Powerpoint presentation
Keeping
UP on Diabetic
Medications and Complications
Apply current clinical practice standards to optimize
pharmacologic treatment outcomes and non-pharmacological management strategies for an adult patient with type 2 diabetes.
Recognize patient situations where the utilization of a
new or novel pharmacological agent appropriately optimizes type 2 diabetes management.
Apply current standards of medical care to perform
appropriate patient assessments, and suggest appropriate monitoring parameters and goals of therapy or an adult patient with type 2 diabetes
Results primarily from insulin resistance in muscle and liver, which could lead to defect in pancreatic insulin secretion
Accounts for 90-95% of diabetes
Prevalence in the US is 7.8% (about 23.6 million and growing)
Formerly known as non-insulin-dependent diabetes (adult-onset diabetes)
Often asymptomatic with a slow onset over 5-10 years
Disturbing increased trends in children and adolescents due to rise in obesity
CDC. National Diabetes Statistics Report 2014: Estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Diseas e Control and Prevention, 2011. Available at
CDC 2014. Summary Health Statistics for U.S. Adults: 2012. Table 8. Available at
FAST FACTS ON DIABETES
Diabetes affects 29 million people—
9.3% of the U.S. population
DIAGNOSED
21.0 million people
UNDIAGNOSED
8.1 million people
American Indian/Native American 17.9%
Non-Hispanic White 7.3%
CDC. National Diabetes Statistics Report 2014: Estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. Available at
CDC 2014. Summary Health Statistics for U.S. Adults: 2012. Table 8. Available at
ADA Data-Native Americans

Every 24 hours…
4557 adults are diagnosed with diabetes.
136 people begin treatment for end-stage
renal disease.
200 nontraumatic lower-limb amputations
641 people die from diabetes, or diabetes
is a contributing cause of their death.
CDC. National Diabetes Statistics Report 2014: Estimates of diabetes and its burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011. Available at
ACCE 2016
<8% - Elderly, frail, brittle
>6.5% - Concurrent serious illness and high hypoglycemic risk
Monotherapy A1c <9%: Metformin
A1c <7.5%: Met, SGLT2i, DPP4i, or
GLP-1 RA
A1c >9%: Dual Therapy –
A1c >7.5%: Dual Therapy –
Metformin + SU, TZD, DPP-4i,
Metformin + (Preferred: DPP-4i,
SGLT2i, GLP-1 RA, Insulin
SGLT2i, GLP-1 RA, Colesevelam,
A1c >10% and symptomatic:
Bromocriptine, Agi, Use caution:
Metformin +Basal + Prandial Insulin SU/GLN, TZD, Insulin)
Triple Therapy Metformin + 2 others
Metformin + 2 others
AACE/ACE Comprehensive Type 2 Diabetes Management Algorithm 2016 Published in Endocr Pract.2016;22:84-113
ACCE 2016
A1c >10% + Symptoms : Metformin + A1c <8%: Basal TDD 0.1-0.2 U/kg Basal Insulin (Start 10 U/day or 0.1-
A1c >8%: Basal TDD 0.2-0.3 U/kg
0.2 U/kg/day) + Add Prandial Insulin
*Add Prandial Insulin or GLP-1 RA if
or GLP-1 RA if goal not achieved (1
goal not achieved (Plus 1, 2, 3 or
meal or Premixed)
Goal <140/90 mmHg
Goal <130/80 mmHg
1st line: ACEi/ARB
1st line: ACEi/ARB
*Add CCB or Thiazide if needed
Dual Therapy: BP >150/90 use ACEi/ARB + CCB, BB, Thiazide
<40 yo w/o ASCVD Risk – no statin
>40 yo – Moderate/High Intensity
AACE/ACE Comprehensive Type 2 Diabetes Management Algorithm 2016 Published in Endocr Pract.2016;22:84-113
Goals of Diabetes Management
Primary goal – prevent onset of acute or chronic complications
Acute complications:
Hypoglycemia, DKA, hyperglycemic hyperosmolar nonketotic
Chronic complications:
Microvascular: Retinopathy, nephropathy, and neuropathy Macrovascular: Cardiovascular, cerebrovascular, and peripheral
vascular diseases
Glycemic therapy goals: A1C <7% and <8% (elderly) FPG or premeal 80-130mg/dL (change in 2015 by ADA) Peak postprandial glucose (1-2 hours after meal) <180mg/dL
ADA 2016 Clinical Practice Recommendations: http://care.diabetesjournals.org/content/39/Supplement_1
Goals of Diabetes Management Cont.
Blood Pressure
BP <150/90 mmHg for >60 years old; <140/90 mmHg for
everyone else (change in 2015 by ADA to match with JNC8)
For younger patients consider treating to <130/80 to prevent
long term complications
No specific LDL-C, HDL-C, or TG goal are currently recommended Lowering LDL-C by 30%-49% in pt with diabetes 40-75 years old
and by at east 50% if 10-year risk of cardiovascular event is >7.5% (ACC/AHA 2013)
ADA 2016 Clinical Practice Recommendations: http://care.diabetesjournals.org/content/39/Supplement_1
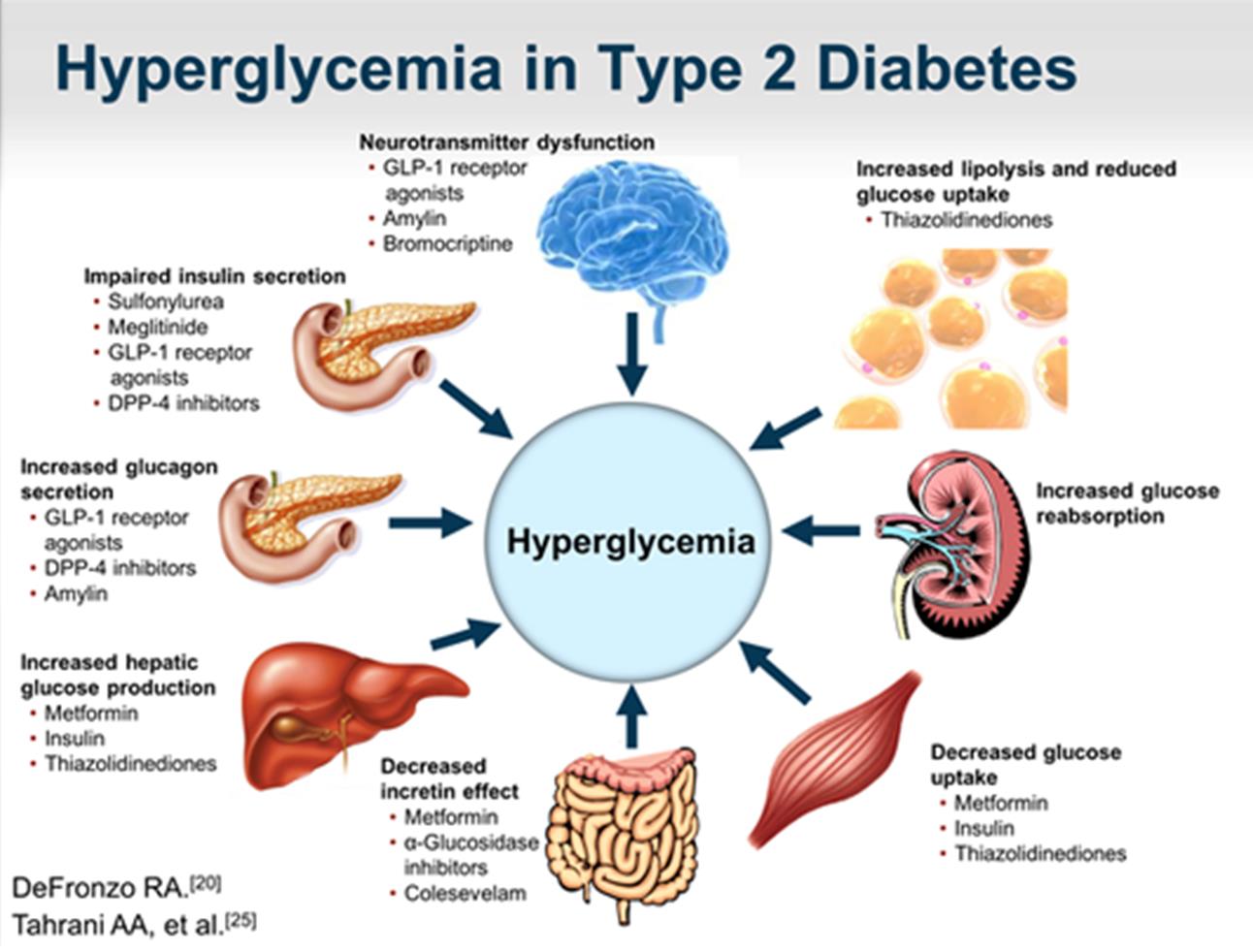
Insulin Deficiency- lack of secretion
Insulin Resistance
Glucagon Secretion
Increased gastric motility
Decrease GLP/GIP
Leptin & Ghrelin
UKPDS: β-Cell Function over Time
Decline to insulin deficiency 12 yrs after Dx!
Insulin loss starts 10 yrs before Dx.
Half gone by Dx.
Insulin loss is part of T2 DM
51% residual secretion
28% residual insulin secretion
Years After Diagnosis
Diabetes 44: 1249-1258, 1995
Primary Mechanism of Action
Decrease hepatic glucose production (HGP)
Increase glucose uptake in muscle
Mean drop in A1c
Side effects:
weight neutral, moderate GI symptoms (N/V, flatulence, diarrhea), B‐12 deficiency
Contraindicated: eGFR<45ml/min due to lactic acidosis, CKD (stage 3B, 4, and 5)
Benefit in CVD pt?
Primary Mechanism of Action
Increase insulin secretion
Glipizide (better option for older adults and those with renal impairment)
Glyburide (BEER's list)
Mean drop in A1c
Dose titration: can increase weekly
Side effects:
Moderate/severe hypoglycemia (more risk in renal disease patients), weight gain
Thiazolidinediones
Primary Mechanism of Action
Increase glucose uptake in muscle and fat
Decrease hepatic glucose production (HGP)
Mean drop in A1c
Slow dose titration and may not see effect until 8-12 weeks
Side effects:
May worsen fluid retention (caution with CHF patients), weight gain, moderate bone
loss, possible bladder cancer risk
a-Glucosidase Inhibitors
Primary Mechanism of Action
Delay carbohydrate absorption from intestine
Mean drop in A1c
Side effects:
Moderate GI symptoms (bloating, flatulence, diarrhea, abdominal pain), rash
Contraindications & Precautions: Inflammatory bowel disease, colonic ulcerations,
intestinal obstruction
Primary Mechanism of Action
Decrease glucagon secretions
Slow gastric emptying
Increase satiety
Mean drop in A1c
0.5-1% (very effective at controlling postprandial glucose)
Side effects:
High risk of hypoglycemia, moderate GI symptoms (N/V, diarrhea,
abdominal pain), fatigue, dizziness, modest weight loss
Bile Acid Sequestrant
Primary Mechanism of Action
Remove bile acid from the body, so lower cholesterol. Mechanism to reduce
serum glucose: unknown (Decrease HGP? Increase incretin levels?)
Colesevelam (Welchol)
Mean drop in A1c
0.5% when added to metformin, sulfonylurea, or insulin
Side effects:
Mild GI symptoms (constipation, indigestion, and nausea), fatigue,
headache, myalgia
Contraindicated in pts with a history of bowel obstruction, TG concentration
Dopamine-2 Agonist
Primary Mechanism of Action
Activates dopaminergic receptors; "reset" biological clock to improve metabolism
Bromocriptine (Cycloset)
Mean drop in A1c
Side effects:
Moderate GI symptoms, fatigue, headache/syncope, dizziness, somnolence, and
possible cardiovascular benefit
Contraindications & Precautions: pt with syncopal migraines; can limit effectiveness
of agents used to treat psychosis or exacerbate psychotic disorder;
Primary Mechanism of Action
Remove bile acid from the body, so lower cholesterol. Mechanism to reduce serum glucose: unknown (Decrease HGP? Increase incretin levels?)
Increase insulin secretion (similar to Sulfonylurea but more rapid onset and shorter duration of activity)
Repaglinide (Caution: level greatly increased when use with gemfibrozil)
Mean drop in A1c
Mild hypoglycemia (more risk for renal disease patients), weight gain, and upper respiratory infection/bronchitis
Glucagon-like peptide 1 receptor agonists (GLP-1 agonist)
Dipeptidyl peptidase 4 inhibitors (DPP-4 Inhibitors)
Sodium glucose cotransporter 2 inhibitors (SGLT2 Inhibitors)
Inhaled Insulin - Afrezza
Concentrated Insulin
U200 and U300
GLP-1 Receptor Agonist
Injectable non-insulin medication for T2DM
Works in the small intestine
Stimulates glucose-dependent stimulation of insulin secretion
Slows gastric emptying
Prevents inappropriate glucagon secretion
Promotes weight loss
GLP-1 Receptor Agonist
Trulicity (dulaglutide) 0.75mg-1.5mg SQ weekly
Tanzeum (albiglutide) 30mg-50mg SQ weekly+
Bydureon (exenatide) 2mg SQ weekly+**
Victoza (liraglutide) 0.6mg-1.8mg SQ daily**
Byetta (exenatide) 5-10mcg SQ bid within 60 minutes of meal**
+Requires dilution and mixing prior to use
** Caution in renal failure
GLP-1 Receptor Agonist
Injection Site Reaction
Pancreatitis
Acute pancreatitis has been reported but the FDA has found
insufficient evidence
Discontinue if pancreatitis occurs Do not start if history of pancreatitis
GLP-1 Receptor Agonists
Contraindicated in pts with or family history of:
Thyroid C-cell type cancer including medullary thyroid carcinoma Multiple endocrine neoplasia syndrome type 2 (MEN 2)
GLP-1 Receptor Agonists
Lower A1c 1-1.5%
Weight loss 2-3 kg
Little hypoglycemia
Disadvantages
Gastrointestinal side effects may limit use 10-50%
Risk of thyroid c-cell tumors
Risk of pancreatitis
Worsening renal function
DPP-4 Inhibitors
Oral medication for T2DM
Inhibits the enzyme DPP-4, prevents the deactivation of GLP-1
Stimulates insulin release from the pancreatic islets Slows gastric emptying Inhibits post-meal glucagon release Reduces food intake
DPP-4 Inhibitors
Januvia (sitagliptin) 100mg po daily*
Onglyza (saxigliptin) 2.5 – 5mg po daily*
Nasina (alogliptin) 25mg po daily*
Tradjenta (linagliptin) 5mg po daily
*Requires renal adjustments All are Pregnancy Category B and can be taken with/without food
DPP-4 Inhibitors
Pancreatitis
All DPP-4 Inhibitors
Hepatic Function
Aloglipitin – baseline LFTs and every 3 months for first year
Saxaglipitin – serious skin reactions All DPP-4 Inhibitors – Stevens-Johnson syndrome
DPP-4 Inhibitors
Cardiovascular Effect
Saxigliptin and aloglipitin – increased risk of hospitalization for
July 2015, Meta-Analysis 14,735 patients with DMT2 and
history of CVD on sitagliptin or placebo showed no differences in CVD outcomes.
DPP-4 Inhibitors
No hypoglycemia (monotherapy) Weight neutral Well tolerated
Disadvantages
May be associated with Pancreatitis Risk of Heart failure (saxagliptin)
SGLT2–Inhibitors
Oral medication for adults with T2DM
Increase glucose excretion by inhibiting sodium-glucose co-
transporter 2 (SGLT2) in the kidney
Independent of insulin Potential for lowering blood pressure and weight loss (2-3 kg)
Canagliflozin may also decrease post-prandial hyperglycemia by
delaying glucose absorption
SGLT2–Inhibitors
Canagliflozin (Invokana)
100mg-300mg po daily before breakfast* Canagliflozin/Metformin (Invokamet) 50/500mg-150/1000mg bid
Dapagliflozin (Forxiga)
5mg-10mg po daily* Dapagliflozin/Metformin (Xigduo XR) 5/500mg-10/2000mg daily
Empagliflozin (Jardiance)
10mg-25mg po daily Empagliflozin/Linaglipitin (Glyxambi) 10mg/5mg daily
*Requires dose adjustment for renal function All ar
e Pregnancy category C and only canagliflozin should be take with regard to a meal but should be taken in the
morning as they have a diuretic effect
SGLT2–Inhibitors
Vulvovaginal Candidiasis
Canagliflozin 10-11%
Dapagliflozin 7-8%
Empagliflozin 4-6%
Urinary Tract Infections
Canagliflozin 4-6%
Dapagliflozin 4-6%
Empagliflozin 9%
Hypoglycemia
Low with monotherapy, elevated when combined with insulin or other oral DM medication
SGLT2–Inhibitors
Diabetic Ketoacidosis FDA Black Box Warning
SGLT2 inhibitors alone or in combination products, may lead to ketoacidosis
Signs or symptoms of ketoacidosis (ex. difficulty breathing, nausea, vomiting, abdominal pain, confusion, unusual fatigue or sleepiness)
Glucose may not be elevated as typically seen with DKA
Major illness, reduced food and fluid intake, and reduced insulin dose may contribute to ketoacidosis in patients taking SGLT2 inhibitors.
Bladder Cancer
Dapagliflozin – 10 cases reported
Prompted FDA to require post-marketing surveillance studies
SGLT2–Inhibitors
Little hypoglycemia (as monotherapy)
Mild weight loss
Reduced Blood pressure
Disadvantages
Vulvovaginal infections
Urinary tract infections
Can't be used in severe renal failure
Risk of bladder cancer (dapagliflozin)

1921 Insulin "Discovered"
1923 Eli Lilly manufactures Insulin
1973 U-100 Insulin Developed
1978 Engineered "human" insulin developed
1986 Insulin Pen Developed
2006 First Approved Inhaled Insulin Developed
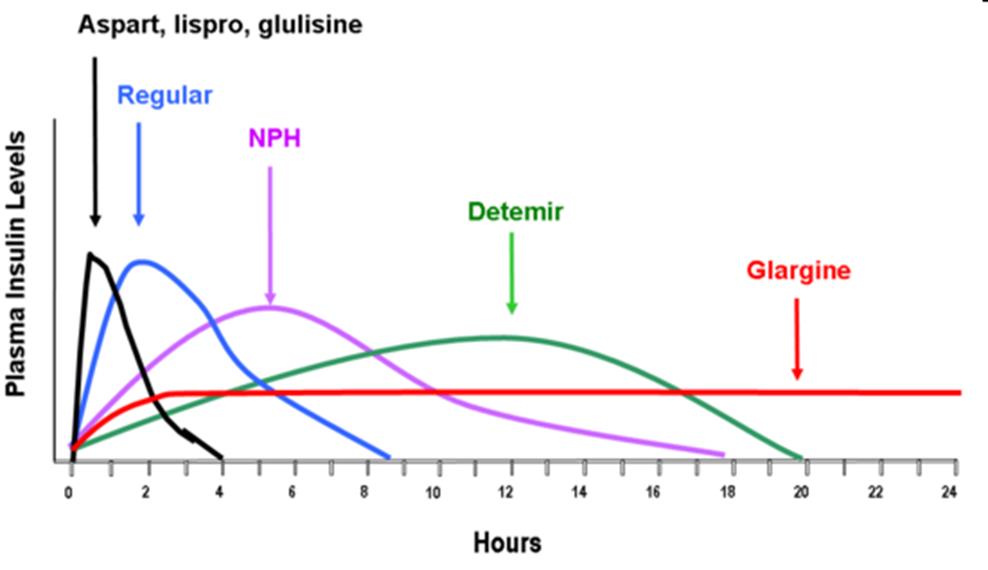
Pharmacokinetics
Primary Mechanism of Action
Stimulate peripheral glucose uptake
Decrease hepatic glucose production (HGP)
Inhibits proteolysis, enhances protein synthesis, and inhibits lipolysis in adipocytes
Short acting (Regular human insulin)
Rapid acting (Novolog, Humalog, Glulisine)
Intermediate acting: NPH
Long acting: Lantus and Levemir (can not be mixed with other insulins)
Mean drop in A1c
1.5-3% (most effective treatment for hyperglycemia)
Side effects:
Hypoglycemia, injection site reaction, rash, weight gain, peripheral edema
Nathan DM, Buse JB, Davidson MB, et al. Diabetes Care. 2009; 32(1): 193-203)
Insulin Medications
Starting Insulin
Starting Insulin
Basal 0.1-0.2 Units/kg/day or 10 units
Adjust 1-2/week by 10-15% or 2-4 units
Goal pre-prandial 80-130mg/dL
Adding Bolus Prandial Insulin
Start if FBG reached, but A1c not controlled or TDD >0.5 U/kg/day
Bolus 4 units or 0.1 U/kg or 10% of Basal dose with largest meal
Adjust 1-2/week by 1-2 U or 10-15%
Add additional "bolus" with >2 meals
Adding Premixed
Divide Basal dose: 2/3 in am and 1/3 in pm
Adjust 1-2/week by 1-2 U or 10-15%
Adjusting Insulin
Blood Sugar
Insulin Dose
Breakfast
Levemir 30 units hs
Levemir 30 units hs
Levemir 30 units hs
FBG controlled – Lunch pre-prandial BS above goal
Start: Bolus Insulin with Breakfast
Dose: 4 units, 0.1 U/kg (weight 100kg = 10 units), 10% = 3 unit
Switching Between Insulin
Intermediate to Basal
Once daily – convert 1:1 Twice daily – Use 80% of dose
Converting to U500
Use 80% of TDD insulin dose, given 30 minutes before meals TDD = 200-300 units/day divide dose bid TDD = 300-750 units/day divide dose tid TDD = 750-2000 units/day divide dose four times daily TDD >2000units/day consider insulin pump
Inhaled Insulin - Afrezza
Concentrated Insulin
U200 U300
Ultra Long-Acting Insulin
Inhaled Insulin - Afrezza
Second Generation Inhaled Insulin
Approved June 2014
Ultra Rapid-acting inhaled insulin for adults at least 18 years of
age with T1DM or T2DM.
Technosphere Insulin, dry powdered insulin for prandial
coverage in non-smoking adults without pulmonary diseases
In patients with T1DM, Afrezza must be used in combination
with long-acting insulin.
Inhaled Insulin – Afrezza
Administration
Give immediately prior to meal, reaches peak plasma in 12-15 minutes
Sealed foil packages refrigerated, once opened may be out of fridge for 10days
Inhaler must be replaced every 15 days
Risk of acute bronchospasm in patients with chronic lung disease
Contraindicated in patients with Asthma, COPD, lung cancer, current smokers or cessation smokers within last 6 months
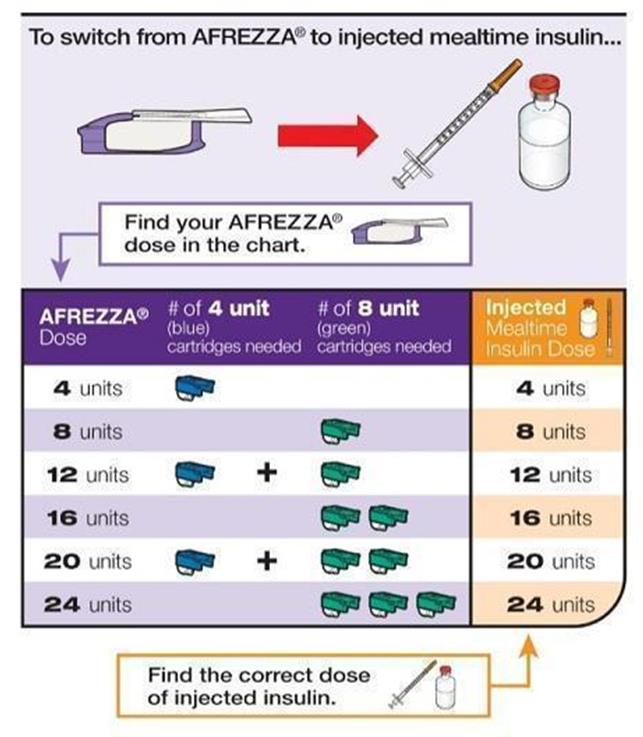
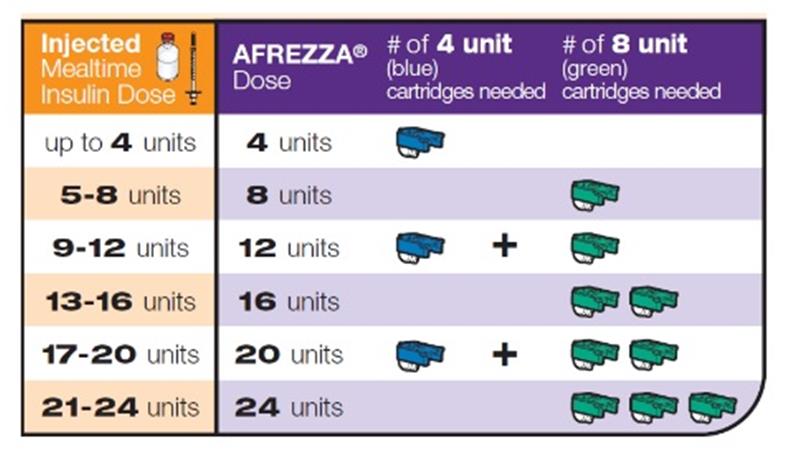
Inhaled Insulin – Afrezza
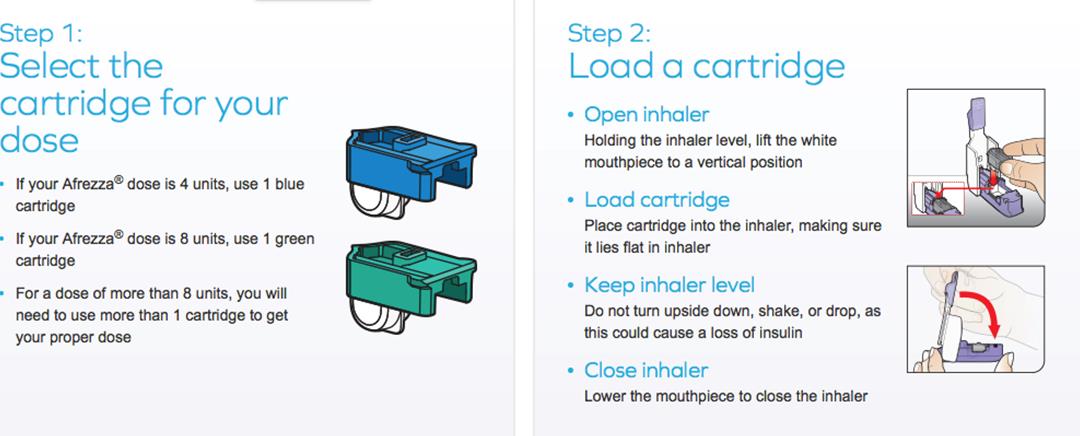
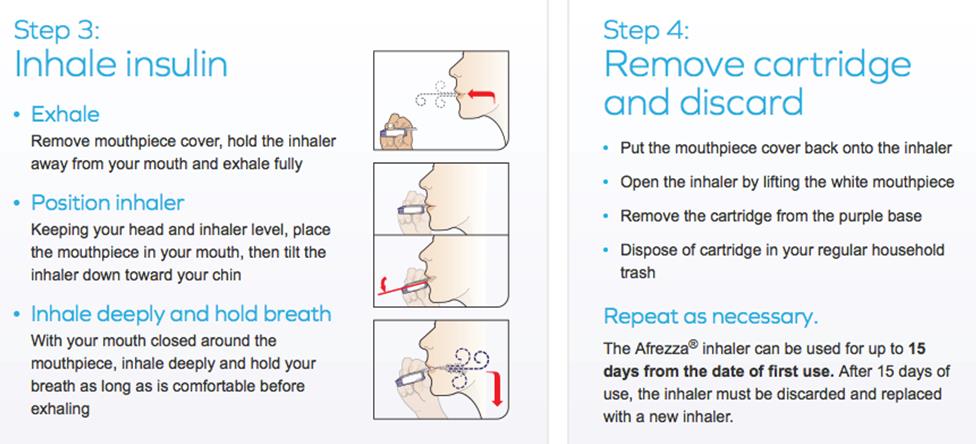
Inhaled Insulin - Afrezza
Inhaled Insulin – Afrezza
Reduces number of insulin injections Can be used immediately before meal
Disadvantages
Contraindicated in pts with respiratory problems, lung cancer or
Multiple inhalations for higher insulin doses Risk of lung function decreasing Dosing increments of 4 and 8 units
Concentrated Insulin U-200
Humalog Kwikpen
Rapid acting mealtime insulin, identical to Humalog U100
except contains 200units/ml
Could be considered for people on >20 units/day of rapid acting
Concentrated Insulin U-300
Toujeo (Insulin glargine)
Available in SoloStar pen, which only delivers up to 80 units per
Longer duration of action than Lantus (slower absorption)
Higher doses may be required to get same glycemic efficacy as Lantus
5 days to achieve maximum benefit, titrate every 3-4 days
Conversions:
Daily Lantus, Levemir or NPH 1:1 conversion
Twice daily Lantus 1:1, Levemir or NPH use 80% of total daily dose
Insulin Degludec
Approved September 2015
Ultra Long-acting insulin approved for adults at least 18 years of
age with T1DM or T2DM.
Available in Flextouch pen U100 or U200 with dosing up to 160
Tresiba [package insert]. Plainsboro, NJ: Novo Nordisk Inc; September 2015.
Insulin Degludec
Dosing and Administration
Once daily SQ dose, start at 10 units, 0.2-0.4mg/kg
Daily Lantus, Levemir or NPH 1:1 conversion
Pharmacokinetics
Half-life 25 hours, Duration of Action 42 hours
Unopened - Refrigerator
Opened – Unrefrigerated, stable for 8 weeks below 86°F
Similar rates of hypoglycemia to Insulin Glargine
Lower rates of nocturnal hypoglycemia compared to Insulin Glargine (not significant)
Tresiba [package insert]. Plainsboro, NJ: Novo Nordisk Inc; September 2015.
Biosimilars are close to the original "reference" drug and
produce similar therapeutic outcomes but are made differently and by different companies and will cost less than the original
First Insulin Biosimilars approved by FDA in 2014
Basaglar – similar to Lantus (under 30 month automatic stay due
to patent infringement filed by Sanofi)
Abasria – European product
New DM Medications On the Horizon
Medication
Expected Approval
Insulin peglispro
LA basal insulin
5-10 units SQ daily
0.8-1.6mg po weekly 2016
10-25mg po weekly
10-40mg po daily
Glucagon receptor
5-200mg po daily
75 yo female has T2DM, her HgA1c is 10.1 with SCr 0.9 and she is on Metformin 1000mg bid and glyburide 10mg . Select the best treatment plan.
Continue metformin, glyburide and add pioglitizone 15mg daily
Continue metformin, glyburide and add sitagliptin 5mg daily
Continue metformin, glyburide and add Levemir 10 units hs
Continue metformin, stop glyburide and add Levemir 10 units hs
35 yo female with T2DM her HgA1c 9.1, BMI 44kg, BP 137/88, SCr
0.7. She is currently on Levemir 45 unit SQ bid, Novolog 10 units SQ AC, Lisinopril 20mg daily, omeprazole 20mg daily, metoclopramide 10mg TID, calcium/vit D. Intolerant to metformin. What would be the best option for her DM?
Add a DPP-IV inhibitor
Add a GLP1-agonist
Add SGLT-2 inhibitor
35 yo female with T2DM her HgA1c 9.1, BMI 44kg, BP 137/88, SCr 0.7. She is currently on Levemir 45 unit SQ bid, Novolog 10 units SQ AC, Lisinopril 20mg daily, omeprazole 20mg daily, metoclopramide 10mg TID, calcium/vit D. Her pooled cohort is 7%. What is the best option for the patient.
Add simvastatin 20mg HS
Wait until she is 40 then add statin
Add atorvastatin 20mg
Add atorvastatin 80mg
35 yo female with T2DM her HgA1c 9.1, BMI 44kg, BP 137/88, SCr 0.7. She is currently on Levemir 45 unit SQ bid, Novolog 10 units SQ AC, Lisinopril 20mg daily, omeprazole 20mg daily, metoclopramide 10mg TID, calcium/vit D. Intolerant to metformin. What else should be done to manage to optimize her therapy?
Add amlodipine 10mg daily
Increase Lisinopril to 30mg daily
Add Vitamin D3 1000units daily
64 yo male on Levemir 50 units SQ HS and Novolog 15 units SQ
AC. The chart displays his BS. How would you adjust his insulin?
Patient Case cont.
Change Levemir to 30 units SQ QAM and HS
Increase Novolog to 17 units SQ AC
Increase Novolog to 17 units qam, 15 units before lunch and dinner
Increase Novolog to 15 units qam and dinner and 17 units before lunch
Metformin is still the first-line medication treatment option for
GLP-1 agonists may be an alternative for patient's who doesn't
tolerate metformin as first-line.
Choosing second or third line DM medications must depend on
patient preference, patient's medical history and cost.
Several new medications are on the horizon but no new novel
Preventing and treating macrovascular and microvascular
complications are vital in reducing mortality and morbidity
Blood Pressure goals for Diabetics are <140/90mmHG
All diabetics should be on statin therapy if tolerated and
maximized to moderate or high intensity depending on their ASCVD risk
ADA 2016 Clinical Practice Recommendations: http://care.diabetesjournals.org/content/39/Supplement_1
Management of hyperglycemia in type 2 diabetes, 2016: a patient-centered approach: update to a position statement
of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2016
AACE/ACE Comprehensive Type 2 Diabetes Management Algorithm 2016 Published in Endocr Pract.2016;22:84-113
Micromedex® Solutions [online]. Updated periodically. Truven Health Analytics, Inc. Accessed July 30, 2015.
Insulin. Facts and Comparisons® eAnswers. Drug Facts and Comparisons® [online]. Updated periodically. Clinical Drug
Information, LLC. Accessed July 30, 2015.
Hajheydari Z, Kashi Z, Akha O, Akbarzadeh S. Frequency of lipodystrophy induced by recombinant human insulin.
European Review for Medical and Pharmacological Sciences. 2011; 15(10): 1196-1201.
Toujeo®, insulin glargine injection 300 Units/mL [package insert]. Sanofi-Aventis, LLC. Bridgewater, NJ. 2015.
Afrezza®, human insulin inhalation powder [package insert]. Sanofi-Aventis, LLC. Bridgewater, NJ. 2015.
Farxiga (dapagliflozin) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company;
Jardiance (empagliflozin) [prescribing information]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc;
American Diabetes Association (ADA). Standards of medical care in diabetes--2015. Diabetes Care. 2015;38(Suppl 1):S1-94.
FDA Safety Communication. FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. Food and Drug Administration website.
Invokana (canagliflozin) [prescribing information]. Titusville, NJ: Janssen Pharmaceuticals, Inc;
2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart
Association Task Force on Practice Guidelines. Circulation, 2013 Nov 12. With Full Report Supplement.
Rising tide of cardiovascular disease in American Indians: the Strong Heart Study Howard BV, Lee ET, Cowan LD, Devereux RB, Galloway JM, Howard WJ, Rhoades ER, Robbins DC, Sievers ML,
Welty TK. Circulation. 1999;99:2389 –2395.
Racial Misclassification and Disparities in Cardiovascular Disease Among American Indians and Alaska Natives Dorothy A. Rhoades. Circulation. 2005;111:1250-1256.
Source: http://up4health.org/wp-content/uploads/2016/06/UP-DM-presentation-2016.pdf
Agence PACA Etude d'incidence N° contrat : 20110337 EVALUATION DES INCIDENCES DU PROJET D'AMENAGEMENT D'UN RESERVOIR D'EAU POTABLE SUR LE SITE DE GARGALON A FREJUS AU REGARD DES OBJECTIFS DE CONSERVATION DES HABITATS ET DES ESPECES D'INTERET COMMUNAUTAIRE DU SITE NATURA 2000
Vi ringraziamo per aver acquistato una fotocamera digitale Olympus. Prima di iniziare ad usare la vostra fotocamera, leggete attentamente queste istruzioni per ottenere i migliori risultati e per una maggior durata della macchina. Conservate il manuale in un posto sicuro per futuro riferimento. Prima di fare fotografi e importanti, vi consigliamo di scattare alcune fotografi e di prova, per acquisire







