Undergraduatelibrary.org
The Effect of the Decontamination of Asthma Spacer Devices on their
Function and their Suitability for Reuse in a Paediatric Emergency
Abstract
Objectives: The aims of this study were to determine the effects of dishwashing and
dishwashing frequency on the efficiency of spacer devices used with ipratropium inhalers by
determining effects on emitted dose.
Introduction: Recent evidence shows that the delivery of bronchodilators via a Metered
Dose Inhaler-spacer device combination is as effective as delivery via a nebuliser in
paediatric asthma emergency cases, with certain added benefits. However uptake of this
practice has been slow due in part to the perceived increased cost associated with use of
the spacer devices. At present spacer devices are recommended for single patient use only
and must either be given to the patient or discarded after use. A previous study indicated
that sufficient decontamination of spacer devices could facilitate their reuse in emergency
departments, thus providing significant cost savings. However, the effect of this
decontamination procedure on spacer performance has to be determined before a change
to practice could be implemented.
Method: Three types of spacer devices were used in conjunction with ipratropium inhalers,
namely: Aerochambers®, Babyhalers® and Volumatics®. The emitted dose from an MDI-
spacer device combination was measured before and after dishwashing. Emitted dose was
measured via a Pharmacopeial method with drug detection being carried out via High
Performance Liquid Chromatography analysis. Two wash cycles were undertaken with two
separate sets of spacers: twice daily washing for five days and once daily washing for ten
Results: In all cases the emitted dose decreased after dishwashing. This decrease in emitted
dose varied from 5-20% depending on spacer type and was statistically significant after ten
washes compared to control (unwashed).
Conclusion: Repeated dishwashing of spacers can lead to a small but statistically significant
decrease in the emitted dose delivered to paediatric patients. This would have to be considered
if the practice was to be implemented into paediatric emergency departments.
Word Count: 4901
Keywords: ipratropium bromide, spacer devices, paediatric emergency department, asthma,
1. Introduction
1.1 Asthma in Ireland
Asthma is a chronic disease which is characterised by inflammation of the airways. This
inflammation causes reversible obstruction of air flow making it difficult for the patient to
breathe and causing them to suffer from breathlessness, coughing and wheezing (Busse
2007). In Ireland up to 20% of children are affected by asthma with 79% of these children
suffering with uncontrolled asthma. This is reflected in the fact that children account for
approximately 55% of the 20,000 cases of asthma that present to emergency departments
every year (Asthma Society of Ireland 2013) (Asthma Society of Ireland 2011). Drugs used in
the treatment of asthma are predominantly delivered by inhalation.
1.2 Drug Delivery in Asthma
Over 70 million people worldwide use a metered dose inhaler (MDI) either alone or in
combination with an inhaler add-on device (Terzano 2001). Inhaler add-on devices or spacer
devices were developed to help patients achieve better coordination between breathing
and inhaler actuation. Spacer devices allow younger patients to tidal breathe and decrease
gastrointestinal deposition of the drug in children who are crying (Barry & O'Callaghan 2003,
Barry et al. 1996, Brown et al. 1990, Hindle & Chrystyn 1994, Lavorini & Fontana 2009,
Morgan et al. 1982, Warner et al. 1998, Wildhaber et al. 1999). Spacer devices also decrease
the velocity of drug particles in turn allowing the evaporation of propellant thereby creating
smaller drug particles which are less likely to impact on the back of the patient's throat and
more likely to be delivered to the patient's lung (Dalby & Suman 2003).
A Cochrane review has now shown definitively that the combination of an MDI and a spacer
device is as effective as the use of wet nebulisation for the delivery of β agonists in children
and adults suffering from mild to moderate exacerbations of asthma with nebulisers still
being recommended for those patients with severe exacerbations (Cates et al. 2009, Mason
et al. 2008) . In addition, the use of an MDI and spacer device is associated with a
significantly shorter length of stay in the emergency department compared to the use of a
nebuliser (Cates et al. 2009, Newman et al. 2002). Although spacer devices have many
advantages as regards drug delivery as described above, there are complications and
disadvantages to their use. Some of the problems commonly experienced with spacer
devices are outlined below.
Plastic and polycarbonate spacers are most widely used, however these can accrue a static
charge on their walls due to their non-conducting nature (Piérart et al. 1999). This can cause
charged drug particles to accumulate on the interior surface of the spacer device, which in
turn decreases drug delivery to the lungs (Barry & O'Callaghan 2003, O'Callaghan et al.
1993). To avoid the build up of any static charge on the inside of the spacer device, the
manufacturers of spacer devices recommend hand-washing the spacer devices with a mild
detergent and allowing them to air dry (NHS Primary Care Trust 2011). An exception to this
is the Aerochamber® which can be washed on the top rack of the dishwasher at
temperatures not exceeding 70°C (Trundell Medical International 2012).
The combination of an MDI and a spacer device is now considered to be of equal efficacy to
wet nebulisation in the treatment of acute asthma exacerbations. Studies have shown a lack
of uptake of this practise in many paediatric emergency departments (Dewar et al. 1999,
Powell et al. 2001, Scott et al. 2009). While the reasons for this are manifold most studies
cite cost as a major barrier to change. There is substantial conflict in the literature as
regards actual cost comparisons of the two methods. Studies which have found that use of
an MDI and spacer device is less costly than wet nebulisation are based mainly on the fact
that use of this combination reduces admissions and time spent in the emergency
department. This may be counteracted by the increased labour costs associated with the
increased time required to deliver the drugs when using a spacer device (Bowton & Haponik
1992, Cates et al. 2009, Dhuper et al. 2011, Doan et al. 2011).
1.3 Reuse of Spacer Devices
This study investigates whether spacer devices could be reused with a view to decreasing
cost associated with their use. A study examining the effectiveness of various washing
techniques on spacer decontamination found that with the correct washing technique cross
contamination with re-use of spacer devices is unlikely to be a major issue (Blackburn et al.
2011). As well as this the risk of any microbiological contamination, or indeed cross-
contamination with the spacer devices would be much less than that which would exist with
the reuse of nebulisers (Scott et al. 2009).
Anecdotal evidence suggests that at present spacer devices are given to the patient to be
taken home with them as the manufacturers recommend that the devices are suitable for
single patient use only. To date no other published studies have looked at the effects of
dishwashing spacers on their performance, but anecdotal reports suggest that this has
indeed been done before in some hospitals at no detriment to the patient concerned. Thus
this study was undertaken to examine whether or not the spacer devices could be safely
reused with a view to decreasing costs associated with their use.
2. Methods
2.1 Spacer Devices
Three types of spacer devices were used in this study; the Babyhaler® and Volumatic® made
by Allen and Hanbury's and Trundell Medical's Aerochamber Yellow®. Four of each spacer
type were used giving a total of twelve spacer devices. Spacer devices were marked with a
unique code using a permanent marker to ensure their identification throughout the study.
2.2 Emitted dose
An emitted dose device connected to a flow controller and vacuum pump represents the
Pharmacopeial standard for quality control testing of inhalers (Copley Scientific 2012, USP
32 2009) and thus this method was chosen. A vacuum flow rate of 28.3L/min was chosen as
this rate is considered to be particularly appropriate for mirroring a child's breathing rate
(Copley Scientific 2012, Dubus et al. 2001, Finlay 1998, Peyron et al. 2005, USP 32 2009). It
was calculated that at a flow rate of 28.3L/min the vacuum pump would have to run for
eight seconds to allow four litres of air to flow through the spacer device. Four litres mimics
average inhalational capacity and is the amount recommended by the manufactures of the
equipment (Copley Scientific 2012). Thus a time of eight seconds was set on the timer.
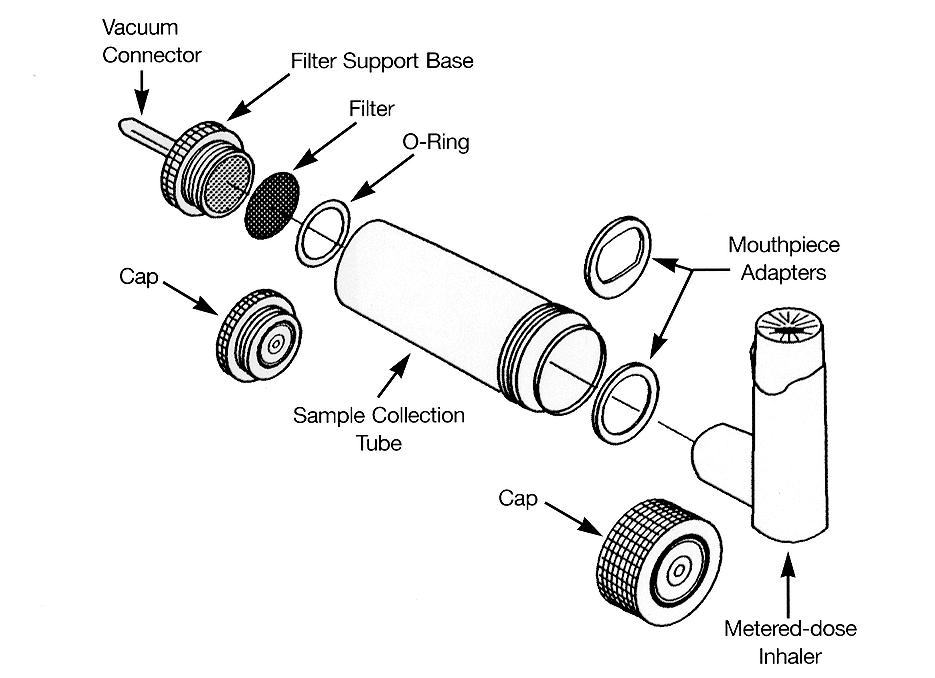
As shown below in Fig. 1 the collection tube is a cylindrical apparatus. A glass fibre filter
measuring 25mm in diameter and with a pore size of 1 micron was placed at one end of the
collection tube. This filter prevented passage of the drug particles into the tubing attached
to the flow controller and thus minimised loss of emitted drug in this way. A rubber
mouthpiece adapter was attached to the other end of the collection tube which allowed for
the attachment of the inhaler or spacer device to the collection tube. This setup is shown in
Figures 1, 2, 3 and 4 below.
Two actuations of the inhaler were fired to waste outside the apparatus to prime the inhaler
and ensure that it was working as recommended by the manufacturers (Boehringer
Ingelheim Limited 2012). A thirty second interval was observed between actuations. This is
representative of the time between actuations when the inhaler is being used in practice,
allowing the patient to tidal breathe and this practise has been documented in the literature
(Dubus et al. 2001). Eight actuations of the inhaler were used per run as this is the amount
that would be used in practice for the treatment of an acute exacerbation of asthma in a
child over six years of age in the emergency department (OLCHC 2012). When the run was
complete, the vacuum pump was switched off and the inhaler and mouthpiece adapter
removed. 10ml of Fisher Scientific HPLC Grade Water was poured into the collection tube.
The collection tube was then sealed and inverted ten times to allow dissolution of the drug
particles. Two 1ml samples were then taken from the collection tube using a sterile 1ml
pipette. Each sample was filled in to a separate glass tube, which was then labelled. The
tubes were stored in the fridge until High Performance Liquid Chromatography (HPLC)
analysis could be undertaken.
(Copley Scientific 2012)
Fig. 1: Diagram showing the apparatus used for measurement of emitted dose.
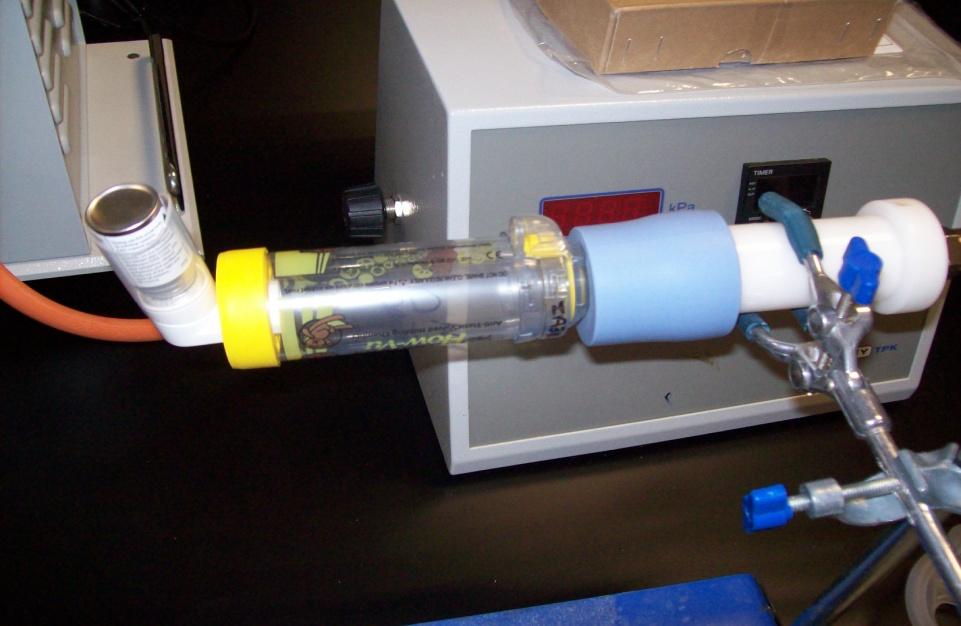

Fig. 2: Experimental setup for the measurement of emitted dose when using an
Aerochamber® spacer device.
Fig. 3: Experimental setup used for measuring the emitted dose when using a Volumatic®

Fig. 4: Experimental setup used for measuring the emitted dose when using a Babyhaler®
2.3 Standard Solutions
A standard of ipratropium bromide was ordered from LRC Standards to facilitate the making
up of standard solutions, the choosing of a method of analysis and the creation of a
standard curve. Standard solutions were made up from a stock solution. The concentrations
of the standard solutions used were 7.5, 10, 15 and 25 µg/ml.
2.4 High Performance Liquid Chromatography
2.4.1 Buffer and Mobile Phase
The HPLC method used was based on that outlined in the literature for the isolation and
detection of ipratropium bromide and related compounds (Simms et al. 1998). Two mobile
phases were used: Mobile Phase A and Mobile Phase B. These were made up from a
common buffer which contained 100mM of KH2PO4 adjusted to pH 4 with orthophosphoric
1600ml buffer.
400ml HPLC Grade Acetonitrile.
550ml buffer. 450ml HPLC Grade Acetonitrile.
The column used for elution was a Phenomenex Luna 5u C18 column of dimensions
100mm X 4.6mm. This column is similar to those used in the literature(Simms et al.
2.4.3 Running the HPLC
The programme used for the elution of the ipratropium was as follows:
An injection volume of 100μl was used as this provided a sufficient amount of drug for
detection. The UV detector was set at 210nm as this is the optimal wavelength for
detection as outlined in the literature (Brambilla et al. 1999, Majoral et al. 2007, Simms
et al. 1998). Two injections from each sample were used. This allowed an average
absorbance to be ascertained, thus increasing accuracy.
A set of standards were run to determine the elution time of ipratropium bromide. The
elution time was determined to be between 5.1 and 5.5 minutes. The standard
solutions were then used to develop a standard curve.
2.4.4 Data Analysis
At the end of each sample set the area under the peak at the relevant elution time was
recorded. This area under the peak was then substituted into the equation obtained
from the standard curve. Thus the micrograms of ipratropium per 1ml of sample were
determined. As the collection tube had initially been washed out with 10ml of water the
value obtained was multiplied by ten to find the total amount in the collection tube; the
emitted dose. This value was then divided by 160μg, the maximum possible dose, and
multiplied by 100 to get the percentage emitted dose.
2.5 Statistical Analysis
The change in the emitted dose from each spacer device after washing was calculated.
Four of each type of spacer device were used in this study. In each group of spacer
devices an outlier was identified. This outlier was excluded from the final calculations.
Thus all results are based on three devices of each spacer type. A one-tailed t-test was
performed on Microsoft Excel and p values were ascertained. 95% confidence intervals
were also calculated and these are included in the results.
The dishwashing cycle was set at 60°C for forty five minutes. The spacers were then
removed from the dishwasher and air dried. The detergent used was a normal
household detergent. The valves on the Babyhaler® were not removed during washing
as this would be unlikely to be done in a busy emergency department in reality. For one
set of spacers the washing took place twice daily for five days. The other set of spacers
were washed once daily for five days, retested and then washed once daily for a further
five days, thus giving 10 days of once daily washing.
3. Results
3.1: Emitted dose of Atrovent® MDI
The emitted dose of the Atrovent® MDI without a spacer device attached was ascertained
using the method described above. This provided evidence that the label claim of the
inhaler was being delivered. The emitted dose of each inhaler used in the study was
recorded so as to ensure that any decrease in emitted dose seen after washing the spacer
devices was not simply due to a decrease in the emitted dose of the inhaler. Table 1 below
shows the average emitted dose for each of the Atrovent® MDI's used as well as the overall
average and standard deviation.
Table 1: Average emitted doses from Atrovent® MDI without spacer devices attached.
Inhaler Number
% Emitted Dose
Standard Deviation
3.2 Effect of Once Daily Washing on Emitted Dose:
In all cases % relates to the emitted dose obtained as a percentage of the maximum possible
3.2.1 Aerochambers®:
The emitted dose of ipratropium was measured using the Atrovent® inhaler attached to the
Yellow Aerochamber® device before the devices had been washed. Emitted dose was
measured after five once daily washes and again after a further five once daily washes giving
ten washes in total. The emitted doses obtained as well as the total decrease in emitted
dose are shown in Table 2 below.
Table 2: Change in emitted dose after once daily washing at five days and ten days. **
Indicates statistical significance with a p<0.01 when emitted dose after 10 once daily washes
is compared with control.
3.2.2 Babyhalers®:
Emitted dose of ipratropium was measured using the Atrovent® inhaler attached to the
Babyhaler® device as described in the method. This testing was undertaken at the same
time points as for the Aerochambers. The emitted doses obtained as well as the total
decrease in emitted dose are shown in Table 3 below.
Table 3: Change in emitted dose noted after once daily washing at five days and ten days. **
Indicates statistical significance with a p<0.01 when emitted dose after 10 washes is
compared with control.
3.2.3 Volumatics®:
Emitted dose of ipratropium was measured using the Atrovent® inhaler attached to the
Volumatic® device as described in the method. Testing was undertaken before washing
(control), after five once daily washes and again after a further five once daily washes giving
ten washes in total. The emitted doses obtained as well as the total decrease in emitted
dose are shown in Table 4 below.
Table 4: Change in emitted dose noted after once daily washing at five days and ten days. **
represents a p<0.01 when emitted dose after 10 washes is compared with control.
3.2.4 Average change in emitted dose:
Table 5 below shows a summary of the information contained in sections 3.2.1-3.2.3 above.
P values are calculated with respect to the emitted dose obtained before any washing was
Table 5: Average change in emitted dose and associated statistical significance compared to
Change from
Spacer Type
Fig. 5: The average emitted dose of each of the three spacer types before washing, post five
washes and post ten washes (n=3 +/- S.D.). Statistical significance is calculated with
reference to the emitted dose before washing. **p<0.01.
3.3 Effect of Twice Daily Washing on Emitted Dose
3.3.1 Aerochambers®:
Emitted dose of ipratropium was measured using the Atrovent® inhaler attached to the
Yellow Aerochamber® device as described in the method. This testing was undertaken
before washing (control) and after five days of twice daily washing. The emitted doses
obtained as well as the total decrease in emitted dose are shown in Table 6 below.
Table 6: Change in emitted dose and associated significance after 5 days of twice daily
washing. * represents p<0.05 and is calculated with respect to the emitted dose obtained on
control testing.
Post 10 washes
Change from
3.3.2 Babyhalers®:
Emitted dose of ipratropium was measured using the Atrovent® inhaler attached to the
Babyhaler® device as outlined in the method section. Emitted dose was measured before
washing (control) and after five days of twice daily washing. The emitted doses obtained as
well as the total decrease in emitted dose are shown in Table 7 below.
Table 7: Change in emitted dose and significance associated with said change after 5 days of
twice daily washing. *represents p<0.05 and is calculated with reference to the emitted
dose of the control.
Post 10 washes
Change from
3.3.3 Volumatics®:
Emitted dose of ipratropium was measured using the Atrovent® inhaler attached to the
Volumatic® spacer device as outlined in the method section. Emitted dose was measured
before washing (control) and after five days of twice daily washing. The emitted doses
obtained as well as the total decrease in emitted dose are shown in Table 8 below.
Table 8: Change in emitted dose and significance associated with said change after 5 days of
twice daily washing. * shows a decrease in emitted dose that is statistically significant
Post 10 washes
Change from
3.3.4 Average change in emitted dose after five days of twice daily washing:
Table 9 gives an overview of the average drop off in emitted dose after five days of twice
daily washing and the statistical significance associated with same. Fig. 6 shows this
decrease graphically.
Table 9 : Average change in emitted dose following five days of twice daily washing.
Average change
Post 5 Days BD Washing (10 washes)
Spacer type
Fig. 6: The emitted dose for each spacer device before washing and the emitted dose post
ten washes. (n=3 +/- S.D.). p<0.05.
4. Discussion
The aim of this study was to determine the effect of dishwashing on spacer device
effectiveness, by measuring the change in emitted dose from the spacer devices. The drug
used in this study was ipratropium, marketed as the Atrovent® MDI. Ipratropium is a short
acting atropine like bronchodilator which is used in patients who are experiencing a mild
exacerbation of asthma where salbutamol alone is not effective or for children with
moderate asthma exacerbations (Kasawar & Farooqui 2010). The combination of an MDI
and spacer device is now recommended for the delivery of β agonists in the treatment of
acute exacerbations of asthma (Cates et al. 2009, Powell et al. 2001, Raucci et al. 1993).
The Aerochambers® used in this study are the only spacers licensed for dishwashing
(Trundell Medical International 2012). Thus, for the other spacer devices the washing
undertaken here was outside of the manufacturers specifications. However, this study would
have been futile had the washing conditions not mirrored what would be done in practise.
Additionally hand-washing alone was shown to be ineffective at decontaminating spacer
devices to a sufficient standard to allow their reuse (Blackburn et al. 2011).
There is a paucity of evidence in the published literature as regards the estimated emitted
dose of ipratropium from the Atrovent® inhaler. Available studies suggest an average
emitted dose of between 83 and 94% from the Atrovent® inhaler (Brambilla et al. 1999). The
values obtained in this study are in agreement with this. Each time a new inhaler was used
the emitted dose was tested to ensure consistency. Owing to the proximity of all results no
account was taken of the slight variability in the emitted dose between inhalers. Although
values for emitted dose from spacer devices vary greatly in the literature, in all cases the
emitted dose from the combination of an MDI and spacer device is lower than that from the
inhaler alone (Barry et al. 1996).
As outlined above, two washing cycles were undertaken to establish whether the amount of
washes per day would affect spacer performance. In the once daily cycle spacers were
tested before washing, after five days of washing and again after a further five days of
washing, thus giving ten washes in total. The reduction in emitted dose after five days of
once daily washing was not statistically significant. However a statistically significant
decrease in emitted dose did occur after a further five days of once daily washing (Fig5).
This trend indicates that there is a connection between number of washes and emitted
dose, with emitted dose decreasing with an increasing number of washes.
A second set of spacers were washed twice daily for five days. Thus while the number of
washes was the same as for those spacers which were washed once daily, the frequency of
washing differed. The spacers were washed using the same washing conditions as those
being washed once daily. The reduction in emitted dose for these spacers was, on average
less than that seen at ten days for those spacers undergoing once daily washing. Of note,
the decrease in emitted dose for the Volumatics® in both wash cycles was approximately
16.6%. This would indicate that while the frequency of washing is not important, a
statistically significant drop off in emitted dose occurs on washing. All spacer devices that
were washed twice daily had statistically significant decreases in emitted dose after ten
washes. Although being washed more regularly did not adversely affect the function of
these spacer devices it is likely that in practise twice daily washing of spacer devices would
not occur as there would be neither the resources to facilitate this nor the usage to warrant
Another, similar study was carried out in parallel to this study using salbutamol delivered via
a Ventolin® inhaler and the same types of spacer devices as in this study. Twice daily
washing of the spacers was undertaken for five days giving a total of ten washes. Washing of
spacer devices took place in the same dishwasher as that used in this study, using the same
washing cycle to allow direct comparison of the results. In the salbutamol study decreases in
emitted dose of 11.7% for Aerochambers®, 9.8% for Babyhalers® and 10.6% for Volumatics®
were observed (Keane et. al Unpublished). This shows that regardless of the drug being used
there is a decrease in emitted dose after dishwashing the spacer devices. It has previously
been noted in the literature that individual drug molecules may have an effect on the
emitted dose and thus this may account for the variability in the diminution in emitted dose
seen with the two drugs (Ahrens et al. 1995, Barry & O'Callaghan 2003). There is also an
amount of intra- and inter spacer variability. This has also been noted in the literature with
factors such as spacer volume, properties of the drug substance and properties of the
inhaler device used being seen to play a key role in this variability (Ahrens et al. 1995, Barry
et al. 1996, Barry & O'Callaghan 2003).
The results of this study show that the washing of spacer devices in a dishwasher does cause
a statistically significant decrease the emitted dose and that this diminution in dose depends
on the number as opposed to the frequency of the washes. However in this case, statistics
alone cannot be used to determine significance. In an emergency situation the dose
administered will be determined both by the age of the child and by the severity of the
symptoms being experienced. However if the patient does not respond then the dose will
be increased until an improvement in the patient's condition is seen (British Thoracic Soceity
2012). Thus to determine the real significance of these results the spacers would have to be
tested on patients to see how greatly, if at all the decrease in emitted dose affects their
response to treatment.
There are also some issues with regard to the practicality of washing and drying the spacer
devices. All of the spacers are identical, thus each spacer would have to be marked with a
unique code to ensure that it would not be washed more than the requisite number of
times. A log of what spacer was washed when would be a solution to this issue. To prevent
the build up of charge on the interior of the spacer device the spacers need to be air dried.
Although air drying of the device was carried out in this study it is difficult to see how this
could be done on a more constant basis as this air drying requires quite a lot of space.
Therefore the practicality of washing the spacers will be restricted by the space available for
drying and the human resources required to monitor the washing log. All of this would also
have to be taken into account when determining the cost effectiveness of washing and
reuse of the spacer devices.
5. Limitations
The method used for determination of the emitted dose in this experiment gives only
quantitative data as regards the emitted dose and does not allow determination of where in
the respiratory tract the drug will be deposited. Thus it may be argued that although the
amount of drug emitted may not change significantly, the properties of the emitted drug
In the study which looked at the decontamination of the spacer devices, spacers were hand
washed before dishwashing. This pre-wash by hand was not carried out in this study as it
would probably not be feasible in a busy hospital environment. This pre-wash would be
unlikely to affect spacer performance but may affect decontamination. Thus the
microbiological study may need to be repeated to confirm that dishwashing alone provides
sufficient decontamination.
The spacer devices in this study were washed only ten times and therefore their use beyond
this point cannot be recommended on the basis of this study.
6. Conclusions/Future Work
The results of this study show that there is a decrease in the emitted dose from spacer
devices post dishwashing. This decrease varies between 5-20%. Although the results
obtained are statistically significant their clinical significance is not yet clear. However
washing the spacer devices on more than ten occasions is likely to further decrease the
emitted dose which may then be detrimental to the patient.
Successful and seamless change from nebulisers to spacers can only be achieved if there is
education of and agreement between all parties involved in this changeover (Dewar et al.
1999, Doherty et al. 2007, Powell et al. 2001, Scott et al. 2009). Staff should regularly be
reminded of these guidelines and an audit undertaken to ensure that said protocol is being
followed appropriately.
Provided it is ascertained that dishwashing alone provides sufficient decontamination of the
spacer devices proper guidelines should be drawn up as regards their reuse as shown in
Spacers should be marked to ensure they are not washed on more than ten occasions. A
rota should be drawn up and marked each time a particular spacer is washed.
Calculation of the incremental cost effectiveness ratio should be undertaken to assess the
cost effectiveness of the MDI and spacer combination.
It should be noted that the guidelines relating to spacer use also apply to the treatment of
asthma exacerbations in adults and thus such cost savings could also be executed in a
hospital dealing predominantly with adult patients (Mason et al. 2008).
Appendix 1
Flow Chart Depicting when Spacer Devices Should be Sent for Dishwashing.
Spacer Device used
Patient Sent Home
Spacer Device will
Spacer Device No
Spacer Device or
Longer Needed by
Doesn't Have One
Already Has One at
patient on the ward
Give Spacer Device
Send Spacer Device
Give Spacer Device
Send Spacer Device
Ahrens, R., Lux, C. & Bahl, T., 1995. Choosing the metered-dose inhaler spacer or holding
chamber that matches the patient ' s need : Evidence that the specific drug being
delivered is an important consideration. Journal of allergy and clinical immunology, 96,
Anon, 2009. U.S. Pharmacopeia,
Asthma Society of Ireland, 2013. Asthma Clinic. Available at:
Asthma Society of Ireland, 2011. Hse. Health Service Executive Asthma Information,
Barry, P W & O'Callaghan, C., 2003. The influence of inhaler selection on efficacy of asthma
therapies. Advanced drug delivery reviews, 55(7), pp.879–923. Available at:
Barry, P W, Robertson, C.F. & O'Callaghan, C., 1993. Optimum use of a spacer device.
Archives of disease in childhood, 69(6), pp.693–4. Available at:
&rendertype=abstract.
Barry, Peter W, Callaghan, C.O. & Royal, L., 1996. Inhalational drug delivery from seven
different spacer devices. Thorax, 51, pp.835–840.
Blackburn, C. et al., 2011. SAEM Abstracts, Plenary Session. Academic Emergency Medicine,
18, p.S84. Available at: http://doi.wiley.com/10.1111/j.1553-2712.2011.01073.x
[Accessed January 24, 2013].
Boehringer Ingelheim Limited, 2012. Atrovent Summary of Product Characteristics. , pp.1–7.
Bowton, L. & Haponik, F., 1992. Substitution of Metered Dose Inhalers for Hand Held
Nebulizers. Chest, 101, pp.305–308.
Brambilla, G. et al., 1999. Modulation of aerosol clouds produced by pressurised inhalation
aerosols. International journal of pharmaceutics, 186(1), pp.53–61. Available at:
British Thoracic Soceity, 2012. British Guidelines on the Management of Asthma,
Brown, P.H. et al., 1990. Do large volume spacer devices reduce the systemic effects of high
dose inhaled corticosteroids? Thorax, 45(10), pp.736–9. Available at:
&rendertype=abstract.
Busse, W., 2007. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and
Management of Asthma–Summary Report 2007. Journal of Allergy and Clinical
Immunology, 120(5), pp.S94–S138. Available at:
Cates, C., Crilly, J. & Rowe, B., 2009. Holding chambers ( spacers ) versus nebulisers for beta-
agonist treatment of acute asthma ( Review ). Cochrane Collaboration, (1).
Copley Scientific, 2012. Quality Solutions for Who are Copley, Available at:
http://www.copleyscientific.com/documents/ww/Inhaler Brochure 2012 (Low
Dalby, R. & Suman, J., 2003. Inhalation therapy: technological milestones in asthma
treatment. Advanced drug delivery reviews, 55(7), pp.779–91. Available at:
Dewar, a L. et al., 1999a. A randomised controlled trial to assess the relative benefits of
large volume spacers and nebulisers to treat acute asthma in hospital. Archives of
disease in childhood, 80(5), pp.421–3. Available at:
&rendertype=abstract.
Dhuper, S. et al., 2011. Efficacy and cost comparisons of bronchodilatator administration
between metered dose inhalers with disposable spacers and nebulizers for acute
asthma treatment. The Journal of emergency medicine, 40(3), pp.247–55. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/19081697 [Accessed November 2, 2012].
Doan, Q., Shefrin, A. & Johnson, D., 2011. Cost-effectiveness of metered-dose inhalers for
asthma exacerbations in the pediatric emergency department. Pediatrics, 127(5),
pp.e1105–11. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21464192 [Accessed
November 20, 2012].
Doherty, S.R. et al., 2007. Evidence-based implementation of adult asthma guidelines in the
emergency department: a controlled trial. Emergency medicine Australasia : EMA,
19(1), pp.31–8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17305658
[Accessed December 4, 2012].
Dubus, J.C., Rhem, R. & Dolovich, M., 2001. Delivery of HFA and CFC salbutamol from spacer
devices used in infancy. International journal of pharmaceutics, 222(1), pp.101–8.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/11404036.
Finlay, W., 1998. Inertial sizing of aerosol inhaled during pediatric tidal breathing from an
MDI with attached holding chamber. International Journal of Pharmaceutics, 168(2),
pp.147–152. Available at:
Hindle, M. & Chrystyn, H., 1994. Relative bioavailability of salbutamol to the lung following
inhalation using metered dose inhalation methods and spacer devices. Thorax, 49(6),
pp.549–53. Available at:
&rendertype=abstract.
Kasawar, G.B. & Farooqui, M., 2010. Development and validation of a stability indicating RP-
HPLC method for the simultaneous determination of related substances of albuterol
sulfate and ipratropium bromide in nasal solution. Journal of pharmaceutical and
biomedical analysis, 52(1), pp.19–29. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/20045275 [Accessed January 23, 2013].
Keane, A et al, The Effect of the Decontamination of Asthma Spacer Devices on their Function
with Salbutamol Sulphate Inhalers and their Suitability for Reuse in a Paediatric Emergency
Department. Unpublished Work.
Lavorini, F. & Fontana, G. a, 2009. Targeting drugs to the airways: The role of spacer devices.
Expert opinion on drug delivery, 6(1), pp.91–102. Available at:
Mason, N. et al., 2008. Nebulisers or spacers for the administration of bronchodilators to
those with asthma attending emergency departments? Respiratory medicine, 102(7),
pp.993–8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18396026 [Accessed
December 4, 2012].
Morgan, M. et al., 1982. SHORT REPORTS Terbutaline aerosol given through pear spacer in
acute severe asthma. British Medical Journal, 285(September), pp.849–850.
Newman, K.B. et al., 2002. A comparison of albuterol administered by metered-dose inhaler
and spacer with albuterol by nebulizer in adults presenting to an urban emergency
department with acute asthma. Chest, 121(4), pp.1036–41. Available at:
NHS Primary Care Trust, 2011. Patient Information Leaflet. , (Mdi).
O'Callaghan, C. et al., 1993. Improvement in sodium cromoglycate delivery from a spacer
device by use of an antistatic lining, immediate inhalation, and avoiding multiple
actuations of drug. Thorax, 48(6), pp.603–6. Available at:
&rendertype=abstract.
Our Lady's Children's Hospital Crumlin, Asthma Treatment Flow Diagram, OLCHC, 2012.
Peyron, I.D. et al., 2005. Development and performance of a new hydrofluoroalkane (HFA
134a)-based metered dose inhaler (MDI) of salmeterol. Respiratory medicine, 99 Suppl
A, pp.S20–30. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15777605 [Accessed
December 4, 2012].
Piérart, F. et al., 1999. Washing plastic spacers in household detergent reduces electrostatic
charge and greatly improves delivery. The European respiratory journal : official
journal of the European Society for Clinical Respiratory Physiology, 13(3), pp.673–8.
Available at: http://www.ncbi.nlm.nih.gov/pubmed/10232445.
Powell, C. V et al., 2001. Successful implementation of spacer treatment guideline for acute
asthma. Archives of disease in childhood, 84(2), pp.142–6. Available at:
&rendertype=abstract.
Raucci, J.C. et al., 1993. Emergency Department Treatment of. Chest, 103, pp.665–672.
Scott, S.D. et al., 2009. Barriers and supports to implementation of MDI/spacer use in nine
Canadian pediatric emergency departments: a qualitative study. Implementation
science : IS, 4, p.65. Available at:
&rendertype=abstract [Accessed December 4, 2012].
Simms, P.J. et al., 1998. The separation of ipratropium bromide and its related compounds.
Journal of pharmaceutical and biomedical analysis, 17(4-5), pp.841–9. Available at:
Terzano, C., 2001. Pressurized metered dose inhalers and add-on devices. Pulmonary
pharmacology & therapeutics, 14(5), pp.351–66. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/11603949 [Accessed December 2, 2012].
Trundell Medical International, 2012. AeroChamber Plus Flow-Vu Patieant Information
Warner, J.O., Naspitz, C.K. & Cropp, G.J. a., 1998. Third International Pediatric Consensus
statement on the management of childhood asthma. Pediatric Pulmonology, 25(1),
pp.1–17. Available at: http://doi.wiley.com/10.1002/%28SICI%291099-
Wildhaber, J.H. et al., 1999. Inhalation therapy in asthma: nebulizer or pressurized metered-
dose inhaler with holding chamber? In vivo comparison of lung deposition in children.
The Journal of pediatrics, 135(1), pp.28–33. Available at:
Source: http://www.undergraduatelibrary.org/system/files/2925_0.pdf
Electronic Modular Switching System Please read this manual before connecting theElectronic Modular Switching System. ELECTRONIC MODULAR SWITCHING SYSTEM Thank you for purchasing the Panasonic Model KX-T206E, Electronic Modular Switching System. Electronic Modular Switching System Proprietary telephone with display
Prof.Dr.med. Stephan H. Duda Herausgeberschaften Gastherausgeberschaft im Themenheft „Primäre Lebertumoren" in „Der Onkologe", zusammen mit P.M. Schlag (2000) Editorial Board Mitglied "Investigative Radiology" (seit Januar 2002) Editorial Board Mitglied "Techniques in Vascular and Interventional Radiology" (seit Januar 2005) 1. Publikationen a.) Wissenschaftliche Originalarbeiten in referierten Journals 1. Biewald, W., S.H. Duda: Die Meatoplastik. Ein Konzept zur Behandlung der kongenitalen distalen



