Oconnell.fas.harvard.edu
Social Status Predicts How Sex Steroid Receptors
Regulate Complex Behavior across Levels of
Biological Organization
Lauren A. O'Connell and Hans A. Hofmann
Institutes for Cellular and Molecular Biology (L.A.O., H.A.H.) and Neuroscience (H.A.H.) and Section ofIntegrative Biology (L.A.O., H.A.H.), The University of Texas at Austin, Austin, Texas 78712
Social status strongly affects behavior and physiology, in part mediated by gonadal hormones,
although how each sex steroid acts across levels of biological organization is not well understood.
We examine the role of sex steroids in modulating social behavior in dominant (DOM) and sub-
ordinate (SUB) males of a highly social fish,
Astatotilapia burtoni. We first used agonists and
antagonists to each sex steroid receptor and found that androgens and progestins modulate
courtship behavior only in DOM, whereas estrogens modulate aggressive behavior independent
of social status. We then examined the hormonal and physiological responses to sex steroid re-
ceptor antagonist treatment and uncovered substantial changes in circulating steroid hormone
levels and gonad size only in SUB, not in DOM. Consistent with status-based physiological sensi-
tivities to drug manipulation, we found that neuropeptide and steroid receptor gene expression
in the preoptic area was sensitive only in SUB. However, when we compared the transcriptomes of
males that received either vehicle or an estrogen receptor antagonist, 8.25% of all genes examined
changed expression in DOM in comparison with only 0.56% in SUB. Finally, we integrate behavior,
physiology, and brain gene expression to infer functional modules that underlie steroid receptor
regulation of behavior. Our work suggests that environmentally induced changes at one level of
biological organization do not simply affect changes of similar magnitude at other levels, but that
instead very few key pathways likely serve as conduits for executing plastic responses across mul-
tiple levels.
(Endocrinology 153: 1341–1351, 2012)
individual's social status. To function in a social
ment, physiology, and molecular responses in the nervous
group, every group member integrates information about
system. Thus, our present understanding of how physiol-
its own internal physiological state with external social
ogy, brain gene expression, and social environment regu-
information into a behavioral output that ultimately pro-
late behavior in social groups is still limited.
motes its fitness. Although behavioral and hormonal re-
Sex steroid hormones are excellent candidates for me-
sponses within social groups are relatively well under-
diating the integration of external and internal informa-
stood in mammals and fish (1–5), researchers have only
tion into an adaptive behavioral response. These hor-
recently begun to examine the molecular events in the
mones vary across many physiological contexts such as
brain that mediate the behavioral responses of individuals
sex, social status, breeding condition, and season (6 –10).
in different social states (6, 7). Despite the importance of
Such variability is important in regulating an animal's be-
integrating external and internal signals within the brain
havioral response to both reproductive and aggressive
into subsequent behavioral changes, studies investigating
contexts (11). Circulating hormones robustly respond to
this integration are limited due to the general difficulty of
social stimulation and have been extensively studied in the
ISSN Print 0013-7227
ISSN Online 1945-7170
Abbreviations: AR, Androgen receptor; AVT, arginine vasotocin; CA, cyproterone acetate;
Printed in U.S.A.
DHT, dihydrotestosterone; DOM, dominant; ER, estrogen receptor; gbw, gram body
Copyright 2012 by The Endocrine Society
weight; GEE, generalized estimating equations; GSI, gonadosomatic index; IST, isotocin;
doi: 10.1210/en.2011-1663 Received August 22, 2011. Accepted November 23, 2011.
11KT, 11-ketotestosterone; 17␣-20-P, 17␣-20-dihydroprogesterone; POA, preoptic area; PR,
First Published Online December 13, 2011
progesterone receptor; qPCR, quantitative PCR; SUB, subordinate.
For editorial see page 1001
Endocrinology, March 2012, 153(3):1341–1351
O'Connell and Hofmann
Social Status Predicts Steroid Hormone Action
Endocrinology, March 2012, 153(3):1341–1351
context of the androgen challenge response to aggressive
compliance with the Institutional Animal Care and Use Com-
interactions, a physiological response conserved across
mittee at The University of Texas at Austin.
vertebrates (11, 12). Sex steroids integrate these social and
Behavior and pharmacology
physiological cues into a molecular response by acting
Male
A. burtoni chosen for this study were stable in social
through either nuclear hormone receptors, which function
status for 1 wk before injections. Only one animal per tank was
as transcription factors and modulate gene expression (re-
manipulated at a given time. Animals were injected ip with 10 l
viewed in Ref. 13), or more quickly through membrane-
mineral oil per gram body weight (gbw) for 2 d (to allow for
bound receptors that trigger a signal transduction cascade
within-individual comparisons) and then with a sex steroid re-
ceptor agonist or antagonist for the next 2 d. Fish were imme-diately placed back into the home tank after injection. One group
To study the proximate mechanisms of male-typical
of males used as the control group for all end-point analyses were
behavior in a social hierarchy, we use the African cichlid
injected with vehicle for all 4 d. Five-minute focal observations
fish,
Astatotilapia burtoni, a model system in social neu-
were conducted between 0800 and 1100 h the following morn-
roscience and behavioral genomics (16, 17).
A. burtoni
ing of each day after injections and for 2 d after injections ended
males have two plastic behavioral phenotypes. Brightly
(Supplemental Fig. 1, published on The Endocrine Society's Jour-nals Online web site at http://endo.endojournals.org) by an ob-
colored dominant (DOM) males aggressively defend ter-
server blind to the treatment. This observation paradigm avoided
ritories where they court and spawn with females. Subor-
nongenomic fast-acting effects of steroid hormones (14). Social
dinate (SUB) males are dull in coloration, reproductively
behaviors recorded are described elsewhere (22) and include ag-
suppressed, and school with females. The brain gene ex-
gressive (chasing and biting), sexual (quivering and leading), ter-
pression profiles of these two phenotypes differ substan-
ritorial (border dispute, carousel, and threats), and subordinate
tially (18). Given the opportunity, SUB will attain DOM
(fleeing) displays.
We chose the following doses based on a dose-response ex-
status in an astonishing transition that is accompanied by
periment in DOM (Supplemental Fig. 2) where three doses of
a concerted change in gene expression in brain and testes
each drug were tested to see which had the largest effect on
as well as a rapid response in circulating steroid hormones
aggressive and/or courtship behavior: 0.4 g/gbw 17-estradiol
(6). However, we know relatively little about how sex
(Steraloids, Newport, RI; n ⫽ 8 SUB and n ⫽ 10 DOM), 0.13
steroid hormone receptors mediate behavior in relation to
g/gbw dihydrotestosterone (DHT; Sigma Chemical Co., St.
Louis, MO; n ⫽ 8 per social status), 0.125 g/gbw 17␣-20-
social status and across levels of biological organization
dihydroprogesterone (17␣-20-P; Steraloids; n ⫽ 8 per social
(including,
e.g. behavior, physiology, and brain gene
status), 1.6 g/gbw ICI182780 (ER antagonist; Sigma; n ⫽ 8 per
social status), 0.83 g/gbw cyproterone acetate (AR antagonist;
We examined the differential role of sex steroid hor-
Sigma; n ⫽ 8 per social status), and 1.6 g/gbw ZK0112993 (PR
mones in the regulation of behavior in these two social
antagonist; Bayer Schering Pharma AG, Berlin, Germany; n ⫽ 8per social status). Dose-response curves were not conducted in
phenotypes with the hypotheses that 1) steroid hormone
SUB, because social suppression by DOM males may mask any
receptors mediate distinct behavioral components and 2)
effect of a drug, and isolating SUB for testing will result in a
the gene regulatory actions of sex steroid hormone recep-
transition to DOM status within hours, a period too short to
tors differ with social status. We first determined how
conduct a dose-response curve manipulating nuclear hormone
androgen receptors (AR), estrogen receptors (ER), and the
receptors. DHT was used, rather than the teleost-specific 11-ketotestosterone (11KT), because DHT binds AR with higher
progesterone receptor (PR) modulate social behavior and
affinity than 11KT (23, 24). All antagonists have been shown to
circulating hormone levels in DOM and SUB within a
bind their respective teleost sex steroid receptors [ICI182780
community setting. Additionally, we examined steroid re-
binds all three ER (25); cyproterone acetate binds both AR (23);
ceptor regulation of gene expression in the preoptic area
and ZK0112993 binds PR (26)].
(POA), a brain region that regulates male aggressive and
On the last day of behavioral observations, we recorded
weight and length of each individual that received an antagonist
sexual behaviors in all vertebrates (19, 20).
or only vehicle and drew blood from the dorsal aorta using hep-arinized 26-gauge butterfly infusion sets (Becton Dickinson,Mountain View, CA). Gonads were removed and weighed for
Materials and Methods
calculation of the gonadosomatic index (GSI: testes mass dividedby body mass times 100). Brains were rapidly dissected, embed-
ded in Tissue-Tek OCT Compound (Sakura Finetek U.S.A., Tor-
A. burtoni from a wild-caught stock population were main-
rance, CA), fresh frozen on dry ice, and stored at ⫺80 C.
tained in a naturalistic community as previously described (21)with eight males and eight females per 110-liter tank. A total of
34 DOM (length, 4.877 ⫾ 0.1264 cm; weight, 3.341 ⫾ 0.2851
Free testosterone, 17-estradiol, and progesterone were mea-
g) and 32 SUB (length, 4.615 ⫾ 0.0851 cm; weight, 2.486 ⫾
sured for each individual using ELISA (Enzo Life Sciences, Farm-
0.1289 g) were used in this study. All work was carried out in
ingdale, NY); Intraassay variation was 9.43, 2.67, and 4.06%,
Endocrinology, March 2012, 153(3):1341–1351
respectively; interassay variation was 4.96, 3.38, and 10.5%,
structed from brain-specific and mixed tissue libraries represent-
respectively. Plasma samples were diluted 1:30 and processed
ing a total of 17,712 cichlid-specific features (30, 69). DOM and
as previously described (27).
SUB males were examined in separate loops, and each array wascompetitively hybridized with a vehicle- or ER antagonist-
Laser microdissection and RNA isolation
treated sample. Two technical replicates were performed, where
Brains were sectioned at 18 m, thaw mounted onto mem-
each sample was labeled with both Cy3 and Cy5 to control for
brane-covered slides (P.A.L.M. Microlaser Technologies AG,
dye bias. The slides were scanned on an Axon 4000B array scan-
Bernried, Germany), and hydrated for 1 min in cold 95, 70, and
ner using GenePix version 6.0 software (Molecular Devices,
50% ethanol, stained with toluidine blue (0.5% toluidine blue,
Sunnyvale, CA) and filtered for microarray printing errors, hy-
1% phenol, 20% ethanol in water) for 2 min, rapidly dehydrated
bridization artifacts, and signals with low intensity. Intensities
in an ascending ethanol series, incubated in xylene for 5 min, and
were lowess-normalized within arrays in R/Bioconductor; only
air dried for 2 min. Sections were visualized on a laser-micro-
features with average intensities 2 SD or more above average
dissection microscope (P.A.L.M. Microlaser Technologies), and
background were considered for further analysis (18). All raw
an area corresponding to the POA (including parvocellular, mag-
and processed data are available at the GEO database (accession
nocellular, and some gigantocellular cells) (28) was excised and
number GSE28508).
captured with the laser (Supplemental Fig. 3). Some technicalvariation likely resulted from the capture of the gigantocellular
POA due to the sporadic distribution of these neurons. Thirty
All analyses, with the exception of the microarray data, were
microliters of Trizol (Invitrogen, Carlsbad, CA) was added to the
conducted in PASW (IBM, Somers, NY). To determine differ-
sample and stored at ⫺80 C until further processing. Total RNA
ences between DOM and SUB,
t tests were used with vehicle-
was isolated with Trizol according to the manufacturer's
treated animals. In all other cases, statistics for DOM and SUB
were run separately. For behavioral data, a generalized estimat-ing equations (GEE) (31) model was used with the behavioral
RNA amplification, reverse transcription, and
measure as the dependent variable, and baseline behavior (d 1
quantitative PCR (qPCR)
and 2) was compared with drug treatment (d 4 – 6) as a within-subject variable; the model was run separately for each drug
Isolated RNA was subjected to one round of amplification
treatment. Hormone measurements and qPCR data were not
using the MessageAmp II system (Ambion, Austin, TX) accord-
normally distributed and were log-transformed, resulting in nor-
ing to manufacturer's instructions. Additionally, samples from
mally distributed residuals. Hormone data were analyzed with
animals that received either vehicle only or ER antagonist treat-
ANOVA using hormone as the dependent variable and drug as
ment were divided after the first round of amplification, with one
the independent variable followed by a Bonferroni
post hoc test.
half being immediately reverse transcribed and the other half
qPCR gene expression data from the experimental groups were
processed for another round of RNA amplification to be used for
compared with the control gene expression levels using ANOVA
microarray analysis. Each RNA sample was treated with deoxy-
followed by Dunnett's
t test, which corrects for multiple testing.
ribonuclease I (Ambion) according to the manufacturer's in-
Significance was considered as
P ⬍ 0.05. Because most of our
structions. The RNA was reverse transcribed using Superscript
data were nonparametric, we calculated correlations using
III reverse transcriptase (Invitrogen) and gene-specific primers
Spearman rank correlation coefficients to explore covariation
(see Supplemental Table 1). Positive controls used 1 ng of whole-
patterns. The microarray data were analyzed in R/Bioconductor.
brain RNA in place of RNA derived from laser-microdissected
Between-group analyses were conducted using the LIMMA
samples and in negative controls the reverse transcriptase was
package (18, 32).
omitted. Excess primers and salts from the transcription reactionwere removed in Microcon YM30 columns (Millipore, Bedford,MA). An aliquot of the sample was used for Ribogreen (Invit-rogen) analysis to determine total cDNA concentration of each
sample. qPCR primers (Supplemental Table 1) were designed toflank exon boundaries using the zebrafish genome as a reference.
Distinct roles of sex steroids in a social hierarchy
For each sample, target gene abundance was measured in trip-
We used a within-subject design by treating DOM and
licate in an ABI PRISM 7900HT real-time PCR cycler (ABI SDS
SUB with specific sex steroid receptor agonists or antag-
version 2.2.1 software) using SYBR Green (Invitrogen). Stan-
onists (Supplemental Fig. 1) and found that sex steroid
dard curves were constructed using known dilutions of cDNA,and amplification efficiency was calculated. For each individual,
receptors differentially modulate aggression and court-
median values from the reference and target gene triplicates were
ship in the two phenotypes (Fig. 1). Although teleost fish
used to calculate the relative transcript abundance of the target
have a single ER␣ and PR, there are two isoforms of AR
gene using the mean normalized expression formula of Simon
(AR␣ and AR) and ER (ERa and ERb) due to a ge-
(29). Each sample was normalized to total cDNA as measured by
nome duplication event in this lineage (33). We selected
drugs that targeted all three ER isoforms or both ARisoforms.
Microarray samples were prepared as previously described
Manipulation of ER changed aggressive behavior, but
(18) and hybridized in a loop design (Supplemental Fig. 4) to a
not courtship behavior, independent of social phenotype.
19K
A. burtoni microarray (GEO platform GPL6416) con-
DOM males display two kinds of aggression: chases are
O'Connell and Hofmann
Social Status Predicts Steroid Hormone Action
Endocrinology, March 2012, 153(3):1341–1351
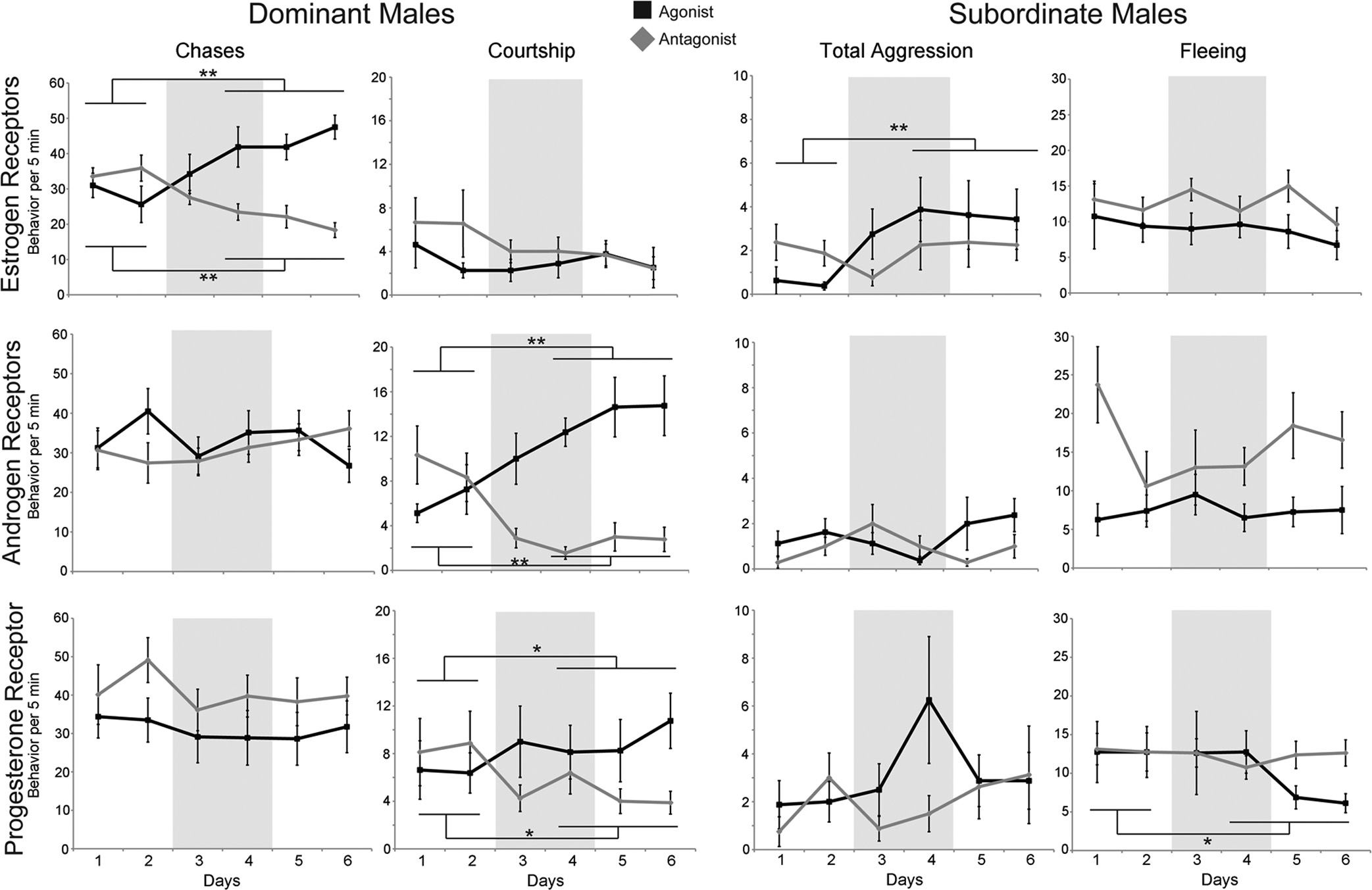
FIG. 1. Effects of sex steroid receptor manipulation vary by social status. The mean behavioral observations of animals given ER (top row), AR
(middle row), or PR (bottom row) manipulations are shown with agonist (black lines) and antagonist (gray lines) treatments for DOM and SUB
males (n ⫽ 8 per status per treatment). Behavior per 5 min is represented on the vertical axis, and day of treatment is on the horizontal axis. Gray
background shading indicates days of drug administration. Data are represented as the mean ⫾ SEM; GEE: **, P ⬍ 0.0001; *, P ⬍ 0.05.
typically directed toward SUB and females in the school,
gressive behavior even under control conditions. ER ma-
whereas territorial defense behavior is typically directed
nipulation did not affect fleeing behavior in SUB (GEE:
toward other DOM in the community. Estradiol treat-
estradiol, P ⫽ 0.24; Wald 2 ⫽ 1.382; ICI182780, P ⫽
ment increased chases in DOM (GEE, P ⫽ 9.3 ⫻ 10⫺9;
0.86; Wald 2 ⫽ 0.033). Courtship and territorial defense
Wald 2 ⫽ 32.984), whereas treatment with the ER-spe-
behavior are almost never observed in SUB and thus are
cific antagonist ICI182780 decreased chases (GEE, P ⫽
not reported.
1.4 ⫻ 10⫺6; Wald 2 ⫽ 23.284). Estradiol treatment also
AR manipulations exclusively affected courtship be-
decreased territorial defense behavior (GEE, P ⫽ 0.045;
havior and had no effect on aggressive behavior in either
Wald 2 ⫽ 4.036; data not shown), although ICI182780
social phenotype. Instead of the teleost-specific androgen
treatment had no effect (GEE, P ⫽ 0.283; Wald 2 ⫽
11KT, we used the nonaromatizable androgen DHT for
1.151; data not shown). ER manipulations in DOM did
pharmacological manipulation of AR, because both 11KT
not affect courtship displays (GEE: estradiol, P ⫽ 0.864;
and DHT produce similar effects in other teleosts (34, 35),
Wald 2 ⫽ 0.03; ICI182780, P ⫽ 0.06; Wald 2 ⫽ 3.539),
yet DHT has a higher binding affinity to both AR␣ and
although there is a nonsignificant trend for the antagonist
AR (23, 24). DOM treated with DHT increased court-
to decrease courtship displays. Aggressive displays (a sum
ship displays (GEE, P ⬍ 0.0002; Wald 2 ⫽ 13.794),
of chases and bites) were also increased in SUB treated
whereas treatment with an AR antagonist, cyproterone
with estradiol (GEE, P ⫽ 1.8 ⫻ 10⫺4; Wald 2 ⫽ 13.993),
acetate (CA), decreased courtship behavior (GEE, P ⫽
which was unexpected given the severe social suppression
8.5 ⫻ 10⫺6; Wald 2 ⫽ 19.823). Manipulation of AR did
SUB are subjected to by DOM. Not surprisingly, treat-
not alter chases (GEE: DHT, P ⫽ 0.22; Wald 2 ⫽ 1.502;
ment of SUB with an ER antagonist did not result in a
CA, P ⫽ 0.20; Wald 2 ⫽ 1.624) or territorial defense be-
behavioral change (GEE, P ⫽ 0.8; Wald 2 ⫽ 0.064), pos-
havior (GEE: DHT, P ⫽ 0.20; Wald 2 ⫽ 1.653; CA, P ⫽
sibly due to a floor effect, because SUB rarely display ag-
0.09; Wald 2 ⫽ 2.890; data not shown) in DOM. Similarly,
Endocrinology, March 2012, 153(3):1341–1351
no behavioral change in total aggression (GEE: DHT, P ⫽0.55; Wald 2 ⫽ 0.360; CA, P ⫽ 0.55; Wald 2 ⫽ 0.355) orfleeing (GEE: DHT, P ⫽ 0.81; Wald 2 ⫽ 0.058; CA, P ⫽0.75; Wald 2 ⫽ 0.100) was observed in SUB.
PR manipulations showed effects similar to those of
AR, as we observed changes in courtship behavior dis-played by DOM. We used 17␣-20-P as a PR agonistbecause it cannot be converted readily into testosteroneand has higher binding affinity to the teleost PR than pro-gesterone (24). DOM treated with 17␣-20-P increasedcourtship displays (GEE, P ⫽ 0.040; Wald 2 ⫽ 4.233),whereas the PR antagonist ZK112993 decreased court-ship displays (GEE, P ⫽ 0.041; Wald 2 ⫽ 4.168). PRmanipulation in DOM did not affect chasing (GEE: 17␣-20-P, P ⫽ 0.148; Wald 2 ⫽ 2.091; ZK112993, P ⫽0.053; Wald 2 ⫽ 3.751) or territorial defense behavior(GEE: 17␣-20-P, P ⫽ 0.08; Wald 2 ⫽ 3.100;ZK112993, P ⫽ 0.752; Wald 2 ⫽ 0.100; data notshown). Similar to DOM, total aggression in SUB did notchange with PR manipulation (GEE: 17␣-20-P, P ⫽0.14; Wald 2 ⫽ 2.173; ZK112993, P ⫽ 0.68; Wald 2 ⫽0.172). Interestingly, SUB treated with 17␣-20-P dis-played less fleeing behavior (GEE, P ⫽ 0.001; Wald 2 ⫽11.195), but their behavior did not change after treatmentwith the PR antagonist (GEE, P ⫽ 0.52; Wald 2 ⫽ 0.409).
Dissociation of behavior from hormones and
physiology
To investigate whether our manipulations of sex ste-
roid receptor signaling affected circulating levels of sexsteroid hormones in relation to social status, we measuredfree circulating 17-estradiol, testosterone, and proges-terone in the plasma of animals that received a receptor
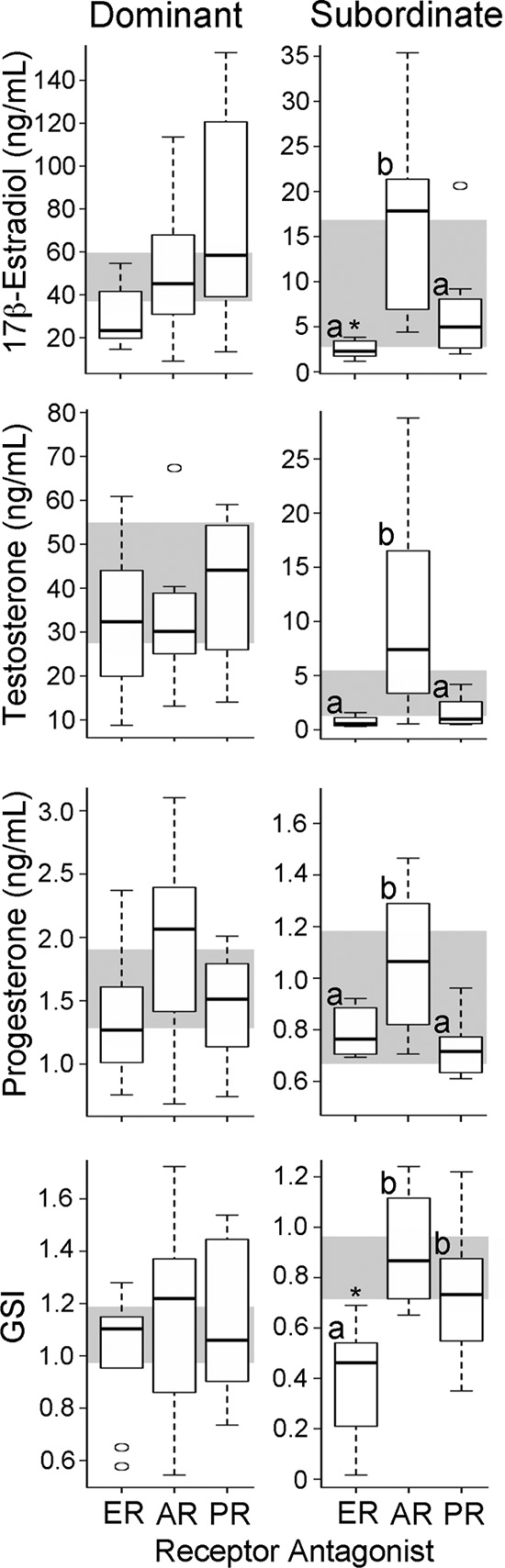
FIG. 2. Physiology and circulating hormone levels change in SUB but
antagonist or vehicle only (Fig. 2); individuals that re-
not DOM males after steroid receptor manipulation. Free 17-estradiol, testosterone, and progesterone levels, as well as GSI, are
ceived an agonist were not used for hormone measure-
depicted by box and whisker plots for DOM (left panels) and SUB (right
ments or gene expression studies. As expected, control
panels) males (n ⫽ 8 per status per treatment). Each box represents
DOM had higher circulating testosterone (t
groups receiving a sex steroid receptor antagonist (x-axis), and the gray
3.7; P ⫽
0.005) and 17-estradiol (t ⫽
background bar depicts the first and third quartiles for control animals.
3.8; P ⫽ 0.003) levels than
Letters indicate significance between groups; *, significant difference
control SUB as previously reported (36). Progesterone lev-
from control with Bonferroni post hoc test.
els did not differ between social states, although there wasa nonsignificant trend to higher levels in DOM (t
progesterone, F(3,30)
3.471, P ⫽ 0.03]. Specifically, SUB
2.053; P ⫽ 0.065). Surprisingly, we found that in DOM
treated with ER antagonists had lower levels of 17-es-
treated with a receptor antagonist, circulating levels did
tradiol (Dunnett's t test P ⫽ 0.017) and testosterone (Dun-
not change for any of the sex steroids compared with con-
nett's t test P ⫽ 0.032) compared with controls. Thus, on
trols [ANOVA: 17-estradiol, F
1.796, P ⫽ 0.169;
a physiological level, SUB responded even though they
0.289, P ⫽ 0.83; progesterone,
showed little behavioral response to steroid receptor ma-
0.289, P ⫽ 0.436], despite the clear behavioral
nipulations within a social community.
responses we observed to receptor antagonist manipula-
The GSI, a measure of relative testes mass, was higher in
tion. Even more striking, SUB displayed distinct changes in
control DOM compared with control SUB (t
2.3; P ⫽
circulating hormone levels with treatment of receptor an-
0.039). Steroid receptor antagonists did not affect the GSI of
tagonists [ANOVA: 17-estradiol, F
7.106, P ⫽
0.231; P ⫽ 0.853], yet in SUB, ER antagonist
4.23 ⫻ 10⫺4; testosterone, F
7.106, P ⫽ 0.001;
treatment resulted in a significant reduction in GSI [F(3,32)
O'Connell and Hofmann
Social Status Predicts Steroid Hormone Action
Endocrinology, March 2012, 153(3):1341–1351
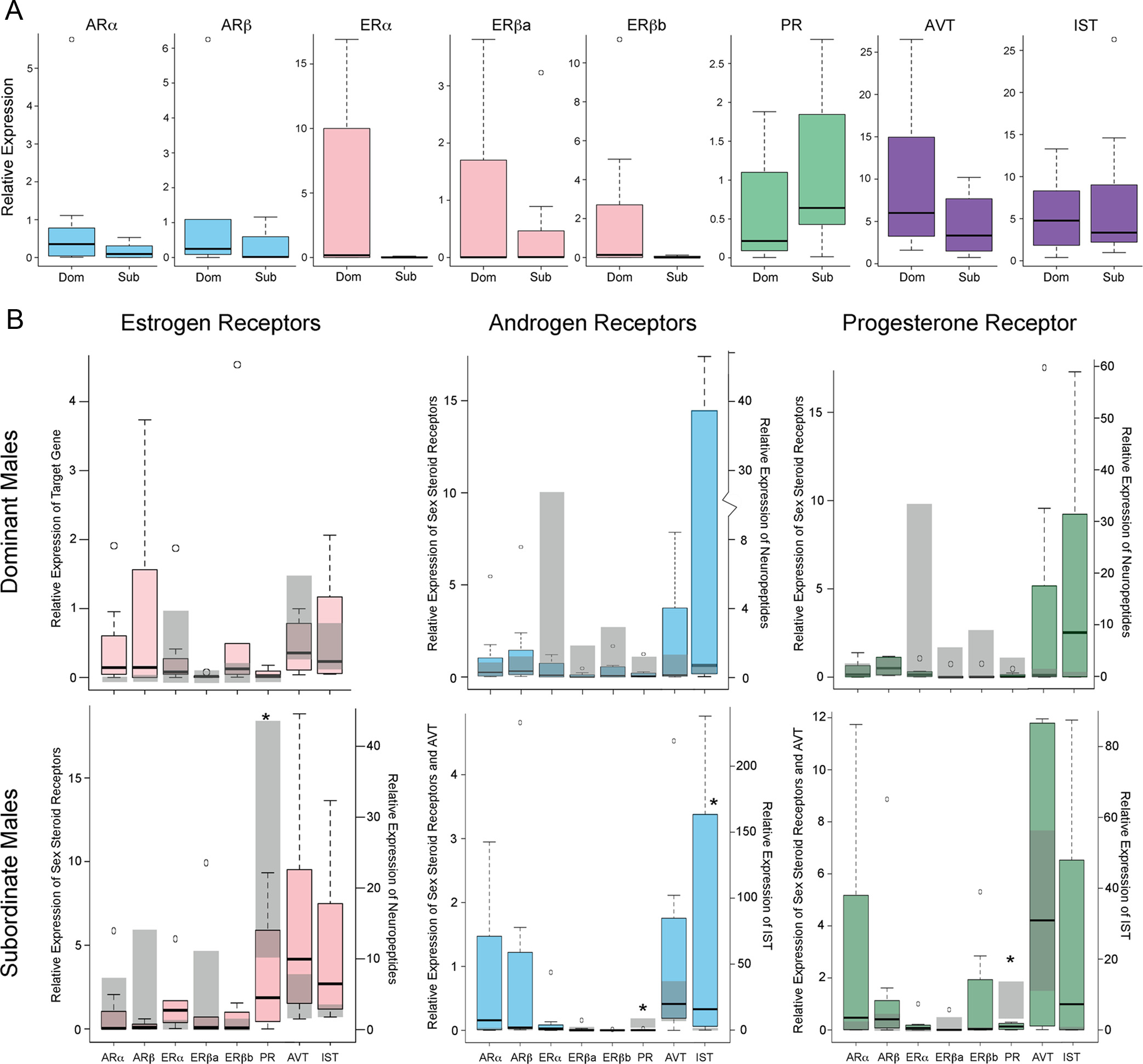
FIG. 3. Expression of candidate genes in the POA. A, Expression levels of steroid hormone receptors and neuropeptides in control treated DOM
and SUB males are shown as box and whisker plots. B, Changes in gene expression after treatment of an ER (left panel), AR (middle panel), or PR
(right panel) antagonist in DOM (top row) and SUB (bottom row) males is shown with colored box and whisker plots. Gray background bars depict
the first and third quartiles for control animals. *, P ⬍ 0.05, Dunnett's t test.
sured mRNA levels of all sex steroid receptors as well as
SEM: ICI182780 0.391 ⫾ 0.080; control 0.872 ⫾ 0.066], a re-
the nonapeptides arginine vasotocin (AVT) and isotocin
markable physiological change in such a short time.
(IST), because all these neuroendocrine pathways playimportant roles in modulating social behavior across
Sex steroid receptor-mediated gene expression
vertebrates (reviewed in Ref. 37).
impinges on social status
To our surprise, control and experimental groups of
To better understand how sex steroid receptors reg-
DOM did not differ in the expression of any of these
ulate social behavior in a hierarchy, we used qPCR to
candidate genes despite the robust changes in social be-
analyze the expression of these receptors and neuro-
havior we had observed, whereas in SUB, mRNA levels
peptides in the POA of males that received either a sex
of several candidate genes differed significantly be-
steroid receptor antagonist or vehicle (Fig. 3). We mea-
tween control and experimental groups. These results
Endocrinology, March 2012, 153(3):1341–1351
are consistent with our findings described above of an
An even more striking picture emerged in SUB, where
apparent dissociation, at least in DOM, of the behav-
the P value distribution was surprisingly devoid of small
ioral from the physiological responses to sex steroid
values (Supplemental Fig. 5B), suggesting a genome-
antagonist treatment. However, it should be noted that
wide suppression of expression variation in this pheno-
microdissection of the POA did not include all gigan-
type. At a P ⬍ 0.05 threshold, only 48 (0.59%) of the
tocellular neurons and thus may have masked potential
features were differentially regulated between the con-
differences (see also Ref. 34). In SUB, PR and IST levels
trol and ER antagonist groups (LIMMA, P ⬍ 0.05),
were sensitive to antagonist treatment [PR: ANOVA,
even though SUB demonstrated significant changes in
5.321 P ⫽ 0.006; IST: ANOVA: F(3,28)
physiological and candidate gene expression measures,
3.820, P ⫽ 0.022]. Specifically, SUB given an ER, AR,
as described above. Finally, the POA transcriptome re-
or PR antagonist decreased expression of PR (Dunnett's
sponses to ER perturbation showed very little overlap
t test: ER antagonist P ⫽ 0.038; AR antagonist P ⫽
between DOM and SUB, because only four genes were
0.005; PR antagonist P ⫽ 0.006), whereas expression of
regulated at P ⬍ 0.05 by ER in both datasets (Supple-
IST increased after exposure to the AR antagonist (Dun-
mental Table 2). These sequences represent novel genes
nett's t test P ⫽ 0.02).
for which no annotations could be found in the
ER regulation of the social transcriptome
Because perturbation of ER exhibited consistent and
significant effects on aggression in both DOM and SUB,
Integration of genes, physiology, and behavior
we then asked to which extent the POA gene network
To examine the relationship between behavior, hor-
regulated by ER differed between social phenotypes. To
mone levels, gonadal state, and gene expression in
investigate the molecular consequences of ER perturba-
DOM and SUB, we used the network analysis platform
tion on a genomic scale, we compared the POA transcrip-
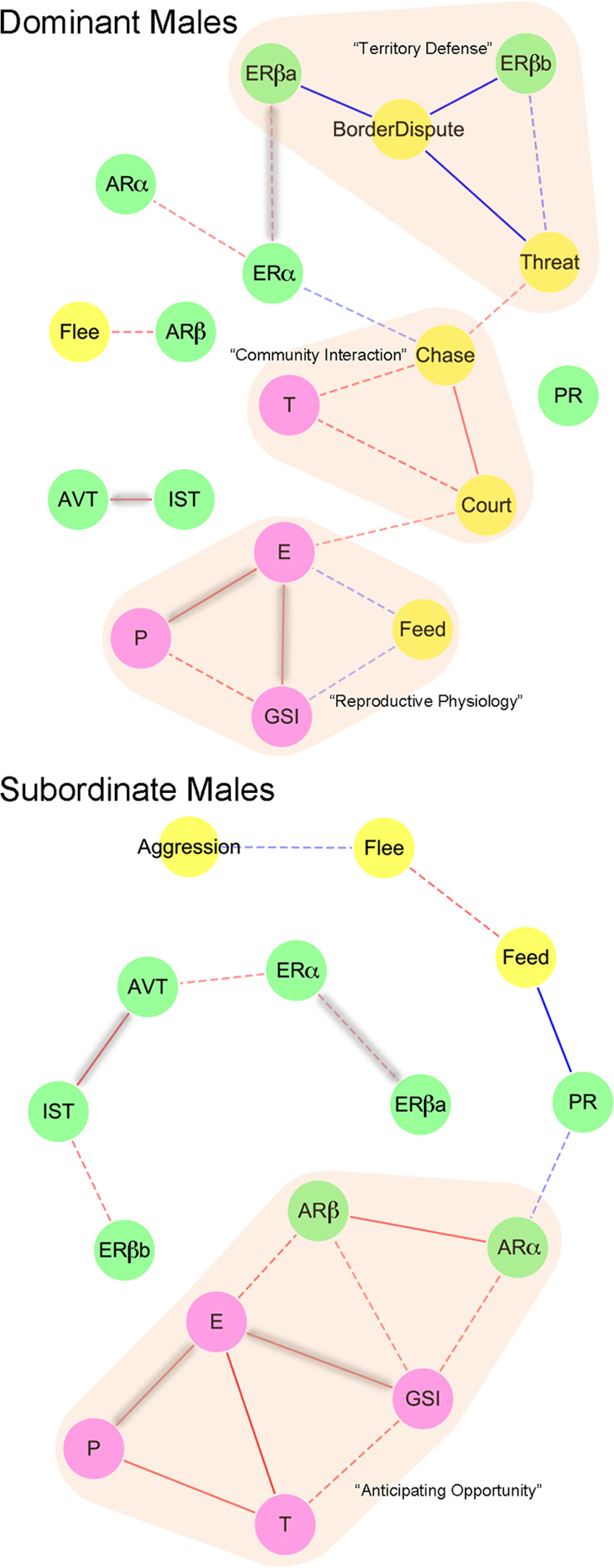
Cytoscape (38) to create association networks based on
tomes of the individuals from the behavioral trials above
Spearman correlation coefficients (Supplemental Ta-
after administration of either ER antagonist or vehicle. In
bles 3 and 4) between measures of behavior, physiology,
DOM, 12,676 (71.6%) of 17,712 array features with
hormones, and POA candidate gene expression of neu-
cichlid sequences yielded above background intensities,
roendocrine genes determined by qPCR in DOM (n ⫽
whereas in SUB, there were only 8,132 (45.9%) such fea-
32) and SUB (n ⫽ 32). As can be seen in Fig. 4, only four
tures, indicating that the POA expresses about 56% more
significant correlations are shared between DOM and
genes in DOM compared with SUB (2 ⫽ 2405.002; P ⬍
SUB males: ER␣ and ERa are highly correlated, as are
0.001). This difference is not a technical artifact, because
AVT and IST, 17-estradiol levels and GSI, and 17-
normalized intensity distributions (as a measure of dy-
estradiol and progesterone levels.
namic range in gene expression) did not differ substan-
This analysis enabled us to propose network modules
tially between the DOM and SUB experiments (Supple-
that may contribute to specific aspects of A. burtoni
mental Fig. 5A). We then compared (separately for DOM
sociality, based on what is known about behavior, phys-
and SUB) the POA transcription profiles of ER antagonist-
iology, and life history of this species. In DOM, border
treated animals with those of vehicle controls, although
disputes and threat displays are directed only toward
none of the differences survived false-discovery correction
other DOM (39). Our results suggest that these behav-
for P ⬍ 0.05 in either social phenotype, which is not sur-
ior patterns are tightly regulated by ERa and ERb
prising given such a subtle perturbation. However, when
signaling and together form a territory defense module.
we examined the P value distributions for DOM and SUB,
The most striking differences between DOM and SUB
we discovered that small P values were considerably over-represented in the DOM dataset (Supplemental Fig. 5B),
were the interactions of hormones and physiology with
indicating widespread gene regulation. We chose P ⬍ 0.05
behavior and expression of sex steroid receptors in the
as an acceptable significance threshold, because our aim
POA. In DOM, testosterone, in association with chas-
was to compare broad-scale patterns of gene regulation
ing and courtship, represents a community interaction
across phenotypes. Unexpectedly, despite the almost com-
module, because these behavior patterns are directed
plete absence of physiological or candidate gene expres-
exclusively at the community of females and SUB. How-
sion responses to ER antagonist treatment in DOM males,
ever, in SUB, testosterone is associated with the other
1,047 (8.25%) of the 12,676 array features that provided
steroid hormones and GSI as well as AR subtype ex-
signal above background were differentially regulated
pression, suggesting that circulating steroid hormones
between control vs. ER antagonist (LIMMA, P ⬍ 0.05).
are reflective of gonadal state. Finally, we found strong
O'Connell and Hofmann
Social Status Predicts Steroid Hormone Action
Endocrinology, March 2012, 153(3):1341–1351
Distinct roles for steroid hormone pathways in
social behavior
Androgens and estrogens have been intensively studied
for decades in the context of sexual behavior and aggres-sion because they dynamically influence behavior (40 –42). Within teleosts, the role of androgens in regulatingsexual behavior appears to be consistent across species(43, 44). Of particular relevance to our study is recentwork in mammals by Juntti et al. (45), who showed thatmice with a conditional neuronal AR knockout displayeddeficits in sexual behavior, yet the number of aggressivedisplays did not differ compared with wild-type mice. Re-search in other nonmammalian tetrapods also suggeststhat AR is important in male-typical sexual behavior, in-cluding amphibians (46), birds (47), and reptiles (48). Theconversion of testosterone to estrogen by aromatase is nec-essary for male aggression in mammals and birds (49, 50)as well as for reproductive behavior in some species (51).
Male ER␣ knockout mice also display deficits in sexualbehavior (52), although this may be due to organizationaleffects of estrogen in the developing male brain (53).
Estrogenic and androgenic modulation of behavior
varies based on environmental cues (9, 54). However, inA. burtoni males, which breed year-round, variation in ARand ER modulation of behavior likely arises from socialrather than seasonal cues. We have shown here that tes-tosterone modulates sexual behavior in DOM and thatestrogens regulate aggression independent of social status.
Although we cannot rule out the conversion of DHT to3-diol (55), we are confident that the androgenic regu-lation of sexual behavior is AR dependent, because theeffects of the AR antagonist were opposite to those ofDHT. We obtained these results using males that were wellestablished in their respective social states and by treatingthem with an agonist or antagonist for several days. How-ever, when SUB are provided with an opportunity to as-cend in social status, aggressive behavior and androgensrise within 30 min of transition, although estrogens do notincrease for several days (7). Androgens may play a short-
FIG. 4. Integrating across levels of biological organization: behavior,
term role in modulating aggressive behavior during social
physiology, hormones, and gene expression. A covariance network isrepresented in DOM (top) and SUB (bottom) males. Edges represent
instability (see also Refs. 8 and 11), whereas estrogens may
significant positive (red) or negative (blue) correlations between
maintain aggressive motivation over the long term if and
behavior (yellow), POA gene expression (green), and physiology
when the social environment has stabilized. These differ-
(purple). Edges highlighted in gray are common across social states,and dashed lines did not survive false discovery rate correction.
ent temporal roles of androgens and estrogens may also be
Functional modules are highlighted in orange. T, Testosterone; E,
a consequence of the relatively slow up-regulation of brain
estradiol; P, progesterone.
aromatase by AR (56). Interestingly, we found that treat-ment of SUB with DHT did not change aggressive behav-
correlations between 17-estradiol, progesterone, GSI,
ior, although different results may have been obtained if
and feeding in DOM, which could be indicative of a
SUB were treated with 11KT rather than DHT. Further-
module governing reproductive physiology.
more, the dose used to treat SUB was determined based on
Endocrinology, March 2012, 153(3):1341–1351
a dose-response curve conducted in DOM, and therefore
social opportunities to ascend to DOM status, as previous
an alternate dose may also have produced a different be-
work has shown a rapid increase in circulating androgens
havioral response. Nonetheless, our results suggest that to
in response to an opportunity to become territorial (6).
ascend in social status, SUB need both an androgen surge
Additionally, several interactions are shared by DOM and
as well as a social opportunity, such as a vacant territory
SUB (e.g. AVT and IST or ER␣ and ERa), suggesting that
and/or the removal of suppression by DOM males.
some genes are coregulated in a similar manner indepen-
The role of progesterone in the adult male brain is less
dent of social status. Finally, although this kind of net-
understood than the actions of androgens and estrogens,
work analysis is clearly useful for integrating data across
although several studies indicate that PR is important in
levels of biological organization to visualize large-scale
male-typical sexual behavior in mammals (57, 58) and
biological patterns, it is important to emphasize that cor-
reptiles (59). Our results also show that PR facilitates
relation of nodes does not necessarily imply causation and
courtship behavior in A. burtoni DOM. In SUB, however,
that it will be necessary to experimentally manipulate the
treatment with a PR antagonist unexpectedly decreased
proposed modules to fully characterize their function in
fleeing behavior, which might suggest that the perception
regulating behavior in a complex social environment.
or evaluation of social (i.e. threatening) cues is modulated
We found several interesting patterns with steroid re-
by PR. Work in humans has shown that progesterone,
ceptor antagonist treatment and downstream gene expres-
along with cortisol, increases in avoidance situations that
sion in the POA, particularly the suppression of IST by AR
signify fear of rejection (60). Furthermore, treatment with
in SUB. Neuropeptides of the oxytocin family have drawn
17␣-20-P has antianxiety effects in rodents (61). It is thus
a lot of attention in the mammalian literature by regulating
possible that PR regulates social cognition in a manner
trust (63) and affiliative behavior (64). In A. burtoni, SUB
that is conserved across vertebrates, although more studies
school (i.e. affiliate) with other SUB and females, yet when
across a wide range of taxa will be needed to test this
given the opportunity to ascend to dominance (a relatively
hypothesis. The work in humans also points to the im-
solitary station), schooling behavior is suppressed, possi-
portance of the stress axis in social status. Although we did
bly via down-regulation of the IST pathway. This idea is
not measure cortisol levels or glucocorticoid receptor ex-
consistent with a study in goldfish that demonstrated a
pression, this pathway does indeed play a pivotal role in A.
role for IST in regulating social approach behavior (65).
burtoni behavior (62).
Another interesting relationship is that of androgens,
estrogens, and expression of their receptors in the POA,
Integrating brain gene expression with behavior
which seem to play very different roles depending on an
individual's position in the social hierarchy. SUB have
We have used a covariation network analysis to orga-
lower expression of ER compared with DOM, and the
nize data across levels of biological organization and pro-
lack of a transcriptome response in SUB compared with
pose functional modules that allow us to generate new
DOM follows this pattern. Inhibition of ER mechanisms
hypotheses about the neuroendocrine and molecular basis
in SUB after administration of an ER antagonist, in com-
of social behavior. Here we discuss correlations between
bination with low circulating testosterone levels, and the
behavior, physiology, and gene expression in the POA, a
resulting absence of brain AR activation, appears to lead
brain region highly conserved in function across verte-
to a remarkable genome-wide suppression of both tran-
brates (20), although these patterns may well differ when
scriptional activity and variation in the POA.
other brain regions are analyzed. Our results suggest dis-tinct modules that may govern different aspects of behav-
Searching for patterns across levels of biological
ior within a social group. DOM display behavior patterns
toward other DOM that are closely associated with ex-
In an era when molecular tools have become available
pression of ER, which is supported by our pharmacolog-
for nontraditional model systems to explore the under-
ical results where ER manipulations altered these territory
pinnings of complex behavioral phenotypes, few research-
defense behaviors. Interactions with other community
ers have analyzed a particular phenotype across many lev-
members, including females and SUB, appear to involve a
els of biological organization. Our results show for the
different module that is dominated by androgens. We hy-
first time how in individuals of the same species, biological
pothesize that this is due to the dynamic response of an-
responses to a perturbation can differ across levels of bi-
drogens to social cues (6, 11, 12) that may then modulate
ological organization as well as between phenotypes. We
courtship or chasing behavior. SUB are quite different, in
are not aware of any other study to date that has integrated
that their hormones are tightly linked to gonadal state and
behavior, hormones, physiology, candidate gene expres-
AR expression, which may enable them to quickly seize
sion, and transcriptome profiling within the same individ-
O'Connell and Hofmann
Social Status Predicts Steroid Hormone Action
Endocrinology, March 2012, 153(3):1341–1351
uals, yet a complete understanding of the mechanisms un-
social status in the African cichlid, Astatotilapia burtoni. Behav
derlying behavioral variation across individuals might
Brain Res 166:291–295
3. Burmeister SS, Jarvis ED, Fernald RD 2005 Rapid behavioral and
require the kind of integration across multiple levels of
genomic responses to social opportunity. PLoS Biol 3:e363
biological organization we have attempted here. Further-
4. Greenwood AK, Wark AR, Fernald RD, Hofmann HA 2008 Ex-
more, given that mRNA and protein need not always be
pression of arginine vasotocin in distinct preoptic regions is associ-ated with dominant and subordinate behaviour in an African cichlid
concordant (66), it will be important to include analyses of
fish. Proc Biol Sci 275:2393–2402
the proteome and small RNAs in future studies as well.
5. Gesquiere LR, Learn NH, Simao MC, Onyango PO, Alberts SC,
Our results show that phenotypic changes at one level
Altmann J 2011 Life at the top: rank and stress in wild male baboons.
Science 333:357–360
of biological organization do not predict changes of com-
6. Maruska KP, Fernald RD 2010 Behavioral and physiological plas-
parable magnitude at another level, although it is impor-
ticity: rapid changes during social ascent in an African cichlid fish.
tant to note that gene expression may be more reflective of
Horm Behav 58:230 –240
7. Oliveira RF, Hirschenhauser K, Carneiro LA, Canario AV 2002
behavior than circulating hormone levels. Our expecta-
Social modulation of androgen levels in male teleost fish. Comp
tion at the beginning of this study was that a behavioral
Biochem Physiol B Biochem Mol Biol 132:203–215
response to sex steroid receptor manipulations would be
8. Trainor BC, Rowland MR, Nelson RJ 2007 Photoperiod affects
accompanied by corresponding changes in circulating
estrogen receptor ␣, estrogen receptor  and aggressive behavior.
Eur J Neurosci 26:207–218
hormone levels, candidate gene expression, and transcrip-
9. Goymann W 2009 Social modulation of androgens in male birds.
tome activity, regardless of social status. However, we
Gen Comp Endocrinol 163:149 –157
found marked differences in DOM and SUB responses at
10. Wingfield JC, Hegner RE, Dufty AM, Jr., Ball GF 1990 The "chal-
lenge hypothesis": theoretical implications for patterns of testoster-
various levels. These discrepancies could, in part, be ex-
one secretion, mating systems, and breeding strategies. Am Nat 136:
plained by differences in gene expression of sex steroid
receptors in some brain regions (67) or in the testes (68),
11. Hirschenhauser K, Oliveira RF 2006 Social modulation of andro-
gens in male vertebrates: meta-analyses of the "challenge hypothe-
by effects of the community structure on the behavioral
sis." Anim Behav 71:265–277
expressions of DOM and SUB males, or most likely a com-
12. Gleason ED, Fuxjager MJ, Oyegbile TO, Marler CA 2009 Testos-
bination of all these factors.
terone release and social context: when it occurs and why. FrontNeuroendocrinol 30:460 – 469
13. Wahli W, Martinez E 1991 Superfamily of steroid nuclear receptors:
positive and negative regulators of gene expression. FASEB J
14. Remage-Healey L, Bass AH 2006 A rapid neuromodulatory role for
We thank Lin Huffman, Aubrey Kelly, Mary Ramsey, Deena
steroid hormones in the control of reproductive behavior. Brain Res1126:27–35
Walker, and Keith Whitaker for helpful comments on earlier
15. Mani SK, Portillo W, Reyna A 2009 Steroid hormone action in the
versions of this manuscript; Lin Huffman, Suzy Renn, and Anna
brain: cross-talk between signalling pathways. J Neuroendocrinol
Sessa for valuable advice on the microarray analysis; and mem-
bers of the Hofmann laboratory for discussions. The PR antag-
16. Hofmann HA 2003 Functional genomics of neural and behavioral
plasticity. J Neurobiol 54:272–282
onist was a kind gift from Bayer Schering Pharma AG, Berlin
17. Robinson GE, Fernald RD, Clayton DF 2008 Genes and social be-
havior. Science 322:896 –900
18. Renn SC, Aubin-Horth N, Hofmann HA 2008 Fish and chips: func-
Address all correspondence and requests for reprints to: Dr.
tional genomics of social plasticity in an African cichlid fish. J Exp
Hans A. Hofmann, Section of Integrative Biology, The Univer-
Biol 211:3041–3056
sity of Texas at Austin, 1 University Station C0930, Austin,
19. Newman SW 1999 The medial extended amygdala in male repro-
Texas 78712. E-mail: [email protected].
ductive behavior. A node in the mammalian social behavior net-work. Ann NY Acad Sci 877:242–257
This work was supported by National Science Foundation
20. O'Connell LA, Hofmann HA 2011 The vertebrate mesolimbic re-
Doctoral Dissertation Improvement Grant 1011253 to L.A.O.,
ward system and social behavior network: a comparative synthesis.
NSF Grant 0843712, the Alfred P. Sloan Foundation, and a
J Comp Neurol 519:3599 –3639
Dwight W. and Blanche Faye Reeder Centennial Fellowship in
21. Munchrath LA, Hofmann HA 2010 Distribution of sex steroid hor-
mone receptors in the brain of an African cichlid fish, Astatotilapia
Systematic and Evolutionary Biology and Institute for Cellular
burtoni. J Comp Neurol 518:3302–3326
and Molecular Biology Fellowship to H.A.H.
22. Fernald, RD 1977 Quantitative behavioural observations of Hap-
Disclosure Summary: The authors have nothing to disclose.
lochromis burtoni under semi-natural conditions. Anim Behav 25:643– 653
23. Wells K, and Van Der Kraak G 2000 Differential binding of endog-
enous steroids and chemicals to androgen receptors in rainbow trout
and goldfish. Environ Toxicol Chem 19:2059 –2065
24. Sperry TS, Thomas P 1999 Characterization of two nuclear andro-
1. Newman ML, Sellers JG, Josephs RA 2005 Testosterone, cognition,
gen receptors in Atlantic croaker: comparison of their biochemical
and social status. Horm Behav 47:205–211
properties and binding specificities. Endocrinology 140:1602–1611
2. Parikh VN, Clement TS, Fernald RD 2006 Androgen level and male
25. Hawkins MB, Thomas P 2004 The unusual binding properties of the
Endocrinology, March 2012, 153(3):1341–1351
third distinct teleost estrogen receptor subtype ERa are accompa-
Schlinger BA 2007 Androgen and the elaborate courtship behavior
nied by highly conserved amino acid changes in the ligand binding
of a tropical lekking bird. Horm Behav 51:62– 68
domain. Endocrinology 145:2968 –2977
48. Tokarz RR 1986 Hormonal regulation of male reproductive behav-
26. Pinter J, Thomas P 1997 The ovarian progestogen receptor in the
ior in the lizard Anolis sagrei: a test of the aromatization hypothesis.
spotted seatrout, Cynoscion nebulosus, demonstrates steroid spec-
Horm Behav 20:364 –377
ificity different from progesterone receptors in other vertebrates.
49. Schlinger BA, Callard GV 1990 Aromatization mediates aggressive
J Steroid Biochem 60:113–119
behavior in quail. Gen Comp Endocrinol 79:39 –53
27. Kidd CE, Kidd MR, Hofmann HA 2010 Measuring multiple hor-
50. Trainor BC, Kyomen HH, Marler CA 2006 Estrogenic encounters:
mones from a single water sample using enzyme immunoassays. Gen
how interactions between aromatase and the environment modulate
Comp Endocrinol 165:277–285
aggression. Front Neuroendocrinol 27:170 –179
28. Braford Jr MR, Northcutt RG 1983 Organization of the dienceph-
51. Adkins-Regan E, Garcia M 1986 Effect of flutamide (an antiandro-
alon and pretectum of ray-finned fishes. In: David RG, Northcutt
gen) and diethylstilbestrol on the reproductive behavior of Japanese
RG, eds. Fish neurobiology. Vol 2. Ann Arbor, MI: University of
quail. Physiol Behav 36:419 – 425
Michigan Press; 117–163
52. Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De
29. Simon P 2003 Q-Gene: processing quantitative real-time RT-PCR
Vries GJ 1997 Masculine sexual behavior is disrupted in male and
data. Bioinformatics 19:1439 –1440
female mice lacking a functional estrogen receptor alpha gene. Horm
30. Salzburger W, Renn SC, Steinke D, Braasch I, Hofmann HA, Meyer
Behav 32:176 –183
A 2008 Annotation of expressed sequence tags for the East African
53. Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF 2006 Roles
cichlid fish Astatotilapia burtoni and evolutionary analyses of
of estrogen receptors alpha and beta in differentiation of mouse
cichlid ORFs. BMC Genomics 9:96
sexual behavior. Neuroscience 138:921–928
31. Liang K, Zeger SL 1986 Longitudinal data analysis using generalized
54. Wingfield JC, Lynn S, Soma KK 2001 Avoiding the ‘costs' of tes-
linear models. Biometrika 73:13–22
tosterone: ecological bases of hormone-behavior interactions. Brain
32. Smyth GK 2005 Limma: Linear models for microarray data. In:
Behav Evol 57:239 –251
Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W, eds. Bioin-
55. Mouriec K, Gueguen MM, Manuel C, Percevault F, Thieulant ML,
formatics and computational biology solutions using R and Biocon-
Pakdel F, Kah O 2009 Androgens upregulate cyp19a1b (aromatase
ductor. New York, NY: Springe; 397– 420
B) gene expression in the brain of zebrafish (Danio rerio) throughestrogen receptors. Biol Reprod 80:889 – 896
33. Hoegg S, Brinkmann H, Taylor JS, Meyer A 2004 Phylogenetic
56. Abdelgadir SE, Resko JA, Ojeda SR, Lephart ED, McPhaul MJ, Roselli
timing of the fish-specific genome duplication correlates with the
CE 1994 Androgens regulate aromatase cytochrome P450 messenger
diversification of teleost fish. J Mol Evol 59:190 –203
ribonucleic acid in rat brain. Endocrinology 135:395–401
34. Zakon HH, Dunlap KD 1999 Sex steroids and communication sig-
57. Witt DM, Young LJ, Crews D 1994 Progesterone and sexual be-
nals in electric fish: a tale of two species. Brain Behav Evol 54:61– 69
havior in males. Psychoneuroendocrinology 19:553–562
35. Margiotta-Casaluci L, Sumpter JP 2011 5␣-Dihydrotestosterone is
58. Phelps SM, Lydon JP, O'malley BW, Crews D 1998 Regulation of
a potent androgen in the fathead minnow (Pimephales promelas).
male sexual behavior by progesterone receptor, sexual experience,
Gen Comp Endocrinol 171:309 –318
and androgen. Horm Behav 34:294 –302
36. Maruska KP, Fernald RD 2010 Steroid receptor expression in the
59. Lindzey J, Crews D 1988 Effects of progestins on sexual behaviour
fish inner ear varies with sex, social status, and reproductive state.
in castrated lizards (Cnemidophorus inornatus). J Endocrinol 119:
BMC Neurosci 11:58
37. Goodson JL, Thompson RR 2010 Nonapeptide mechanisms of so-
60. Wirth MM, Schultheiss OC 2006 Effects of affiliation arousal (hope
cial cognition, behavior and species-specific social systems. Curr
of closeness) and affiliation stress (fear of rejection) on progesterone
Opin Neurobiol 20:784 –794
and cortisol. Horm Behav 50:786 –795
38. Kohl M, Wiese S, Warscheid B 2011 Cytoscape: software for visu-
61. Picazo O, Ferna´ndez-Guasti A 1995 Anti-anxiety effects of proges-
alization and analysis of biological networks. Methods Mol Biol
terone and some of its reduced metabolites: an evaluation using the
burying behavior test. Brain Res 680:135–141
39. Baerends GP, Baerends-van Roon JM 1950 An introduction to the
62. Fox HE, White SA, Kao MH, Fernald RD 1997 Stress and domi-
study of the ethology of cichlid fishes. Behavior Suppl 1:1–242
nance in a social fish. J Neurosci 17:6463– 6469
40. Beach FA 1948 Hormones and behavior. New York: Paul B. Hoeber
63. Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E 2005 Oxy-
41. Lehrman DS 1965 Interaction between internal and external envi-
tocin increases trust in humans. Nature 435:673– 676
ronments in the regulation of the reproductive cycle of the ring dove.
64. Insel TR, Shapiro LE 1992 Oxytocin receptor distribution reflects
Sex and Behavior. Beach FA, ed. New York: Wiley; 355–380
social organization in monogamous and polygamous voles. Proc
42. Ball GF, Balthazart J 2004 Hormonal regulation of brain circuits
Natl Acad Sci USA 89:5981–5985
mediating male sexual behavior in birds. Physiol Behav 83:329 –346
65. Thompson RR, Walton JC 2004 Peptide effects on social behavior:
43. Salek SJ, Sullivan CV, Godwin J 2001 Courtship behavior of male
effects of vasotocin and isotocin on social approach behavior in male
white perch, Morone americana: evidence for control by androgens.
goldfish (Carassius auratus). Behav Neurosci 118:620 – 626
Comp Biochem Physiol A Mol Integr Physiol 130:731–740
66. Vogel C, Abreu Rde S, Ko D, Le SY, Shapiro BA, Burns SC, Sandhu
44. Sebire M, Allen Y, Bersuder P, Katsiadaki I 2008 The model anti-
D, Boutz DR, Marcotte EM, Penalva LO 2010 Sequence signatures
androgen flutamide suppresses the expression of typical male stick-
and mRNA concentration can explain two-thirds of protein abun-
leback reproductive behaviour. Aquat Toxicol 90:37– 47
dance variation in a human cell line. Mol Syst Biol 6:400
45. Juntti SA, Tollkuhn J, Wu MV, Fraser EJ, Soderborg T, Tan S,
67. Burmeister SS, Kailasanath V, Fernald RD 2007 Social dominance
Honda S, Harada N, Shah NM 2010 The androgen receptor governs
regulates androgen and estrogen receptor gene expression. Horm
the execution, but not programming, of male sexual and territorial
Behav 51:164 –170
behaviors. Neuron 66:260 –272
68. Maruska KP, Fernald RD 2011 Plasticity of the reproductive axis
46. Behrends T, Urbatzka R, Krackow S, Elepfandt A, Kloas W 2010
caused by social status change in an african cichlid fish. II. Testicular
Mate calling behavior of male South African clawed frogs (Xenopus
gene expression and spermatogenesis. Endocrinology 152:291–302
laevis) is suppressed by the antiandrogenic endocrine disrupting
69. Renn SCP, Aubin-Horth N, Hofmann HA 2004 Biologically mean-
compound flutamide. Gen Comp Endocrinol 168:269 –274
ingful expression profiling across species using heterologous hybrid-
47. Fusani L, Day LB, Canoine V, Reinemann D, Hernandez E,
ization to a cDNA microarray. BMC Genomics 5, 42
Source: http://oconnell.fas.harvard.edu/files/oconnell/files/oconnellhofmann2012.pdf
Dental Care for the Patient with Bipolar Disorder • David B. Clark, BSc, DDS, MSc (Oral Path), MRCDC • Chronic mental illness and its treatment carry inherent risks for significant oral diseases. Given the shift in treatmentregimens from the traditional institutionally based approach to more community-focused alternatives, generaldental practitioners can expect to see and be asked to treat patients with various forms of psychiatric disorders. Onesuch group consists of patients with bipolar disorder (including type I bipolar disorder or manic-depressive disor-der). The purpose of this paper is to acquaint the dental practitioner with the psychopathological features of bipo-lar disorder and to highlight the oral health findings and dental management considerations for these patients.Bipolar disorder is considered one of the most treatable forms of psychiatric illness once it has been diagnosedcorrectly. Through a combination of pharmacotherapy, psychotherapy and life-adjustment skills counselling, thesepatients are better able to understand and cope with the underlying mood swings that typify the condition and inturn to interact more positively and progressively within society as a whole. Both the disease itself and its variouspharmacologic management modalities exact a range of oral complications and side effects, with caries, peri-odontal disease and xerostomia being encountered most frequently. It is hoped that after reading this article thegeneral dental practitioner will feel more confident about providing dental care for patients with bipolar disorderand in turn to become a vital participant in the reintegration of these patients into society.
Spécialité ADIP L04 LA PLONGEE DEPUIS UN BATEAU JEAN-CLAUDE TAYMANS JCT Consulting – Moby Dick Diving School Page 1/29 2, Rue Mouzin – 7390 Wasmuël - Belgium La plongée depuis un bateau La plongée est une activité à risque. Elle ne peut être pratiquée que par des personnes correctement formées, bien entraînées et en bonnes conditions physiques et mentales. Le non-respect des règles peut conduire à des blessures graves, des invalidités permanentes ou à la mort. Il vous incombe personnellement d'en évaluer les risques. Ne comptez pas sur les données de cet ouvrage pour garantir votre sécurité. Avant d'entrer dans l'eau, vous devez exercer votre propre jugement quant aux dangers et difficultés que vous allez rencontrer. A vous de faire une évaluation réaliste des conditions de plongée, de la difficulté du site et de votre condition physique !



