenic
genic enzyme activities. Indeed, previous studies reported that
genes has been interpreted as a compensatory response to inhibit-
clotrimazole inhibited ovarian P450 aromatase and testicular P450
ing enzyme activities (
Cyp17 17␣-hydroxylase and 17,20 lyase enzyme activities in in vitro
and would also explain
microsomal assays at concentrations ranging from 0.01 M to
that no significant effect of clotrimazole on 11-KT blood concentra-
1.7 M depending on the model used and the enzyme activity
tions was measured (this study, (In this setting
we assume that clotrimazole initially induced a decrease of testicu-
We assume that this inhibitory action
lar androgen output and hence a depression of circulating androgen
on cytochrome P-450 dependent steroidogenic enzymes explains,
levels, which triggered the compensatory response, resulting in an
D. Baudiffier et al. / Toxicology 298 (2012) 30–39
Fig. 3. (A) Cyp17-I and (B) Cyp11c1 labeling on zebrafish testes by fluorescent immunohistochemistry following a 7 days exposure to clotrimazole. Immunostaining was
observed in all individuals and localized in Leydig cells (N = 4–5 fish per condition). The present pictures are representative of all individuals. Red: P450c17 and P450c11B
immunostaining, blue: Hoechst. Scale bars represent 50 m (red). (For interpretation of the references to color in this figure legend, the reader is referred to the web version
increased de novo synthesis of steroidogenic enzymes, allowing to
4.2. The effect of clotrimazole on testicular steroidogenesis is
attain normal circulating androgen levels. Such transient depres-
likely regulated by pituitary follicle-stimulating hormone
sion of circulating 11-KT was recently reported after 1 and 4 days
of exposure of fish to 30 and 300 g/L of ketoconazole while 11-KT
To support the hypothesis that the increased activity of the
concentrations return to a normal level after 8 days
steroidogenic system may be due to a compensatory response of
Physiologically, a transient decrease in circulating andro-
feedback loops within the HPG-axis to overcome the fungicide-
gen levels seems a signal similar to reduced signaling via the AR,
mediated inhibition of enzyme activities, we studied the expression
which may explain the similarity of the response to the AR antag-
of genes encoding for gonadotropin -subunits as well as the
onist flutamide and to clotrimazole. However, it should be noted
testicular expression of gonadotropin receptors. At the pituitary
that high inter-individual variability was observed in the group
level, induction of genes encoding for the -subunit of Fsh, but
of fish exposed to the highest concentration of clotrimazole, per-
not of Lh, was observed in clotrimazole-exposed fish. However,
haps reflecting that the physiological limits of compensation have
probably due to the variability of expression between individuals
been reached. For instance, some fish had very high levels of 11-
within groups, these effects did not reach statistical significance.
KT (overcompensated), while others had very low levels. In the
Concomitantly, a significant up-regulation of the fshr, but not the
latter, up-regulation of enzyme expression may be insufficient to
lhcgr, gene expression was measured in testes of clotrimazole-
balance the pharmaceutical's inhibitory effect on enzyme activ-
exposed fish. Moreover, fshr expression was strongly correlated
with the expression of testicular genes related to steroidogenesis in
D. Baudiffier et al. / Toxicology 298 (2012) 30–39
Fig. 4. In vivo effect of clotrimazole on (A) pituitary gonadotropins  subunit and (B) testicular gonadotropin receptors gene expressions following a 7 days exposure. Data are
represented as mean ± SEM and expressed as fold change in mRNA expression from the control (N = 11–19 fish per condition). Relative mRNA expression was determined as
the ratio of target gene mRNA/2 g RNA. Each sample was analyzed in duplicate. Asterisks indicated a significant difference compared to control group (*p ≤ 0.05, **p ≤ 0.01).
clotrimazole-exposed groups (As regards lhcgr
recently discovered member of the Igf family that is only present
mRNA levels, no clear correlation with steroidogenic genes was
in fish and is expressed specifically in gonadal tissue (
found. In agreement with these findings, it has been shown that Fsh
In zebrafish, igf3 expression was localized in Sertoli cells and
is a potent steroidogenic hormone in fish, acting through the Fshr,
testicular expression of igf3 is up-regulated by zebrafish Fsh, but not
which in fish is also expressed by Leydig cells. In Japanese eel and
by Lh (Nobrega, Morais, de Waal, Bogerd and Schulz; unpublished
zebrafish, recombinant Fsh, but not Lh, induced star and cyp17a1
data). Our results, showing an up-regulation of igf3 expression in
gene expressions in testicular explants system (
testes of clotrimazole-exposed fish, are thus also consistent with
Activation of Fsh-/Fshr signaling path-
way in response to a decrease in androgen production is further
In addition to these molecular and cellular changes, we previ-
illustrated by a recent work in which unilateral gonadectomy in
ously reported an increased proportion of spermatogonia type A
male African catfish lead to induction of fshr gene expression and
in clotrimazole-exposed fish under similar conditions of exposure
steroid production capacity of the remaining testis. In consequence,
(However, in this study no significant differ-
the final concentration of androgens was unchanged
ence as regards piwil1 gene expression, a spermatogonial marker
(was seen between treatment groups (
Taken together, these data suggest that Fsh-/Fshr-signaling
Nonetheless, considering the role of Fsh-stimulated androgen pro-
played an important role in mediating the clotrimazole-induced
duction and the role of androgens in stimulating the first steps of
compensatory response of the steroidogenic Leydig cells. Interest-
spermatogenesis (as well as the stimulation
ingly in mammals, treatment of male with azole compounds such
of germ cell mitosis by other Igf peptides we hypothe-
as anastrozole and letrozole led to increase concentrations of circu-
size that the effects seen at the histological level on spermatogonial
lating gonadatropins and testosterone
proliferation in our previous study (may have
This supports the view that disrup-
been a direct consequence of the Fsh-mediated effect on the testes
tion of the HPG axis by azoles is well-conserved among vertebrates
of zebrafish exposed to clotrimazole.
with the notable difference that in fish Fsh seems to play a predom-
In conclusion, this study provides further evidence of the
inant role in stimulating steroidogenesis.
endocrine disrupting potency of clotrimazole in fish as demon-
In addition to affecting steroidogenesis in Leydig cells, clotri-
strated by the elevated expression of steroidogenesis-related genes
mazole exposure up-regulated the levels of igf3 mRNA. Igf3 is a
in testicular tissue. By studying a functional network of genes
D. Baudiffier et al. / Toxicology 298 (2012) 30–39
Fig. 5. Correlation between fshr expression and star, cyp17a1 and cyp11c1 expressions in control group and clotrimazole-exposed-groups. Spearman rank number is indicated
Asterisks indicated a significant correlation (*p ≤ 0.05, **p ≤ 0.01).
Fig. 6. In vivo effect of clotrimazole on Sertoli cell marker expressions following a 7 days exposure. Data are represented as mean ± SEM and expressed as fold change in
mRNA expression from the control. Relative mRNA expression was determined as the ratio of target gene mRNA/2 g total RNA (N = 12–19 fish per condition). Asterisks
indicated a significant difference compared to control group (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
operating in the pituitary-gonad axis, we show that clotrimazole
triggered in a model vertebrate compensatory responses of the HPG
induced a cascade of molecular and cellular events at the pituitary
axis. This approach could be extended to other azole compounds
and testicular level that is compatible with assuming an important
acting as inhibitor of P450-steroidogenic enzymes in in vitro assays
role for Fsh (1) in stimulating Leydig cell steroidogenesis to com-
pensate the inhibitory action of clotrimazole on 11-KT synthesis
and (2) in inducing the expression of the Fsh-regulated igf3 gene in
Sertoli cells. Studying this gene network along the pituitary-gonad
Conflict of interest statement
axis appears useful and relevant to elucidate further the mode
of action of clotrimazole and it demonstrated that this chemical
D. Baudiffier et al. / Toxicology 298 (2012) 30–39
Hill, A.J., Teraoka, H., Heideman, W., Peterson, R.E., 2005. Zebrafish as a model ver-
tebrate for investigating chemical toxicity. Toxicol. Sci. 86, 6–19.
Hinfray, N., Baudiffier, D., Leal, M.C., Porcher, J.M., Ait-Aissa, S., Le Gac, F., Schulz,
This work was funded by a grant of the French ministry of
R.W., Brion, F., 2011. Characterization of testicular expression of P450 17 alpha-
Ecology P189-NEMO to FB, the French National Research Agency
hydroxylase, 17,20-lyase in zebrafish and its perturbation by the pharmaceutical
(Project NEED, CES 2008-011) to FB and by the European Union
fungicide clotrimazole. Gen. Comp. Endocrinol. 174, 309–317.
Hinfray, N., Porcher, J.M., Brion, F., 2006. Inhibition of rainbow trout (Oncorhynchus
LIFECYCLE projects no FP7-222719 to RWS. DB was supported by a
mykiss) P450 aromatase activities in brain and ovarian microsomes by various
doctoral fellowship from ANRT and INERIS. The authors would like
environmental substances. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 144,
to thank Dr. Alexis Fostier (INRA, Rennes, France) for its comments
of this manuscript.
Huggett, J., Dheda, K., Bustin, S., Zumla, A., 2005. Real-time RT-PCR normalization;
strategies and considerations. Genes Immun. 6, 279–284.
Kazeto, Y., Kohara, M., Miura, T., Miura, C., Yamaguchi, S., Trant, J.M., Adachi, S.,
Yamauchi, K., 2008. Japanese eel follicle-stimulating hormone (Fsh) and luteiniz-
Appendix A. Supplementary data
ing hormone (Lh): production of biologically active recombinant fsh and Lh by
Drosophila S2 cells and their differential actions on the reproductive biology.
Supplementary data associated with this article can be found, in
Biol. Reprod. 79, 938–946.
Lacey, C., Basha, S., Morrissey, A., Tobin, J.M., 2012. Occurrence of pharmaceutical
the online version, at .
compounds in wastewater process streams in Dublin. Ireland. Environ. Monit.
Assess. 184, 1049–1062.
Laville, N., Balaguer, P., Brion, F., Hinfray, N., Casellas, C., Porcher, J.M., Ait-Aissa,
S., 2006. Modulation of aromatase activity and mRNA by various selected
pesticides in the human choriocarcinoma JEG-3 cell line. Toxicology 228,
Ankley, G.T., Cavallin, J.E., Durhan, E.J., Jensen, K.M., Kalh, M.D., Makynen, E.A.,
Thomas, L.M., Wehmas, L.C., Villeneuve, D.L., 2012. A time-course analysis
Leal, M.C., de Waal, P.P., Garcia-Lopez, A., Chen, S.X., Bogerd, J., Schulz, R.W., 2009.
of effects of the steroidogenesis inhibitor ketoconazole on components of
Zebrafish primary testis tissue culture: an approach to study testis function ex
the hypothalamic-pituitary-gonadal axis of fathead minnows. Aquat. Toxicol.
vivo. Gen. Comp. Endocrinol. 162, 134–138.
114–115, 88–95.
Lemaire, G., de Sousa, G., Rahmani, R., 2004. A PXR reporter gene assay in a sta-
Ankley, G.T., Jensen, K.M., Kahl, M.D., Makynen, E.A., Blake, L.S., Greene, K.J., Johnson,
ble cell culture system: CYP3A4 and CYP2B6 induction by pesticides. Biochem.
R.D., Villeneuve, D.L., 2007. Ketoconazole in the fathead minnow (Pimephales
Pharmacol. 68, 2347–2358.
promelas): reproductive toxicity and biological compensation. Environ. Toxicol.
Liu, C.S., Zhang, X.W., Deng, J., Hecker, M., Al-Khedhairy, A., Giesy, J.P., Zhou, B.S.,
Chem. 26, 1214–1223.
2011. Effects of prochloraz or propylthiouracil on the cross-talk between the
Arukwe, A., 2006. Toxicological housekeeping genes: do they really keep the house?
HPG, HPA, and HPT axes in zebrafish. Environ. Sci. Technol. 45, 769–775.
Environ. Sci. Technol. 40, 7944–7949.
Loir, M., 1994. In vitro approach to the control of spermatogonia proliferation in the
Ayub, M., Levell, M.J., 1989. The effect of ketoconazole related imidazole drugs
trout. Mol. Cell. Endocrinol. 102, 141–150.
and antiandrogens on [H-3] R1881 binding to the prostatic androgen receptor
Miller, W.L., 1988. Molecular-biology of steroid-hormone synthesis. Endocr. Rev. 9,
and [H-3]5-alpha-dihydrotestosterone and [H-3] cortisol binding to plasma-
proteins. J. Steroid Biochem. Mol. Biol. 33, 251–255.
Monod, G., Demones, A., Fostier, A., 1993. Inhibition of ovarian microsomal
Ayub, M., Levell, M.J., 1987a. Inhibition of testicular 17-alpha-hydroxylase and
aromatase and follicular estradiol secretion by imidazole fungicides in rainbow-
17,20-lyase by imidazole drugs. J. Endocrinol. 112, 42-42.
trout. Mar. Environ. Res. 35, 153–157.
Ayub, M., Levell, M.J., 1987b. Inhibition of testicular 17-alpha-hydroxylase and
Moore, J.T., Kliewer, S.A., 2000. Use of the nuclear receptor PXR to predict drug
17,20-lyase but not 3-beta-hydroxysteroid dehydrogenase-isomerase or 17-
interactions. Toxicology 153, 1–10.
beta-hydroxysteroid oxidoreductase by ketoconazole and other imidazole
Navas, J.M., Chana, A., Herradon, B., Segner, H., 2004. Induction of cytochrome
drugs. J. Steroid Biochem. Mol. Biol. 28, 521–531.
P4501A (CYPIA) by clotrimazole, a non-planar aromatic compound. Compu-
Brown, A.R., Bickley, L.K., Le Page, G., Hosken, D.J., Paull, G.C., Hamilton, P.B., Owen,
tational studies on structural features of clotrimazole and related imidazole
S.F., Robinson, J., Sharpe, A.D., Tyler, C.R., 2011. Are toxicological responses
derivatives. Life Sci. 76, 699–714.
in laboratory (inbred) zebrafish representative of those in outbred (wild)
Ohta, T., Miyake, H., Miura, C., Kamei, H., Aida, K., Miura, T., 2007. Follicle-stimulating
populations?—a case study with an endocrine disrupting chemical. Environ. Sci.
hormone induces spermatogenesis mediated by androgen production in
Technol. 45, 4166–4172.
Japanese Eel, Anguilla japonica. Biol. Reprod. 77, 970–977.
Burns, K.H., Matzuk, M.M., 2002. Minireview: genetic models for the study of
OSPAR, 2005. OSPAR Background document on clotrimazole. ISBN: 1-94426-38-7.
gonadotropin actions. Endocrinology 143, 2823–2835.
Parker, K.L., Schimmer, B.P., 1995. Transcriptional regulation of the genes encoding
Campbell, B., Dickey, J.T., Swanson, P., 2003. Endocrine changes during onset of
the cytochrome P-450 steroid hydroxylases. Vitam. Horm. 51, 339–370.
puberty in male spring chinook salmon, Oncorhynchus tshawytscha. Biol. Reprod.
Peschka, M., Roberts, P.H., Knepper, T.P., 2007. Analysis, fate studies and monitoring
69, 2109–2117.
of the antifungal agent clotrimazole in the aquatic environment. Anal. Bioanal.
de Boer, H., Verschoor, L., Ruinemans-Koerts, J., Jansen, M., 2005. Letrozole nor-
Chem. 389, 959–968.
malizes serum testosterone in severely obese men with hypogonadotropic
Schulz, R.W., de Franca, L.R., Lareyre, J.J., Legac, F., Chiarini-Garcia, H., Nobrega,
hypogonadism. Diabetes Obes. Metab. 7, 211–215.
R.H., Miura, T., 2010. Spermatogenesis in fish. Gen. Comp. Endocrinol. 165,
Eil, C., 1992. Ketoconazole binds to the human androgen receptor. Horm. Metab. Res.
24, 367–370.
Schulz, R.W., Van Dijk, W., Chavez-Pozo, E., Garcia-Lopez, A., de Franc¸a, L.R.,
Filby, A.L., Thorpe, K.L., Maack, G., Tyler, C.R., 2007. Gene expression profiles reveal-
Bogerd, J., 2012. Sertoli cell proliferation in the adult testis is induced
ing the mechanisms of anti-androgen-and estrogen-induced feminization in
by unilateral gonadectomy in African catfish. Gen. Comp. Endocrinol.
fish. Aquat. Toxicol. 81, 219–231.
Garcia-Lopez, A., Bogerd, J., Granneman, J.C.M., van Dijk, W., Trant, J.M., Taranger,
Schuster, I., 1985. The interaction of representative members from 2 classes of
G.L., Schulz, R.W., 2009. Leydig cells express follicle-stimulating hormone recep-
antimycotics – the Azoles and the allylamines – with cytochromes-P-450 in
tors in African catfish. Endocrinology 150, 357–365.
steroidogenic tissues and liver. Xenobiotica 15, 529–546.
Garcia-Lopez, A., de Jonge, H., Nobrega, R.H., de Waal, P.P., van Dijk, W., Hem-
Sipes, N.S., Padilla, S., Knudsen, T.B., 2011. Zebrafish—as an integrative model for
rika, W., Taranger, G.L., Bogerd, J., Schulz, R.W., 2010. Studies in zebrafish
twenty-first century toxicity testing. Birth Defects Res. Part C: Embryo Today
reveal unusual cellular expression patterns of gonadotropin receptor messenger
Rev. 93, 256–267.
ribonucleic acids in the testis and unexpected functional differentiation of the
Skolness, S.Y., Durhan, E.J., Garcia-Reyero, N., Jensen, K.M., Kahl, M.D., Makynen, E.A.,
gonadotropins. Endocrinology 151, 2349–2360.
Martinovic-Weigelt, D., Perkins, E., Villeneuve, D.L., Ankley, G.T., 2011. Effects
Garcia-Valcarcel, A.I., Tadeo, J.L., 2011. Determination of azoles in sewage sludge
of a short-term exposure to the fungicide prochloraz on endocrine function
from Spanish wastewater treatment plants by liquid chromatography-tandem
and gene expression in female fathead minnows (Pimephales promelas). Aquat.
mass spectrometry. J. Sep. Sci. 34, 1228–1235.
Toxicol. 103, 170–178.
Goetz, A.K., Rockett, J.C., Ren, H.Z., Thillainadarajah, I., Dix, D.J., 2009. Inhibition of
Turner, K.J., Morley, M., Atanassova, N., Swanston, I.D., Sharpe, R.M., 2000.
rat and human steroidogenesis by triazole antifungals. Syst. Biol. Reprod. Med.
Effect of chronic administration of an aromatase inhibitor to adult male
55, 214–226.
rats on pituitary and testicular function and fertility. J. Endocrinol. 164,
Hecker, M., Newsted, J.L., Murphy, M.B., Higley, E.B., Jones, P.D., Wu, R., Giesy, J.P.,
2006. Human adrenocarcinoma (H295R) cells for rapid in vitro determination of
Villeneuve, D.L., Blake, L.S., Brodin, J.D., Greene, K.J., Knoebl, I., Miracle, A.L., Mar-
effects on steroidogenesis: hormone production. Toxicol. Appl. Pharmacol. 217,
tinovic, D., Ankley, G.T., 2007a. Transcription of key genes regulating gonadal
steroidogenesis in control and ketoconazole- or vinclozolin-exposed fathead
Heneweer, M., van den Berg, M., Sanderson, J.T., 2004. A comparison of human H295R
minnows. Toxicol. Sci. 98, 395–407.
and rat R2C cell lines as in vitro screening tools for effects on aromatase. Toxicol.
Villeneuve, D.L., Miracle, A.L., Jensen, K.M., Degitz, S.J., Kahl, M.D., Korte, J.J., Greene,
Lett. 146, 183–194.
K.J., Blake, L.S., Linnum, A.L., Ankley, G.T., 2007b. Development of quantitative
Henry, M.J., Sisler, H.D., 1984. Effects of sterol biosynthesis-inhibiting (Sbi) fungi-
real-time PCR assays for fathead minnow (Pimephales promelas) gonadotropin
cides on cytochrome-P-450 oxygenations in fungi. Pestic. Biochem. Physiol. 22,
beta subunit mRNAs to support endocrine disruptor research. Comp. Biochem.
Physiol. C: Toxicol. Pharmacol. 145, 171–183.
D. Baudiffier et al. / Toxicology 298 (2012) 30–39
Vinggaard, A.M., Hnida, C., Breinholt, V., Larsen, J.C., 2000. Screening of selected
Zhang, X.W., Hecker, M., Jones, P.D., Newsted, J., Au, D., Kong, R., Wu, R.S.S., Giesy,
pesticides for inhibition of CYP19 aromatase activity in vitro. Toxicol. Vitro 14,
J.P., 2008. Responses of the medaka HPG axis PCR array and reproduction to
prochloraz and ketoconazole. Environ. Sci. Technol. 42, 6762–6769.
Vinggaard, A.M., Nellemann, C., Dalgaard, M., Jorgensen, E.B., Andersen, H.R., 2002.
Zmora, N., Kazeto, Y., Kumar, R.S., Schulz, R.W., Trant, J.M., 2007. Production of
Antiandrogenic effects in vitro and in vivo of the fungicide prochloraz. Toxicol.
recombinant channel catfish (Ictalurus punctatus) FSH and LH in S2 Drosophila
Sci. 69, 344–353.
cell line and an indication of their different actions. J. Endocrinol. 194,
Wang, D.S., Jiao, B.W., Hu, C.J., Huang, X.G., Liu, Z.H., Cheng, C.H.K., 2008. Discovery
of a gonad-specific IGF subtype in teleost. Biochem. Biophys. Res. Commun. 367,
Zohar, Y., Munoz-Cueto, J.A., Elizur, A., Kah, O., 2010. Neuroendocrinology of repro-
duction in teleost fish. Gen. Comp. Endocrinol. 165, 438–455.
Source: http://m.damien-baudiffier.fr/var/f/b4/P6/b4P6Cg3aLZHljQ9hNx8oMIfEtqWpyDUevJzwYKSrOF_n501csB.pdf
SECURITY DETAILS Pages: 1 to 7 VINFERMATON (SPECIAL COCKROACHES) ISSUE DATE: 09/09/2004 SECURITY DETAILS Complying with de European Committee 91/155/EEC, last modification 2001/58/EC (07/27/01) and R.D. 255/2003. 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND OF THE COMPANY. Name of the product: VINFERMATON (SPECIAL COCKROACHES)
Global Listed Infrastructure Monthly Review and Outlook As at June 2014 Welcome to the latest monthly update on the First State Global Infrastructure, providing a review of the Fund and latest outlook Key highlights: Global listed infrastructure capped the 2013/2014 financial year with a further increase in June. The Fund climbed by 0.9%; its seventh consecutive month of positive returns. The regulated utility and energy pipeline sectors hummed with M&A activity and corporate re-structurings. Fund positioning remains tilted towards "growth" oriented sectors such as toll roads, ports and railways. Underweight exposure has been maintained towards "income" sectors such as regulated utilities and energy pipelines that







