Microsoft word - review of trial data.doc
An examination of existing trial data on the treatment
of prostate cancer using external beam radiotherapy
combined with hormone therapy.
Professor L.J.S.Bradbury Ph.D.
1.0 Introduction. For low or intermediate risk prostate cancer patients, external beam radiotherapy is one of the common forms of treatment available to them. In the UK, this treatment is most commonly given in fractions of 2Gy with a total dose of 70Gy. This dose combination seems to have emerged over time as being a reasonable compromise between curative efficacy and the avoidance of too severe acute and long-term side effects. However, improvements in linear accelerator technology in the form of three-dimensional conformal beam radiotherapy and intensity modulated radiotherapy have led to a situation where higher doses can be delivered without increasing these side effects. In addition, it is clear that the use of hormone therapies in conjunction with radiotherapy also improves disease free survival rates. This paper discusses the evidence in favour of changing the present UK standard practice to a more aggressive radiotherapy regime but one with a shorter period of hormone use. Firstly, in sections 2 and 3, it is shown that the use of LHRH agonists like goserelin (i.e. Zoladex as its trade name) used in conjunction with external beam radiotherapy leads to very significant improvement in disease free survival. In particular, it is the use during and after the radiotherapy (concurrent and adjuvant use) that leads to the largest improvement and this improvement can be achieved by a period of treatment measured in months rather than the years previously thought necessary. There seems to be less benefit (if any) from the neo-adjuvant use of hormones but nonetheless it is probably worthwhile if only to reduce the prostate size to a more compact target. The reduction in risk of disease recurrence from the adjuvant use of hormones seems to be around the 50% level. The question of the relative merits of bicalutamide (i.e. Casodex as its trade name) as an alternative to goserelin is briefly discussed in section 4. Section 5 then discusses the effect of the overall radiotherapy dose on disease free survival and it is argued that there is a worthwhile gain to be achieved by increasing the dose to between 76 and 80Gy which should not lead to a significant increase in side effects provided either 3-D conformal or IMRT techniques are used. Compared with a 70Gy dose, this would lead to an improvement in the five-year disease free survival probability for an intermediate risk patient from around the 70% level to the region of 80% from the dose increase alone and a further improvement to around 90% from the use of adjuvant hormones. Some comments on possible synergy between hormones and radiotherapy are given in section 6 and the possible advantages of a lower level of radiation being applied to the whole pelvic region is discussed in section 7.
Finally, in section 8, it is concluded that the existing UK practice should be replaced by one in which between 76 and 80Gy of radiation in 2Gy fractions should be the routinely given in conjunction with either Zoladex or Casodex admininstered in neo-adjuvant, concurrent and adjuvant use but with the period of adjuvant use reduced to a period of around six months. There is also the possibility of further improvements in treatment efficacy by increasing the acute dose fraction (hypofractionation) but this will be discussed in a separate paper. 2.0 External beam radiotherapy with and without the use of goserelin
as an adjuvant hormone treatment – the disease free survival data.
The published data on the influence of adjuvant hormone treatment on external beam radiotherapy (ebrt) refers invariably to the use of goserelin as the primary hormone treatment with an anti-androgen such as bicalutamide as the secondary hormone treatment used to block testosterone ‘flare'. However, there has been no consensus about the period of hormone therapy necessary to achieve the optimum benefit. But, recent work by D'Amico et al (2004) taken in conjunction with the EORTC trial reported by Bolla et al (2002) and the RTOG 92-02 trial (Sandler et al(2003) and Hanks et al(2003)) now provides quite powerful evidence that the period of hormone treatment can be much shorter than previously envisaged. In particular, the merit of comparing the D'Amico results with Bolla's is that they were very comparable trials with the exception of the period of the adjuvant hormone therapy. Both trials used an ebrt dose of 70Gy delivered over similar periods of six or seven weeks at a rate of 2Gy per day. Table 1 lists other important features of these two trials. In the case of Bolla's results, only the data for ‘intermediate' risk patients has been used as these are closely similar to the patients used in the D'Amico trial.1 The ebrt only arms of the trial groups did not apparently receive any form of hormone therapy.
Table 1-Comparison of the androgen suppression therapy regimes used in the D'Amico and
the Bolla trials.
One of the areas of difference between the two trials is the definition of treatment ‘failure'. In Bolla's case, disease progression on the basis of PSA measurements was defined as a PSA greater than 1.5ng/ml with two successive increases. In D'Amico's case, failure was defined as a PSA of greater than 1 ng/ml with an increase in PSA of more than 0.2ng/ml on two consecutive measurements. This is a weakness in making comparisons but it has to be hoped that the general conclusions are not overly affected by the definition of ‘failure'. In the RTOG 92-02 trial, hormone therapy was used in both arms of the trial but, in one case, androgen suppression therapy (AST) was restricted to neo-adjuvant and concurrent use whereas in the other arm, this was followed by two years of adjuvant 1 The average starting PSA in the D'Amico trial was 11ng/ml with an average Gleason score of 7. In Bola's trial, ‘intermediate' risk meant that patients had two out of three criteria, namely (i) PSA>=10, (ii) Gleason>6 and (iii) staging worse than T1c.
hormone therapy. The trial group contained patients with much higher PSA values and Gleason scores than either the D'Amico or the Bolla trials. There was also some variation in the ebrt dose given but it averaged around 70 Gy to the prostate although, in high risk patients, this was supplemented by additional radiation to the whole pelvic region. Table 2 gives the details of the two RTOG 92-02 AST regimes.
Table 2- Comparison of the AST regimes used in the two arms of the RTOG 92-02 trial.
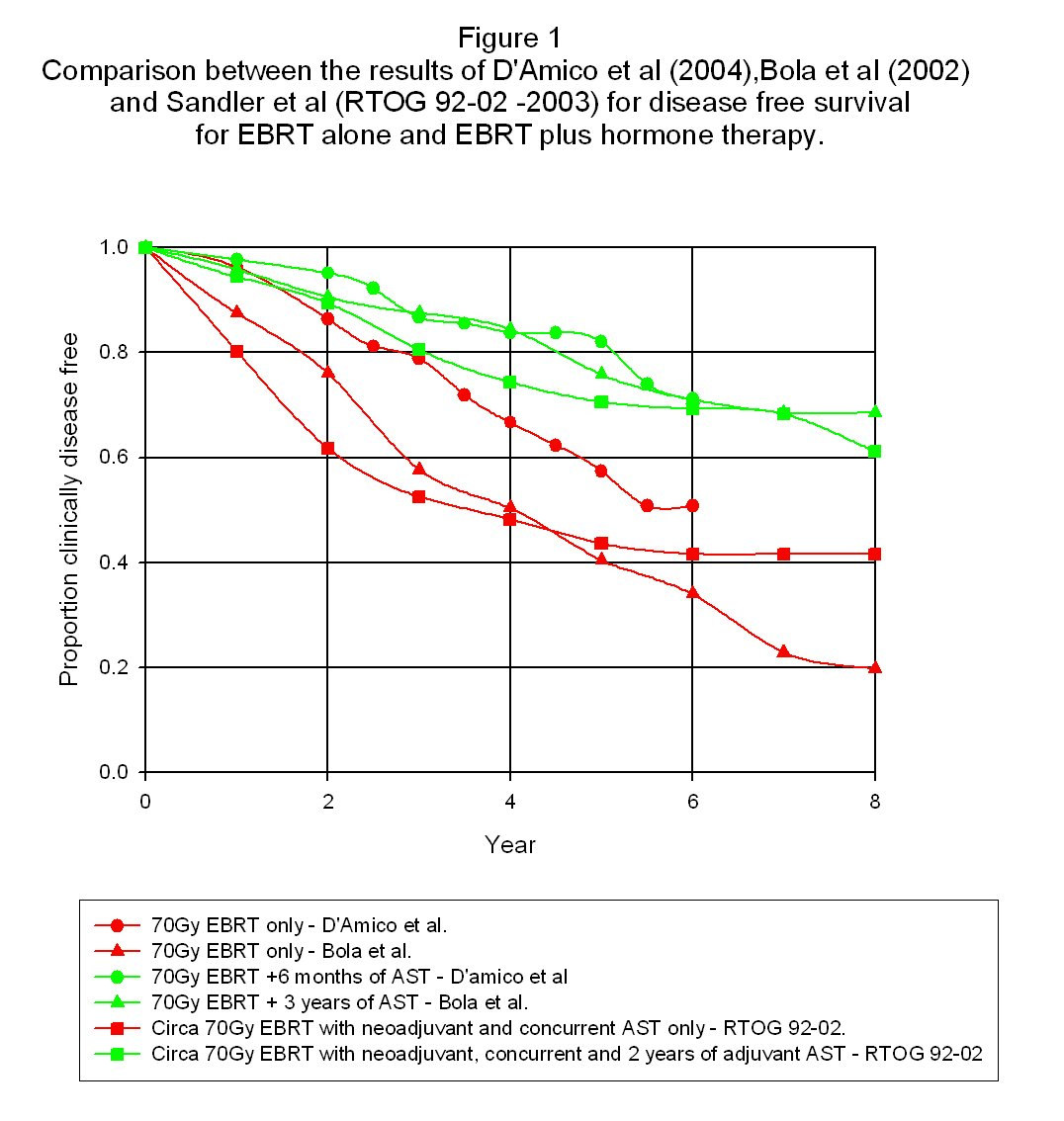
The definition of treatment failure in the RTOG 92-02 trail was the ASTRO definition of three consecutive PSA rises. Figure 1 shows the results for disease free survival as a function of time for all these trial results.
The most obvious point about these results is that in spite of the many differences between the trials and the definitions used of treatment failure, the results convincingly cluster into two groups – namely those without adjuvant hormones and those with adjuvant hormones. This confirms the clear advantage in the use of adjuvant hormone therapy as far as disease free survival is concerned. Very roughly, it looks as if failure free survival using 70Gy of radiation for a broad spectrum of
patients without adjuvant therapy is around 40% after between six and eight years whereas the addition of the adjuvant therapy increases this to around 60-70%. However, as will be discussed in section 5, disease free survival is a strong function of the initial patient ‘state' and so these figures are of very little individual significance. The second point of note is that there does not seem to be any particular difference in the effectiveness of adjuvant hormone use between the 2-month, 2-year or 3-year results. Since the use of goserelin leads to a significant loss of quality of life for patients, it would be an important advantage if its use could be restricted to a much shorter timescale than was previously imagined to be necessary. It would also lead to a cost saving in the treatment! If one wanted to adopt a cautious approach to reducing the period of using goserelin, it would seem that there is little doubt that, say, six months use of hormone therapy after the end of ebrt would ensure substantially the full advantage of adjuvant hormones. The third point of note is that there does not seem to be any clear evidence in the above trial data of an improvement as far as disease free survival is concerned in the use of neo-adjuvant hormone treatment. This is not to say that such a benefit does not exist but it is certainly lost in the general scatter of the data produced both by genuine variability in the data and also from other factors like differing definitions of the disease free state and differing characteristics of the patient populations used. By contrast, the effect of adjuvant therapy seems clear irrespective of these causes of variability and so one of the slightly surprising conclusions from this is that adjuvant hormone use has a bigger therapeutic impact than neo-adjuvant hormone use. Of course, the reduction in the size of the prostate due to neo-adjuvant hormone use may have other advantages in terms of providing a more compact target with a greater separation from surrounding organs like the rectum thus resulting in lower collateral radiation damage. On the question of neo-adjuvant hormone therapy, it should be noted that Crook et al (2004) found no difference in disease-free survival at a five-year follow-up period between three months and eight months of neo-adjuvant hormone use in the form of Zoladex. Unfortunately, there was no arm in the trial without any hormones at all. It is also worth noting that Beyer et al (2005) actually found a survival disadvantage from the use of neo-adjuvant hormones when used in conjunction with permanent seed brachytherapy. Thus, the value of neo-adjuvant hormone use is far from clear. 3.0 External beam radiotherapy with and without the use of goserelin
as an adjuvant hormone treatment – the overall survival data.
The three trials discussed in the previous section all reported overall survival data but, for present purposes, it is sufficient to include here just the results for the D'Amico and Bolla trials. Figure 2 is a copy of their survival data taken from the original reports. It is important to note that because prostate cancer affects older men who are at a comparatively high risk of death from other causes, the impact of a ‘successful' prostate cancer treatment will be weaker on overall survival statistics than its impact on disease free survival data. Nevertheless, both the D'Amico and the Bolla data show an overall survival benefit. The Bolla data shows a lower survival rate than the
D'Amico data but it should be noted that Bolla's data in figure 2 now includes the high risk patients and this is probably why these results are worse than the D'Amico data. Although Bolla separates the disease free survival data into the three groups of low, intermediate and high risk, he does not do this in his paper for the overall survival characteristics so that a direct comparison with the D'Amico data is not possible. A final comment on the D'Amico data is that, in both arms of the trial, death from other causes was greater than prostate cancer deaths. For ebrt alone, there were 23 deaths out of 103 participating patients at six years but only six of these were from prostate cancer. In the ebrt+hormone arm, there were 12 deaths out of 98 participating patients with no deaths from prostate cancer.
Figure 2. The overall survival data of D'Amico and Bolla.
4.0 External beam radiotherapy with and without the use of Casodex
as the only adjuvant hormone treatment.
Because its toxicity is less than that of Zoladex (goserelin), the use of Casodex (bicalutamide) given at a daily rate of 150mg is an attractive alternative adjuvant hormone therapy. However, although the large Casodex trial involving some 8,000 patients reported by See et al (2002) in the Journal of Urology contained about 1,300 patients treated with ebrt, these ebrt results have not yet been reported separately in a detailed form. However, Payne et al(2003) and Payne(2004) reports a general figure from this trial that the risk of disease progression was reduced in the ebrt arm of the trial by 42% through the use of Casodex. It is not obvious that the interaction between radiation and prostate cancer cells will the same when cells are deprived of testosterone as compared to when their testosterone receptors are ‘blocked' by bicalutamide but the figure given by Payne seems to show that the effect is similar. 5.0 The effect of total dose on the efficacy of ebrt. The dilemma in using radiation as a treatment for cancer is that of walking the line between having a high enough dose to achieve good tumour control and yet not so high as to cause unacceptable adverse side effects. Additionally, there is some variability in an individual's tolerance to radiation, which further complicates the matter. In the case of prostate cancer, general practice over the last decade or so
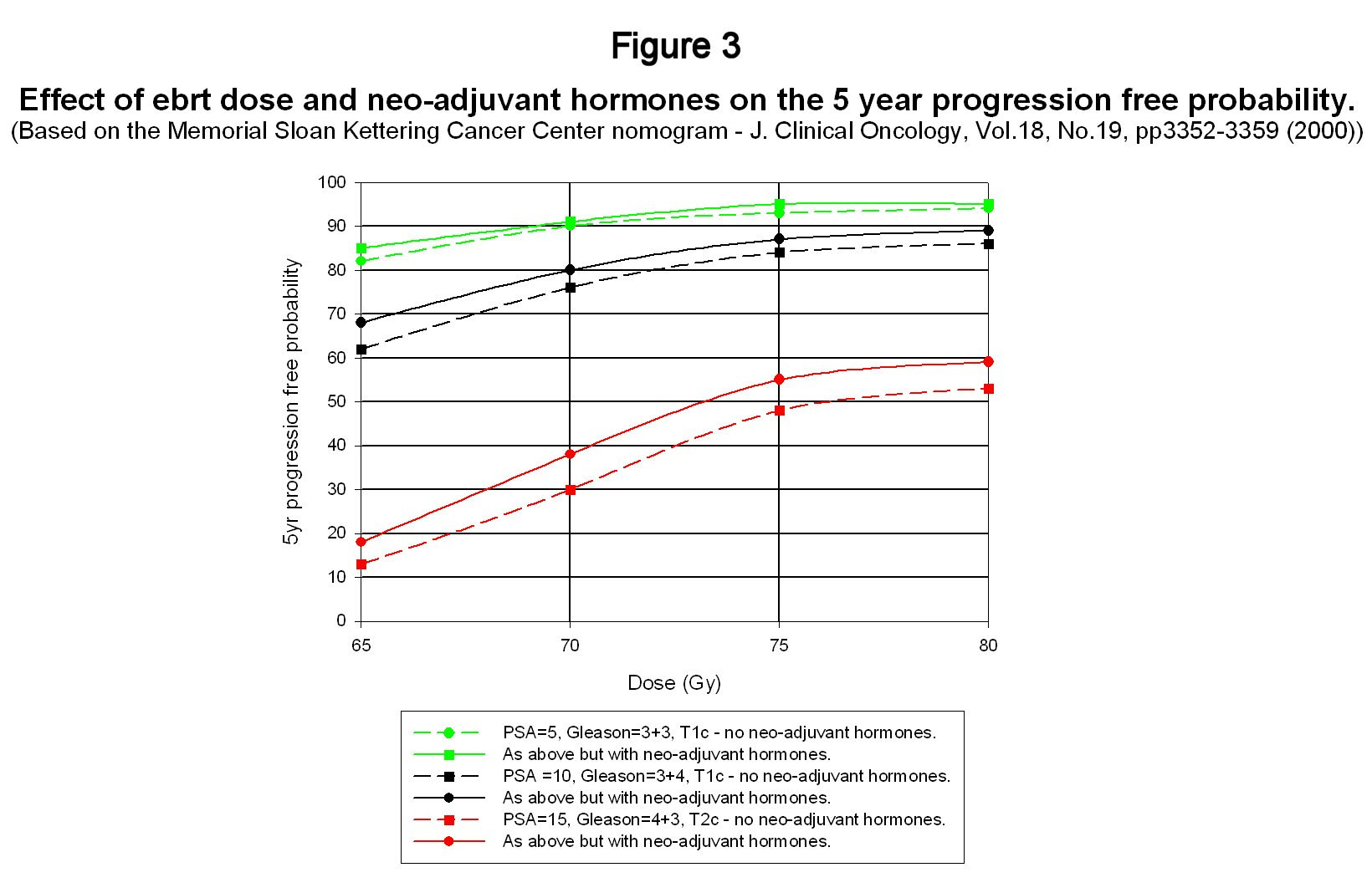
seems to have settled on a figure of about 70 Gy delivered at around 2Gy per day. However, progress in recent years with beam collimation in linear accelerators coupled with the development of mathematical techniques to optimise radiation dose profiles has enabled dose levels to be increased without increasing adverse side effects. The technique of three-dimensional conformal radiotherapy has now been further incrementally improved by beam intensity modulated techniques and dose levels as high as 86.4 Gy have been tested at the Memorial Sloan-Kettering Cancer Center (Zelefsky et al (2002)) without significant changes to acute or long-term side-effects. This group has also developed nomograms for predicting the outcome of various forms of treatment for prostate cancer and these have been collated into a single Microsoft Access database that can be download from the Memorial Sloan-Kettering website2 (www.mskcc.org/mskcc/html/10088.cfm). For present purposes, the nomogram for predicting the outcome of ebrt is the one that is of interest – see Kattan et al (2000). This nomogram was based on data from 1042 patients at Memorial Sloan-Kettering and then compared with a second independent set of patient data from the Cleveland Clinic, Ohio. The real value of this nomogram is not its absolute accuracy – which might easily be disputed – but in its prediction of trends. Although there has been a huge amount of other data published on dose effects, this nomogram provides a compact means of looking at the influence of a variety of factors on treatment outcome. Figure 3 shows predictions for 5 year disease free survival as a function both of total dose with and without neoadjuvant and concurrent hormones and also as a function of initial patient ‘risk' factors. Adjuvant hormones were not used in the data from which this nomogram was compiled. Failure is based on the ASTRO definition. The confidence intervals have not been shown to avoid cluttering up the graph.
2 This database covers not only ebrt (with and without neo-adjuvant hormone therapy) but also radical prostatectomy and brachytherapy (including adjuvant ebrt). The database also has built into it the Partin tables and a facility for calculating the PSA velocity and doubling time for an arbitrary number of PSA readings. It also includes a life expectancy calculator and a prostate volume calculator! It is very simple to use too.
This figure shows a number of interesting trends. The first is the very strong influence of the initial risk factors on disease free survival probability. The prospects for low and intermediate risk patients are comparatively good with conventional 70Gy treatment but the outlook when PSA rises above 10 coupled with a palpable tumour and high Gleason score diminishes rapidly. The second point is that the improvement in outlook by increasing the dose from 70Gy to around 76 to 80Gy is significant and there have been a number recent reports in addition to the Zelefsky work that strongly indicate that this increase in dose can be achieved without a significant impact on side effects provided that advanced three-dimensional conformal or intensity modulated techniques are used. The third point to note is that whereas the nomogram predicts an improvement in disease free survival from the use of neo-adjuvant hormones (Sloan Kettering use goserelin), the effect is not large particularly for low and intermediate risk patients – particularly when compared to adjuvant use. This has already been discussed in reference to the results shown in figure 2 and once again casts doubt on the value of neo-adjuvant hormone use. The final point concerns the consistency of the nomogram predictions with the trial data discussed in section 2. The whole process of treatment and the measurement of its efficacy is not a precise science and so it is important not to be too transfixed by precise numbers. Nevertheless, one would not want glaring inconsistencies between them either. On the face of it, the Sloan-Kettering nomogram seems to be a little optimistic in its predictions compared with the trial data shown in figure 2. However, as has already been mentioned, treatment outcome is very sensitive to the initial ‘risk' factors. The D'Amico data was drawn from a narrower range of patients based on initial risk factors than the other trials and, roughly speaking, his average initial conditions were a PSA of 11, a Gleason score of 7 and a tumour grading of T2a. His five year survival free probability with ebrt alone was somewhere between 50% and 60%. The Sloan-Kettering nomogram gives a probability of 58% (95% confidence limits 48%-68%)! For interest, the nomogram predicts an improvement in disease free probability with neo-adjuvant hormones to 64% (95%confidence limits 54% to 74%) whereas D'Amico's results with 2 months of adjuvant therapy as well gives about a 75-80% disease free probability. Of course, making comparisons with one data point is a risky business – to put it mildly – but the nomogram seems reasonably consistent with the trial data of section 2. As more data becomes available from maturing trials, it should be possible to extend the nomogram to longer time scales and also include the influence of adjuvant hormone therapy. However, as will be discussed in the second review paper, it is almost certainly better to develop an outcome ‘predictor' using a framework that is not entirely empirical. 6.0 Is there synergy between radiotherapy and androgen deprivation? It is generally agreed that radiation works in killing tumours by causing irreparable double strand breaks (DSB's) in the DNA molecule, which lead to cell death at mitosis (i.e. cell division) although it is also thought that cell death can in some instances occur prior to cell division (apotosis). By contrast androgen deprivation or
androgen blocking is not thought to lead to the death of prostate cells directly but rather that it slows down cell replication. The question of the interaction between chemotherapy and radiotherapy is briefly discussed in an article by Stewart and Bartelink (Exploitable mechanisms in combined radiotherapy and chemotherapy) in the book Basic Clinical Radiobiology (ed. G.G.steel (2002)). In this article, they discuss interactive and non-interactive combined treatments. One of the interesting conclusions from the observation that adjuvant therapy seems more effective than neo-adjuvant therapy is that this shows almost certainly that there is interaction between the hormone therapy and the radiation and that this interaction is different between neo-adjuvant hormones and adjuvant hormones. The interaction in the neo-adjuvant use of hormones is supposed to be due to tumour size reduction and may be a volume effect. In the case of adjuvant use, the effect is larger and it seems that cancer cells deprived of testosterone are somehow more susceptible to radiation damage than would otherwise be the case. 7.0 Targeting in prostate cancer external beam radiotherapy. With modern ebrt techniques, it is possible to ‘sculpture' the isodose contours to a much greater extent than was possible in the past with the principle benefit of reducing the damage to surrounding organs. In the case of the prostate, it is the rectum and bladder that are the most likely to suffer damage. However, in analogy to surgical margins, it is generally necessary to irradiate a region surrounding the prostate because, as the Partin tables demonstrate, the spread of cancer beyond the gland and into the seminal vesicles and lymph nodes is a significant risk apart from low risk patients with low PSA's and Gleason scores. Roach et al (2003) in RTOG trial 9413 took this further by routinely exposing the trial patients to whole-pelvic radiotherapy with a total dose of 50.4Gy followed by a prostate only ‘boost' of 19.8 Gy. The precise details of the fields covered are given in their report. They claimed a 10% improvement in disease free survival at 4 years without a statistically significant increase in acute or late complications – although it has to be said that the whole-pelvic group did have consistently higher numbers of acute and late gastrointestinal side-effects compared to the control group.3 8.0 Concluding remarks. From an examination of existing data, it does seem that comparatively minor changes to the UK practice for the ebrt treatment of prostate cancer could be made which should lead to a worthwhile improvement in disease free progression without incurring either an increase in side effects (either acute or late) or an increase in treatment cost. The protocol would be as follows:- (i) Provided conformal beam or intensity modulated beam radiotherapy facilities are available, an ebrt dose of 76-80 Gy delivered at 2 Gy per day should replace the existing 70 Gy standard dose4.
3 Acute gastrointestinal (GI) problems were reported in 14 out of 643 patients in the whole pelvic group compared to 5 in the 640 control group. Late grade 3+ GI side effects were reported in 12 patients in the whole pelvic group compared to 5 in the prostate only group. The differences in the urinary side effects were much smaller than this. 4 Other recent reports also conclude that a somewhat more aggressive approach to prostate cancer treatment in terms of radiation dose is now appropriate.
(ii) Goserelin with an anti-androgen should be administered as it is now in neo-adjuvant, concurrent and adjuvant phases but with the period of adjuvant use reduced to a shorter period than is currently the case. The adjuvant period apparently could be reduced to as little as two months without a significant loss of therapeutic benefit but a conservative approach might be to reduce this period to, say, somewhere between four and six months. (iii) The question of a targeting protocol is less obvious until more data are available. In addition to these points, there is also the important question of the relative merits of Zoladex compared to Casodex as the hormone arm of the treatment. Interim results suggest that the effects of these two drugs are similar but detailed results on the interaction with ebrt from the large Casodex trial have not yet been published. In any case, if the total period of hormone therapy is reduced to the order of a year or less then the adverse side effects of goserelin could probably be borne with more stoicism than the much longer periods currently thought necessary. Finally, it should be noted that there is a prospect of improving further the treatment efficacy of ebrt by increasing the acute fractions to around 3Gy and reducing the overall dose to around 60-63Gy (hypofractionation) but the arguments about this more radical change will be discussed in a later review paper.
References.
D'Amico, Manola, Loffredo, Renshaw, DellaCroce & Kantoff (2004). 6-month androgen suppression plus radiation therapy alone for patients with clinically localised prostate cancer. JAMA, vol.292, no.7, pp.821. Beyer, D.C., McKeough, T. and Thomas, T. (2005) Impact of short course hormonal therapy on overall and cancer specific survival after permanent prostate brachytherapy Int.J.Radiation Oncology Biol Phys., vol 61, No.5, pp.1299-1305. Bolla, Collette, Blank, Warde, Dubois, Mirimanoff, Storme, Bernier, Kuten, Sternberg, Mattalaer, Torecilla, Pfeffer, Cutajar, Zurlo & Piersrt (2002). Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. The Lancet, Vol.360, July 13, pp. 103 Crook J., Ludgate C., Malone S., Lim J., Perry G., Eapen L., Bowen J., Robertson S., and Lockwood G. (2004) Report of a multicenter Canadian phase III randomised trial of 3 months vs. 8 months neo-adjuvant androgen deprivation before standard-dose radiotherapy for clinically localized prostate cancer. Int.J.Radiation Oncology Biol Phys., vol 60, No.1, pp.15-23.
Hanks, Pajak, Porter, Grignon, Brereton, Venkatesan, Horwitz, Lawton, Rosenthal,
Sandler & Shipley (2003).
Phase III trial of long-term adjuvant androgen deprivation after neo-adjuvant
hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the
prostate: the RTOG protocal 92-02.
J. of Clinical Oncology, Vol.21, No.21, pp.3972-3978
Kattan, Zelefsky, Kupelian, Scardino, Fuks & Leibel (2000)
Pre-treatment nomogram for predicting the outcome of three-dimensional conformal
radiotherapy in prostate cancer.
J. Clinical Oncology, Vol.18, No.19, pp.3352-3359.
Payne, H (2004)
The value of delaying disease progression with bicalutamide (‘Casodex)150mg in
locally advanced prostate cancer.
European Journal of Hopsital Pharmacists, Vol.5, pp.42-44.
Payne H., Tyrrell C., See W.A., McLeod D.G., Wirth M.P., Iversen P., Garside L.
(2003)
Bicalutamide 150 mg as adjuvant to radiotherapy significantly increases progression-
free survival in early prostate cancer
Int. J. Radiation Oncology Biol Phys., Vol. 57, Issue 2 (Supplement), S173-S174 57
Pilepich M.V., Winter K., Lawton A., Krisch R.E., Wolkov, H.B., Movsas B.,
Hug E.B., Absell, S.O., and Grignon, D. (2005)
Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma –
long-term results of phase III RTOG 85-31.
Int.J.Radiation Oncology Biol Phys., vol 61, No.5, pp.1285-1290.
Roach, DeSilvio, Lawton, Uhl, Machtay, Seider, Rotman, Jones, Asbell, Valicenti,
Han, Thomas & Shipley (2003).
Phase III Trial Comparing Whole-Pelvic Versus Prostate-Only Radiotherapy and
Neoadjuvant Versus Adjuvant Combined Androgen Suppression: Radiation Therapy
Oncology Group 9413.
J. of Clinical Oncology, Vol.21, No.10.
Sandler, Pajak, Hanks, Porter, DeSilvio & Shipley (2003).
Can biomechanical failure (ASTRO definition) be used as a surrogate endpoint for
prostate cancer survival in phase III localised prostate cancer clinical trials?
Analysis of RTOG 92-02 data.
Powerpoint presentation from website
http://www.fda.gov/cder/drug/cancer_endpoints/ProstatePresent/Sandler.ppt.
See, Wirth, McLeod, Iversen, Klimberg, Gleason, Chodak, Montie, Tyrell, Wallace,
Delaere, Vaage, Tammela, Lukkarinen, Persson, Carroll & Kolvenbag (2002).
Bicalutamide as immediate therapy either alone or as an adjuvant to standard care of
patients with localised or locally advanced prostate cancer: first analysis of the early
prostate cancer program.
J. of Urology, Vol. 168, pp.429-435.
Steel G.G. (Editor)(2002) Basic Clinical Radiobiology – 3rd Edition. Arnold, London Zelefsky, Fuks, Hunt, Yamada, Marion, Ling, Amols, Venkatraman & Leibel (2002). High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int.J.Radiation Oncology Biol Phys., vol 53, No.5, pp.1111-1116. Memorial Sloan Kettering Cancer Center website. Prostate Nomogram for Microsoft Access. http://www.mskcc.org http://www.mskcc.org/mskcc/html/10088.cfm
Source: http://www.prostate-cancer-radiotherapy.org.uk/Pdf%20files/Review%20of%20trial%20data.pdf
Distributed in Australia by Orthotic & Prosthetic Centre Pty Ltd, Trading as OPC Health 151-159 Turner Street PORT MELBOURNE VIC 3207 T: 03 9681 9666 F: 03 9681 9366E: [email protected] OPC HEALTH ABN: 26 454 494 673 ACN: 005 863 525 SECTION 1 – Chemical Product and Company Identification
NOMBRE DEL MEDICAMENTO: Arava 10 mg, Arava 20 mg, Arava 100 mg comprimidos recubiertos con película COMPOSICIÓN CUALITATIVA Y CUANTITATIVA: Arava 10 mg. Cada comprimido contiene 10 mg de leflunomida. Excipiente(s) con efecto conocido: cada comprimido contiene 78 mg de lactosa monohidrato Arava 20 mg. Cada comprimido contiene 20 mg de leflunomida. Excipiente(s) con efecto conocido: cada comprimido contiene 72 mg de lactosa monohidrato. Arava 100 mg. Cada comprimido contiene 100 mg de leflunomida. Excipiente(s) con efecto conocido: cada comprimido contiene 138,42 mg de lactosa monohidrato. FORMA FARMACÉUTICA: Comprimido recubierto con película. Arava 10 mg: comprimido recubierto con película blanco o blanquecino, redondo, con la inscripción ZBN en una cara. Arava 20 mg: comprimido recubierto con película amarillento a ocre, triangulares, con la inscripción ZBO en una cara. Arava 100 mg: comprimido recubierto con película blanco o blanquecino, redondo, con la inscripción ZBP en una cara. DATOS CLÍNICOS. Indicaciones terapéuticas: La leflunomida está indicada para el tratamiento de pacientes adultos con: artritis reumatoide activa como un "fármaco antirreumático modificador de la enfermedad" (FARME), artritis psoriásica activa. El tratamiento reciente o concomitante con FARMEs hepatotóxicos o hematotóxicos (por ejemplo, metotrexato) puede producir un aumento del riesgo de aparición de reacciones adversas graves; por tanto, en estos casos, el inicio del tratamiento con leflunomida debe considerarse en función del balance beneficio/riesgo. Más aún, el sustituir la leflunomida por otro FARME sin realizar el procedimiento de lavado, puede incrementar el riesgo de aparición de reacciones adversas graves incluso durante un largo período de tiempo después del cambio. Posología y forma de administración: El tratamiento se debe iniciar y supervisar por especialistas con experiencia en el tratamiento de artritis reumatoide y artritis psoriásica. Los niveles de alanina transaminasa (ALT) o transaminasa piruvato glutamato sérico (SGPT) y un recuento hemático completo, incluyendo un recuento diferencial de leucocitos y un recuento de plaquetas, deben determinarse simultáneamente, y con la misma frecuencia en las siguientes situaciones: antes de iniciar el tratamiento con leflunomida, cada dos semanas durante los primeros seis meses de tratamiento, y posteriormente, cada ocho semanas. Posología: En artritis reumatoide: el tratamiento con leflunomida se inicia normalmente con una dosis de carga de 100 mg una vez al día durante 3 días. La omisión de la dosis de carga puede disminuir el riesgo de reacciones adversas. La dosis de mantenimiento recomendada es de 10 mg a 20 mg de leflunomida una vez al día dependiendo de la gravedad (actividad) de la enfermedad. En artritis psoriásica: el tratamiento con leflunomida se inicia con una dosis de carga de 100 mg una vez al día durante 3 días. La dosis de mantenimiento recomendada es de 20 mg de leflunomida una vez al día. El efecto terapéutico normalmente empieza después de 4 ó 6 semanas y puede mejorar posteriormente hasta los 4 ó 6 meses. No hay un ajuste de dosis recomendable en pacientes con insuficiencia renal leve. No se requiere realizar un ajuste de la dosis en los pacientes con edad superior a 65 años. Población pediátrica: No se recomienda la utilización de Arava en pacientes menores de 18 años, ya que no se ha establecido la eficacia y la seguridad en la artritis reumatoide juvenil (ARJ). Forma de administración: Los comprimidos de Arava deben ingerirse enteros con suficiente líquido. La ingesta de alimentos no modifica la absorción de la leflunomida. Contraindicaciones: Hipersensibilidad al principio activo (especialmente con historial previo de síndrome de Stevens-Johnson, necrólisis epidérmica tóxica, eritema multiforme) o a alguno de los excipientes. Pacientes con insuficiencia hepática. Pacientes con estados de inmunodeficiencia grave, por ejemplo, SIDA. Pacientes con afectación significativa de la función de la médula ósea o con anemia, leucopenia, neutropenia o trombocitopenia importante debida a causas distintas de la artritis reumatoide o psoriásica. Pacientes con infecciones graves. Pacientes con insuficiencia renal de moderada a grave, debido a que la experiencia clínica de la que se dispone en este grupo de pacientes es insuficiente. Pacientes con hipoproteinemia grave, por ejemplo en el síndrome nefrótico. Mujeres embarazadas o mujeres en edad fértil que no utilicen un método anticonceptivo eficaz durante el tratamiento con leflunomida y después de finalizar el mismo mientras los niveles plasmáticos del metabolito activo estén por encima de 0,02 mg/l. Antes de iniciar el tratamiento con leflunomida, debe descartarse el embarazo. Mujeres que se encuentren en periodo de lactancia. Advertencias y precauciones especiales de empleo: No se aconseja la administración conjunta con FARMEs hepatotóxicos o hematotóxicos (por ejemplo metotrexato). El metabolito activo de leflunomida, A771726, tiene una vida media larga, generalmente de 1 a 4 semanas. Pueden producirse efectos adversos graves (por ejemplo: hepatotoxicidad, hematotoxicidad o reacciones alérgicas, ver más abajo), aunque se haya interrumpido el tratamiento con leflunomida. Por tanto, cuando aparezcan estos efectos adversos o si por cualquier otro motivo se

