Prodovite.biz
Functional Foods in Health and Disease 2015; 5(9): 292-303
Page 292 of 303
Research Article
The effect of VMP35 supplement ingredients encapsulated in a novel
Phospholipid Prodosome SK713 SLP nutrient delivery technology observed as
a result of changes in properties of live human blood
B. William Downs 1, Steven Kushner 2, Ted Aloisio3, Frans J. Cronjé4, and Kenneth
1Department of Research and Development Victory Nutrition International Lederach, USA; 2ALM Research and Development Oldsmar, USA; 3Veritas Health Woodbridge, Canada; 4University of Stellenbosch Cape Town, South Africa; 5Department of Psychiatry and McKnight Brain Institute, University of Florida, College of Medicine, Gainesville, Florida, USA
*
Corresponding author: Kenneth Blum PhD, Department of Psychiatry and McKnight Brain
Institute, University of Florida, College of Medicine, Gainesville, Florida, USA
ABSTRACT
Background: In North America digestive malfunction in terms of disintegration, dissolution,
and absorption of food and nutrients, is a widespread malady. Malabsorption is also an
exacerbating factor in most chronic degenerative diseases that might benefit from dietary
supplementation. The purpose of this experiment was to evaluate absorption following the
sublingual administration of VMP35 SK713 encapsulated nutraceutical formulation.
Method: Changes in peripheral blood smears from 38 subjects were observed using peripheral
live blood cell imaging (LBCI) with phase contrast microscopy. Observation of changes in
properties of live blood was made and compared to placebo and baseline at five and 30 minutes
after administration.
Results: Compared to baseline and control, the VMP35 formulation SK713 SLP technology
effected positive changes in the blood. They were demonstrated by morphological,
hematological and rheological changes observed five minutes from the intake and were sustained
for at least 30 minutes post intake.
Conclusions: These observable changes showed that the SK713 SLP system can make a key
contribution to increasing the potential benefits of dietary supplementation for those patients
with compromised digestive processes. We encourage additional research on the effects of this
novel neutraceutical formulation and delivery system.
Keywords: digestive malfunction live blood cell imaging, peripheral blood smear, cell
aggregation, rheology, phospholipids, nutrient encapsulation, multinutrient complex.
Functional Foods in Health and Disease 2015; 5(9): 292-303
Page 293 of 303
BACKGROUND
According to the Center for Disease Control, after accidents, digestive disorders are the number
one reason for emergency room visits in the US [1, 2]. Digestive-malfunction in terms of
impaired disintegration, dissolution, and absorption of food and nutrients is a widespread
malady; it is also an exacerbating factor in most chronic degenerative diseases. Prescription and
OTC drugs for digestive distress are among the most popular medications in the US,
emphasizing the importance of this disorder. Effective digestion of food, food constituents, and
for some, dietary supplements are essential for maintaining health.
Liposome structures are safe, biodegradable, and biocompatible [3]. In addition, the
liposome structure can be adequately and rapidly absorbed by the sublingual mucosal membrane. In doing so, they can accommodate both water-soluble and fat-soluble vitamins, trace minerals, and naturally occurring phytonutrients including flavonoids, terpenes, and saponins [4].
The purpose of this experiment was to observe changes in live blood to evaluate absorption
following the sublingual administration of VMP35 SK713 encapsulated nutraceutical formulation.
MATERIALS
SK713 Soy Lecithin Phospholipid (SK713 SLP)
SK713 Soy Lecithin Phospholipid (SK713 SLP) nutrient Prodosome encapsulation technology
employs a unique three-step manufacturing process. Step one is the production of the SK713
SLP compound and its impregnation and saturation with solar-dried electrolytes that supply
free ions. This process amplifies the ionic properties on each level of the multi-lamellar sphere.
Step two is an advanced wet milling pre-treatment of the nutrient components to be
combined with SK713 SLP. The final step involves specific blending methods for combining
the components into a thoroughly homogeneous nutraceutical compound. Effective
multilamellar liposomal encapsulation requires precise control over the SK713 SLP
manufacturing as well as the pretreatment and preparation of specific nutrient compounds. The
process produces a finished nutraceutical that can be utilized for oral or topical delivery
applications [5].
One of the major components of SK713 SLP is a high grade specially prepared soy lecithin
material that contains a minimum of 85% phosphatidylcholine (>85PC), an essential phospholipid. While most lecithin products contain only 19-21% PC [6], the high PC content in SK713 SLP ensures thorough formation of liposomes. In addition to acting as biological capacitors and protecting the VMP35 supplement ingredients (see Table 1), multilamellar liposome phospholipids offer many other health-related benefits [7]. The multilamellar or multisphered-multilayered-clustoidal structure of SK713 SLP, unlike standard liposome technology, is capable of enveloping a diverse range of nutrients simultaneously. Through experimentation, the structure of SK713 SLP was found to form vesicles made up of hundreds of concentric lipid bilayers. The lipid bilayers range in size from 100 nanometers to 500 micrometers and are made up of a few dozen to several thousand molecules [4]. The SK713 SLP multilamellar liposomes form spontaneously as the electrostatic and adsorptive properties lower surface tension (surfactant). The net result is thorough and complete phospholipid encapsulation (or entrapment) of nutritional ingredients within multiple layers of nano to low micrometer-sized
Functional Foods in Health and Disease 2015; 5(9): 292-303
Page 294 of 303
spheres. This electrostatic encapsulation is effective for encapsulating and transporting both water and fat-soluble nutritional ingredients including phytonutrients within the same spherical structure.[8,9].
Table 1. SK713 SLP Encapsulated VMP35 Multivitamin, Mineral & Phytonutrient Formula
Per Serving
Unit of Measure
Vitamin A (Retinyl Palmitate)
Vitamin C (Ascorbic acid)
Vitamin D3 (Cholecalciferol)
Vitamin E (Alpha-tocopheryl Succinate)
Vitamin B1 (Thiamin HCl)
Vitamin B2 (Riboflavin)
Vitamin B3 (Niacin)
Vitamin B6 (Pyridoxine HCl)
Vitamin B12 (Cyanocobalamin)
Pantothenic acid (d-calcium pantothenate)
Iodine (potassium iodide)
Magnesium citrate
Copper gluconate
Manganese sulfate
Chromium chloride
Potassium citrate
Choline bitartrate
White pine cone extract
BiAloe Concentrated 200:1 Water Extract
VMP35 1:1 Herbal Blend:
Astragalus extract 1:111 Ginger extract 1:1 Green tea extract 1:1 Fo-ti extract 1:1 Hawthorne berry extract 1:1 Elderberry extract 1:1 Eleuthero extract 1:1 Chamomile extract 1:1 Citrus bioflavonoids (from rose hips) 1:1 Gotu kola extract 1:1
Functional Foods in Health and Disease 2015; 5(9): 292-303
Page 295 of 303
Many nutritional compounds, especially inorganic minerals and resinous phytonutrients, do
not dissolve well in water. Prior to SK713 SLP processing, all materials are pre-processed using
a low sheer tri-blender jet-compression-particle-processing technology. Previously insoluble
materials can now be blended into the aqueous base made of the high-grade lecithin (>85%PC)
combined with alcohol. The SK713 SLP material can then be blended into the liquid nutritional
compound. Importantly for human consumption, the transport vehicles or spheres are comprised
of all natural GRAS ingredients (Generally Recognized As Safe).
Multilamellar Sphere Components
The SK713 SLP multilamellar sphere contains large quantities of electrolytes and hydroxyl-rich
botanicals that contribute bioflavonoids and assist in maintaining healthy pH, proper hydration,
and the transport and utilization of vital nutrients (see Table 1). The SK713 SLP sphere is also a
zwitterion, methyl donor, and potential alkalizing buffer [10]. Zwitterions carry both positive and
negative charges. They may lower the energy requirement for transporting molecules thereby
enhancing absorption by spreading the nutrient out over a larger surface area [3], are soluble in
water and highly soluble in solvents. The SK713 SL phospholipid spheres also have a natural
‘adhesive' property that enhances the ability of the body to absorb their nutritional contents.
METHODS
Changes in properties of live blood, following the sublingual administration of VMP35 SK713
encapsulated nutraceutical formulation were observed, recorded and compared to placebo and
baseline, at five and 30 minutes after administration.
Live blood cell imaging
Live blood cell imaging (LBCI) was performed by Veritas Health in Woodbridge, Ontario,
Canada, using an Olympus BX-30 light microscope with a Phase Contrast Condenser to visualize
samples. A 150-watt lightbox with fiber optic cable assembly was used to highlight the specimen
against a gray field and increase the range of intermediate shades. The lighting produces a high
level of cell definition, clearer morphology and can distinguish features of some cell walls. The
lens configuration was 10x eyepiece and 100x-oil -immersion objective magnification, to
achieve approximately 1000 times magnification. Oil immersion achieved finer resolution and
brightness.
Peripheral blood smear
Peripheral blood smear (PBS) was performed by puncturing the finger with a Bayer Single-Let
Disposable Lancet 23G 2.25mm sterile single-use lancing device. A small amount of capillary
blood was allowed to exude and collect spontaneously on the fingertip without squeezing the
finger. The blood was transferred directly onto a microscope slide without touching the slide
with the finger. The slides used were pre-cleaned standard 1 inch by 3 inches with a thickness of
1 mm supplied by Electron Microscopy Sciences. The slide was covered quickly and gently with
a cover glass without pressure to protect blood cells from damage. The cover glass was pre-
cleaned #1 22mmx40mm with 0.13 to 0.17 mm thickness supplied by Electron Microscopy
Sciences. The corners of the cover glass were tapped carefully to disperse surface tension and
Functional Foods in Health and Disease 2015; 5(9): 292-303
Page 296 of 303
create an even layer for viewing. The slide was then transferred directly to the microscope for
viewing. Evaluation of blood properties began in less than 30 seconds after the blood was taken
from the finger. Consistent blood extraction and handling procedures were followed to avoid
artifacts.
Study Design and Subjects
This observational, prospective, controlled cross-over study was designed to evaluate the effect
of trans-mucosal administration of SK713 SLP encapsulated VMP35 MNC (active) as compared
to baseline and commercially available bottled water (control). This study was approved by the
Path Foundation NY IRB on April 25, 2013 (#13-009) and all subject signed a consent. Changes
in peripheral blood smears (PBS) were observed using Live Blood Cell Imaging and Phase
Contrast Microscopy [11]. The 38 subjects were recruited at random from medical health clinics
during interviews in Woodbridge and Perth Ontario Canada (see Table 2). There were ten males
and 28 females ranging in age from 12 years to 82 years with an average age for males of 49
years and for females of 46.8 years as seen in Table 2. Subjects were assigned randomly into one
of three groups and underwent PBS LBCI as seen in Table 3.
Table 2. Selected Subjects Participating in Live Blood Cell Imaging
Participant
Ethnicity
Self-Reported Health Issues
High Blood Pressure (BP)
Osteoporosis, Arthritis
Digestion Problems
Attention Deficit Disorder.
Thyroid, Severe Pain
Functional Foods in Health and Disease 2015; 5(9): 292-303
Page 297 of 303
Participant
Ethnicity
Self-Reported Health Issues
Depression, Thyroid, Hormone
High BP, Diabetes, Heart
Severe Periodontal Disease
High Blood Pressure
Bladder Cancer, Cll
Table 3. Blood tests timing.
Groups (n)
Baseline
5 minutes after water
5 minutes after
30 minutes after
VMP35 MNC
VMP35 MNC
Group 1 (control)
Group 1 (active)
Group 2 (active)
Group 3 (active)
Group 3 (active)
Total tests
After the baseline, PBS Group 1 (n=8) consumed 30 mL water with a follow-up PBS taken
at 5 min. Both active groups Group 2 (n= 26) and Group 3 (n=7) consumed 30 mL of VMP35 liquid MNC with a follow-up PBS at 5 min. Thereafter, Group 2b had an additional PBS taken at 30 minutes. Group 1 then consumed 30 mL of VMP35 MNC and had a PBS at 5 min after intake.
A non-blinded comparison was done between the baseline and subsequent PBS samples.
Pictures were taken of each phase of the study, for each group changes in morphological, hematological and rheological characteristics were recorded. Videos (not submitted for publication) of the changes confirmed rheological changes following the VMP35 MNC intake.
Group 1: Water Control group consisting of 8 individuals (3 blood samples each): a. Baseline blood test before consuming water b. 2nd Blood Test 5 Minutes After Water c. 3rd Blood Test 5 Minutes After VMP35 MNC. Group 2: Active Group consisting of 23 individuals (2 blood samples each): a. Baseline blood test before VMP35 MNC b. 2nd Blood test 5 Minutes After VMP35 MNC. Group 3: Active Group consisting of 7 individuals (3 blood samples each)
Functional Foods in Health and Disease 2015; 5(9): 292-303
Page 298 of 303
a. Baseline blood test before VMP35 MNC b. 2nd Blood test 5 Minutes After VMP35 MNC c. 3rd Blood Test 30 Minutes After VMP35 MNC. The subjects in Group 1 were first given 30 mL of commercially bottled water (control).
Group 2 and 3 were given 30 mL of VMP35 liquid MNC (active). At 5 minutes, all participants
were subjected to a PBS LBCI. Group 3 was reassessed at 30 minutes. Group 1 was then crossed
over taking 30 mL of VMP35 liquid MNC and assessed at 5 minutes.
RESULTS
Control Group: No changes were observed between the baseline and the 5-minute samples in the
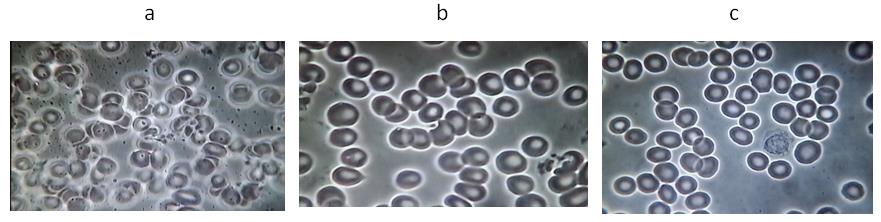
control group. See an example in Figure 1(a) and (b).
Active Groups: Substantial differences were observed between the baseline and 5-minute
samples in the active groups. Examples can be seen in: Group 1 see Figure 1(b) and (c) (Post-
control active group) and Group 2 see Figure 2 (a) and (b), Figure 3 (a) and (b), and Figure 4 (a)
and (b).
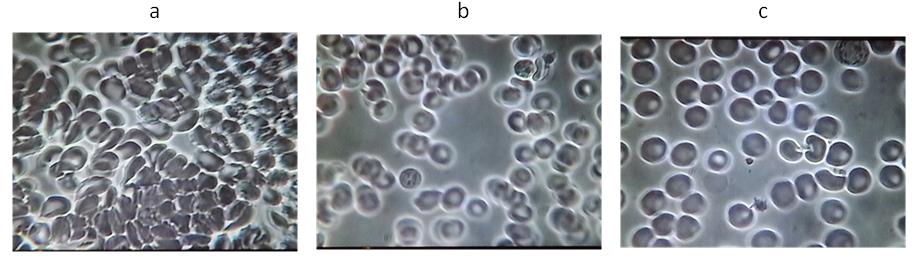
Thirty minute post-active group: Substantial differences were observed between the baseline, 5
minute, then 30-minute post-active group (Group 3). See examples in Figure 5 (a), (b) and (c),
and Figure 6 (a), (b) and (c).
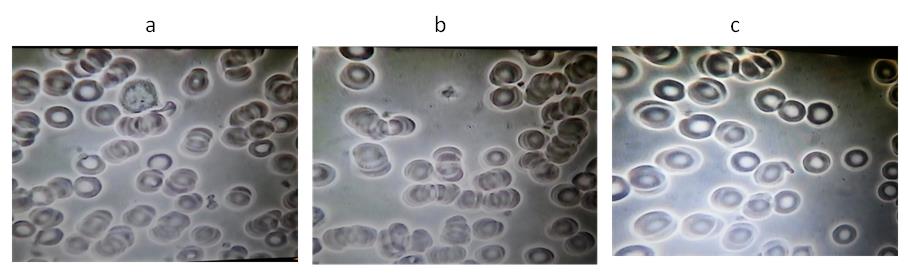
Figure 1. A representative example from Group 1: Subject #45
(a) Baseline before water. (b) Five minutes after taking 30ml water. (c) Five minutes after
taking 30ml VMP35 MNC.
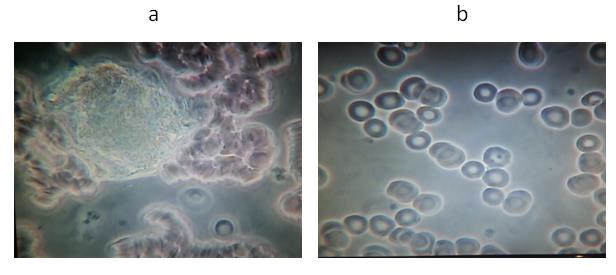
Figure 2. A representative example from Group 2: Subject # 10
(a) Baseline blood test before VMP35 MNC. (b) Five minutes after taking 30 ml VMP35 MNC.
Functional Foods in Health and Disease 2015; 5(9): 292-303
Page 299 of 303
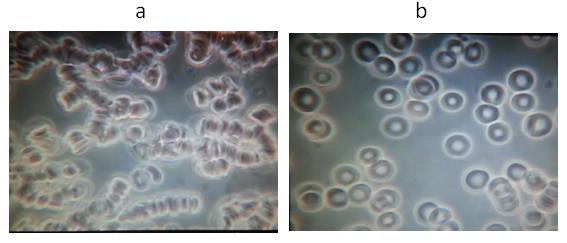
Figure 3. A representative example from Group 2: Subject # 11
a Baseline blood test before VMP35 MNC. b Five minutes after taking 30 ml VMP35 MNC.
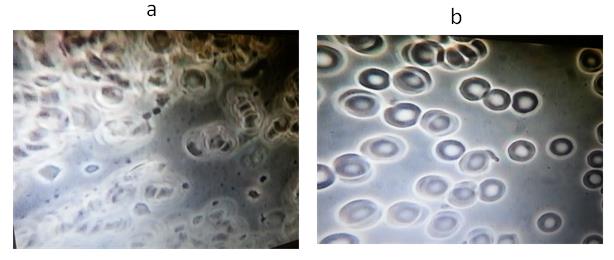
Figure 4. A representative example from Group 2: Subject # 40
(a) Baseline blood test before VMP35 MNC. (b) Five minutes after taking 30 ml VMP35 MNC.
Figure 5. A representative example from Group 3: Subject # 47
(a) Baseline blood test before VMP35 MNC. (b) Five minutes after taking VMP35MNC. (c)
Thirty minutes after taking 30ml VMP35 MNC.
Figure 6. A representative example from Group 3: Subject # 49
(a)Baseline blood test before VMP35 MNC. (b) Five minutes after taking 30 ml VMP35MNC. (c) Thirty minutes after taking VMP35 MNC.
Functional Foods in Health and Disease 2015; 5(9): 292-303
Page 300 of 303
Baseline and control
Baseline and the 5-minute samples in the control group observations included aggregation and
immobility – a sludge effect; malformation and damage and extensive hypo-chromic state (i.e. an
oversized ‘donut hole' evidencing reduced hemoglobin). At baseline, extensive ‘debris' in the
plasma and ‘dwarfed' white blood cells (WBCs) were also observed. A cross-section of ages and
a variety of conditions (see Table 2) were represented by the subjects so that the individual
baseline blood smear examples shown in the figures do not look similar.
Post supplement RBC improvements after 5 minutes
Post-supplementation RBC improvements after 5 minutes included a breakup of aggregation and
splaying out of RBCs on the slide; improved RBC spherical formation and a progressive
reduction (with time) of hypochromicity. Other post supplementation observations include
improved movement and ability to flow (rheology) of RBCs in plasma, evidence of improved
hydration, reduced viscosity, and reduced surface tension.
Post supplement RBC improvements after 30 minutes
Group 3 PBSs were evaluated at 5 minutes and 30 minutes post intake of the VMP35 MNC and
similarly showed marked improvement in biomarkers from baseline. Observations of
supplementation post intake, at five and 30 minutes also showed improved hemoglobin
concentration and a reduction in plasma debris. The plasma looked cleaner, possibly due to a
reduced quantity of sequestrants (of unknown origin), which are greater in number in blood with
greater cellular aggregation and reduced hydration. See examples in Figures 5 and 6. Overall,
improvements in the splayed arrangement, size, form, density and distribution of RBCs
following the intake of the VMP35 MNC were observed.
DISCUSSION
In this experiment, RBC and blood rheology improvements were observed demonstrating that
VMP35 nutraceutical encapsulated within the multilamellar SK713 SLP Prodosome architecture
was delivered and absorbed by sublingual trans-mucosa within 5 minutes.
PBS LBCI offers a unique ability to observe the rapid onset of hematological changes in
response to interventions [12]. The objective of using PBS LBCI in this study was for it to serve as a time-sensitive marker of biological perturbation and as a visual analytical tool for the degree of response to the delivered bioactive nutrients. The central finding was that changes occurred within 5 minutes of administration of VMP35 MNC and were sustained for at least 30 minutes. Conversely, no such changes were found when the equivalent volume of water was ingested by the control group, which adds credibility to the baseline findings and demonstrates reproducibility, in the absence of active intervention. On the other hand, the prompt, sustained and progressive findings in the active groups; Group 2 at five minutes and Group 3, at 5 and 30 minutes supports the validity of the observations. This conclusion is further strengthened by the observations of similar findings in Group 1 during the active cross-over phase.
Bioactive effects were observed. This evaluation demonstrates that the SK713 SLP delivery
technology exerts rapid positive effects on morphological, hematological, and rheological properties of the blood. This rapid response also suggests that the SK713 SLP technology
Functional Foods in Health and Disease 2015; 5(9): 292-303
Page 301 of 303
efficiently delivers nutrients into the blood via the sublingual mucosa in less than 5 minutes from the intake.
Although blood levels of the major bioactive ingredients in the blood are required to
determine the bioavailability and bioactivity this speedy sublingual delivery may, however, overcome digestive inefficiencies of those with various underlying digestive pathologies [5, 8, 13-14]. We are also proposing that the entire SK713 SLP process could also help the remaining nutrients disperse over a larger surface area within the small intestine. The low-sheer tri-blender jet compression technology decreases the particle size of larger and more granular or resinous materials. The smaller particle size of a particular nutrient will allow this nutrient to cover a broader surface area once it reaches the small intestine. We speculate that the SK713 SLP spheres provide protection of the encapsulated nutrient contents within the multilamellar structures against the harsh acidic environment of the stomach. Our notion is that this protection enables the nutrients within the spheres to reach the small intestine intact, which should promote greater nutritional synergy in absorption and utilization [13].
Interestingly, variations in this type of delivery technology have been the focus of many
pharmaceutical companies to enhance drug delivery [13, 14]. In addition, a liposomal delivery system been shown previously to improve the bioavailability of alendronate in rats [15], this Prodosomal (more advanced liposomal delivery) technology was devised to simultaneously envelope a diverse range of nutrients including more granular or resinous materials.
Limitations
In this experiment PBS LBCI is not intended for any diagnostic evaluations as this imaging
technology has not been considered appropriate for such applications. However, using PBS and
LBCI, RBC and blood rheology, improvements and changes in the LBC morphology were
observed at the intervals tested. There was no measurement of the level of the major bioactive
ingredient in the plasma or red blood cells at times 0, 5 and 30 minutes. Future research that
assesses levels of the major bioactive ingredients in the blood is required to determine levels of
absorption, bioavailability and bioactivity, not measured in this study. The design of any future
studies will be double blinded and include quantification using statistical analysis.
CONCLUSIONS: The results of this observational study provide support that a Phospholipid
Prodosome SK713 SLP nutrient delivery technology compared to placebo rapidly and effectively
delivered nutraceuticals into the blood. As such, the SK713 SLP system, following required
additional research, makes an important contribution towards increasing the potential benefits of
dietary supplements.
We encourage additional research on this novel delivery system and the positive collateral
changes observed in other properties of the blood must be noted and merit future investigation.
Abbreviations: Live Blood Cell Imaging (LBCI), Peripheral Blood Smear (PBS), Multinutrient
complex SK713, Soy Lecithin Phospholipid (SLP), Red Blood Cells (RBC), Generally
Recognized As Safe (GRAS), white blood cells (WBCs)
Functional Foods in Health and Disease 2015; 5(9): 292-303
Page 302 of 303
Competing interests: B. William Downs, Stephen Kushner, and Kenneth Blum have ownership
interest in Victory Nutrition International. Ted Aloisio did not have any financial interest when
carrying out this non-blinded study. Ted Aloisio's spouse became an independent representative
of Victory Nutrition. There are no other conflicts to report.
Author Contributions: The design of the study was a team effort primarily involving B.
William Downs, Stephen Kushner and Ted Aloisio. The technology was developed by Stephen
Kushner; with the assistance of B. William Downs. Ted Aloisio performed the experiments. The
interpretation of these data was further evaluated by Kenneth Blum, PhD, and Frans J. Cronjé.
The manuscript was written by both B. William Downs and Stephen Kushner. Kenneth Blum
extended the writing and provided the basis of the final manuscript. The final submitted
manuscript was approved by all authors prior to submission.
Acknowledgments and Funding: The authors would like to thank Margaret A. Madigan for her
expert edits of the entire manuscript. The authors would like to thank the research staff of
Victory Nutrition International, Inc, as well as Synaptamine, Inc. Ted Aloisio contributed
reagents/materials /imaging tools.
REFERENCES
1. US Dept. of Health and Human Services. Center for Disease Control and Prevention,
National Center for Health Statistics. National hospital ambulatory medical care survey: 2011
emergency
department
[http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf] (accessed 18th September, 2014)
2. Nawar EW, Niska RW, Xu J: National hospital ambulatory medical care survey: 2005
emergency department summary. Advance data 2007, 1-32.
3. Koerner MM, Palacio LA, Wright JW, Schweitzer KS, Ray BD, Petrache HI:
Electrodynamics of lipid membrane interactions in the presence of zwitterionic buffers. Biophysical journal 2011, 101:362-369.
4. Keller BC: Liposomes in nutrition. Trends in Food Sci Techn 2001, 12: 25-31. 5. Shoji Y, Nakashima H: Nutraceutics and delivery systems. J Drug Target 2004; 12:385-
6. Scholfield CR. Composition of soybean lecithin. J Amer Oil Chem Soc 1981; 58:889-
7. Ambroziak A, Cichosz G: [Milk phospholipids as nutraceutic]. Pol Merkur Lekarski
8. Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y,
Samiei M, Kouhi M, Nejati-Koshki K: Liposome: Classification, preparation, and applications. Nanoscale Res Lett 2013, 8: 102.
9. Helfrich W: Distributions of vesicles: The role of the effective rigidity of membranes. J
Phys 1984, 47(2): 321-329.
Functional Foods in Health and Disease 2015; 5(9): 292-303
Page 303 of 303
10. Bouchard, G.; Pagliara, A.; Carrupt, P.A.; Testa, B.; Gobry, V.; Girault, H.H.
Theoretical and experimental exploration of the lipophilicity of zwitterionic drugs in the 1,2-dichloroethane/water system. Pharmaceutical research 2002, 19:1150-1159.
11. Popescu G, Park Y, Choi W, Dasari RR, Feld MS, Badizadegan K: Imaging red blood
cell dynamics by quantitative phase microscopy. Blood cells, molecules & diseases 2008, 41:10-16.
12. Popescu G, Ikeda T, Best, CA, Badizadegan K, Dasari RR, Feld MS:. Erythrocyte
structure and dynamics quantified by hilbert phase microscopy. Journal of biomedical optics 2005, 10. doi:10.1117/1.2149847.
13. Akbarzadeh A, Samiei M, Joo SW, Anzaby M, Hanifehpour Y, Nasrabadi HT, Davaran
S: Synthesis, characterization and in vitro studies of doxorubicin-loaded magnetic nanoparticles grafted to smart copolymers on a549 lung cancer cell line. J Nanobiotechnology 2012, 10: 46. doi: 10.1186/1477-3155-10-46.
14. Valizadeh A, Mikaeili H, Samiei M, Farkhani SM, Zarghami N, Kouhi M, Akbarzadeh
A, Davaran S: Quantum dots: Synthesis, bioapplications, and toxicity. Nanoscale Res Lett 2012, 28: 7, 480. doi: 10.1186/1556-276X-7-480.
15. Han HK, Shin HJ, Ha DH. Improved oral bioavailability of alendronate via the
mucoadhesive liposomal delivery system. Eur J Pharm Sci. 2012, 46(5):500-507. doi: 10.1016/j.ejps.2012.04.002.
Source: http://www.prodovite.biz/wp-content/uploads/2016/02/Prodovite_Clinical_Study-with-Live-Blood-Pictures.pdf
Breast Cancer Res Treat (2012) 131:939–947 Individually tailored treatment with epirubicin and paclitaxelwith or without capecitabine as first-line chemotherapyin metastatic breast cancer: a randomized multicenter trial T. Hatschek • L. Carlsson • Z. Einbeigi • E. Lidbrink • B. Linderholm •B. Lindh • N. Loman • M. Malmberg • S. Rotstein • M. So¨derberg •M. Sundquist • T. M. Walz • M. Hellstro¨m • H. Svensson • G. A
Piano Nazionale Vaccini (aggiornamento 2005) Sommario Pag. 3 Introduzione Pag. 5 La priorità nelle scelte delle strategie vaccinali: criteri generali Pag. 7 Parte Prima 1. Malattie prevenibili con la vaccinazione: cosa è cambiato negli ultimi 5 anni Pag. 10 2. Obiettivi di salute da raggiungere con le vaccinazioni e strategie di scelta Pag. 14





