Testmenu.com


ANTIMICROBIAL AND
CLINICAL MICROBIOLOGY
GUIDEBOOK
Second edition
July 2015
Page 1 of 65
TABLE OF CONTENTS
Contacts
Clinical Microbiology
Organism Identification Flowcharts
Antimicrobial Concepts and Tips (future work) Antimicrobial Restrictions and Utilization Guidelines (future work)
Antimicrobial Clinical Practice Guidelines
Antibiotic lock therapy (future) Ethanol lock therapy (future)
Clinical Pathway for Clostridium difficile infection (future)
Community-acquired pneumonia in adults (future)
Nosocomial pneumonia in adults (future)
Invasive Candidiasis (future)
Prophylaxis in surgery (future)
Sepsis in adults (future)
Skin and Soft Tissue Infections (future)
Antimicrobial Therapeutic Interchanges (future) Antimicrobial IV to PO Conversion Protocols (future)
Antimicrobial Drug-Food Interaction Chart (future)
Page 2 of 65
Page 3 of 65
ANTIMICROBIAL AND CLINICAL MICROBIOLOGY GUIDEBOOK
This is the First Edition of the Antimicrobial and Clinical Microbiology Guidebook at Lawrence Memorial Hospital. The development of this guidebook has been a joint effort of the Antimicrobial Stewardship Program, Infectious Disease and Prevention, Pulmonology Specialist Group, Pharmacy, and the Microbiology Department. The purpose of the booklet is to optimize antimicrobial usage and patient outcomes for infectious disease-related issues.
Every effort has been made to ensure that the information is complete, accurate, and up to date; however, this booklet does not serve as a substitute for clinical judgment or consultation with experts in Infectious Diseases. Application of this information to each clinical situation is the responsibility of the practitioner.
Megan Germer, pharmD
Amanda Gudgell, D.O.
Holly Hamilton, MLS(ASCP)cm
Humbelina Harper, MLS(ASCP)cm
Mary Ann Henthorne, MLS(ASCP)cm
Megan Kehrli, pharmD
Christopher Penn, M.D.
Jeff Pierce, pharmD
The Antimicrobial Stewardship Program Contacts:
Christopher Penn, M.D. 843-5160
Amanda Gudgell, D.O. 505-3205
Infection Prevention 505-3158
Holly Hamilton, LIS coordinator 505-2649
Mary Ann Henthorne, Microbiology 505-6177
Jeff Pierce 505-6442
Page 4 of 65
Page 5 of 65
Page 6 of 65
Key phrases from the Microbiology Laboratory
Suggested findings
Gram positive cocci in clusters
Staphylococcus species
Gram positive cocci in pairs and chains
Streptococcus species or Enteroccoccus species
Gram positive cocci in pairs
Streptococcus pneumoniae
Small pleomorphic Gram negative coccobacilli
Haemophilus species
Pleomorphic Gram positive bacilli
Corynebacterium species or Propionobacterium species
Branching Gram positive bacilli
Actinomyces or Nocardia species
Acid fast bacilli
Mycobacterium species
Budding yeast or pseudohyphae
Fungal elements or hyphal elements
Gram negative fusiform bacilli
Fusobacterium species
Susceptibility Testing
Susceptibility testing is an in vitro assay that allows us to detect resistance to antimicrobial agents that may be used to treat an infection. It is important to note however, that clinical outcome may be dependent on various patient specific factors such as immune status or surgical treatment that are not reflected in laboratory tests.
All methods of susceptibility testing are based on diffusion or dilution.
A. Semi-Automated Susceptibility Testing
Semi-automated antimicrobial susceptibility testing is performed using the Vitek 2 system which is based on broth microdilution. This system allows the laboratory to rapidly perform identification and susceptibility testing on most common pathogens (e.g. Staphylococci, Enterobacteriaceae, Enterococci, Pseudomonas species, etc…). The antibiotics tested vary based upon the Vitek panel used and the antibiotics that are currently on the LMH formulary. The Vitek panels are chosen and agreed upon by the Infectious Disease specialist, the pharmacy and the microbiology laboratory. The microbiology laboratory reports antibiotics from most antibiotic classes that are appropriate for the specific organism tested. For example, if the laboratory recovers an isolate from urine, the results from the following antibiotic classes are reported: penicillin, Beta lactam/Beta lactamase combination, cephems, carbapenems, aminoglycosides, fluoroquinolones, nitrofurantoin, and trimethoprim-sulfamethoxazole. Selective reporting of antibiotics for each organism group is reviewed and approved by the Antimicrobial Stewardship Committee and the medical director of the laboratory.
Page 7 of 65
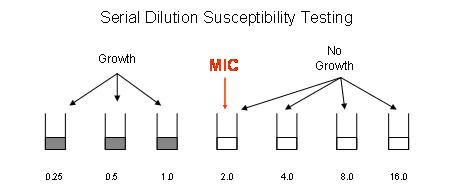
The results obtained from the Vitek system are based on the minimum inhibitory concentration (MIC). The MIC is defined as the lowest concentration of antibiotic that completely inhibits growth of the specific organism being tested. For example, in figure 1 below, the organism being tested grew in wells containing 0.25, 0.5, and 1.0 ug/ml of antibiotic. The lowest concentration of antibiotic (MIC) that completely inhibits growth was 2.0 ug/ml. Figure 1:
The MIC is then interpreted (S=susceptibile, I=Intermediate, or R=resistant) using FDA approved guidelines. These guidelines are based on many studies, including clinical, pharmacokinetic/pharmacodynamic, and microbiological studies. It is important to be aware that, although there are many examples of bacteria and antibiotics for which we have FDA approved guidelines for reporting of automated susceptibility results, there are some bacteria for which FDA approved guidelines do not exist. If susceptibility testing for a specific organism cannot be reported using the automated Vitek method, disc diffusion testing may be performed and results will be reported based on CLSI (Clinical and Laboratory Standards Institute) standards if available. If there are no interpretive standards for automated or disc diffusion testing, the physician may notify the microbiology laboratory (505-6177) and request the isolate be submitted to a reference laboratory for testing.
B. Disk Diffusion
The LMH microbiology laboratory does routinely perform disk diffusion (Kirby Bauer) antimicrobial susceptibility testing for Pseudomonas aeruginosa isolates from cystic fibrosis patients, beta-lactamase positive Haemophilus species, and other fastidious organisms. Disk diffusion allows for measurement of the zone of growth inhibition. See figure 2.
Page 8 of 65
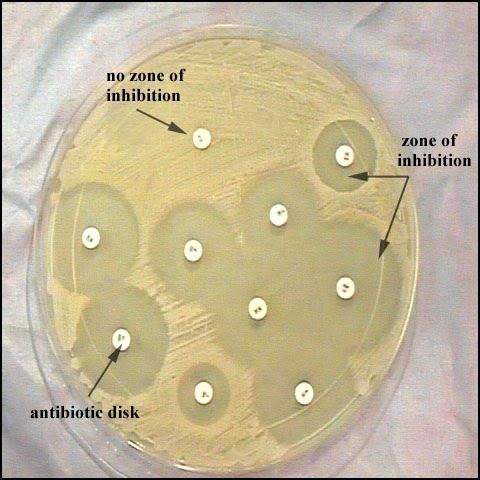
The CLSI provides interpretive standards for reporting an organism as S, I, or R based on the zone of inhibition. The main difference between disk diffusion testing and MIC testing is that disk diffusion provides clinicians with qualitative results, whereas MIC testing provides the clinicians quantitative results. Knowing the MIC can help clinicians incorporate pharmacodynamic/pharmacokinetic principles into the design of the treatment regimen. For instance, if the clinician wants to use ceftriaxone to treat meningitis due to Streptococcus pneumoniae, we need to achieve a concentration in the cerebrospinal fluid (CSF) of approximately four times the MIC for about 40% of the dosing interval due to the time-dependent/concentration-independent nature of the drug. Therefore, if the MIC of the S. pneumoniae isolate to ceftriaxone is 0.25 ug/ml, we want a concentration of at least 1 ug/ml in the CSF for 40% of the dosing interval. The size of the zone of inhibition does not give us the MIC, and so in this specific case disk diffusion is not helpful. Also, zone sizes cannot be compared between drugs. Just because drug A has a larger zone size than drug B, it does not mean that drug A will work better. Zone sizes must be correlated back to the CLSI interpretive standards in order to determine susceptibility or resistance.
C. E-test
The LMH laboratory does perform E-test susceptibility testing for daptomycin on all MRSA and VRE isolates from non-respiratory tract sites per the Infectious Disease practitioner. Daptomycin susceptibility testing is available by E-test upon special request by ID or Pharmacy. Penicillin susceptibility testing is also available by E-test for unusual Strep species.
Page 9 of 65

The E-test is an agar based method that uses a plastic strip with antibiotic concentrations in variable size plastic disks on its underside. When placed on an agar surface pre-inoculated with the bacterial isolate, the diffusing antibiotic creates a concentration gradient in the agar. Decreasing concentrations of antibiotic from the top of the strip to the bottom create an ellipsoid diffusion pattern around the strip. The resultant elliptical zone of inhibition allows the MIC to be read at the point where the zone crosses the E-test strip (figure 3). Figure 3:
D. Special Susceptibility Testing Issues:
Extended-spectrum Beta-lactamases (ESBL's)
ESBL's are Beta-lactamases that are capable of hydrolyzing expanded-spectrum
cephalosporins (ceftriaxone, cefotaxime, and ceftazidime) as well as cefepime and
aztreonam. ESBL's can be isolated from many different Enterobacteriaceae species but are
most commonly isolated from Klebsiella pneumoniae, Klebsiella oxytoca, E. coli, and Proteus
mirabilis.
Using the Vitek, isolates that carry ESBL's can initially be intermediate or resistant to one or
all of the extended spectrum cephalosporins, cefepime or aztreonam. This is due to the fact
that there are many different ESBL's with different substrate specificities. The Vitek gram
negative panel includes an ESBL confirmatory test and all ESBL positive isolates are verified.
If a particular isolate is confirmed as resistant or intermediate to any of the extended-
spectrum cephalosporins, cefepime or aztreonam, the following statement will be included
in the report: "Isolate is an ESBL producing strain resistant to all penicillins, cephalosporins,
and aztreonam."
ESBL positive E coli isolates from urinary tract specimens will be tested for fosfomycin
susceptibility per the Infectious Disease practioner.
The Nanosphere Gram Negative Blood culture assay can detect the CTX-M beta-lactamase
resistance mechanism.
Page 10 of 65
CTX-M is a beta-lactamase that has potent activity against cefotaxime. The mechanism of
resistance is plasmid-mediated.
Because of the significant public health implications, the spread of CTX-M beta-lactamase
producers merits close monitoring.
Carbapenemases:
Carbapenemases are beta-lactamases that are capable of hydrolyzing all beta-lactams,
including the carbapenems. Carbapenemases can be isolated from many different
Enterobacteriaceae species.
Carbapenemases are particularly dangerous resistance mechanisms, since they can
inactivate a wide range of different antibiotics. Enterobacteriaceae isolates that produce
carbapenemase have been referred to as "Infection Control Emergencies." Bacteria that
produce carbapenemases are often referred to in the news as "superbugs" because
infections caused by them are extremely difficult to treat.
For all acute care facilities, CDC and HICPAC recommend an aggressive infection control
strategy, including managing all patients with carbapenem resistant Enterobacteriaceae
(CRE) using contact precautions.
There are several important mechanisms of carbapenem resistance that increases the risk
for dissemination:
1. KPC: Klebsiella pneumoniae carbapenemase (KPC) refers to the production of a
carbapenemase enzyme, blakpc. The gene that encodes the blakpc enzyme is carried on a mobile piece of genetic material (transposon), which can easily be passed from one organism to the other.
2. NDM: New Dehli Metallo-beta-lactamase-1 refers to the production of a
carbapenemase referred to as NDM-1 enzyme. The most common bacteria that produce this enzyme are gram negatives such as E coli and Klebsiella pneumoniae, but the gene for NDM blaNDM-1 can spread from one strain of bacteria to another by horizontal gene transfer.
3. IMP: IMP-type carbapenemases are plasmid mediated Class B metallo-beta-lactamases
that can be found in both enteric Gram-negative organisms and in Pseudomonas and Acinetobacter species.
4. VIM (Verona integron-encoded metallo-beta-lactamase): The VIM family of
carbapenemases occur mostly in Pseudomonas aeruginosa and P. putica and vary rarely
Page 11 of 65
in Enterobacteriaceae. The VIM enzymes are integron-associated and hydrolyse all beta-lactams and can evade all beta-lactam inhibitors.
5. OXA (Oxacillinase): The OXA group of beta-lactamases occur mainly in Acinetobacter
The Nanosphere Gram Negative Blood Culture assay can detect the following carbapenem resistance mechanisms: KPC, NDM, VIM, OXA, and IMP. Infectious disease consultation is suggested when one of these resistance mechanisms is detected. If the Vitek susceptibility profile suggests the presence of a carbapenemase producing organism, the microbiology department will repeat testing and will perform a Modified Hodge test on the isolate. The following flowchart was developed for testing based on CDC recommendations. It is important to note that if an isolate is suspected to be a carbapenem resistant Enterobacteriaceae (CRE), the Infectious Disease specialist and the Infection Preventionist will be notified immediately to ensure the patient is placed in isolation.
Page 12 of 65
Practical Testing Scheme to Detect KPC and NDM-Producing Enterobacteriaceae
(CRE-Carbapenem Resistant Enterobacteriaceae)
Clinical isolate of Enterobacteriaceae
(not indicated for Proteus, Providencia or Morganella)
susceptibility testing:
ETP or IMP MIC > 1 and/or MEM MIC > 2.0.
No further testing. Presumptive KPC
Notify Infection Control. Repeat MIC susc and perform Modified Hodge Test (MHT) and KB susc for ETP, MEM, and IMP.
Notify IC immediately of suspected CRE to isolate patient.
If ETP is the only drug displaying reduced sensitivity, further testing is not
necessary. If IMP and/or MEM, and beta lactams also
Isolate is positive for carbapenemase
display resistance,
production Send isolate to ARUP for
send isolate to ARUP
PCR confirmation ARUP test #
for PCR, test code
Page 13 of 65
Inducible clindamycin-resistance in Staphylococcus and Streptococcus species:
Erythromycin resistance within staphylococci is typically mediated through two distinct
mechanisms. The first mechanism entails protection of the ribosome from erythromycin
and clindamycin through methylation (referred to as MLSB resistance). This mechanism may
be constitutive (conferring resistance to both erythromycin and clindamycin) or inducible
(conferring resistance only to erythromycin). Published clinical reports have demonstrated
that S. aureus isolates carrying an inducible MLSB resistance gene should be considered
resistant to clindamycin even if the in vitro result considers the isolate susceptible to
clindamycin. The second resistance mechanism is conferred through efflux of erythromycin
out of the cell through specific pumps (encoded by the msrA gene). Staphylococcal isolates
carrying the MsrA efflux pump are resistant only to erythromycin and not clindamycin.
The Vitek performs an inducible clindamycin resistance (ICR) test (otherwise known as D-
test) automatically on Staph species, group A Beta Strep and group B Beta Strep. If the test
is positive for ICR, the clindamycin result is not reported and a comment is attached to the
report "isolate presumed resistant to clindamycin by ICR testing."
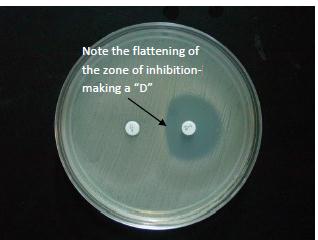
Isolates of Streptococcus pneumoniae, and other Beta Streptococcus species (as specified by
CLSI) may require a manual D-test performed (Figure 4). If the D-test is positive the
clindamycin result is not reported and a comment is attached to the report "isolate
presumed resistant to clindamycin by ICR testing."
Figure 4
(positive D-test by
manual method):
Page 14 of 65
Inducible Methicillin Resistance in Staph aureus:
Methicillin resistance in S. aureus is generally due to the presence of the mecA gene located
on the staphylococcal cassette chromosome mec(SCCmec). The mecA gene codes for
production of an altered penicillin binding protein PBP2', also referred to as PBP2a. PBP2'
has a low binding affinity for beta-lactam antibiotics. Because oxacillin and other beta-
lactams cannot bind to the altered PBP2' site, these antibiotics are ineffective. The ‘gold
standard' for the detection of MRSA is molecular methodology using either polymerase
chain reactions (PCR) or nucleic acid amplification. Molecular methods for detection of
genes such as mecA that code for resistance are being used in laboratories with increasing
frequency. However, routine susceptibility testing by microdilution or disk diffusion is still
the method of choice for resistance determination in most laboratories.
Until recently, our laboratory used microdilution and PBP2' latex methods to detect MRSA.
In January 2012, the Cepheid SSTI MRSA/MSSA cartridge for use on the GeneXpert platform
was added to the laboratory's test menu. In addition to a routine wound culture, we began
to offer a special site culture with PCR for MRSA/MSSA. PCR results are available in just over
one hour from the time of specimen receipt in the laboratory. PCR testing performed on
these specimens is followed up with routine culture and susceptibility testing.
Our laboratory has previously identified S aureus isolates from outpatient wound specimens
that were positive for the mecA gene by PCR that were found to be sensitive to oxacillin by
follow-up routine susceptibility testing. Upon further investigation, these isolates were
found to demonstrate resistance in vitro, after exposure to a beta-lactam (cefoxitin)
antibiotic. In other words, the S aureus isolates demonstrated "inducible methicillin
resistance."
The prevalence of this inducible methicillin resistant S aureus strain (Ridom spa type T175)
has been found to be low however susceptibility testing alone can miss these strains. It is
important to be aware that this strain has historically been found in our community. Also, it
is important to note that if an inducible MRSA isolate were isolated from a serious infection,
such as sepsis, and the infection were treated with first-generation cephalosporins or semi-
synthetic penicillin, the patient may fail therapy.
Page 15 of 65
INTRINSIC RESISTANCE TABLES
Intrinsic resistance is the innate ability of a bacterial species to resist activity of a particular antimicrobial agent through its inherent structural or functional characteristics. Such natural resistance can be due to: lack of affinity of the drug for the bacterial target, inaccessibility of the drug into the bacterial cell, extrusion of the drug by chromosomally encoded active exporters, or innate production of enzymes that inactivate the drug.
Below are lists of organisms with their respective intrinsic antimicrobial resistance.
Antimicrobial agents
Enterococcus faecalis
Enterococcus faecium
Enterococcus gallinarum/
Enterococcus casseliflavus
*Warning: For Enterococcus spp., cephalosporins, aminoglycosides (except for high level resistance
screening), clindamycin, and trimethoprim-sulfamethoxazole may appear active in vitro, but are not
effective clinically.
Note: Gram-positive bacteria are also intrinsically resistant to aztreonam, polymyxin B/colistin and naladixic acid.
Antimicrobial Agents
S. aureus
There is no intrinsic resistance in these species.
S. lugdenensis
S. epidermidis
S. haemolyticus
S. saprophyticus
S. capitis
S. cohnii
S. xylosus
Page 16 of 65
1. Gram positive bacteria are also intrinsically resistant to aztreonam, polymyxin B/colistin and
2. MRSA and oxacillin resistant coagulase negative staphylococci are considered resistant to other
beta-lactam agents, (penicillins, beta-lactam/beta-lactamase inhibitor combinations, cephems (with the exception of ceftaroline), and carbapenems.
Antimicrobial Agent
Citrobacter freundii
Citrobacter koseri
Enterobacter aerogenes
Enterobacter cloacae
Escherichia coli
There is no intrinsic resistance to beta-lactams in this organism
Escherichia hermannii
Hafnia alvei
Klebsiella pneumoniae
Morganella morganii
Proteus mirabilis
No intrinsic resistance to penicillins and cephalosporins
Proteus penneri
Proteus vulgaris
Providencia rettgeri
Providencia stuartii
Salmonella and Shigella spp
No intrinsic resistance to beta-lactams in these organisms
1ST AND 2ND generation cephalosporins may appear active in vitro, but are not effective clinically.
Serratia marcescens
Yersinia enterocolitica
Note: Cephalosporins III, cefepime, aztreonam, ticarcillin-clavulanate, piperacillin-tazobactam, and the carbapenems are not listed, because there is no intrinsic resistance in Enterobacteiaceae.
Enterobacteriaceae are also intrinsically resistant to clindamycin, daptomycin, glycopeptides, (vancomycin), linezolid, macrolides (erythromycin, clarithromycin, azithromycin), quinupristin-dalfopristin, and rifampin.
Page 17 of 65
Antimicrobial Agents
teriaceae
er
A.
s complex
a cepacia
as
1. *Acinetobacter baumannii/calcoaceticus may appear to be susceptible to ampicillin-sulbactam due
to the activity of sulbactam with this species.
2. # Stenotrophomonas maltophilia is intrinsically resistant to tetracycline but not to doxycycline or
3. Note: Nonfermentative gram-negtive bacteria are also intrinsically resistant to cephalosporin I
(cephalothin, cefazolin), cephalosporin II (cefuroxime), cephamycins (cefoxitin, cefotetan), clindamycin, daptomycin, fusidic acid, glycopeptides (vancomycin, teicoplanin), linezolid, macrolides (erythromycin, azithromycin, clarithromycin), penicillin, quinupristin-dalfopristin, and rifampin.
Other organisms:
Organisms
Natural Resistance Against
Anaerobic bacteria
Aerobic bacteria
Lactobacilli and Leuconostoc Vancomycin Aerococcus urinae
Su lfonamides and Netilmicin
Cardiobacterium hominis
Cli ndamycin
Page 18 of 65
CLSI Cumulative Antimicrobial Susceptibility for Bacteroides fragilis Group Organisms
Anaerobic
Organisms
Susceptible
Resistant
B.fragilis
B.ovatus
B.vulgatus
B.uniformis
B.eggerthii
teroides
distasonis
B.frag group
Not B.frag
B.frag group
(all above
species)
Data was provided by CLSI and generated from 4 referral laboratories: Tufts, Boston MA; Loyola, Maywood IL; RM Alden Research Lab, Culver City, CA; International Health Management Associates, Inc., Schaumburg, IL. (2010-2012)
Testing was performed by agar dilution.
Resistance to metronidaxole occurs infrequently.
Intermediate category is not shown, but can be derived by subtraction of %S and %R from %100.
Page 19 of 65
CLSI Cumulative Antimicrobial Susceptibility for Anaerobic organisms other than Bacteroides fragilis
Anaerobic
Organisms
Susceptible
Resistant
Prevotella
Fusobac-
nucleatum-
necrophorum
Anaerobic
positive
Veillonella
Clostridium
perfringens
C.difficile
Clostridium
Data was provided by CLSI and generated from 4 referral laboratories: Tufts, Boston MA; Loyola, Matwood IL; RM Alden Research Lab, Culver City, CA; International Health Management Associates, Inc., Schaumburg, IL. (2010-2012)
Testing was performed by agar dilution.
Intermediate category is not shown, but can be derived by subtraction of %S and %R from %100.
Development of Resistance and Testing of Repeat Isolates: Isolates that are initially susceptible may
become intermediate or resistant after initiation of therapy. Therefore, subsequent isolates of the same
species from a similar body site should be tested in order to detect resistance that may have developed.
This can occur within as little as three to four days and has been noted most frequently in Enterobacter,
Citrobacter, and Serratia spp. with third-generation cephalosporins; in P. aeruginosa with all
antimicrobial agents, and staphylococci with quinolones. For S. aureus, vancomycin-susceptible isolates
may become vancomycin intermediate during the course of prolonged therapy.
Page 20 of 65
In certain circumstances, testing of subsequent isolates to detect resistance that may have developed might be warranted earlier that within three to four days. The decision to do so requires knowledge of the specific situation and the severity of the patient's condition.
Interpretation of Microbiology Reports
Semi-quantitative Terminology (rare/few/moderate/many):
The amount of each type of organism seen is quantified when reading gram stains using the following
interpretive criteria:
Description No. per oil immersion field (×1000)
Moderate
Of note, when a report says, "rare gram negative bacilli" it does not mean rare as in unusual, it means rare as in very few.
Contaminant vs. Pathogen:
Normally Sterile
PATHOGENS
Any organism isolated
LIKELY CONTAMINANTS
Coagulase negative staphylococci Alpha-hemolytic streptococci Bacillus spp. Corynebacterium spp. (except C. jeikeium) Propionibacterium acnes
TISSUE AND BODY FLUIDS
Normally Sterile
PATHOGENS-Any organism isolated; use judgment to evaluate the possibility of normal flora
being present in relation to the source of specimen.
NORMAL FLORA:
coagulase negative Staphylococci
Page 21 of 65
Non-hemolytic streptococci Alpha-hemolytic streptococci Diphtheroids
SKIN
NORMAL FLORA:
coagulase negative staphylococci Propionibacterium acnes Diphtheroids Alpha-hemolytic streptococci Bacillus spp.
PATHOGENS
Neisseria gonorrhoeae Beta-hemolytic streptococci (GBS will be held if present for susceptibility testing if needed) Predominant growth of Yeast or S aureus
URINE
Should be sterile. Cultures with mixed flora will be reported as such if contamination is suspected.
PATHOGENS
Enterobacteriaceae Enterococcus spp. Pseudomonas spp. and other non-fermenters Group B Streptococcus (Streptococcus agalactiae) S. aureus and S. saprophyticus Yeast Aerococcus urinae
STOOL (CULTURES NO LONGER PERFORMED IN-HOUSE):
ENTERIC PATHOGEN TESTING BY PCR/HYBRIDIZATION:
Shigella spp. Salmonella spp. Campylobacter group (C. coli, C. jejuni, and C. lari) Yersinia enterocolitica Vibrio group (V. cholera and V. parahaemolyticus) Shiga-toxin producing strains of E coli (both shiga toxin 1 and 2) Norovirus (GI and GII) Rotavirus A
Note: If cultures for Aeromonas or Plesiomonas are needed, specific orders for these organisms will
need to be requested and these specimens will be referred to the reference laboratory.
RESPIRATORY TRACT
PATHOGENS:
Group A Streptococcus (Streptococcus pyogenes) Group C Streptococcus (large colony) Group G Streptococcus (large colony) Arcanobacterium haemolyticum
Page 22 of 65
Streptococcus constellatus subsp. pharyngis Streptococcus pneumoniae Haemophilus influenzae Moraxella catarrhalis (predominant) Enterobacteriaceae (lower respiratory tract) Pseudomonas spp. and other non-fermenters Burkholderia cepacia Yeast (predominant) S. aureus (predominant)
Specimen Requirements
Specimen requirements can be found on the hospital Intranet as follows:
Select "Documents" in left column, select "other documents", select "lab" and then select "Specimen Requirements.pdf."
Timing of Reports
Preliminary/Final Reports:
Cultures are examined each day and preliminary reports are generated on a daily basis that include any
information or presumptive identification of organisms isolated that would be helpful to the physician.
When the culture is completed, a final report is released.
Gram Stain:
Surgical specimens, sterile body fluids and bronch washings and brushings-within 1 hour of receipt in lab
Organism identification:
The organism will be identified within 24 hours of isolation of an organism unless it is an unusual or fastidious organism and/or requires further work-up to confirm.
Susceptibility results:
The susceptibility results will be reported within 24-48 hours of isolation of an organism unless it is an unusual or fastidious organism and/or requires further work-up to confirm identification.
The hospital-wide antibiogram can be found on the intranet by selecting the physicians tab directly above the current inpatient census. Select "Antibiogram." It is updated every 6 months and approved by the antibiotic stewardship committee.
Urinalysis and Urine Culture
Indicators of Infection from a Urinalysis:
Positive leukocyte esterase Positive nitrite >5 WBC's/hpf
Page 23 of 65
Urine Culture:
A urine culture must ALWAYS be interpreted in the context of a urinalysis and patient symptoms.
Ideally, a urine culture would not be performed unless a urinalysis indicated a possible infection.
Typically, catheterized patients can become colonized within 48 hours of catheterization. The only
patient populations for which it is recommended to screen for and treat asymptomatic bacteriuria are
pregnant women and patients scheduled for genitourinary surgical procedure.
Urine cultures are held for 2 days before finalizing as "No Growth."
Guidelines for the Prevention of Perinatal Group B Streptococcal (GBS) Disease published in Nov. 2010 note that the presence of group B Streptococcus in amounts of > 10,000 CFU/ml during any trimester is considered indicative of heavy maternal colonization of GBS in the vaginal flora. (i.e. Maternal GBS bacteriuria at any point during pregnancy is a recognized risk factor for early-onset GBS disease and therefore has been included as an indication for intrapartum antibiotic prophylaxis.)
If GBS is present as the predominant organism, susceptibility will be performed and reported. If susceptibility testing is not performed and the patient is female, the organism will be held for 2 weeks and the following comment is attached to the report: "Presence of group B Strep in urine samples of pregnant individuals during any trimester is indicative of heavy maternal GBS colonization if the patient is pregnant and penicillin allergic, notify the laboratory if susceptibility testing is required. Disregard if susceptibility testing was performed."
Stool Testing
Stool for WBC's: The presence of fecal leukocytes suggests inflammation of the bowel. This should lead
the physician to evaluate for the cause of inflammation and consider selective cultures for the most
common invasive pathogens such as: Campylobacter spp., Salmonella spp., Shigella spp., and
enterohemorrhagic E coli 0157:H7.
Enteric pathogen (EP) testing: Stool cultures (in-house) were discontinued in July 2015. Testing of
liquid and soft stool specimens is now performed on the Verigene Nanosphere system. This assay
utilizes amplification (PCR) and hybridization to qualitatively detect and identify common
gastrointestinal pathogens.
The EP assay detects and identifies the following enteric bacteria/toxins and viruses: Campylobacter group (C. coli, C. jejuni, and C. lari), Salmonella species, Shigella species, Vibrio group (V. cholera and V. parahaemolyticus), Yersinia enterocolitica, Shiga toxins 1 and 2 (STEC), Norovirus and Rotavirus. If the sample is positive for Vibrio, Salmonella, Shigella or STEC, the specimen will be sent to KDHE for further identification.
Note: This assay is FDA-approved for testing of liquid or soft stools. Testing of formed stools is not indicated.
If Plesiomonas shigelloides or Aeromonas spp. are suspected, separate orders are required for referral to the reference laboratory.
Page 24 of 65
Note: It is inappropriate to order enteric pathogens testing on patients who have been hospitalized for
more than 3 days and then develop diarrhea. In these situations, studies have shown that the most
common pathogen is C.difficile and PCR testing should be ordered.
Stool should be tested for Clostridium difficile on patients over 6 months of age with clinically significant diarrhea and a history of antibiotic exposure. Current College of American Pathologists (CAP) guidelines indicate that a provider should consider C. difficile testing as an alternative to routine microbiologic studies for inpatients over 6 months of age who have test requests for routine enteric pathogens. Additionally, CAP clinical guidelines indicate that no more than 2 stool specimens/patient should be accepted without prior consultation with the provider who can explain the limited yield provided by additional specimens.
Clostridium difficile testing by PCR:
The Cepheid Gene XPert C diff/epi assay is the test currently used for diagnosing C. difficile infection (CDI) at LMH. This assay is a qualitative in vitro diagnostic test for rapid detection of toxin B gene sequences and for the presumptive identification of 027/NAP1/BI strains (epidemic, "epi"), of toxigenic Clostridium difficile from unformed (liquid or soft) stool specimens collected from patients suspected of having CDI.
The test utilizes automated real-time polymerase chain reaction (PCR) to detect toxin gene sequences associated with toxin producing C. difficile. The assay is intended as an aid in the diagnosis of CDI.
Detection of 027/NAP1/B1 strains of C. difficile by the assay is presumptive and is solely for epidemiological purposes and is not intended to guide or monitor treatment for CDI. 027/NAP1/B1 strains of C. difficile have been referred to as "hypervirulent." These strains exhibit increased toxin production and are thought to produce more spores leading to enhanced persistence in the environment.
Testing will not be performed on formed stool (stool that does not take the shape of the container)
unless a rare case of ileus is suspected. In cases of suspected ileus, a formed stool may be tested by
special request of the physician.
The C. difficile assay should not be used to assess response to therapy. Patients can continue to test positive after treatment.
Blood Cultures
A minimum of two sets (four bottles; one set = one aerobic bottle + one anaerobic bottle) should usually be obtained. The suggested volume of blood per bottle for adults is 10 ml.
Ordering one set may lead to confusion if the culture is positive for an organism that is commonly a contaminant. For example, if one set is ordered and is positive for coagulase-negative staphylococci (CoNS), a common contaminant, it is difficult to determine if this represents contamination or infection. However, if two sets are ordered, and only one is positive for CoNS, this most likely represents contamination.
Page 25 of 65
Please specify the desired sites of the blood draw (e.g., one from line, one peripherally). Ideally, blood cultures should be drawn before the first dose of antibiotics, but antibiotics should not be withheld because of a delay in getting cultures drawn. Although it is common practice to wait 30-60 minutes between blood cultures, there is little data to support this practice, and we do not recommend it.
If a vascular catheter is thought to be a potential site of infection, blood should be drawn from the catheter and the periphery. Site and time of phlebotomy should be noted. The differential time to positivity can help in assessing whether the catheter is the likely source.
Differential Time to Positivity (DTP)
a. A positive line culture result is obtained at least 2 hours earlier than a positive peripheral
blood culture result.
b. Typically, the diagnosis of a line-associated infection can be made according to the following
criteria: presence of an intravascular device, at least one positive blood culture obtained from a peripheral site, clinical manifestations of infection, and no other apparent source for bloodstream infection
Blood Cultures are held for 5 days prior to reporting the culture as final for no growth.
Blood cultures are continuously monitored by the BacTAlert 3D analyzer. If a positive bottle is detected, a gram stain is prepared and the bottle is sub-cultured at that time. The result of the gram stain is called to the physician immediately.
If the Gram stain indicates the presence of a possible staphylococci (Gram positive cocci in clusters), PCR testing is performed on the Gene XPert analyzer for the presence or absence of targets specific for S aureus and MRSA.
If the PCR assay detects the presence of S aureus but does not detect the presence of the targets specific for MRSA (mecA gene and SCCmec cassette), the result will be reported as: "positive for S aureus by PCR, MRSA negative. " A comment is attached to this result stating: "the mecA gene which most often determines methicillin resistance is not present, susceptibility testing to follow." It is important to note that there are rare cases of methicillin resistance due to mechanisms other than the mecA gene such as mecC and hyper-Beta-lactamase production.
If the Gram stain indicates the presence of possible streptococci (Gram positive cocci in pairs or chains), micro-array testing will be performed on the Verigene analyzer for the presence or absence of targets specific for:
Streptococccus pyogenes (group A Beta Strep)
Streptococcus agalactiae (group B Beta Strep)
Streptococcus pneumoniae (Streptococcus mitis can cross react with S pneumo target)
Streptococcus anginosus group
Streptococcus species
Enterococcus faecalis
Page 26 of 65
Enterococcus faecium
Listeria species
The assay can also detect the vanA and vanB vancomycin resistance mechanisms if Ec faecalis or Ec faecium are detected.
If the Gram stain indicates the presence of Gram negative bacilli (rods), micro-array testing will be performed on the Verigene analyzer for the presence or absence of targets specific for:
Acinetobacter species
Citrobacter species
Enterobacter species
Klebsiella oxytoca
Klebsiella pneumoniae
Proteus species
Pseudomonas aeruginosa
The assay can also detect the CTX-M, KPC, NDM, OXA, IMP and VIM resistance mechanisms if an organism is detected.
Respiratory Cultures
Lower respiratory tract: Appropriate specimens to identify pathogens causing disease of the lower
respiratory tract (tracheitis, bronchitis, pneumonia, lung abscess, and empyema) include expectorated
and induced sputum, endotracheal tube aspirations, bronchial brushings, washes, or alveolar lavages
collected during bronchoscopy and pleural fluid.
Sputum evaluations are performed on all sputum specimens using the gram stain to assess for quality
(lack of contaminating oral respiratory tract flora and epithelial cells). An acceptable specimen generally
yields less than 10 squamous epithelial cells per low power field. The presence of 25 or more
polymorphonuclear leukocytes per low power field together with few squamous epithelial cells, implies
an excellent specimen.
If the specimen is of poor quality, a comment is attached to the gram stain report, "Suggests orophapharyngeal contamination, interpret accordingly." The number of white blood cells may not always be relevant, because many patients are severely neutropenic and specimens from these patients will not show white blood cells on gram stain examination. If the specimen is from a patient (from either the ED or an inpatient floor) with a diagnosis of pneumonia and the specimen is poor quality, a new specimen may be requested.
Upper respiratory tract: Appropriate specimens to identify pathogens causing upper respiratory tract
infections include samples from the nasopharynx, throat, oral ulcerations, and inflammatory material
from the nasal sinuses.
Page 27 of 65
Specific pathogens or normal respiratory flora are quantified in the culture report using the terms –many, -moderate, or –few.
All negative rapid strep tests for group A Strep are followed up with culture for group A, C, F, and G Beta hemolytic Streptococcus, Arcanobacterium haemolyticum and Streptococcus constellatus ssp. pharyngis.
Legionella and S pneumo antigen testing: It is important to note that as an alternative to culture for
Legionella, the laboratory offers a highly sensitive rapid EIA for the detection of L. pneumophilia antigen
in the urine of patients suspected of having legionellosis. The laboratory offers a similar rapid EIA test
for the detection of S. pneumoniae antigen in the urine of patients suspected of having pneumococcal
pneumonia or sepsis. Note: A negative result does not rule out the possibility of infection but indicates
that the antigen may be below the limits of detection for this assay.
Genital Tract Cultures
Genital tract cultures are performed in the microbiology laboratory to determine the etiology of various clinical syndromes, including vulvovaginitis, genital ulcers, urethritis, cervicitis, endometritis, salpingitis, and ovarian abscess in females and urethritis, epididymitis, prostatitis, and genital ulcers in males.
Genital tract cultures are held for 3 days before finalizing the report.
Bacterial Vaginosis: The gram stain is the preferred method for detecting bacterial vaginosis (BV).
While it is true that Gardnerella vaginalis (one of the prevalent organisms in BV) is easily cultured, it can
be present in greater than 60% of normal patients. The presence of "clue cells" an indication of BV, can
only be determined from a gram stain and culturing the anaerobic bacteria associated with BV is too
costly and time-consuming, therefore the culture should not be used for the diagnosis of bacterial
vaginosis.
In our laboratory graded criteria for evaluation of the gram stain is used for determining the presence or absence of suspected bacterial vaginosis. One of the following interpretations is attached to the gram stain report: "No Evidence of Bacterial Vaginosis" (Grade 1); "Intermediate for Bacterial Vaginosis" (Grade 2); or "Bacterial Vaginosis Indicated upon Smear Review." If the specimen quality was poor a comment will be attached to the report indicating "Submitted specimen does not have sufficient vaginal material to evaluate for bacterial vaginosis."
GBS screens: Our laboratory offers two separate orders for GBS screens on pregnant females at 35 to
37 weeks of gestation. STREPB is the pneumonic developed at LMH for GBS screening on non-allergic
patients. STRPBS is the pneumonic developed at LMH for GBS screening on penicillin-allergic patients to
ensure that if the test is positive susceptibility testing will be performed.
Chlamydia/GC testing: The laboratory offers in-house testing using real-time PCR for the detection of
Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) from both endocervical swabs and urine
specimens. A specimen adequacy control has been added to this test to detect human cells and DNA
which ensures the specimen quality is acceptable.
Results are reported as Detected or Not Detected for each specific organism. An Invalid result can be due to the presence of interfering substances or it can be due to the absence of human cells or DNA.
Page 28 of 65
This test should not be used for the evaluation of suspected sexual abuse or for other medico-legal indications. This assay has not been evaluated in patients less than 14 years of age.
A negative test result does not exclude the possibility of infection because test results may be affected by improper specimen collection, concurrent antibiotic therapy or the number of organisms in the specimen which may be below the sensitivity of the test.
The test takes 90 minutes to complete. Specimens collected from patients in the Emergency Department will be performed stat. Specimens received from clinics will be performed on a routine basis ensuring results in less than 24 hours Monday through Friday.
The sensitivities and specificities of the various specimen types are as follows:
Endocervical Swabs Female Urine Male Urine
CT NG CT NG CT NG
Sensitivity 96.0% 100% 98.1% 94.4% 98.5% 98.3%
Specificity 99.6% >99.9% 99.8% >99.9% 99.8% 99.9%
Specimens submitted to the laboratory for acid fast culture will be sent to the Kansas Department of Health and Environment for testing. Our laboratory will perform an acid fast stain and report results within 24 hours of receipt. The state laboratory will perform a culture and fluorescent stain for Mycobacterium spp. and will concentrate specimens as necessary.
Specimen requirements:
Sputum: Spontaneously produced sputum is the specimen of choice. A good sputum is 5-10 mls with a
minimal amount of oral or nasal secretion. Three early morning specimens, refrigerated until processed,
are desirable. These specimens should be collected on three separate days. Respiratory Therapy may
collect an induced specimen using inhalation treatment or nebulization.
Urine: Early morning clean-voided specimens are preferred. Specimens should be submitted daily for at
least 3 days. Refrigeration prior to processing is necessary. Direct smears are not prepared on urine
specimens. Pooled specimens are not desirable because of excessive dilution, higher contamination and
difficulty in concentrating.
Tissue and Body Fluids: All body fluids, exudates, and tissue should be submitted in sterile containers.
Large amounts are preferable. Small amounts of exudates may require moistening with sterile saline
and tissues may be sent in a small amount of sterile saline.
Gastric Lavages: Optimal time for gastric lavage is early in the morning before meals. The objective of
gastric lavage is to obtain sputum that may have been swallowed during the night. The specimen should
be obtained at least 8 hours after the patient has eaten or taken oral drugs. The procedure should be
limited to senile, non-ambulatory patients, children younger than 3 years of age, and patients who fail to
Page 29 of 65
produce sputum by aerosol induction. Specimens should be collected as a series of three specimens collected on separate days.
A preliminary report is received from the state within 3-5 days. A final report, if negative, is issued at 8 weeks. Positive interim reports from DNA probes may be available in 3-5 days. Positive culture results will take 2-4 weeks.
The patient's physician is notified immediately if any report is positive for AFB.
The state will NOT perform sensitivities on M. tuberculosis isolates. If susceptibility testing is requested for Mycobacterium spp. other than M. tuberculosis, notify the microbiology lab at 505-6177 so that KDHE can be requested to send the isolate to us so that our laboratory can forward it to the reference laboratory for susceptibility testing.
Virology
Virus detection- There are four general ways in which viral infections can be detected: culture, direct
viral antigen detection, serology, or nucleic acid detection.
Culture: Specimens for culture will be sent to the reference laboratory. The site of collection must be
noted for the reference laboratory to perform testing. The specimen should preferably be submitted in
viral transport media (a sterile container is acceptable) and delivered to the laboratory as soon as
possible.
CSF for Enterovirus: Enterovirus testing on CSF is available in-house on the Cepheid Gene XPert
analyzer. The XPert EV assay is a reverse transcription polymerase chain reaction (RT-PCR) using the
Gene XPert Dx System for the presumptive qualitative detection of Enterovirus (EV) RNA in
cerebrospinal fluid (CSF) specimens from individuals with signs and symptoms of meningitis. This test, in
conjunction with other laboratory results and clinical information, may be used as an aid in the
laboratory diagnosis of enterovirus infection in patients with a clinical suspicion of meningitis or
meningoencephalitis.
Expected turnaround time for the enterovirus assay is 3.5 hours form time of receipt of the CSF in the laboratory.
Influenza A/B and H1N1 by PCR: PCR testing for Influenza A, Influenza B, and the 2009 H1N1 strain are
available in-house. The specimen requirement is a nasopharyngeal swab in viral media (UTM kit) or a
nasopharyngeal aspirate. The specimen should be refrigerated if there is a delay in delivery to the
laboratory. The assay takes 1.5 hours to perform. Testing of inpatients for Influenza must be ordered
by PCR. Note: Rapid testing for influenza A/B is available for outpatients, however, PCR testing can be
ordered on these patients if specified by the physician.
Direct Respiratory Viral Antigen Detection: Rapid tests are available in the laboratory for Respiratory
Syncytial Virus (RSV) and Influenza A/B. The specimen of choice is a nasopharyngeal swab in viral media
(UTM kit).
Rotavirus Detection: Rotavirus is a major cause of acute gastroenteritis, especially in children 6 to 24
months in age. In addition, rotavirus infections can produce severe illness as well as asymptomatic
infection in adults. The incubation period of rotavirus infection is usually one to three days, followed by
gastroenteritis with an average duration of five to eight days. Virus titers in stool reach a maximum
Page 30 of 65
shortly after the onset of illness, then decline. Due to inadequacies in existing culture methods, human rotavirus is not routinely isolated from rotavirus-containing specimens.
The Enteric Pathogens (EP) molecular assay detects Rotavirus A in liquid or soft stool. The specimen requirement is a stool specimen in a clean container or in Cary Blair preservative.
Norovirus Detection: Noroviruses are highly contagious and cause on average 19-21 million cases of
acute gastroenteritis each year ranking norovirus in the top five pathogens for enteric illnesses.
The Enteric Pathogens (EP) molecular assay detects Norovirus GI and GII in liquid or soft stool. The specimen requirement is a stool specimen in a clean container or in Cary Blair preservative.
Mycology
Specimen Collection: The ideal specimens for fungal isolation are either tissue, sterile body fluid, or
blood. If a tissue specimen is to be tested for the presence of fungi, it is important that part of the
specimen is sent to the microbiology laboratory before the specimen is fixed in formalin for histological
examination. Blood to be tested for fungus should be added to a separate blood culture bottle that can
be held for 30 days.
Timing of Reports: Moulds may take 3-4 weeks to grow, whereas yeasts grow rather rapidly and can
usually be identified within 3-5 days. Specimens will be finalized at 4 weeks.
Susceptibility Testing: Susceptibility testing is performed by the reference laboratory only upon special
request. If susceptibility testing is needed, please contact the microbiology laboratory at 505-6177. If
susceptibility testing is needed for mould, a list of antifungals for testing must be specified for the
reference laboratory to perform testing.
Cryptococcus Antigen Testing in CSF/serum: The laboratory offers a highly sensitive rapid lateral flow
assay that can be performed on serum or CSF. This assay is more sensitive than culture, India ink, latex
agglutination and enzyme immunoassay (EIA). The assay will detect antigens for Cryptococcus species
complex (Cryptococcus neoformans and Cryptococcus gattii). This test is not meant to be used as a
screening test for the general population. It should only be done when clinical evidence suggests the
diagnosis of cryptococcal disease.
Ova and Parasites: An O&P test should be ordered on patients presenting with a history of chronic
diarrhea (> 10 days). It is not appropriate to order an O&P test if the patient develops diarrhea while in
the hospital. Due to the low incidence of most parasitic infections in the United States and Kansas, stool
specimens for Ova and Parasite are routinely tested only for Giardia lamblia and Cryptosporidium
parvum through an EIA test. However, if the patient has a travel history that includes regions of the
world where parasitic infections are endemic, microscopic evaluation for ova and parasites can be
ordered. Please contact the microbiology lab if this is the case (505-6177). These specimens will then be
submitted to the Kansas Department of Health and Environment laboratory for further testing.
Timing of Reports: A Giardia/Cryptosporidium antigen test is available every day of the week and is
offered on all shifts.
Malaria Exam: Specimens submitted for malaria exam are tested by both a Rapid
immunochromatographic assay in addition to smear exam. Malaria smears are performed in the
Page 31 of 65
microbiology laboratory, however, malaria may be found by the hematology department from the differential smear. Thin and thick smears are both examined using the Giemsa stain. All smears positive for malaria are reviewed and percent of parasitemia is determined by the pathologist. If a previously unknown positive is found, the specimen will be sent to the CDC for further testing.
References:
University of Nebraska Antimicrobial and Clinical Microbiology Guide Book, 2nd Edition, 2010, Omaha, NE.
Guidance for Control of Infections with Carbapenem-Resistant or Carbapenemase-Producing Enterobacteriaceae in Acute Care Facilities, CDC MMWR, 58(10);256-260, March 20, 2009,
Bailey and Scott's Diagnostic Microbiology, 13th Edition, Mosby Inc. St. Louis, Missouri, 2014.
"Prevention of Perinatal Group B Streptococcal Disease", Morbidity and Mortality Weekly Report, HHS and CDC, Vol. 59, No. RR-10, Nov. 19, 2010.
Manual of Clinical Microbiology, 10th Ed., Washington, D.C., ASM Press, 2011.
Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement, M100-S25, Clinical and Laboratory Standards Institute, January 2015.
Page 32 of 65
LMH Antimicrobial Formulary
July 2015
(Key: $: <$25; $$: $26-$100; $$$: $101-$150; $$$$: >$150)
Antifungals
Azole Antifungals
Fluconazole 200 mg premix (DIFLUCAN ) 200 mg 100 mL Bag
Fluconazole 400 mg premix (DIFLUCAN ) 400 mg 200 mL Bag
Fluconazole inj (DIFLUCAN ) 100 mg 50 mL SDV
Fluconazole susp (DIFLUCAN ) 200 mg 5 mL suspension
Fluconazole tab (DIFLUCAN ) 100 mg 1 tablet
Itraconazole (SPORANOX ) 100 mg 1 capsule
Ketoconazole (NIZORAL ) 200 mg 1 tablet
Voriconazole (VFEND ) 50 mg 1 tablet
Voriconazole (VFEND ) 200 mg 1 tablet
Micafungin (MYCAMINE ) 50 mg 5 mL injection
Micafungin (MYCAMINE) 100 mg 5 mL injection
Misc Antifungals
Betameth/mupir/miconazole oint(- )
Flucytosine (ANCOBON ) 500 mg 1 capsule
Terbinafine (LamISIL ) 250 mg 1 tablet
Polyenes
Amphotericin B Inj (AMPHOTERICIN B ) 50 mg 1 vial injection
Amphotericin B LIPOSOMAL(Ambisome ) 50 mg 12.5 mL injection
Amebicides
Paromomycin (PAROMYCIN ) 250 mg 1 capsule
Amikacin Inj.(AMIKACIN SULFATE ) 500 mg 2 mL inj
Aentamicin (baby/peds) PF (GENTAMICIN) 20 mg 2 mL inj
Page 33 of 65
Gentamicin (GARAMYCIN ) 80 mg 2 mL injection
Gentamicin (GENTAMICIN) 40 mg 1 mL inj
Gentamicin (GENTAMICIN SULFATE, INJECTABLE ) 40 mg 1 mL injection
Neomycin (NEOMYCIN SULFATE ) 500 mg 1 tablet
Streptomycin 1 g 1 EA injection
Tobramycin Inhalation (TOBI ) 300 mg 5 mL Inhaler
Tobramycin (NEBCIN ) 40 mg 1 mL injection
Tobramycin (NEBCIN ) 80 mg 2 mL injection
Carbapenems
Ertapenem (INVanz ) 1,000 mg 1 vial injection
ImiPENem-cilastatin (PRIMAXIN IV ) 250 mg 1 vial injection
ImiPENem-cilastatin (PRIMAXIN IV ) 500 mg 1 vial injection
MEROpenem (MERREM ) 500 mg 1 vial injection
MEROpenem (MERREM ) 1,000 mg 1 vial injection
First generation Cephalosporins
Cefadroxil (DURICEF ) 500 mg 1 capsule
CeFAZolin duplex bag(ANCEF ) 1,000 mg 50 mL duplex bag
CeFAZolin premix bag (ANCEF ) 1,000 mg 50 mL Bag
CeFAZolin vial (ANCEF ) 1,000 mg 1 vial inj
Cephalexin 200ml susp (KEFLEX ) 250 mg 5 mL suspension
Cephalexin susp (KEFLEX ) 125 mg 5 mL suspension
Cephalexin (KEFLEX ) 500 mg 1 capsule
Second generation Cephalosporins
Cefaclor 187/5ml susp (Ceclor ) 187 mg 5 mL suspension
Cefaclor susp (CECLOR ) 250 mg 5 mL suspension
Cefaclor susp (CECLOR ) 125 mg 5 mL suspension
Cefaclor (CECLOR) 250 mg 1 capsule
Cefotetan premix bag (CEFOTAN ) 1 g 50 mL Bag
Cefotetan premix bag (CEFOTAN ) 2 g 50 mL Bag
Cefotetan vial (CEFOTAN ) 2 g 1 vial injection
CefoTEtan vial(CEFOTAN ) 1,000 mg 1 vial injection
CefoTEtan vial (CEFOTAN ) 2,000 mg 1 vial injection
Page 34 of 65
Cefotetan (CEFOTAN ) 1 g 1 vial injection
Cefotetan (CEFOTAN ) 2 g 50 mL injection
CefOXItin duplex (MeFOXin ) 2,000 mg 50 mL duplex bag
CefOXitin (MeFOXin ) 1,000 mg 1 vial inj
CefOXitin (MeFOXin ) 2,000 mg 1 vial inj
Cefprozil susp (CEFZIL ) 125 mg 5 mL suspension
Cefprozil (CEFZIL ) 500 mg 1 tablet
CeFUROxime (CEFTIN) 250 mg 1 tablet
CeFUROxime (ZINACEF ) 1,500 mg 1 vial injection
Third generation cephalosporins
Cefixime (SUPRAX ) 400 mg 1 tablet
Cefotaxime (CLAFORAN ) 1 g 1 vial injection
CefoTAXime (CLAFORAN ) 1,000 mg 1 vial inj
Cefpodoxime susp (VANTIN ) 50 mg 5 mL suspension
Cefpodoxime (VANTIN ) 200 mg 1 tablet
CefTAZidime (FORTAZ ) 1,000 mg 1 vial injection
CefTRIAXone premix (ROCEPHIN ) 1,000 mg 50 mL Bag
CefTRIAXone premix (ROCEPHIN ) 2,000 mg 50 mL Bag
CefTRIAXone vial (ROCEPHIN ) 250 mg 1 vial inj
CefTRIAXone vial (ROCEPHIN ) 500 mg 1 vial injection
CefTRIAXone vial (ROCEPHIN ) 1,000 mg 1 vial inj
CefTRIAXone vial (ROCEPHIN ) 2,000 mg 1 vial inj
Ceftriaxone vial (ROCEPHIN ) 2,000 mg 1 vial injection
CefTRIAXone (ROCEPHIN ) 1 g 0 injection
CefTRIAXone (ROCEPHIN ) 500 mg 1 vial injection
CefTRIAXone (ROCEPHIN ) 1,000 mg 1 vial injection
Fourth generation cephalosporins
CefePIME (MAXIPIME ) 1 g 1 vial inj
CefePIME (MAXIPIME ) 2 g 1 vial inj
Gl ycylcyclines
Tigecycline (TYGACIL ) 50 mg 1 vial IV Piggyback
Page 35 of 65
Dapsone 100 mg 1 tablet
Dapsone 25 mg 1 tablet
Lincomycin derivatives
Clindamycin oral (CLEOCIN ) 150 mg 1 capsule
Clindamycin premix bag (CLEOCIN) 300 mg 50 mL Bag
Clindamycin premix bag (CLEOCIN) 600 mg 50 mL Bag
Clindamycin premix bag (CLEOCIN ) 900 mg 50 mL Bag
Clindamycin susp (CLEOCIN PEDIATRIC ) 75 mg 5 mL suspension
Clindamycin (CLEOCIN PHOSPHATE ) 300 mg 2 mL inj
Clindamycin (CLEOCIN PHOSPHATE ) 600 mg 4 mL injection
Clindamycin (CLEOCIN PHOSPHATE ) 900 mg 6 mL injection
Macrolides
Azithromycin Inj (ZITHROMAX IV ) 500 mg 1 vial inj
Azithromycin (ZITHROMAX ) 100 mg 5 mL suspension
Azithromycin(ZITHROMAX ) 200 mg 5 mL suspension
Azithromycin(ZITHROMAX ) 250 mg 1 tablet
Clarithromycin(BIAXIN ) 125 mg 5 mL suspension
Clarithromycin(BIAXIN ) 250 mg 1 tablet
Clarithromycin(BIAXIN ) 250 mg 5 mL suspension
Clarithromycin(BIAXIN ) 500 mg 1 tablet
Erythromycin EC (ERYTHROMYCIN BASE ) 250 mg 1 cap EC Capsule
Erythromycin EC Tablet (ERY-TAB ) 500 mg 1 tab EC Tablet
Erythromycin susp (Eryped 200 ) 200 mg 5 mL Susp
Erythromycin susp (ERYTHROMYCIN) 200 mg 5 mL Susp
Erythromycin (- ) 500 mg 1 vial REC Injection
Erythromycin (- ) 500 mg 10 mL inj
Erythromycin (- ) 1,000 mg 10 mL inj
Erythromycin(Eryped ) 200 mg 5 mL Susp
Erythromycin(ERY-TAB ) 250 mg 1 tab DR Tab
Fidaxomicin(Dificid ) 200 mg 1 tablet
Miscellaneous antibiotics
Atovaquone (MEPRON ) 750 mg 5 mL Susp
Aztreonam Inj (AZACTAM ) 500 mg injection
Aztreonam Inj (AZACTAM ) 1,000 mg 1 vial injection
Page 36 of 65
Aztreonam Inj (AZACTAM ) 2,000 mg 1 vial injection
Bacitracin irrigation (- ) 0 250 mL Irrigation
Bacitracin (BACITRACIN ) 50,000 unit 1 vial injection
Chloramphenicol (CHLOROMYCETIN ) 1,000 mg 1 vial injection
Colistimethate (- ) 150 mg 1 vial injection
DAPTOmycin (CUBICIN ) 1 mg 0.02 mL inj
Daptomycin (Cubicin ) 500 mg 1 vial Insert
Erythromycin-sulfisoxazole susp (Eryped ) 0 5 mL suspension
Linezolid premix bag (ZYVOX ) 600 mg 300 mL Bag
Linezolid (ZYVOX ) 600 mg 1 tablet
Pentamidine INHALATION(NEBUPENT ) 300 mg 1 vial suspension
Sulfamethoxazole-trimethoprim DS(BACTRIM DS ) 1 tab DS Tab
Sulfamethoxazole-trimethoprim inj (BACTRIM ) 160 mg 10 mL inj
Sulfamethoxazole-trimethoprim inj (BACTRIM ) 2,400 mg 30 mL inj
Sulfamethoxazole-trimethoprim ss (BACTRIM ) 1 tablet
Sulfamethoxazole-trimethoprim susp(SULFATRIM ) 20 mL Susp
Vancomycin vial (advantage)(Vancomycin ) 500 mg 1 vial injection
Vancomycin vial (advantage)(- ) 1 g 1 vial injection
Vancomycin vial(VANCOMYCIN HCL ) 500 mg 5 mL inj
Vancomycin vial(VANCOMYCIN HCL ) 1,000 mg 10 mL inj
Penicillins
Aminopenicillins
Amoxicillin susp (TRIMOX ) 250 mg 5 mL suspension
Amoxicillin (AMOXICILLIN ) 250 mg 1 capsule
Ampicillin cap (PRINCIPEN ) 250 mg 1 capsule
Ampicillin susp (PRINCIPEN ) 250 mg 5 mL suspension
Ampicillin vial (AMPICILLIN ) 125 mg 1.2 mL injection
Ampicillin vial (AMPICILLIN ) 250 mg 1 mL injection
Ampicillin vial (AMPICILLIN ) 500 mg 2 mL injection
Ampicillin vial (AMPICILLIN ) 1,000 mg 3.5 mL injection
Page 37 of 65
Ampicillin vial (AMPICILLIN ) 2,000 mg 1 vial injection
Beta-lactamase inhibitors
Amoxicillin-clavulanate susp(AUGMENTIN ) 200 mg 5 mL suspension
Amoxicillin-clavulanate susp(AUGMENTIN ) 400 mg 5 mL suspension
Amoxicillin-clavulanate susp(AUGMENTIN ) 600 mg 5 mL suspension
Amoxicillin-clavulanate(- ) 0 5 mL Powder
Amoxicillin-clavulanate(AUGMENTIN ) 0 1 tablet
Amoxicillin-clavulanate (AUGMENTIN ) 0 5 mL Powder
Amoxicillin-clavulanate (AUGMENTIN ) 500 mg 1 tablet
Amoxicillin-clavulanate (AUGMENTIN ) 875 mg 1 tablet
Ampicillin-sulbactam (UNASYN ) 1,500 mg 1 vial injection
Ampicillin-sulbactam (UNASYN) 3,000 mg 1 vial injection
Piperacillin-tazo premix (ZOSYN ) 2.25 g 50 mL Bag
Piperacillin-tazo premix (ZOSYN ) 3.375 g 50 mL Bag
Piperacillin-tazo (ZOSYN ) 2.25 g 10 mL inj
Piperacillin-tazo (ZOSYN ) 3.375 g 10 mL inj
Natural penicillins
Penicillin (IM ONLY)(BICILLIN L-A ) 1.2 MU 2 mL LA inj
Penicillin GK (Penicillin G Potassium ) 1 MU 2 mL inj
Penicillin G potassium 3 MU 50 mL Bag
Penicillin G sodium vial(- ) 5 MU 10 mL inj
Penicillin susp(Veetids ) 125 mg 5 mL REC Powder
Penicillin susp(Veetids ) 250 mg 5 mL REC Powder
Penicillin VK tab(- ) 250 mg 0.5 tablet
Penicillinase resistant penicillins
Dicloxacillin (DICLOXACILLIN SODIUM ) 500 mg 1 capsule
Nafcillin (- ) 1,000 mg 10 mL injection
Nafcillin (NAFCIL ) 2,000 mg 10 mL injection
Nafcillin (NAFCIL ) 1,000 mg 1 vial injection
Quinolones
Page 38 of 65
ciprofloxacin premix bag(CIPRO I.V. ) 400 mg 200 mL Bag
Ciprofloxacin (CIPRO ) 100 mg 1 tablet
Ciprofloxacin (CIPRO ) 250 mg 1 tablet
Ciprofloxacin (CIPRO ) 500 mg 1 tablet
Ciprofloxacin (CIPRO ) 500 mg 5 mL Susp
Ciprofloxacin (CIPRO ) 750 mg 1 tablet
Levofloxacin premixed bag (LEVAQUIN ) 250 mg 50 mL Bag
Levofloxacin premixed bag (LEVAQUIN ) 500 mg 100 mL Bag
Levofloxacin premixed bag (LEVAQUIN ) 750 mg 150 mL Bag
Levofloxacin (LEVAQUIN ) 250 mg 1 tablet
Levofloxacin (LEVAQUIN ) 500 mg 1 tablet
Levofloxacin (LEVAQUIN ) 750 mg 1 tablet
Moxifloxacin (AVELOX ) 400 mg 1 tablet
sulfaDIAZINE(- ) 500 mg 1 tablet
sulfaSALAzine(Azulfadine ) 500 mg 1 tablet
Demeclocycline (DECLOMYCIN ) 150 mg 1 tablet
Doxycycline inj (VIBRAMYCIN) 100 mg 1 vial injection
Doxycycline susp (VIBRAMYCIN) 25 mg 5 mL suspension
Doxycycline (VIBRAMYCIN) 100 mg 1 tablet
Minocycline (MINOCIN ) 100 mg 1 vial injection
Urinary anti-infectives
Fosfomycin (MONUROL ) 3 g suspension
Nitrofurantoin monohydrate (MACROBID ) 100 mg 1 capsule
Nitrofurantoin (MACROBID ) 50 mg 1 capsule.
Antimalarial agents
Antimalarial quinolines
quiNINE(Qualaquin ) 324 mg 1 capsule
Page 39 of 65
Miscellaneous antimalarials
Pyrimethamine (DARAPRIM ) 25 mg 1 tablet
QuiNINE (QuiNINE sulfate ) 260 mg 1 tablet
Quinine(QUININE SULFATE ) 324 mg 1 capsule
Antituberculosis agents
Nicotinic acid derivatives
isoniazid(INH ) 300 mg 1 tablet
pyrazinamide(- ) 500 mg 1 tablet
Rifamycin derivatives
Rifampin inj (RIFADIN IV ) 600 mg 10 mL inj
Rifampin (RIFADIN ) 300 mg 1 capsule
Antiviral agents
Adamantane antivirals
amantadine syrup(Symmetrel ) 50 mg 5 mL Syrup
amantadine(symmetrel ) 100 mg 1 capsule
Antiviral chemokine receptor antagonist
maraviroc(Selentry ) 300 mg 1 tablet
Antiviral combinations
abacavir-lamivudine (EPZICOM ) 0 1 tablet
emtricitabine-tenofovir 200/300 mg (TRUVADA ) 0 1 tablet
Integrase strand transfer inhibitor
Raltegravir (ISENTRESS ) 400 mg 1 tablet
Miscellaneous antivirals
Foscarnet (FOSCAVIR ) 24 mg 1 mL injection
Foscarnet (FOSCAVIR ) 6,000 mg 250 mL injection
Palivizumab (SYNAGIS ) 50 mg 0.5 mL injection
Palivizumab (SYNAGIS) 100 mg 1 mL injection
Neuraminidase inhibitors
Page 40 of 65
Oseltamivir (TAMIFLU ) 60 mg 5 mL suspension
Oseltamivir (TAMIFLU ) 75 mg 1 capsule
Zanamivir (RELENZA ) 5 mg 0 Powder
efavirenz(SUSTIVA ) 200 mg 0 Cap
efavirenz(Sustiva ) 600 mg 1 tablet
nevirapine(VIRAMUNE ) 50 mg 5 mL Susp
nevirapine(Viramune ) 200 mg 1 tablet
Lamivudine(EPIVIR ) 150 mg 0 Tab
LamiVUDine (EPIVIR ) 150 mg 1 tablet
LamiVUDine-zidovudine (COMBIVIR ) 0 1 tablet
Stavudine (ZERIT) 40 mg 1 capsule
Zidovudine (RETROVIR ) 200 mg 20 mL injection
Zidovudine (RETROVIR ) 2,400 mg 240 mL Syrup
Zidovudine (RETROVIR ) 100 mg 1 capsule
Protease inhibitors
Indinavir (CRIXIVAN ) 400 mg Cap
Indinavir (CRIXIVAN ) 400 mg 1 capsule
Nelfinavir (VIRACEPT ) 250 mg 1 tablet
Purine nucleosides
Acyclovir Inj (ZOVIRAX ) 500 mg 1 vial inj
Acyclovir susp (ZOVIRAX ) 800 mg 20 mL Susp
Acyclovir (ZOVIRAX ) 200 mg 1 capsule
Acyclovir (ZOVIRAX) 800 mg 1 tablet
Cidofovir (VISTIDE ) 375 mg 5 mL injection
Famciclovir (FAMVIR) 250 mg 1 tablet
Famciclovir (FAMVIR ) 500 mg 1 tablet
Ganciclovir (CYTOVENE ) 500 mg 1 vial injection
Ribavirin Inh soln (VIRAZOLE ) 6 g 0 Inhaler
ValACYclovir (VALTREX ) 500 mg 1 tablet
Page 41 of 65
Antimicrobial Clinical Practice Guidelines
Dosing for Vancomycin
Empiric Dosing
1. Set Loading dose 25 mg x Actual Body Weight (Max 2 g) = _ mg
2. Set Maintenance dose = 15-20 mg/ kg x Actual Body Weight (max dose of 2 g to start, based on severity of infection) – Round all doses to nearest 250 mg
Determine CrCl and determine frequency
Dosing in morbidly obese patients:
1. Empiric dosing should be dosed on total body weight at 15mg/Kg q12 hrs for patients with normal renal function. Maximum dose 2 grams, rounded to the nearest 250mg. For patients with impaired renal function, refer to frequency table above.
2. Trough levels should be drawn on all obese patients. Vancomycin is cleared much faster in this population vs the non-obese population. Hence, the need for q8hr dosing is not unusual when renal function is normal.
3. Estimated creatinine clearance should be calculated using the Salazar-Corcoran equation, which has shown to be a better predictor of CrCl in obese patients when compared to the Cockroft-Gault equation.
Dosing in dialysis patients:
Individualize patient doses based on weight, indication, dialysis schedule, and dialysis filter. At LMH, the filters are high flux.
Pts with residual kidney function (RRF): Dose per weight/indication, monitor every 3 days, redose per weight when levels are less than 10mcg/ml. Drug should be given post dialysis and levels should be drawn 1 hour post dialysis to compensate for Vancomycin redistribution.
Page 42 of 65
Anuric: Dose per weight, monitor every 5 days, redose per weight when levels are less than 10 mcg/ml.
Intradialytic administration at KDS is generally the following:
High flux Hemodialysis (HF HD) – in patients less than 50Kg, 1 gram IV loading over the last hour of HD and 500mg IV in the last hour of dialysis during each of the following HD sessions. Draw level on the first treatment following the patient's weekend. Call nephrologist if level is <5 or >20. For patients 50 to 70Kg, initial dose is 1.5grams, maintenance is 750mg. For patients greater than 70Kg, initial dose is 2 grams over last 1.5 hours of dialysis, maintenance doses are 1 gram.1,2
Monitoring Guidelines
Therapeutic Plasma Concentrations
LMH Normal – Trough 5-15 mcg/ml LMH Critical Value: > 25 mcg/ml
Recommended Goals – Troughs should be obtained just prior to the next dose at steady-
state conditions
Mild to Moderate Infections
Severe Infections
Goal: 10-15 mcg/ml
Goal: 15 to 20 mcg/ml (if over 20 and less than
25, use clinical judgement)
Examples: Empiric therapy, skin and skin
Examples: Endocarditis, osteomylitis,
structure infections
meningitis, bacteremia and hospital acquired
pneumonia caused by Staphylococcus
Monitoring Guideline
Obtain a serum creatinine weekly, more frequently when given with nephrotoxic medications
Monitor CBC, Tmax to evaluation drug effectiveness.
Monitoring Serum Vancomycin
Monitoring is NOT recommended in the following settings:
o Patients treated for less than five days. o Patients receiving oral vancomycin, unless treated for greater than 3 weeks at 1 gram per
day with diminished renal function.
o Patients with stable renal function who are treated empirically for up to 14 days for mild to
moderate infections.
Page 43 of 65
Monitoring trough serum concentrations IS recommended in the following settings:
Patients who will need continued IV treatment (>five days) for moderate to severe infections.
Prolonged (>14 days) treatment.
Serious or life-threatening infections.
End-stage renal disease in patients who are likely to receive more than one dose.
Co-administration of nephrotoxic drugs (e.g., aminoglycosides, amphotericin B, cyclosporine).
Extremes of body weight - (Morbidly obese patients)
Patients with rapidly changing or unpredictable renal function.
Monitoring peak serum concentrations IS not recommended1
References:
1. Rybak M, Lomaestro, B, Rotschafer J, et al., Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists Am J Health-Syst Pharm. 2009; 66:82-98
2. Pallotta,KE, Manley, HJ. Vancomycin Use in Patients Requiring Hemodialysis: A Literature Review Seminars in Dialysis, Vol21, No1 (January-February) 2008 pp.63-70.
3. KDS Vancomycin dosing for Dr. Solcher and Dr. Duvuur - April 2009
4. Cockroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1975;16:31-41
LMH DEPARTMENT OF PHARMACY
ANTI-INFECTIVES RENAL DOSING CHART
NORMAL DOSE
RENAL DOSE ADJUSTMENTS
Acyclovir IV
5 mg/kg IV Q8 hours
Frequency to Q12 hours
Frequency to Q24 hours
50% normal dose IV Q24
Use ideal body weight
10 mg/kg IV q8 hours
Q24 hours schedule dose to
be given after dialysis
Dose and Renal Adjustments Vary by Indication. Please refer to appropriate drug reference.
Extended Interval Dosing:
Extended Interval Dosing:
15 mg/kg IV Q24 hours
40-59: 15 mg/kg IV Q36 hours
Note: Consultation with ID
Note: draw level 6-14 hours
30-39: 15 mg/kg IV Q48 hours
Page 44 of 65
& Pharmacy recommended. after 1st dose
<30 AVOID, use conventional
Conventional Dosing:
Conventional Dosing:
5-7.5 mg/kg/dose Q8 hours
40-60: 5-7.5 mg/kg Q12 hours
Note: Peak & trough levels
20-39: 5-7.5 mg/kg Q24 hours
should be monitored
<20: 5-7.5 mg/kg then monitor
HD: 5-7.5 mg/kg Q48-72h,
monitor level prior to HD to determine dosing needs
250-500 mg PO Q8 hours
10-30: 500 mg PO Q12 hours
<10: 500 mg PO Q24 hours
HD: 500 mg PO q24 hours
schedule dose to be given after dialysis
Amoxicillin/ Clavulanate
875 mg PO Q12 hours
10-30: 500/125 mg PO Q12 hours
<10: 250 mg PO Q12 hours
500 mg PO Q8 hours
HD: 250-500 mg PO q24 hours
schedule dost to be given after dialysis
< 30 XR formulation NOT
1000/62.5 mg PO q12h (XR
recommended with CrCl < 30
NORMAL DOSE
RENAL DOSE ADJUSTMENTS
Continuous Infusion:
***** Continuous Infusion
8-12 g IV over 24
30-49: 8 g IV over 24 hours
10-29: 6-8 g IV over 24 hours
<10: Use Traditional Dosing
HD: Use Traditional Dosing
Conventional Dosing:
***** Conventional Dosing
1-2 g IV Q4 hours
30-49: 1-2 g IV Q6 hours
Note: 2g dose is
10-29: 1-2 g IV Q12 hours
recommended for
<10: 1-2 g IV Q12 hours
endocarditis, meningitis and
HD: 1-2 g IV Q12 hours
bacteremia
1.5 g IV Q6 hours
30-49: 1.5-3 g IV Q8 hours
15-29: 1.5-3 g IV Q12 hours
5-14: 1.5-3 g IV Q24 hours
Page 45 of 65
HD: 1.5-3 g IV Q24 hours –
Usual: 500mg PO x 1, then 250 mg PO Q24 hours x 4-10 days
or 500 mg PO Q24 hours x 3 days or 1 gm PO as a single dose
Note: Dose and duration vary by indications. Please see appropriate reference .
1-2 g IV Q8 hours
10-29: 50% of usual dose at usual
Interval to q6 hours for severe
<10: 25% of usual dose at usual
infections (esp. P.aeruginosa)
HD: 500 mg IV Q12 hours
ER: 500mg PO Q12 hours
<10: Reduce dose by 50%
RR: 250-500 mg PO Q8-12h
HD: Reduce dose by 50% - Give
1000 mg PO Q12 hours
25-50: 500 mg PO Q12 hours
Indication based alternative
10-25: 500 mg PO Q24 hours
dosing: Please see appropriate
<10: 500 mg PO Q36 hours
reference
10-30: Frequency to Q12 hours
<10: Frequency to Q24 hours
Note: >= 80 kg or severe
HD: Frequency to Q24 hours
infections may use 2 g
schedule dose to be given after dialysis
NORMAL DOSE
RENAL DOSE ADJUSTMENTS
1 g IV Q8-12 hours
30-49: 1 g IV Q12 hours
10-29: 1 g IV Q24 hours
<10: 1 g IV Q24 hours
HD: 1 g IV Q24 hours - Give AFTER
CNS infections or febrile
CNS Infections or febrile
neutropenia
30-49: 2 g IV Q12 hours
10-29: 2 g IV Q24 hours
<10: 1 g IV Q24 hours
HD: 1 g IV Q24 hours - Give AFTER
400 mg PO Q24 hours
21-60: 75% dose PO Q24 hours
or 200 mg PO Q12 hours Note: alternate dosing
<20 50% dose PO Q24 hours
Page 46 of 65
available for STD, please see
HD: 75% dose PO Q24 hours,
appropriate drug information
schedule dose to be given
reference.
1-2 g IV Q4-8 hours
10-50: Administer Q6-12 hours
<10 Administer Q24 hours
Note: Dose and frequency
HD: 1-2 g IV Q24 hours, schedule
vary by indications. Please
dose to be given after dialysis
see appropriate drug information reference.
1 g IV Q12 hours
10-30: Frequency to Q24 hours
Note:>=80 kg may use 2 g
<10: Frequency to Q48 hours
HD: 25% dose IV Q24 hours non-
HD days, 50% dose on HD days, schedule dose after dialysis
10-49: 1 g IV Q8 hours
Note:>=80 kg may use 2 g
<10: 1 g IV Q12 hours
200-400 mg PO Q12 hours
<30: 200-400 mg PO Q24 hours
HD: 200-400 mg PO 3 x week after
NORMAL DOSE
RENAL DOSE ADJUSTMENTS
Sinusitis: 250-500mg PO Q12
<30: Give 50% of dose
HD: Give 50% of dose schedule
dose to be given after
500 mg PO Q24 hours
COPD exac/bronchitis:
500 mg PO Q12 hours Skin: 250 mg PO Q12 hours or 500 mg PO Q12-24 hours
600 mg IV Q12 hours
31-50: 400 mg IV Q12 hours
15-30: 300 mg IV Q12 hours
<15: 200 mg IV Q12 hours
HD: 200mg IV Q12 hours
30-49: 1 g IV Q12 hours
Page 47 of 65
<30: 1 g IV Q24 hours
CNS infections: Use 2g dose
HD: Load with 1 g then give 1 g
with same frequency
after each HD session
Meningitis or CNS Infection: 2 g IV
No renal adjustments needed
Q12 hours For all indications other than CNS: 1 g IV Q24 hours
Cefuroxime IV
750mg-1.5g IV Q8 hours
10-20: 750 mg IV Q12 hours
Note: Frequency can be
<10: 750 mg IV Q24 hours
increased to Q6 hours for life
HD: 750 mg IV after each
threatening infections
dialysis session
Cefuroxime PO tab
250-500 mg PO Q12 hours
<10: 250-500 mg Q24 hours
Note: tablet dosing not
HD: 250-500 mg PO q24 hours
equivalent to suspension, we
schedule dost to be given
do not carry suspension
500 mg PO Q6 hours
10-30: 500 mg PO Q8 hours
<10: 500 mg PO Q12 hours
HD: 250-500 mg PO q24 hours
schedule dost to be given after dialysis
Ciprofloxacin IV
400 mg IV Q12 hours
<30 200-400 mg IV Q24 hours
HD: 200 mg IV Q24 hours
400 mg IV Q8 hours
schedule dose to be given after dialysis
NORMAL DOSE
RENAL DOSE ADJUSTMENTS
Ciprofloxacin PO
500 mg PO Q12 hours
10-29: 250 mg PO Q12 hours
<10: 250 mg PO Q24 hours
HD: 250 mg PO Q24 hours
schedule dose to be given after dialysis
500 mg PO Q12 hours
10-29: 250 mg PO Q12 hours
<10: 250 mg PO Q24 hours
HD: 250 mg PO Q24 hours
schedule dose to be given after dialysis
Clindamycin IV
600 mg IV Q8 hours, 900 mg IV Q8
No renal adjustment needed
hours for necrotizing faciitis Note: For patients >100 kg 900 mg IV Q8 hours may be used
Page 48 of 65
Clindamycin PO
150-450 mg PO q6-8 hours (varies
Note: For patients >100 kg 450 mg
by indication, please use
PO Q6 hours may be used
appropriate reference)
4-6 mg/kg IV Q24 hours
<30: 4-6 mg/kg IV Q48 hours
Note: Not to be used for
HD: 4-6 mg/kg IV Q48 hours
infections in the lungs; 4
schedule dose to be given
mg/kg for UTI/SSSTI
125-500mg PO Q6 hours
No adjustment needed
Doxycycline IV or PO
No adjustment needed
1000 mg IV Q24 hours
<30: 500 mg IV Q24 hours
HD: 500 mg IV Q24 hours
schedule dose to be given after dialysis
Erythromycin IV
500-1000 mg IV Q6 hours
<10: 50% PO/IV dose at same
Erythromycin PO
Base: 250-500mg PO Q6-12 h
HD/CAPD: Dose the same as CrCl <10
EES: 400-800mg PO Q6-12 h
NORMAL DOSE
RENAL DOSE ADJUSTMENTS
***** Initial Treatment:
Initial: 15 mg/kg PO Q24 hrs
10-50: 15 mg/kg PO Q24-36 hours
Retreatment: 25 mg/kg PO
<10: 15 mg/kg PO Q48 hours
Q24 hours x 60 days, then 15
HD: Administer dose after
Note: Should not be used
alone; Monthly eye exams
***** Retreatment:
recommended on high dose
10-50: 25 mg/kg PO Q24-36 hours
regimen; max dose 2.5 gm
<10: 25 mg/kg PO Q48 hours
HD: Administer dose after
250-500 mg PO Q8 hours 40-59: same dose Q12 hours
500 mg PO Q8 hours for VZV
20-39: same dose Q24 hours
Note: no clear adjustment
<20: 50% dose Q24 hours
Page 49 of 65
recommendations in CAPD
HD: 50% after each dialysis session
200 mg PO Q12 hours
No adjustment needed
Fluconazole IV or PO
*Invasive candidiasis: 800 mg
10-29: Load x1, then 50% PO/IV
(12 mg/kg) load x1, then 400
mg (6 mg/kg) PO/IV Q24
<10: Load x1, then 25% PO/IV
HD: Load x1, then 400 mg (6
mg/kg) after HD 3x/weekly
CAPD: 50% PO/IV Q24 hours
<30: 50% PO/IV Q 24 hours
candidiasis: 200 mg PO/IV Q24 hours for esophageal;
HD: 100% PO/IV after each
100 mg Q24 hours for
dialysis session
CAPD: 50% PO/IV Q24 hours
Note: Some indications may
require different dosing, please refer to appropriate drug reference
Fosfomycin PO
Uncomplicated cystitis: 3 gm
<50: Same dose
x1 Complicated cystitis: 3 gm
<50: 3 gm Q72 hours
NORMAL DOSE
RENAL DOSE ADJUSTMENTS
Ganciclovir IV
Induction:
5 mg/kg IV Q12 hours
50-69: 2.5 mg/kg IV Q12 hours
25-49: 2.5 mg/kg IV Q24 hours
10-24: 1.25 mg/kg IV Q24 hours
5 mg/kg IV Q24 hours
<10: 1.25 mg/kg IV 3 x week
HD: 1.25 mg/kg IV 3 x week
schedule dose to be given after hemodialysis
2.5 mg/kg IV Q24 hours
1.25 mg/kg IV Q24 hours
0.625 mg/kg IV Q24 hours
0.625 mg/kg IV 3 x week
0.625 mg/kg IV 3 x week schedule dose to be given
Page 50 of 65
after hemodialysis
Ganciclovir PO
1000 mg PO Q8 hours
1500 mg PO Q24h or 500 mg PO Q8h
1000 mg PO Q24h or 500 mg PO Q12h
500 mg PO Q24 hours
500 mg PO 3 x week schedule dose to be given afer hemodialysis
Extended Interval Dosing:
Extended Interval Dosing:
7 mg/kg IV Q24 hours
40-59: 7 mg/kg IV Q36 hours
Note: draw level 6-14 hours
30-39: 7 mg/kg IV Q48 hours
Note: Consultation with
after 1st dose
<30 AVOID, use conventional
Pharmacy recommended
Conventional Dosing:
Exclusion to Extended
Conventional Dosing:
40-60: 1-2.5 mg/kg Q12 hours
Interval: Pregnancy,
1-2.5 mg/kg/dose Q8 hours
20-39: 1-2.5 mg/kg Q24 hours
breastfeeding, burns (>20%
Note: Peak & trough levels
<20: 1-2.5 mg/kg then monitor
body), ascites,
monitored, consult pharmacy
Enterococcoal endocarditis,
HD: 2-3 mg/kg Q48-72h,
HD or CrCl < 20 ml/min
monitor level prior to HD to
determine dosing needs
NORMAL DOSE
RENAL DOSE ADJUSTMENTS
Imipenem/ Cilastatin
250-1000mg IV Q6-8 hours
30-70: Decrease daily dose by 50%
& divide Q6-8 hours (round to nearest 250 mg)
Usually 500 mg IV Q6 hours
20-30: Decrease daily dose by 63%
Note: max dose 50mg/kg/day
& divide q8-12 hours (round
or 4g/day
to nearest 250 mg)
6-20: Decrease daily dose by 75%
& divide Q12 hours (round to nearest 250 mg)
Note: Dose varies by severity
<5: Not recommended unless
of infection & organism
HD being started
sensitivity
HD: Decrease daily dose by 75%
& divide q12 hours (round to nearest 250 mg); dose after dialysis on dialysis day
Page 51 of 65
300 mg PO Q24 hours (5 mg/kg PO Q24 hours); max of 300 mg; HD/CAPD: dose after dialysis
200 mg PO Q8-24 hours (histo/cocci/blasto: load with 200 mg Q8 hours x2 days, then 200 mg Q12 hours)
Notes: avoid PPIs/H2 blockers; suspension on empty stomach; capsules with meal or acidic drink; consider monitoring SS trough after 5-7 days (>1 mg/dL, sum of itraconzole and hydroxy-itraconazole)
200-400 mg PO Q24 hours
Note: Dose and frequency vary by indications. Please see appropriate drug information reference.
Levofloxacin IV or PO
Pneumonia/Pseudomonas:
20-49: Pneumonia /Pseudomonas:
750 mg Q24 hours
Other: 500 mg load then
10-19: Pneumonia /Pseudomonas:
500 mg Q48 hours
Other Indications:
Other: 500 mg load then
500 mg Q24 hours
<10: Pneumonia /Pseudomonas:
Other: 250 mg Q48 hours
HD: Pneumonia /Pseudomonas:
Other: 250 mg Q48 hours
NORMAL DOSE
RENAL DOSE ADJUSTMENTS
Linezolid IV or PO
600 mg Q12 hours
No adjustment needed
500 mg IV Q6 hours
25-49: 500 mg IV Q8 hours (UTI:
500 mg Q12 hours; meningitis, etc: 2 gm Q 12 hours)
UTI: 500 mg Q8 hours
10-24: 500 mg IV Q12 hours (UTI:
250 mg Q12 hours; meningitis, etc: 1 gm Q12 hours)
Meningitis, CF, meropenem
<10/HD: 500 mg IV Q24 hours (UTI:
MIC of 4 mg/dL: 2 gm Q8
500 mg Q24 hours;
meningitis, etc: 1 gm Q24 hours)
HD/CAPD dose as CrCl <10, given after
dialysis on dialysis days
Page 52 of 65
Metronidazole IV or PO
500 mg Q12 hours if C difficile <10:
Consider 50% at same
is not suspected
interval if >14 day duration
500 mg Q8 hours if C difficile
HD/CAPD Give after dialysis on
100 mg IV Q24 hours
No adjustment needed
Minocycline PO
100 mg PO Q12 hours
<10: 100 mg Q24 hours
HD: 100 mg Q24 hours
Continuous Infusion: 8-12 g/day
No adjustment needed
Note: 12 g/day recommended for endocarditis Intermittent Dosing: 2 g IV Q4-6 hours
100 mg PO Q12 hours
<60, <50, HD/CAPD: Use is not
HD/CAPD: recommended - will not
reliably reach useful concentrations in urine and will have increased risk of toxicity.
NORMAL DOSE
RENAL DOSE ADJUSTMENTS
<30: Prophylaxis: 75 mg PO Q48
75 mg PO Q12 hours
HD: Treatment/Prophylaxis: 30
75 mg PO Q24 hours
mg PO x 1 before HD then after every other dialysis session x 3 doses
Penicillin G IV
Continuous Infusion:
Continuous Infusion
18-24 million units/day
10-50: 12-18 million units/day
(75% IV at same time interval)
<10 6-12 million units/day (75%
IV at same time interval)
HD: Dose as CrCl <10, given
after dialysis on dialysis days
Page 53 of 65
CAPD: Dose a CrCl <10.
Intermittent Dosing: 2-3
Intermittent Dosing
million units IV Q4-6 hours
10-50: 1-2 million units/day IV Q4-
Note: Doses varies by
<10: 1 million units/day IV Q6
indication. Please refer to
appropriate drug reference.
Penicillin VK PO
500 mg PO Q6 hours
10-50: 500 mg PO Q8 hours
Note: Doses varies by
<10: 500 mg PO Q12 hours
indication. Please refer to
HD: Give dose after dialysis on
appropriate drug reference.
Pneumonia/Pseudomonas:
20-39: Pna/Pseudomonas: 3.375 g
4.5 g IV Q6 hours
Other: 2.25 g IV Q6 hours
<20: Pna/Pseudomonas: 2.25 g
Other Indications:
Other: 2.25 g IV Q8 hours
3.375 g IV Q6 hours or 4.5 g IV
HD: Pna/Pseudomonas: 2.25 g
Other: 2.25 g IV Q8 hours Supplement Dose after HD:
Dose as CrCl< 20
NORMAL DOSE
RENAL DOSE ADJUSTMENTS
Quinipristin/ Dalfopristin
7.5 mg/kg IV Q8-12 hours Note: Synercid should be used with discretion due to cost and to maintain susceptibility profile
Doses vary by indication. Please refer to appropriate drug information reference.
Note: Should not be used alone; screen for drug interactions
100mg PO Q12 hours
<30: 100 mg PO Q24 hours
500 mg PO Q12 hours
<50: Use not recommended
250 mg PO Q24 hours
Severe or Pseudomonas:
30-60: 3.1 g IV Q8 hours
3.1 g IV Q4 hours
10-30: 3.1 g IV Q12 hours
Moderate: 3.1 g IV Q6 hours
<10: 2 g (ticarcillin) IV Q12 hours
UTI: 3.1 g IV Q6-8 hours
<10 & hepatic dysf: 2 g
(ticarcillin) IV Q24 hours
Note: <60kg dose 200-
HD: 2 g (ticarcillin) IV Q12 hours
Page 54 of 65
300mg/kg/day divided q4-6 h
plus 3.1 g after HD session
CAPD: Dose as CrCl <10.
Extended Interval Dosing:
Extended Interval Dosing:
Frequency determined by
levels/Hartford nomogram
7 mg/kg IV Q24 hours
40-59: 7 mg/kg IV Q36 hours
Note: draw level 6-14 hours
30-39: 7 mg/kg IV Q48 hours
Note: Consultation with
after 1st dose
<30 AVOID, use conventional
Pharmacy recommended.
Conventional Dosing:
Conventional Dosing
(empiric, before levels):
1-2.5 mg/kg/dose Q8 hours
40-60: 1-2.5 mg/kg Q12 hours
Note: Peak & trough levels
20-39: 1-2.5 mg/kg Q24 hours
monitored, consult pharmacy
10-20: 1-2.5 mg/kg Q48 hours
<10: 1-2.5 mg/kg Q72 hours
HD: 2-3 mg/kg Q48-72h,
monitor level prior to HD to determine dosing needs
NORMAL DOSE
RENAL DOSE ADJUSTMENTS
MRSA cellulitis (or Skin/skin
15-30: Increase interval to Q24
Sulfamethoxazole PO
structure infection/other
hours or Decrease dose by
(1 Bactrim DS tablet =
160mg(TMP)/800(SMX)
1-2 DS tablets Q12 hours
<15: AVOID or decrease dose
Note: When changing
Other Indications:
50% (ie. DS to SS) AND
from IV to PO use the
1 DS tablet Q12 hours
increase interval to Q48
same trimethoprim
dose as IV
HD: Dose according to dosage
for <15, schedule dose to
be given after dialysis
Pneumocystis treatment:
15-30 50% of usual dose in 2-4
Sulfamethoxazole IV
15-20 mg/kg/day in 3-4
Page 55 of 65
<15 AVOID if possible
Note: Dosing is based on Trimethoprim
Pneumocystis treatment:
component
15-20 mg/kg/dose Q48
Severe Infections:
7-10 mg/kg/day divided in
8-10 mg/kg/day in 2-4 divided
Severe Infections:
8-10 mg/kg/dose Q48 hous
4-5 mg/kg/day divided in 1-
HD: Dose according to dosage
for <15, schedule dose to be given after dialysis
NORMAL DOSE
RENAL DOSE ADJUSTMENTS
Loading Dose: 20 mg/kg IV x 1
40-60: 15-20 mg/kg IV Q24 hours
25-39: 15-20 mg/kg IV Q48 hours
<24: Dosing adjustments per
Note: Consultation with
15-20 mg/kg IV Q8-12 hours
HD: Dosing adjustments per
Pharmacy recommended.
Max Dose 2000 mg
Round to nearest 250 mg
Note: trough levels should be
monitored on patient with expected duration > 3 days
*Dosing, therapeutic goals, and monitoring should be individualized for each patient.
Troughs of 15-20 mcg/mL are recommended for patients with MRSA bloodstream infections, endocarditis, meningitis, pneumonia, osteomyelitis, and septic arthritis.
Page 56 of 65
250-500 mg PO Q8 hours
Note: Renal dose
Note: Dose and frequency
adjustments vary by
vary by indications. Please
indication, please refer to
see appropriate drug
appropriate drug reference
information reference. 2g PO q12h
30-49: 1g PO q12h 10-29: 1g PO q12h
<10: 500mg PO q24h
30-49: 1g PO q12h 10-29: 1g PO q24h
<10: 500mg PO q24h
30-49: no adjustment 10-29: 1g PO q24h
<10: 500mg PO q24h
30-49: no adjustment 10-29: 500mg PO q24h
<10: 500mg PO q24h
30-49: no adjustment 10-29: 500mg PO q24h
<10: 500mg PO q24h
30-49: no adjustment 10-29: 500mg PO q48h
<10: 500mg PO q48h
HD: 500mg PO q48h
CAPD: 500mg PO q48h
NORMAL DOSE
CRCL in ml/min
RENAL DOSE
ADJUSTMENTS
Voriconazole PO
Loading Dose: 400 mg PO x 1, then 200 mg PO Q12 hours
May require dose adjustment in hepatic impairment, consult pharmacy
Note: Consider weight base dosing for obese patients using ADJ BW
Note: Oral formulation is 95% bioavailable
Note: Screen for drug interactions, dose may need adjustment
Hepatic dysfunction (Child
Pugh A or B): 6 mg/kg q12h
x2doses then 50% of
normal daily dose.
Page 57 of 65
Voriconazole IV
Loading dose of 6mg/kg q12h
x2 doses, then 4mg/kg 12h
200mg q12h (100mg q12h if
Renal dysfunction: no adjustment
Therapeutic drug monitoring
If CrCl < 50, accumulation of IV vehicle
is suggested. Voriconazole
may occur (cyclodextrin) – Switch to PO
target trough at steady-state
is 2 - 5.5 mg/L.
Reference: Micromedex, Sanford Guide to Antimicrobial Therapy (42th edition)
Page 58 of 65
INFECTION CONTROL
Contact Information:
IP Telephone – 505-3158
24 hour on-call through intranet text paging system
Medical Director: Dr. Christopher Penn
Director: Ava Trahan 505-3157
Infection Preventionist: 505-3158
Isolation Precautions
Patients with various conditions are placed into isolation to minimize the risk of transmission to other
patients and healthcare workers. A full listing of isolation precautions can be found at the CDC Website
These recommendations are from the Centers
for Disease Control and Prevention (CDC) There are four major classifications of isolation precautions
according to CDC at LMH we add a 5th to highlight correct hand hygiene practice along with cleaning
disinfectant specific for one organism:
Standard Precautions
All patients should be cared for using standard precautions. All patients should be considered to
potentially harbor a bloodborne pathogen. Standard precautions require adherence to hand hygiene
recommendations (at a minimum, hand hygiene using WHO 5 Moments) and use of barrier precautions
(gown, gloves, eye-protection, etc.) for contact with blood or body fluids.
Contact Precautions
Contact precautions are utilized in the care of patients infected or colonized with epidemiologically
important microorganisms that can be transmitted via direct contact or indirect contact with environmental
surfaces and vectors. The most common bacteria requiring contact isolation are MRSA, VRE, and multi-
drug resistant gram-negative bacilli. Precautions include:
Private room (cohorting is considered at times of limited bed availability)
Strict adherence to hand hygiene
Gloves
Gowns if contact is anticipated between the healthcare worker's clothing and the patient or patient-care
environment
Masks/eye protection if patient has respiratory infection and is coughing/being suctioned or has wound
irrigation
Dedicated patient care equipment (stethoscope, scale, etc.)
Contact PLUS Precautions (LMH specific)
Due to prevalence of C. diff infections in the area LMH decided to keep Contact Plus Isolation precautions
to highlight correct hand hygiene as well as effective cleaners.
Precautions include:
Private room (cohorting is considered at times of limited bed availability)
Strict adherence to hand hygiene using soap and water
Gloves
Gowns if contact is anticipated between the healthcare worker's clothing and the patient or patient-care
environment
Masks/eye protection if patient has respiratory infection and is coughing/being suctioned or has wound
irrigation
Dedicated patient care equipment (stethoscope, scale, etc)
Cleaning consideration: Bleach or disinfectant with C. diff claim
Page 59 of 65
Droplet Precautions
Droplet precautions are used for a patient known or suspected to be infected with a pathogen transmitted
by respiratory droplets. The most common organisms requiring use of droplet precautions are
meningococcus, influenza, and pertussis. Precautions include:
Private room (cohorting is considered at times of limited bed availability)
Strict adherence to hand hygiene
Mask (surgical) upon entering room
Airborne Precautions
Airborne precautions are utilized in the care of patients known or suspected to be infected with pathogens
transmitted by airborne droplet nuclei (small particles that can remain suspended in the air). The most
common diseases/pathogens requiring airborne precautions are pulmonary tuberculosis, chickenpox, and
disseminated varicella. Precautions include:
Private room with negative pressure and special ventilation – contact infection control or access services
to get room setup
N-95 respirators are used for care of patients known or suspected to be infected with tuberculosis or other
pathogens that are transmitted via an airborne route (Healthcare workers should be fit-tested before
wearing N-95 respirators or caring for patients in airborne isolation if current testing is not on file).
In addition to the 4 major transmission-based isolation categories above, additional precautions are
utilized in the care of certain immunocompromised patient populations. These precautions are used most
commonly for Oncology patients that are found to be profoundly neutropenic and can be instituted in
other areas of the hospital for similar patients. The specific measures are more fully described in the
Infection Prevention and Control manual posted on the intranet under policy Protective Care Precautions.
These measures include the following:
Private room
Strict adherence to hand hygiene
No persons with respiratory infections or other communicable illnesses should enter
No live plants or flowers
No fresh fruit or vegetables
Isolation Precautions Quick Reference
Symptoms
Isolation
DC Isolation
Wound (uncontained draining wound)
Duration of illness
Diarrhea (acute onset)
Resolution of diarrhea for 24 hours
Headache (severe) and fever
Bacterial meningitis ruled out
Residents/patients from other care facilities:
*Respiratory symptoms
Cultures negative for MRSA
*Cellulitis/wound infection
Cultures negative for MRSA or VRE
MRSA (suspicious i.e. Frequent hospitalizations or gram + cocci identified in culture):
Cultures negative for MRSA
Cultures negative for MRSA
Pediatrics
Page 60 of 65
Etiology known and non-
*Respiratory (fall, winter, spring)
communicable Etiology known and non-
*Rash (unknown etiology)
Duration of illness
Diarrhea stops for 48 hours
Airborne+Contact
Lesions crusted (avg. 5-7 days)
Duration of illness
24 hours after treatment is started
Duration of illness
Duration of illness Until confirmed (H. Influenza & Neisseria meningitides do not DC
MRSA (excluding sputum)
Do not DC Isolation
MRSA (sputum/respiratory secretions)
Do not DC Isolation
Do not DC Isolation Until confirmed if TB (do not discontinue TB until physician ordered
or IC determines)
Duration of illness
Pneumonia
24 hours after treatment
Duration of illness
Duration of illness
Duration of illness
24 hours after treatment
Shingles
Lesions crusted (avg. 5-7 days)
Lesions crusted (avg. 5-7 days)
For most current copy of guide please refer to LMH intranet Infection Prevention & Control Policy Manual
Page 61 of 65
Removing a Patient from Isolation
MRSA and VRE
Patients infected or colonized with MRSA or VRE may remain colonized for prolonged periods and may
continue to shed organisms into the environment, serving as a reservoir for continued transmission.
While it is not recommended to decolonize patients the following may be used as a guide if need arises.
To document that a patient is ―decolonized, obtain screening cultures (rectal swabs for VRE or nares
swabs for MRSA).
-Patient should be off antibiotics for at least 48 hours
-Cultures should be obtained three times, at least one week apart
Tuberculosis
Patients with known or suspected TB can be removed from airborne isolation in the following situations:
-Diagnosis is confirmed to be something other than tuberculosis, and tuberculosis is no longer in the differential diagnosis.
-Three sputum specimens from three separate days are reported as negative for acid fast
bacilli (AFB). b. Smear positive for AFB
-Cultures or other laboratory tests reveal AFB to be other species of AFB (non-
c. Known Pulmonary TB
-Patient is on effective therapy -Patient is clinically improving
-Sputum smear is AFB-negative on three separate days
Guidelines for Prevention of Central Venous Catheter-Related Infections
Insert the central venous catheter (CVC) at the subclavian site unless the site is unavailable or medically contraindicated.
Use full sterile barrier precautions in the insertion of a CVC which includes: Long sleeve sterile gowns Sterile gloves Cap Mask Full-size sterile drape Eye protection (bloodborne pathogen precautions)
Use 2% chlorhexidine with 70% alcohol for local skin antisepsis. Use repeated back and forth strokes of the applicator sponge for approximately 30 seconds. Use of chlorhexidine is contraindicated in infants less than 2 months of age.
If any component of the catheter or kit becomes contaminated, discard and replace with a sterile device or new kit.
After insertion, the site should be covered with a sterile, transparent dressing. Dressings should be changed every 7 days and as needed if the dressing becomes loose, damp, or soiled.
Catheters placed under emergent or non-sterile conditions should be removed and replaced at a new site when the patient's condition al ows.
The catheter hub or connector should be scrubbed vigorously with friction with 70% alcohol for at least 5 seconds before the catheter is accessed.
Page 62 of 65
Remove unnecessary catheters as soon as possible
Full recommendations and supporting literature are available at
Guidelines for Prevention of Nosocomial Pneumonia
Disinfect and sterilize respiratory therapy equipment and ventilator circuits per approved guidelines and recommendations.
Practice good standard infection control precautions. Disinfect hands when moving from a dirty area or task to a clean area or task.
Remove endotracheal tubes, nasogastric tubes (NG), and other devices as soon as
Elevate the head of the bed at 30-45º.
Vaccinate with pneumococcal and influenza vaccines in appropriate individuals.
Full recommendations and supporting literature are available a
Guidelines for Prevention of Surgical Site Infections
Prophylactic antibiotics should be administered no more than 60 minutes prior to skin
If the operation is prolonged, a second dose of antibiotics should be administered depending on the half-life of the antibiotic (cefazolin – administer a second dose at four hours).
Dose prophylactic antibiotics based on the weight of the patient (e.g. 2 grams of cefazolin for patients > 80 kg).
Continue prophylactic antibiotics for no more than 24 hours after the end of the
Do not remove hair at the operative site unless necessary. If hair is removed, used electric clippers and remove hair in the pre-operative area just prior to the procedure. Do not use a razor blade to remove hair.
Thoroughly disinfect the skin at the operative site using an approved disinfectant.
Maintain blood glucose levels (< 200 mg/dl), particularly in diabetic patients.
Maintain normothermia (temperature ≥36°C).
Maintain dressing on a primarily closed incision for 24-48 hours. Do not expose the wound to tap water for at least 48 hours (if healing normally) or longer (for non-intact wound).
Full recommendations and supporting literature are available a
Guidelines for Prevention of Urinary Tract Infections
Indwelling urinary catheters should be used only when absolutely necessary, should not be used solely for personnel convenience, and should be discontinued as soon as possible.
Hand hygiene should be done immediately before and after any manipulation of the catheter site or apparatus.
Use as small a catheter as possible, consistent with good drainage.
Secure indwelling catheters properly after insertion to prevent movement and urethral
A sterile closed drainage system is to be used with indwelling catheters.
The meatal-catheter junction and the entire perineal area shall be washed with a clean washcloth and soap and water daily and as needed.
Indwelling catheters should not be changed at arbitrary fixed intervals. In general, if the urine is flowing freely, the catheter is not encrusted, and the drainage bag is functioning well, then there is no need to change the system.
Full recommendations and supporting literature are available at
Page 63 of 65
Bloodborne Pathogen Exposure
If you experience a potential exposure to BBP you should do the following:
Stop the procedure, or excuse yourself from continuation, as soon as it is feasible and safe for the patient.
Wash the affected area with soap and water.
Report your exposure to your supervisor and enter a MIDAS event report.
Report to Employee Health Nurse or Business Health during hours and Emergency Department after hours.
Follow the instructions given by above providers regarding further evaluation and post-exposure prophylaxis
Reportable Diseases
REPORTABLE DISEASES IN KANSAS for health care providers, hospitals, and laboratories
(K.S.A. 65-118, 65-128, 65-6001 - 65-6007, K.A.R. 28-1-2, 28-1-4, and 28-1-18. Changes effective as of
4/28/2006)
- Indicates that a telephone report is required by law within four hours of suspect or confirmed cases to
KDHE toll-free at 877-427-7317
- Indicates that an isolates must be sent to: Division of Health and Environmental Laboratories
Forbes Field, Building #740, Topeka, KS 66620-0001
Phone: (785) 296-1633
Acquired Immune Deficiency Syndrome (AIDS)
Amebiasis
Anthrax
Arboviral disease (including West Nile virus, Western Equine encephalitis (WEE) and St. Louis encephalitis (SLE))
- indicate virus whenever possible
Botulism
Brucellosis
Campylobacter infections
Chancroid
Chlamydia trachomatis genital infection
Cholera
Cryptosporidiosis
Cyclospora infection
Diphtheria
Ehrlichiosis
Escherichia coli O157:H7 (and other shiga-toxin producing E. coli, also known as STEC)
Giardiasis
Gonorrhea
Haemophilus influenza, invasive disease
Hantavirus Pulmonary Syndrome
Hemolytic uremic syndrome, postdiarrheal
Hepatitis, viral (acute and chronic)
Hepatitis B during pregnancy
Human Immunodeficiency Virus (HIV) (includes Viral Load Tests)
Influenza deaths in children <18 years of age
Legionellosis
Leprosy (Hansen disease)
Listeriosis
Lyme disease
Page 64 of 65
Malaria
Measles (rubeola)
Meningitis, bacterial
Meningococcemia
Mumps
Pertussis (whooping cough)
Plague (Yersinia pestis)
Poliomyelitis
Psittacosis
Q Fever (Coxiella burnetii)
Rabies, human and animal
Rocky Mountain Spotted Fever
Rubella, including congenital rubella syndrome
Salmonellosis, including typhoid fever
Severe Acute Respiratory Syndrome (SARS)
Shigellosis
Smallpox
Streptococcal invasive, drug-resistant disease from Group A Streptococcus or Streptococcus pneumoniae
Syphilis, including congenital syphilis
Tetanus
Toxic shock syndrome, streptococcal and staphylococcal
Transmissible Spongioform Encephalopathy (TSE) or prion disease (includes CJD)
Trichinosis
Tuberculosis, active disease
Tuberculosis, latent infection
Tularemia
Varicella (chickenpox)
Viral hemorrhagic fever
Yellow fever
In addition, laboratories must report:
• Viral load results of reportable diseases
• ALL blood lead levels, as of 12/2002 (KCLPPP/ABLES)
• CD4+ T-lymphocyte count < 500/ μl or CD4+ T-lymphocytes <29% of total lymphocytes
Outbreaks, unusual occurrence of any disease, exotic or newly recognized diseases, and suspect acts
of terrorism should be reported within 4 hours by telephone to the Epidemiology Hotline: 877-427-
Mail or fax reports to your local health department and/or to:
KDHE Office of Surveillance and Epidemiology, 1000 SW Jackson, Suite 210, Topeka, KS 66612-1274
Fax: 877-427-7318 (toll-free)
Page 65 of 65
Source: https://www.testmenu.com/lmh/TestDirectory/SiteFile?fileName=sidebar%5CLMH%20ID%20guidebook%207%202015.pdf
Epidemiologic Reviews ª The Author 2008. Published by the Johns Hopkins Bloomberg School of Public Health. All rights reserved. For permissions, please e-mail: [email protected]. Advance Access publication July 16, 2008 Dementia of the Alzheimer Type Jessica J. Jalbert1, Lori A. Daiello1,2, and Kate L. Lapane1 1 Department of Community Health – Epidemiology, Warren Alpert School of Medicine at Brown University, Providence, RI.2 Alzheimer's Disease and Memory Disorders Center, Rhode Island Hospital, Providence, RI.
(Incorporated in Hong Kong with limited liability) (Stock Code: 363) ANNOUNCEMENT OF 2006 ANNUAL RESULTS HIGHLIGHTS Profit before taxation Profit attributable to shareholders Proposed final dividend HK30 cents Earnings per share BUSINESS REVIEW The Board of Directors of Shanghai Industrial Holdings Limited (the "Company") is pleased to announce that audited turnover increased 13.7% to HK$6,851 million. Profits attributable to shareholders increased remarkably by 22.4% to HK$1,258 million. Basic earnings per share increased by 21.5% to HK$1.30. Net profits from recurring operations1 of the businesses of infrastructure facilities, medicine and consumer products amounted to HK$371 million, HK$163 million and HK$595 million, accounting for 33.2%, 14.6% and 53.2% of the Group's Net Business Profit before Exceptional Items2 respectively.





