Anm20-6(06)河村
Annals of Nuclear Medicine Vol. 20, No. 6, 417–424, 2006
Synthesis and evaluation of vesamicol analog (−
)-o-[11C]methylvesamicol
as a PET ligand for vesicular acetylcholine transporter
Kazunori KAWAMURA,*,** Kazuhiro SHIBA,*** Hideo TSUKADA,****
Shingo NISHIYAMA,**** Hirofumi MORI*** and Kiichi ISHIWATA*
*Positron Medical Center, Tokyo Metropolitan Institute of Gerontology
**SHI Accelerator Service Ltd.
***Advanced Science Research Center, Kanazawa University
****Central Research Laboratory, Hamamatsu Photonics K.K.
(−)-
o-Methylvesamicol ((−)-OMV) exhibited
in vitro a high affinity for vesicular acetylcholinetransporter (VAChT) (
Ki, 6.7 nM) and a relatively low affinity for sigma1 receptor (
Ki, 33.7 nM).
We prepared (−)-[11C]OMV by a palladium-promoted cross-coupling reaction using [11C]methyliodide, in a radiochemical yield of 38 ± 6.9% (n = 3), a radiochemical purity of 98 ± 2.3% (n = 5),and a specific activity of 11 ± 7.0 TBq/mmol at 30 minutes after EOB (n = 5). Then, we evaluated
in vivo whether (−)-[11C]OMV has properties as a PET radioligand for mapping VAChT. In rats,the brain uptake of (−)-[11C]OMV was 1.1%ID/g at 5 minutes postinjection, and was retained of ahigh level for 60 minutes. The brain uptake was significantly inhibited by the co-injection (500nmol/kg) of cold (−)-OMV (58–66%), (−)-vesamicol (57–65%), and two sigma receptor ligandswith modulate affinities for VAChTs: SA4503 (56–71%) and haloperidol (39–64%) in all of thebrain regions, including the cerebellum with a low density of VAChTs, but not of sigma1-selectiveligand (+)-pentazocine. However, the pretreatment with a large excess amount of (±)-pentazocine(50 µmol/kg) reduced the uptake in a different manner in the brain regions: 25% reduction in thestriatum with a high density of VAChTs, and a 50–55% reduction in the other regions with a lowerdensity of VAChTs.
Ex vivo autoradiography using (−)-[11C]OMV showed a similar regional braindistribution of [3H](−)-vesamicol. In the PET study of the monkey brain, the regional braindistribution pattern of (–)-[11C]OMV was different from that of [11C]SA4503. The uptake of (−)-[11C]OMV was relatively higher in the striatum, was reversible, and an apparent equilibrium statewas found at 20–40 minutes. In conclusion, (−)-[11C]OMV exhibited appropriate brain kineticsduring the time frame of 11C-labeled tracers and bound mainly to VAChTs; however, the bindingto sigma1 receptors was not disregarded. Therefore, (−)-[11C]OMV-PET together with help of[11C]SA4503-PET may evaluate VAChTs.
Key words: (−)-
o-[11C]methylvesamicol, VAChT, PET, vesamicol
has been established as a reliable marker for presynapticcholinergic terminals. Consequently, a selective radio-
THE VESICULAR ACETYLCHOLINE TRANSPORTER (VAChT) is
ligand for VAChT may be used as a tool for studying the
localized exclusively in cholinergic neurons1–4 and thus
function of cholinergic neurons with positron emissiontomography (PET) and single photon emission computedtomography (SPECT).
Received November 30, 2005, revision accepted May 16,
For reprint contact: Kiichi Ishiwata, Ph.D., Positron Medical
been reported to bind to VAChTs on presynaptic acetyl-
Center, Tokyo Metropolitan Institute of Gerontology, Naka-cho
choline storage vesicles.5,6 Therefore, many radioligands
1–1, Itabashi-ku, Tokyo 173–0022, JAPAN.
based on vesamicol have been developed for mapping
VAChTs with PET7–13 and SPECT.14–18 Substituted
Vol. 20, No. 6, 2006
positions and optical isomerization of the vesamicol de-
Table 1 In vitro affinities of (–)-OMV, (–)-vesamicol and three
rivatives altered their affinities for VAChTs and sigma
sigma receptor ligands for vesicular acetylcholine transporter
receptors.11,19 Shiba
et al
. synthesized (−)-
o-methyl-
(VAChT) and sigma receptors
vesamicol ((−)-OMV), wihch exhibited a high affinity
for VAChTs (
Ki, 6.7 nM) and a relatively low affinity for
1 receptor
in vitro (
Ki for sigma1, 33.7 nM;
Ki for
sigma2, 266 nM) (Table 1).20 The VAChT/sigma1 recep-
tor selectivity of (−)-OMV (5.0) would not be sufficient
for VAChTs; however, in general, a
Ki = 33.7 nM is not
optimal if one is seeking PET and SPECT radioligands.
Recently, Efange
et al
. reported [18F](+)-4-fluoro-
benzyltrozamicol ([18F](+)-FBT) as a potential PET
ligand for mapping VAChTs.10 (+)-FBT was found to
Data from Shiba et al.20
have a high affinity (
Ki, 0.22 nM) for VAChTs and a lower
*Values are the average of three experiments.
affinity for sigma1 receptors (
Ki, 21.6 nM) and sigma2
#Rat cerebral membranes were incubated with [3H](−)-vesamicol
receptors (
Ki, 35.9 nM).21 In the
in vivo study, [18F](+)-
in 50 mmol/
l Tris-HCl (pH 7.8) for 60 minutes at 37°C in the
FBT showed a high uptake and a slow rate of washout
presence of 200 nmol/
l DTG to mask the sigma receptors. †Rat
from the striatum of rats10 and monkeys.21 Compared with
cerebral membranes were incubated with [3H](+)-pentazocine
(+)-FBT, (−)-OMV has a lower affinity for VAChTs, a
in 50 mmol/
l Tris-HCl (pH 7.8) for 90 minutes at 37°C. $Rat liver
slightly lower affinity for sigma
membranes were incubated with [3H]DTG in 50 mmol/
l Tris-
1 receptors and much
lower affinity for sigma
HCl (pH 7.8) for 90 minutes at 37°C in the presence of 0.001
2 receptors. Therefore, it is consid-
ered that (−)-
o-[11C]methylvesamicol ((−)-[11C]OMV)
mmol/
l (+)-pentazocine to mask the sigma1 receptors.
shows a faster rate of washout from the striatum than[18F](+)-FBT without affinity for sigma receptors. Thebrain kinetics that the receptor-ligand binding reaches the
Institute of Gerontology.
apparent equilibrium state during the time-scale for PET
Two male rhesus monkeys (
Macaca mulatta, 7 years
measurement is preferable for quantitative analysis. Neg-
old, 6.4 and 6.6 kg) were used for the PET measurements.
ligible binding of (−)-[11C]OMV to sigma receptors is
They were trained to sit on a chair twice a week for more
also expected. Here, we prepared (−)-[11C]OMV by sub-
than three months. The study was performed in accor-
stitution of the trimethylstannyl group with [11C]methyl
dance with recommendations of the US National Institute
iodide in a palladium-promoted cross-coupling reaction
of Health and the guidelines of the Central Research
(Fig. 1)22 and evaluated
in vivo in rats whether (−)-
Laboratory, Hamamatsu Photonics K.K.
[11C]OMV has properties as a PET radioligand for map-ping VAChT, or whether it shows affinity for both VAChTs
Radiolabeling of (−)-
[11C]OMV
and sigma receptors. We also performed PET imaging of
A solution of tris(dibenzylideneacetone)dipalladium(0)
the monkey brain with (−)-[11C]OMV, compared with the
(2.8–3.4 mg, 3.0–3.6 µmol), tri(
o-tolyl)phosphine (3.7–
PET imaging with [11C]SA4503 that has been developed
4.0 mg, 12–13 µmol) in
N,N-dimethylformamide (DMF)
as a selective PET ligand for mapping sigma1 recep-
(0.2 m
l) was prepared in a dry septum equipped vial and
heated for a few minutes (until the color in the solutionchanged to yellow), and then added to the mixture of
MATERIALS AND METHODS
copper chloride (1.2–3.0 mg, 12–30 µmol), potassiumcarbonate (1.7–2.5 mg, 12–18 µmol) and (−)-
o-
trimethylstannyl-vesamicol (0.6 mg) in DMF (0.2 m
l).
(−)-Vesamicol and haloperidol were purchased from Sigma
[11C]Methyl iodide was produced from [11C]CO2 with an
Chemical (St. Louis, MO). SA4503 and (+)-pentazocine
automated system (Sumitomo Heavy Industries, Tokyo,
were provided from M's Science (Kobe, Japan). (±)-
Japan) and was trapped in the mixture of DMF (0.4 m
l)
Pentazocine (PENTAGIN® injection) was purchased
with air cooling. The reaction mixture was heated at 80°C
from Sankyo (Tokyo, Japan). (−)-OMV and (−)-2-(4-(2-
for 3 minutes. After adding 1.2 m
l of high-performance
liquid chromatography (HPLC) eluent [acetonitrile/ 50
trimethylstannyl-vesamicol) were synthesized as described
mmol/
l ammonium acetate, (30/70, v/v)], the reaction
previously.20 All chemicals were obtained from commer-
mixture was passed through the glass filter (20 µm) and
cial sources.
followed by injection onto the preparative HPLC: YMC-
Male Wistar rats were obtained from Tokyo Labora-
Pack ODS-A column (10 mm inner diameter (i.d.) × 250
tory Animals (Tokyo, Japan). Animal experiments were
mm length, YMC, Kyoto, Japan) with a mobile phase of
carried out in compliance with the Guidelines for Animal
acetonitrile/ 50 mmol/
l ammonium acetate (30/70) at a
Care and Use Committee of the Tokyo Metropolitan
flow rate of 5.0 m
l/minute (UV detector at 260 nm). The
Kazunori Kawamura, Kazuhiro Shiba, Hideo Tsukada, et al
Annals of Nuclear Medicine
Fig. 1 Synthesis of (−)-[11C]OMV.
retention time of (−)-[11C]OMV was 17 minutes. The
1 minute at 4°C to obtain the plasma, which was denatured
fraction of (−)-[11C]OMV was collected and evaporated
with a 1/3 equivalent volume of 20% trichloroacetic acid
to dryness. The residue was dissolved in physiological
(TCA) in acetonitrile. The mixture was centrifuged in the
saline. The final product was analyzed by HPLC using a
same condition, and the precipitate was re-suspended in
TSKgel super-ODS column (4.6 mm i.d. × 100 mm
0.5 m
l of 10% TCA in acetonitrile followed by centrifu-
length, Toso, Tokyo, Japan) with a mobile phase of
gation. This procedure was repeated three times. The
acetonitrile/ 50 mmol/
l acetic acid/ 50 mmol/
l ammonium
brain was homogenized in 1 m
l of 20% TCA in acetoni-
acetate (25/37.5/37.5, v/v/v) at a flow rate of 1.0 m
l/
trile/water (1/1, v/v). The homogenate was treated as
minute (UV detector at 260 nm). The retention time of
described above. The combined supernatant was ana-
(−)-[11C]OMV was 4.4 minutes.
lyzed by HPLC with a radioactivity detector (Radiomatic150TR, Packard, Meriden, CT). A Radial-Pak C18 col-
Tissue distribution in rats
umn equipped in an RCM 8 × 10 module (8 mm × 100 mm,
(−)-[11C]OMV (9.7 MBq/2.2 nmol) was intravenously
Waters, Milford, MA) was used with a mixture of 35%
injected into Wistar rats (7 weeks old, 210–250 g). Rats
acetonitrile and 65% 50 mmol/
l acetic acid/sodium ac-
were sacrificed by cervical dislocation 5, 15, 30 and 60
etate (1/1) at a flow rate of 2 m
l/minute.
minutes after injection (n = 4). The blood was collected byheart puncture, and tissues were harvested and weighed.
Ex vivo
autoradiography in rats
The 11C radioactivity in the samples was measured with
Ex vivo autoradiography of the brain was carried out in
an auto-gamma scintillation counter. The tissue uptake of
rats. (−)-[11C]OMV (111 MBq/7.2 nmol) was intrave-
11C was expressed as the percentage of the injected dose
nously injected into the rat (8 weeks old, 270 g). The rat
per gram of tissue (%ID/g).
was sacrificed by cervical dislocation at 30 minutes after
To determine the specific binding, we performed the
injection. The brain was rapidly dissected, frozen, and
blocking experiment by co-injection with cold (−)-OMV,
coronally cut into 20 µm thick sections using a cryotome
(−)-vesamicol, SA4503 (sigma1 receptor ligand), halo-
(Bright Instrument, Huntingdon, UK). The brain sections
peridol (non-selective sigma receptor ligand) and (+)-
were dried on a hot plate at 60°C and were apposed to a
pentazocine (sigma1 receptor ligand), and by pretreat-
storage phosphor screen (PhosphorImager SI system,
ment with (±)-pentazocine (PENTAGIN® injection)
Molecular Dynamics, Sunnyvale, CA) for 2 hours.
(non-selective sigma receptor ligand). A mixture of (−)-[11C]OMV (9.9 MBq/0.68 nmol) and one of blockers,
PET measurement in the monkey brain
except for (±)-pentazocine, at a dose of 500 nmol/kg in 0.2
A monkey was fixed on a PET camera, a model SHR-7700
m
l physiological saline/dimethyl sulfoxide (1/1, v/v) was
(Hamamatsu Photonics K.K., Hamamatsu, Japan), which
injected into the rats. In the other group of rats, 50 µmol/
acquires 31 slices at a center-to-center interval of 3.6 mm
kg of (±)-pentazocine was intravenously administrated 10
with a resolution of 2.6 mm full width at half maximum in
minutes prior to the tracer injection (14 MBq/ 0.87 nmol).
the transaxial plane. (−)-[11C]OMV (745 MBq/ 8.8 nmol)
The rats were sacrificed by cervical dislocation 30 min-
was injected into the monkey through a posterior tibial
utes after injection (n = 4–6, 7–8 weeks old, 230–270 g).
vein cannula. The PET scanning was performed for 61
The sample collection and measurement were performed
minutes with 6 time frames at 10 second intervals, 6 time
as described above.
frames at 30 seconds, 12 time frames at 1 minute, fol-lowed by 15 time frames at 3 minutes. In the other
Metabolite study in rats
monkey, the PET study with [11C]SA4503 (537 MBq/8.4
(−)-[11C]OMV (111–118 MBq/7.2–25 nmol) was intra-
nmol) was performed in the same way. Regions of interest
venously injected into rats (7–8 weeks old, 240–270 g),
were placed on the striatum, occipital cortex, frontal
and 15 (n = 3) or 30 (n = 1) minutes later they were
cortex, temporal cortex and cerebellum. The decay-cor-
sacrificed by cervical dislocation. Blood was removed by
rected radioactivity was expressed as the percentage of
heart puncture using a heparinized syringe, and the brain
the injected dose per m
l tissue volume (%ID/m
l).
was removed. The blood was centrifuged at 7,000 × g for
Vol. 20, No. 6, 2006
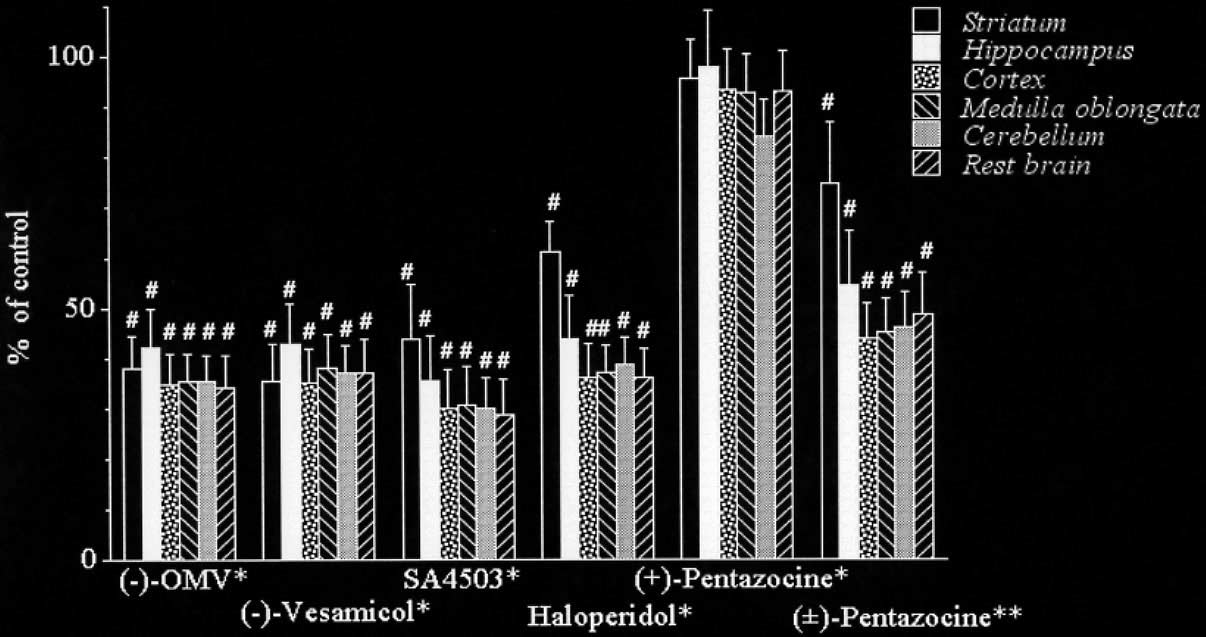
Table 2 Tissue distribution of radioactivity after intravenous injection of (–)-[11C]OMV in rats
Radioactivity level (%ID/g)*
*Radioactivity levels are represented as the mean % injection dose per gram of tissue ± S.D. (n = 4).
Fig. 2 The blocking effects of (−)-OMV, (−)-vesamicol, SA4503, haloperidol, (+)-pentazocine and (±)-
pentazocine on the regional brain distribution of (−)-[11C]OMV in rats at 30 minutes after intravenous
injection. *Co-injected dose with (−)-OMV, (−)-vesamicol and (+)-pentazocine was 500 nmol/kg.
**Pretreatment dose with (±)-pentazocine was 50 µmol/kg. Radioactivity levels are represented as the
mean % injection dose per gram of tissue (%ID/g) ± S.D. (n = 4–6). #p < 0.005, Student's t-test compared
with the control.
(3.4%ID/g) and kidney (2.3%ID/g), while the uptake at60 minutes was high in the pancreas (2.2%ID/g) and
Radiolabeling of (−)-[11C]OMV
small intestine (1.8%ID/g). The uptake in the brain
(−)-[11C]OMV was synthesized by methylation of the
showed a tendency to decrease over 60 minutes, and the
radioactivity levels of (−)-[11C]OMV decreased in the
iodide in a palladium-promoted cross-coupling reaction
blood, heart, lung, kidney and muscle over 60 minutes.
(Fig. 1).22 The total synthesis time was about 30 minutes.
The uptake levels in the pancreas, spleen and small in-
The decay corrected radiochemical yield was 38 ± 6.9%
testine increased until 15 minutes and then decreased
(n = 3) calculated from [11C]methyl iodide, and the
for 60 minutes. In the liver, the uptake slightly increased
radiochemical purity determined by analytical HPLC was
for 60 minutes.
98 ± 2.3% (n = 5). The specific activity was 11 ± 7.0 TBq/
The regional distribution of (−)-[11C]OMV in the brain
mmol (n = 5) at 30 minutes after the end of bombardment.
and the blocking effects of (−)-OMV, (−)-vesamicol, andthree sigma receptor ligands on the uptake were investi-
Tissue distribution in rats
gated at 30 minutes after the tracer injection. In the control
The tissue distribution of the radioactivity after injection
group, the uptake values of the striatum, hippocampus,
of (−)-[11C]OMV into rats is summarized in Table 2. The
cerebral cortex, medulla oblongata, cerebellum and the
initial uptake of (−)-[11C]OMV was high in the lung
rest of brain were 1.07 ± 0.14, 0.97 ± 0.12, 1.16 ± 0.12,
Kazunori Kawamura, Kazuhiro Shiba, Hideo Tsukada, et al
Annals of Nuclear Medicine
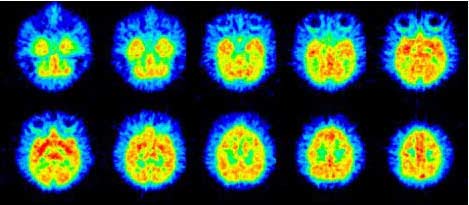
Fig. 3 Ex vivo autoradiography of the coronal sections of the rat brain at 15 minutes after injection of
(−)-[11C]OMV.
Fig. 4 The brain images of (−)-[11C]OMV of a conscious monkey. The PET images of (−)-[11C]OMV
were acquired for 45 minutes starting at 60 minutes after injection.
1.21 ± 0.12, 0.97 ± 0.10 and 1.08 ± 0.12, respectively. As
Metabolite study in rats
shown in Figure 2, the uptake of (−)-[11C]OMV was
Metabolite analysis was carried out in the brain and
significantly decreased by the co-injection of (−)-OMV
plasma at 15 minutes and 30 minutes after injection. The
(34–42% of control), (−)-vesamicol (35–43% of control),
recovery of the radioactivity in the HPLC analysis was
SA4503 (29–44% of control), and haloperidol (36–61%
essentially quantitative. At 15 minutes after injection of
of control) at a dose of 500 nmol/kg in all of the brain
(−)-[11C]OMV, the percentages of the unchanged form in
regions. The blocking effects of (−)-OMV and (−)-
the brain and in the plasma were 96 ± 1.3% and 58 ± 0.5%
vesamicol were equivalent in all of the regions (57–66%
(n = 3), respectively, and at 30 minutes after injection, that
blockade of control), whereas those of each sigma recep-
in the brain and in the plasma were 98% and 58% (n = 1),
tor ligand were smaller in the striatum (56% by SA4503
and 39% by haloperidol) than in the other regions (65–71% by SA4503 and 66–74% by haloperidol). On the
Ex vivo autoradiography in rats
other hand, co-injection of (+)-pentazocine at the dose of
Figure 3 shows the coronal images of the rat brain visual-
500 nmol/kg did not reduce the uptake of (−)-[11C]OMV
ized by ex vivo autoradiography with (−)-[11C]OMV at 15
in any of the brain regions, although the uptake in the
minutes after tracer injection. A slightly higher 11C den-
cerebellum seemed to be reduced. However, pretreatment
sity was observed in the striatum, pyramidal cell layer of
with a large excess of (±)-pentazocine (50 µmol/kg)
the hippocampus, hypothalamus, thalamus and nuclei of
significantly decreased the uptake of (−)-[11C]OMV in all
the cranial motor nerves. A moderate 11C density was
of the brain regions, and the effect was relatively small in
observed in the cortex and amygdaloid.
the striatum (25% reduction) as compared to that in theother regions (50–55%).
PET measurement in the monkey brainFigure 4 shows PET images of (−)-[11C]OMV in the
Vol. 20, No. 6, 2006
Fig. 5 Time-activity curves of the regional brain tissues and the uptake ratio of tissue-to-cerebellum
after intravenous injection of (−)-[11C]OMV (A) and [11C]SA4503 (B) in a conscious monkey using
PET. The radioactivity levels are expressed as the percent of injected dose per ml tissue volume.
monkey brain (images of (−)-[11C]SA4503 not shown24).
the present study is whether (−)-[11C]OMV specifically
The uptake of (−)-[11C]OMV was relatively higher in the
binds to VAChTs (Ki, 6.7 nM),20 but not to sigma1
striatum. In Figure 5, the time-activity curves of (−)-
receptors (Ki for sigma1, 33.7 nM; Ki for sigma2, 266
[11C]OMV and [11C]SA4503 showed different regional
nM)20 in vivo.
distribution patterns. (−)-[11C]OMV showed the highest
The density of VAChTs was the highest in the striatum
uptake in the striatum and lowest uptake in cerebellum,
and low in the cerebellum,11,28,29 whereas sigma1 recep-
while the regional differences in the uptake of (−)-
tors are distributed more uniformly in the brain.30 In a
[11C]SA4503 were small. The binding of the two tracers
blocking study, (−)-OMV, (−)-vesamicol and two sigma1
was reversible, and an apparent equilibrium state was
receptor ligands (SA4503 and haloperidol) having a mod-
found at 20–40 minutes for (−)-[11C]OMV and at 5–15
erate affinity (Ki = 50.2 and 41.4 nM, respectively)20 for
minutes for [11C]SA4503.
VAChT at the dose of 500 nmol/kg significantly reducedthe brain uptake of (−)-[11C]OMV (60–70% of specific
binding), but the same dose of sigma1 receptor ligand, (+)-pentazocine, having a low affinity (Ki = 315 nM)20 for
Synthesis of (−)-[11C]OMV was achieved by a palladium-
VAChT, did not. Haloperidol was sometimes used to
promoted cross-coupling reaction with [11C]methyl
evaluate whether the VAChT radioligands bind to sigma
iodide.22 (−)-[11C]OMV was prepared in a sufficient
receptor.10,12,31 The moderate affinity of haloperidol for
radiochemical yield (38 ± 6.9%) for the in vivo studies.
VAChTs (Ki = 41.4 nM)20; however, reasonably blocked
The specific radioactivity was relatively low (11 ± 7.0
the binding of the radioligands to VAChTs. Previously,
TBq/mmol at 30 minutes after the end of bombardment),
we reported that [3H](+)-pentazocine showed a lower
compared with the other 11C-labeled tracers prepared in
brain uptake and lower specific binding by the modulation
our laboratory, such as [11C]SA4503.23 However, this
of P-glycoproteins.32 Then, we performed a further block-
specific radioactivity is sufficient for the in vivo studies.
ing study by pretreatment with a large excess dose of (±)-
The reason for the low specific radioactivity might be
pentazocine (50 µmol/kg), where the uptake of (−)-
explained by the fact that a cross-coupling between one of
[11C]OMV significantly decreased in all of the brain
the methyl groups on the tin and the nucleoside part can
regions (44–75% of control). A low affinity of (+)-
occur yielding (−)-OMV.26
pentazocine (Ki = 315 nM)20 at the dose of 50 µmol/kg
Vesamicol and its analogs have been reported to bind to
may also block the binding of (−)-[11C]OMV to VAChTs.
VAChTs and sigma receptors.27 The issue addressed in
When comparing the blocking effects among the tissues
Kazunori Kawamura, Kazuhiro Shiba, Hideo Tsukada, et al
Annals of Nuclear Medicine
investigated, the uptake of (−)-[11C]OMV in the striatum
Aid for Creative Scientific Research of the Japan Society for the
was decreased to a lesser extent by the co-injection of
Promotion of Science.
SA4503 (44% of control) and haloperidol (61% of con-trol), as well as by the pretreatment with a large excess of
(±)-pentazocine (75% of control). Although selectiveligands for VAChT or sigma receptors were not applied to
1. Schafer MK, Weihe E, Varoqui H, Eiden LE, Erickson JD.
Distribution of the vesicular acetylcholine transporter
the blocking studies, (−)-[11C]OMV mainly binds to
(VAChT) in the central and peripheral nervous systems of
VAChTs by considering the fact that the striatum has a
the rat. J Mol Neurosci 1994; 5: 1–26.
high density of VAChTs and a low density of sigma1
2. Schafer MK, Weihe E, Erickson JD, Eiden LE. Human and
receptors,30 and a part of the reduction by sigma receptor
monkey cholinergic neurons visualized in paraffin-embed-
ligands reflects the binding of (−)-[11C]OMV to sigma1
ded tissues by immunoreactivity for VAChT, the vesicular
receptors with a moderate affinity (Ki = 34 nM).20 On the
acetylcholine transporter. J Mol Neurosci 1995; 6: 225–
other hand, in the cerebellum, a 70% reduction of uptake
of (−)-[11C]OMV by SA4503 and a lesser reduction by
3. Gilmor ML, Nash NR, Roghani A, Edwards RH, Yi H,
(−)-OMV and (−)-vesamicol reflect that (−)-[11C]OMV
Hersch SM, et al. Expression of the putative vesicular
mainly binds to the sigma
acetylcholine transporter in rat brain and localization in
1 receptors because of the low
density of VAChT. With regard the non-specific binding
cholinergic synaptic vesicles. J Neurosci 1996; 16: 2179–2190.
of (−)-[11C]OMV, 30–35% of the total uptake maximally
4. Weihe E, Tao-Cheng JH, Schafer MK, Erickson JD, Eiden
remained in the blocking study. These levels of the non-
LE. Visualization of the vesicular acetylcholine transporter
specific binding of (−)-[11C]OMV were similar to those of
in cholinergic nerve terminals and its targeting to a specific
[11C]SA4503,24 suggesting that the non-specific binding
population of small synaptic vesicles. Proc Natl Acad Sci
would not hamper the specific signals of the tracer in the
USA 1996; 93: 3547–3552.
human brain by PET.25
5. Bahr BA, Parsons SM. Acetylcholine transport and drug
In an imaging study with ex vivo autoradiography in
inhibition kinetics in Torpedo synaptic vesicles. J Neurochem
rats, the regional brain distribution of (−)-[11C]OMV was
1986; 46: 1214–1218.
similar to that of [3H](−)-vesamicol,33 but not to that of
6. Marshall IG, Parsons SM. The vesicular acetylcholine
[11C]SA4503.23 In the PET measurement of the monkey
transport system. Trends Neurosci 1987; 10: 174–177.
brain, the uptake of (−)-[11C]OMV was relatively higher
7. Kilbourn MR, Jung YW, Haka MS, Gildersleeve DL, Kuhl
DE, Wieland DM. Mouse brain distribution of a carbon-11
in the striatum, being rich in VAChTs, and the regional
labeled vesamicol derivative: presynaptic marker of cholin-
distribution pattern of (−)-[11C]OMV was similar to that
ergic neurons. Life Sci 1990; 47: 1955–1963.
of [18F](+)-4-fluorobenzyltrozamicol as a PET ligand for
8. DeGrado TR, Mulholland GK, Wieland DM, Schwaiger M.
VAChT sites,34 but different from that of [11C]SA4503 as
Evaluation of (−)[18F]fluoroethoxybenzovesamicol as a new
a PET ligand for the sigma1 receptor.24 In the striatum, the
PET tracer of cholinergic neurons of the heart. Nucl Med
density of VAChT was high,29 and that of the sigma1
Biol 1994; 21: 189–195.
receptor was low.35 Therefore, the findings suggest that
9. Rogers GA, Stone-Elander S, Ingvar M, Eriksson L, Par-
the binding of (−)-[11C]OMV to VAChTs is a major
sons SM, Widen L. 18F-labelled vesamicol derivatives:
contribution to the PET images in the striatum.
syntheses and preliminary in vivo small animal positron
In conclusion, (−)-[11C]OMV was prepared in suffi-
emission tomography evaluation. Nucl Med Biol 1994; 21:
cient yield and with specific activity for in vivo studies by
10. Efange SM, Mach RH, Khare A, Michelson RH, Nowak
methylation of (−)-o-trimethylstannyl-vesamicol with
PA, Evora PH. p-[18F]fluorobenzyltrozamicol ([18F]FBT):
[11C]methyl iodide in a palladium-promoted cross-cou-
molecular decomposition-reconstitution approach to
pling reaction. (−)-[11C]OMV showed a high brain uptake
vesamicol receptor radioligands for positron emission to-
and appropriate brain kinetics during the time frame of
mography. Appl Radiat Isot 1994; 45: 465–472.
11C-labeled tracers, and bound mainly to VAChTs; how-
11. Efange SM, Mach RH, Smith CR, Khare AB, Foulon C,
ever, the binding of the tracer to sigma1 receptors due to
Akella SK, et al. Vesamicol analogues as sigma ligands.
a low affinity (Ki for sigma1, 33.7 nM)20 was not disre-
Molecular determinants of selectivity at the vesamicol
garded. Therefore, (−)-[11C]OMV-PET together with help
receptor. Biochem Pharmacol 1995; 49: 791–797.
of [11C]SA4503-PET may evaluate VAChTs.
12. Mulholland GK, Wieland DM, Kilbourn MR, Frey KA,
Sherman PS, Carey JE, et al. [18F]fluoroethoxy-benzo-vesamicol, a PET radiotracer for the vesicular acetylcho-
line transporter and cholinergic synapses. Synapse 1998;30: 263–274.
The authors thank Dr. QingGeLeTu for his technical assistance
13. Efange SM, Nader MA, Ehrenkaufer RL, Khare AB, Smith
in the autoradiography study, and also thank the staff of Positron
CR, Morton TE, et al. (+)-p-([18F]fluorobenzyl)spiro-
Medical Center, Tokyo Metropolitan Institute of Gerontology
trozamicol [(+)-[18F]spiro-FBT]: synthesis and biological
and the staff of Central Research Laboratory, Hamamatsu
evaluation of a high-affinity ligand for the vesicular acetyl-
Photonics K.K. This work was supported in part by Grant-in-
choline transporter (VAChT). Nucl Med Biol 1999; 26:
Vol. 20, No. 6, 2006
24; 745–752.
14. Jung YW, Van Dort ME, Gildersleeve DL, Wieland DM. A
25. Mishina M, Ishiwata K, Ishii K, Kitamura S, Kimura Y,
radiotracer for mapping cholinergic neurons of the brain. J
Kawamura K, et al. Function of sigma1 receptors in
Med Chem 1990; 33: 2065–2068.
Parkinson's disease. Acta Neulologica 2005; 112: 103–107.
15. Efange SM, Michelson RH, Khare AB, Thomas JR. Synthe-
26. Samuelsson L, Långström B. Synthesis of 1-(2′-deoxy-2′-
sis and tissue distribution of (m-[125I]iodobenzyl)trozamicol
([125I]MIBT): potential radioligand for mapping central
([11C]FMAU) via a still cross-coupling reaction with
cholinergic innervation. J Med Chem 1993; 36: 1754–1760.
[11C]methyl iodide. J Label Compd Radiopharm 2003; 46:
16. Shiba K, Mori H, Matsuda H, Tsuji S, Kuji I, Sumiya H,
et al. Synthesis of radioiodinated analogs of 2-(4-phenyl-
27. Custers FG, Leysen JE, Stoof JC, Herscheid JD. Vesamicol
piperidino)cyclohexanol (vesamicol) as vesamicol-like
and some of its derivatives: questionable ligands for selec-
agent. Nucl Med Biol 1995; 22: 205–210.
tively labelling acetylcholine transporters in rat brain. Eur
17. Khare AB, Langason RB, Parsons SM, Mach RH, Efange
J Pharmacol 1997; 338: 177–183.
SM. N-(3-Iodophenyl)trozamicol (IPHT) and related in-
28. Frey KA, Wieland DM, Kilbourn MR. Imaging of monoam-
hibitors of vesicular acetylcholine transport: synthesis and
inergic and cholinergic vesicular transporters in the brain.
preliminary biological characterization. Nucl Med Biol 1999;
Adv Pharmacol 1998; 42: 269–272.
26: 609–617.
29. Efange SM. In vivo imaging of the vesicular acetylcholine
18. Bando K, Taguchi K, Ginoza Y, Naganuma T, Tanaka Y,
transporter and the vesicular monoamine transporter. FASEB
Koike K, et al. Synthesis and evaluation of radiolabeled
J 2000; 14: 2401–2413.
piperazine derivatives of vesamicol as SPECT agents for
30. Bouchard P, Quirion R. [3H]1,3-di(2-tolyl)guanidine and
cholinergic neurons. Nucl Med Biol 2001; 28: 251–260.
[3H](+)pentazocine binding sites in the rat brain: autoradio-
19. Shiba K, Yano T, Sato W, Mori H, Tonami N. Characteriza-
graphic visualization of the putative sigma1 and sigma2
tion of radioiodinated (−)-ortho-iodovesamicol binding in
receptor subtypes. Neuroscience 1997; 76: 467–477.
rat brain preparations. Life Sci 2002; 71: 1591–1598.
31. Efange SM, von Hohenberg K, Khare AB, Tu Z, Mach RH,
20. Shiba K, Ogawa K, Ishiwata K, Yajima K, Mori H. Synthe-
Parsons SM. Synthesis and biological characterization of
sis and binding affinities of methyl vesamicol analogs for
stable and radioiodinated (+/−)-trans-2-hydroxy-3-P[4-(3-
the acetylcholine transporter and sigma receptor. Bioorg
Med Chem 2006; 14: 2620–2626.
IBVM). Nucl Med Biol 2000; 27: 749–755.
21. Mach RH, Voytko ML, Ehrenkaufer RL, Nader MA, Tobin
32. Kawamura K, Kobayashi T, Matsuno K, Ishiwata K. Differ-
JR, Efange SM, et al. Imaging of cholinergic terminals
ent brain kinetics of two sigma1 receptor ligands, [3H](+)-
using the radiotracer [18F](+)-4-fluorobenzyltrozamicol: in
pentazocine and [11C]SA4503, by P-glycoprotein modula-
vitro binding studies and positron emission tomography
tion. Synapse 2003; 48: 80–86.
studies in nonhuman primates. Synapse 1997; 25: 368–380.
33. Shiba K, Mori H, Matsuda H, Tsuji S, Tonami N, Hisada K.
22. Suzuki M, Doi H, Björkman M, Andersson Y, Långström B,
In vivo characterization of radioiodinated 2-(4-phenyl-
Watanabe Y, et al. Rapid coupling of methyl iodide with
piperidino)cyclohexanol (vesamicol) analogs: potential
aryltributylstannanes mediated by palladium(0) complexes:
radioligand for mapping presynaptic cholinergic neurons.
a general protocol for the synthesis of 11CH3-labeled PET
Nucl Med Biol 1995; 22: 823–828.
tracers. Chem Eur J 1997; 3: 2039–2042.
34. Gage HD, Voytko ML, Ehrenkaufer RL, Tobin JR, Efange
23. Kawamura K, Ishiwata K, Tajima H, Ishii S, Matsuno K,
SM, Mach RH. Reproducibility of repeated measures of
Homma Y, et al. In vivo evaluation of [11C]SA4503 as a PET
cholinergic terminal density using [18F](+)-4-fluorobenzyl-
ligand for mapping CNS sigma1 receptors. Nucl Med Biol
trozamicol and PET in the rhesus monkey brain. J Nucl Med
2000; 27: 255–261.
2000; 41: 2069–2076.
24. Kawamura K, Kimura Y, Tsukada H, Kobayashi T,
35. Mash DC, Zabetian CP. Sigma receptors are associated
Nishiyama S, Kakiuchi T, et al. An increase of sigma1
with cortical limbic areas in the primate brain. Synapse
receptors in the aged monkey brain. Neurobiol Aging 2003;
1992; 12: 195–205.
Kazunori Kawamura, Kazuhiro Shiba, Hideo Tsukada, et al
Annals of Nuclear Medicine
Source: http://mail.hobbycook.jp/files/paper/anm/ams206/ANM20-6-06.pdf
GUIDELINES FOR THE MANAGEMENT OF THE INFANT WITH NEONATAL ABSTINENCE SYNDROME Background Neonatal Abstinence Syndrome (NAS) is a syndrome of drug withdrawal observed in infants of mothers physically dependent on drugs. Also known as neonatal withdrawal syndrome or passive addiction, NAS is a condition resulting from exposure in utero or postnatal exposure to opioids and other illicit drugs. It is more common in infants born to opioid-dependent women than in infants born to women dependent on other drugs or alcohol.1
Bull Cancer 2004 ; 91 (5) : E 81-112 Resistance to microtubule-binding agents Resistance to Microtubule-Targeted Cytotoxins in a K562 Leukemia Cell Variant Associated with Altered Tubulin Expression and Polymerization Charles Dumontet, Jean-Pierre Jaffrezou, Etsuko Tsuchiya, George E. Duran, Gang Chen, W. Brent Derry, Leslie Wilson, Mary Ann Jordan, and Branimir I. Sikic

