V-scheiner.brunel.ac.uk
The Significance of Hazardous Chemicals in Wastewater Treatment
Works Effluents
Michael Gardner1, Sean Comber2, Mark D Scrimshaw3, Elise Cartmell4, John Lester4, Brian
Ellor5
1 Corresponding author, Atkins Limited, 500, Park Avenue, Aztec West, Almondsbury, Bristol BS32
4RZ, UK. Tel: +44(0)7834 506 966 Fax: +44 1454 663333; email:
[email protected] 2 Plymouth University, Drake Circus, Plymouth, PL4 8AA, UK
3 Brunel University, Uxbridge, UB8 3PH, UK
4 Cranfield University, Cranfield, MK43 0AL, UK
5 UK Water Industry Research, 1 Queen Anne's Gate, London, SW1H 9BT, UK
Abstract
The advent of increasingly stringent and wider ranging European Union legislation relating to water
and the environment has required regulators to assess compliance risk and to respond by formulating
appropriate pollution control measures. To support this process the UK Water Industry has completed
a national Chemicals Investigation Programme (CIP), to monitor over 160 wastewater treatment
works (WwTWs) for 70 determinands. Final effluent concentrations of zinc, polynuclear aromatic
hydrocarbons (fluoranthene, benzo(a)pyrene, benzo(b)fluoranthene, benzo(k)fluoranthene,
benzo(g,h,i)perylene and indeno(1,2,3-cd)pyrene), "penta" congeners (BDEs) 47 and 99, tributyltin,
triclosan, erythromycin, oxytetracycline, ibuprofen, propranolol, fluoxetine, diclofenac, 17β-estradiol
and 17α-ethinyl estradiol exceeded existing or proposed Environmental Quality Standards (EQSs) in
over 50% of WwTWs. Dilution by receiving water might ensure compliance with EQSs for these
chemicals, apart from the BDEs. However, in some cases there will be insufficient dilution to ensure
compliance and additional management options may be required.
Key words – priority substance, regulation, wastewater, effluent, chemicals
1. Introduction
Recent European Union (EU) legislation in the field of water and the environment has extended the
scope of pollution control measures required to protect surface waters (EC, 2000, 2008, 2012). This
has been driven by the improved understanding of the environmental impact of hazardous chemicals
contained in wastewater effluents upon receiving waters and the flora and fauna they support. These
effects, particularly those associated with endocrine disruption (Sumpter, 2009), have received much
attention in the last decade. Endocrine disruption in the aquatic environment was first reported by
Dodds et al. (1938) and the impact of organic micropollutants and heavy metals have been known for
number of years (Bedding et al. 1982; Lester et al. 1980) and have been the subject of legislation for
an extensive period in the UK (Bedding et al. 1983; Lester, 1983). However, there has been a need
within the EU to update and harmonize existing legislation (EC, 2000, 2008, 2012) including
regulations to control of the introduction of more recently recognized hazardous chemicals (Behera et
al. 2009; Gabriel et al. 2012; González et al. 2012; Martínez Bueno et al. 2012; Martin Ruel et al.
2012; Rodil et al. 2012). European Union Directives including the Water Framework Directive (WFD)
(EC, 2000) and the Priority Substances Daughter Directive (EC, 2008) have defined Environmental
Quality Standards (EQSs) for substances that hitherto have not been subject to detailed scrutiny and
control. Environmental Quality Standards, in the form of annual average and in some cases maximum
admissible concentrations, have been set at EU level for over 30 substances. A further tranche of
more than 20 standards for chemicals designated as ‘specific pol utants' under Annex VIII of the WFD
is under consultation at the UK national level. The wide range of chemicals involved (Bedding et al.
1982; Gonzalez et al. 2012; Kolpin et al. 2002) and the analytical difficulty of working at ng/L levels
(Buisson et al., 1984) in a complex matrix such as wastewater (Robertson and Lester, 1994) make
this a challenging proposition. In order to address this, the UK water industry has initiated an
ambitious programme of investigations the Chemicals Investigation Programme (CIP) which is
coordinated by the UK Water Industry Research (UKWIR) organisation as part of the UK National
Environment Programme. The CIP operates three phases:
C1 - Investigations to assess risk from chemicals
Final effluents from 162 WwTWs from England, Scotland and Wales were collected and
analysed to determine the concentrations of chemicals discharged to receiving waters and
their compliance with identified quality criteria.
C2 - Investigations to assess WwTWs performance
Assessments of 28 WwTWs were completed to evaluate the treatment performance across
primary, secondary and some tertiary treatment processes.
C3 – Source investigations
Overall nine urban catchments across the UK have been studied to assess catchment
sources of the CIP specified chemicals discharged to sewer.
This study reports the findings of the first phase (C1) and for further information on the additional CIP
phases 2 and 3 see supplementary material. The objectives of phase 1 are:
To identify chemicals of concern and their concentrations in final effluents; To assess the range of concentrations between treatment works in different areas and
between works of different types;
To evaluate the compliance risk posed by chemicals with respect to water quality standards; To determine an order of priority amongst chemicals for the possible implementation of
control measures.
The work reported here provides a broadly representative picture of hazardous chemicals from
WwTWs throughout the UK as illustrated in Figure 1. This will allow for the formulation of appropriate
control measures, to meet limit values that are either new or which might be more stringent than
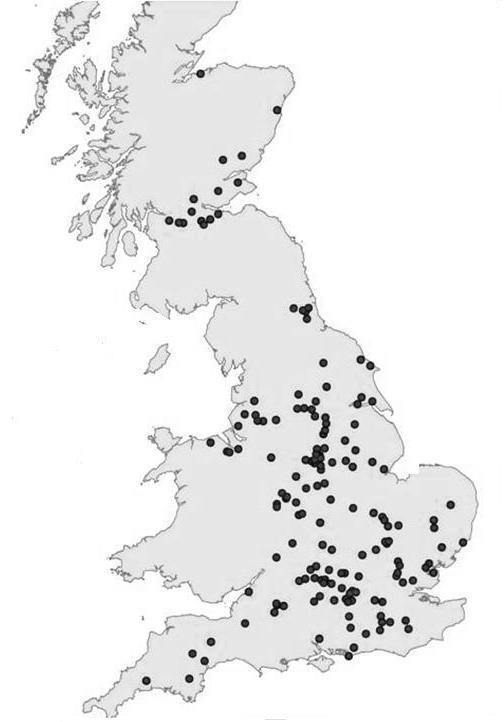
The locations of WwTW selected for phase 1 of the CIP for monitoring chemicals in
final effluents.
2. Materials and Methods
Design of the sampling programme
Figure 2 shows the respective profiles of WwTWs chosen for the phase 1 final effluent investigations
and those of all UK treatment works, il ustrating, through the lightly shaded columns in the "all works"
diagram, that the profile of WwTW sizes matched that of the total UK profile well, with 70% of the
national population being served by works in the size range included in this part of the CIP
programme. Of those excluded, the greater proportion of the population is served by a small number
of extremely large WwTWs, with a population equivalent (PE) of >500,000 which were omitted from
the programme because such sites tend to have known and specific industrial inputs that would have
to be dealt with separately on a site by site basis. Inclusion of such works did not therefore accord
with the aim of characterising the broader national picture. At the opposite end of the scale, the CIP
does not provide a fully proportional representation of very small works (PE <5,000). However, these
works, although numerous, do not treat the wastewater from a large proportion of the population, are
generally subject to larger dilution with receiving water (it might not be cost beneficial or feasible for
some measures to be implemented at smaller works in any case). This therefore demonstrates that
the selection of WwTWs was representative of UK WwTWs works in general, with the treatment
processes operated at these WwTWs divided approximately equally between trickling filters and
activated sludge based processes (supplementary Table 1).
Sample collection
Final effluents from 162 WwTWs were sampled either 14 or 28 times over a period of one year at
each site. Works with lower dilution in receiving waters were sampled more frequently to increase
confidence in site specific information. Grab samples were taken at different times during the day,
with at least 15% of samples taken out of normal working hours (evenings or at weekends). Grab
sampling was the chosen approach due to concerns over sample stability for stored composites. In
addition, since compliance is usually assessed by means of grab sampling, knowledge about the
variance of such samples was seen as of value in itself. Samples for the determination of metals were
collected with polyethylene samplers, filtered (0.45 µm) on site then acidified and stored in
polyethylene (samples for mercury determinations were stored in glass or PTFE and preserved with
acid dichromate (Feldman, 1974)). Samples for the determination of trace organic substances were
collected with stainless steel samplers, stored in glass and transported at 4ºC to the laboratories.
Preservation with 3 ml of 30% hydrochloric acid and 0.25g of copper nitrate per litre was used for
steroid estrogen samples. Storage for all organic samples was a maximum 5 days at 4ºC.
Analysis and quality control
A test of sample stability in settled crude sewage and final effluents was undertaken (Gardner et al ,
2012) to validate the sample storage period of 5 days under refrigeration, before the end of which it
was specified that analysis must have been initiated. Additional spiked quality control samples were
included to assess the impact of storage.
The programme covered more than 70 target chemicals, including 10 metals (total and dissolved), 22
EU Priority or Priority Hazardous Substances, 16 chemicals of emerging concern (herbicides,
consumer chemicals and pharmaceuticals – see supplementary material) along with 16 supporting
determinands including those that are measures of wastewater quality, treatment performance and
the prediction of metal speciation. The required limits of detection (LODs) for the target chemicals
were based on the EQS values in 2009 and are listed in Table 1. However, as of January 2012 further
profile of works size in CIP C1
Profile of all UK works
population equivalent in 1000s
population equivalent in 1000s
Comparison of population profiles of WwTWs included in the survey of chemicals in final effluents with all UK works. The grey shading illustrates that the study had a lower proportion of small works than are present nationally.
Table 1 Chemicals addressed in the Chemicals Investigation Programme
Detection
required
Detection
Substance
required
Substance
nickel (dissolved)
lead (dissolved)
copper (dissolved)
benzo(b)fluoranthene
benzo(k)fluoranthene
zinc (dissolved)
benzo(g,h,i)perylene
indeno(1,2,3-cd)pyrene
cadmium (dissolved)
glyphosate (N-(phosphonomethyl)glycine)
aminomethylphosphonic acid
mercury (dissolved)
triclosan (2,4,4'-trichloro-2'-hydroxydiphenyl ether)
iron (dissolved)
aluminium (dissolved)
aluminium (total)
aluminium (reactive)
silver (dissolved)
diethylhexylphthalate
2,4,4'-tribromodiphenyl ether (PBDE28)
2,2',4,4'-tetrabromodiphenyl ether (PBDE47)
2,2',4,4',5-pentabromodiphenyl ether (PBDE99)
2,2',4,4',6-pentabromodiphenyl ether (PBDE100)
2,2',4,4',5,5'-hexabromodiphenyl ether (PBDE153)
17α ethinylestradiol
2,2',4,4',5,6'-hexabromodiphenyl ether (PBDE154)
nonylphenol ethoxylates (1)
Nonylphenol 4-nonylphenol
nonylphenol ethoxylates (2)
Tributyltin compounds (Tributyltin-cation)
Nonylphenol ethoxylates (3)
proposals on EQSs, amending Directives 2000/60/EC as regards priority substances in the field of
water policy were made by the European Commission (EC, 2012), and therefore the data reported
here are considered in light of these proposals. Table 1 also identifies all chemicals and their
abbreviation codes used in summary figures in the supplementary information. Comparable groups of
determinands were studied by Hope et al (2012) and Martin Ruel et al. (2012).
In developing the CIP, consideration was given to establishing the required characteristics for
analytical performance. The LOD, precision and bias were defined on the basis of achieving adequate
precision at or near the detection limit of interest. These required LODs were set for determinations
made in wastewater final effluent to be at least as low as the EQS or other limit value of likely interest
such as a predicted no effect value (PNEC). A notional limit for analytical error was agreed for organic
chemicals of ± 50% (25% random error, 25% systematic error) and ± 20% for metals (10% random,
10% systematic) or the required LOD, whichever was larger. Accredited analytical laboratories were
required to submit performance test information to demonstrate that they met the analytical
performance targets.
Participating laboratories, contracted by the water companies responsible for delivery of the
investigations were required to submit performance test information to substantiate their claim to meet
the analytical performance targets. A programme of interlaboratory proficiency tests was also set up
with a commercial provider of such services. The tests relied on a combination of routine proficiency
tests provided as part of the ongoing proficiency testing programme (Aquacheck, Bury, UK). With
respect to data analysis, no statistical outliers were rejected, although approximately 20-30 highly
discrepant results out of a total of over 200,000 were queried and rejected from the dataset. Results
reported as less than the limit of detection were substituted with a value ½ the reporting limit as
specified in by EU reporting regulations (EC, 2009). The coherence of the data set and absence of
substantial interlaboratory and marked inter-regional effects adds weight to the evidence that bias in
procedures of sampling and analysis does not significantly affect the primary interpretation of the data
with respect to prioritisation of substances.
Data analysis
The annual average values for each chemical at each WwTWs sampled are presented in the results
tables as percentiles which provide a breakdown of the reported concentrations across all of the
WwTWs. The concentrations reported represent the percentage of WwTWs where the average
concentration measured was at or below the figure presented. For example, for dissolved Ni, 50% of
WwTWs returned an average concentration in their effluent of greater than 4.3 µg/L (Table 2).
Percentile values underlined represent a concentration greater than either an EQS or PNEC value.
3. Results
Works compliance with sanitary and nutrient consents
The results of the programme are summarised in the supplementary Information in the form of box
and whisker diagrams showing results for al WwTWs for each of 40 regulated substances.
Throughout the sampling period all the works were operating within expected design parameters and
were compliant against sanitary determinand consents for biochemical oxygen demand (BOD) and
suspended solids (SS), with 95 % of the works compliant with the traditional 20 mg/L and 30 mg/L
permits for BOD and SS respectively.
Table 2 Percentile values for average concentration of metals (µg/L) Values underlined in bold indicate the percentile greater than existing and proposed standards.
Freshwater EQS
Percentile µg/L
1000 Defra Direction 2010*
"BLM adjusted PNEC"
"BLM adjusted PNEC"
"BLM adjusted PNEC"
"BLM adjusted PNEC"
all results <0.5µg/l
all results <0.5µg/
"BLM adjusted PNEC"
at an undue prop ortion of les
mpossible to estimate a
d unders core suggest an exceedance of an EQS or PNEC
(based on WFD EU and UK values) where multiple EQS apply (e.g. hardness related EQS for some metals) the most stringent value has been used AA annual average, MAC maximum admissible concentration PHS
Priority hazardous substance
Priority substance
Hardness based, these are lowest for <40mg CaCO3/L
Based on a bioavailable fraction
"BLM adjusted PNEC" - based on biotic ligand models available to Environment Agency of England and Wales for pH 7.8, total hardness 125 mg CaCO3/L, 5mg/L DOC
Residual final effluent concentrations and their relevance to EQSs
Summary results for metals are given in Table 2, along with the relevant EQSs. Samples were
analysed for both total and dissolved metals; the dissolved fraction is of direct concern for compliance
with EQS values specified for receiving waters (for aluminium, the reactive form (Gardner et al, 2008)
is relevant to current discussion of standards in the UK). The quantity of metal associated with
suspended solids is of concern as insoluble metal bound to solids can accumulate in sediments. In
addition, there is also the possibility that metals might re-partition into the dissolved phase. This
emphasises the need to maintain good removal of SS in WwTWs to reduce metal loads being
discharged. It is apparent that concentrations of Al, Fe, Cr, Hg and Ag in the effluents were in all
cases below the proposed or existing EQS or PNEC values. The metals for which concentrations
were observed to be above the standards were Cd, Cu, Ni, Pb and Zn.
In the case of the four metals, Cu, Ni, Pb and Zn, the UK will be employing bioavailability-based EQS
values (Comber et al., 2008) incorporating DOC correction for Pb or the Biotic Ligand Model (BLM) for
Cu, Ni and Zn (DeSchamphelaere and Janssen, 2004), which will be used to determine compliance
with standards on a site-specific basis. In Table 2, alongside the relevant standards, a "BLM adjusted
PNEC" has also been derived based on biotic ligand models available to the Environment Agency of
England and Wales (EA) for waters with a pH 7.8, total hardness 125 mg CaCO3/L and 5mg/L
dissolved organic carbon (DOC). These illustrative EQS values have been selected based on a
relatively worst case scenario of water with low concentrations of DOC. The final effluent
characteristics of relevance to the BLM are shown in Table 3.
Table 3 Percentile values for average concentration of metals (mg/L)
Sanitary determinands and nutrients
Percentile mg/L
Substance
Total Suspended solids
Total oxididised nitrogen
Biochemical oxygen demand
Chemical oxygen demand
Total phosphorus
Soluble reactive phosphate
Parameters required for application of biotic ligand models
Percentile mg/L
Substance
Total organic carbon
Dissolved organic carbon
Making these assumptions for the derivation of the BLM adjusted PNEC it can be seen that for
dissolved Pb, average concentrations in effluents from all of the WwTWs were below the BLM
adjusted PNEC, indicating that using the BLM will result in compliance. However, dilution will be
required for Cu and Ni to meet the BLM adjusted PNEC at 10% of sites. For dissolved Zn
concentrations a greater degree of dilution will be required at a higher proportion (50%) of sites, and
the extent of this is currently the subject of further investigation because bioavailability will vary
according to conditions downstream after mixing, rather than simply on the basis of its form in
effluent. As already highlighted the metals for which concentrations occurred above the EQS were
Cd, Cu, Ni, Pb and Zn. When the BLM adjusted PNECs are applied control measures or dilution will
be required for Zn in 50% of the works and for Cd, Cu and Ni in 10% of the works.
Concentrations of regulated and emerging organic chemicals in final effluents
The data for the organic chemicals in the final effluents of the WwTW were compared with existing
and proposed EQS (Table 4). Where concentrations in final effluents exceeded existing or proposed
standards, there will need to be dilution in receiving waters or treatment at the works to ensure that
rivers comply with the relevant standards. In comparison with data for metals in Table 2, it is apparent
from Table 4 that many more organic chemicals in the effluents exceed EQS or PNEC standards.
Indeed, over 50% of WwTWs will be currently be reliant on dilution to limit their impact on the
receiving waters. However, in the case of the herbicides bentazone, glyphosate (along with its
metabolite aminomethyl phosphonic acid - AMPA) and mecoprop the final effluent concentrations at
over 50% of the sites were an order of magnitude below any EQS or PNEC. For glyphosate and
AMPA, the highest reported values were only observed at a limited number (around 5%) of WwTWs.
This might be taken to show that in general, there are limited inputs of agricultural herbicides to the
sewer system, and it may be that the higher values for glyphosate are related to their domestic urban
use and subsequent runoff which does enter the sewer system following rainfall events.
Concentrations of anthracene and naphthalene, for which proposed EQSs are not as stringent as
other PAHs, appear not to be of concern with regard to impact on the quality of receiving waters.
However, in relation to the EQS proposed in January 2012 (EC, 2012), and listed in Table 4, the final
effluent concentrations at over 50% of WwTWs are of concern for fluoranthene, benzo(a)pyrene,
benzo(b)fluoranthene, benzo(k)fluoranthene and at 90% of WwTWs for benzo(g,h,i)perylene and
indeno(1,2,3-cd)pyrene. These 2012 proposed EQSs focus on biota but include implied water quality
standards. They are significantly more stringent than were listed in the 2008 EC document (L348/84
EC, 2008), which ranged from 0.002 µg/L for the sum of benzo(g,h,i)perylene and indeno(1,2,3-
cd)pyrene, up to 0.05 µg/L for benzo(a)pyrene. If concentrations of PAHs in receiving waters are
going to comply with the proposed EQS value, it is likely that dilutions in receiving waters may need to
be between ten and one hundredfold.
Table 4 Percentile values for average concentration of organic substances (µg/L) Values underlined in bold indicate the percentile greater than existing and proposed standards.
Percentile µg/L
Freshwater EQS µg/L
99% of values reported as <2 µg/l
benzo(a)pyrenePHS
benzo(b)fluoranthenePHS
benzo(k)fluoranthenePHS
benzo(ghi)perylenePHS
indeno123(cd)pyrenePHS
ethinylestradiolPS
Missing data indicate tha
t an undue proportion of less than results makes it impossible to estimate a
percentile. PHS Priori
ty hazardous substance. PS
Priority substance. Ind
on the EC list as an Indicator of other, more dangerous PAH a The sum of congeners 28, 47, 99, 100, 153 and 154. PNECs are in many cases notional values based on recommendation of the Environment Agency of England and Wales
For the BDEs, the majority of results for BDE28, 100, 153 and 154 were predominantly <0.0005 µg/L,
for congeners BDE47 and BDE99 concentrations were frequently detected above this value. The
BDEs 100, 153 and 154 are relatively minor components of commercial penta BDE formulations and
therefore it is not surprisingly that they were detected at lower concentrations and frequencies
compared with BDE 47 and 99 which represent 40% and 50% of the commercial product composition,
respectively. Considering the present WFD EQSs for BDEs of 0.0005 µg/L for the sum of six BDE
congeners (28, 47, 99, 100, 153, 154), concentrations in effluents exceeded this in 50% of cases and
an approximate 10 times dilution would be required to meet the EQS downstream. However, the 2012
proposed limits for BDEs by the European Commission (EC. 2012) now imply an annual average
water EQS of 4.9x10-8 µg/L (again the principal focus is on biota), which is four orders of magnitude
lower. This means that no surface waters in the UK receiving effluent would be likely to comply, as
required dilutions would need to be in the order of 1:10,000 or greater.
For the purposes of classifying the compounds determined in the CIP programme into clear groups, it
is worthwhile considering bisphenol-A, DEHP, EDTA, nonylphenol, tributyltin, and triclosan as a set of
compounds that will come from domestic and possibly urban / industrial activities. Looking at the
national picture, Table 4 indicates that in around 10% of WwTWs, DEHP may be of concern, with
concentrations up to twice that of the standard required in receiving waters. For bisphenol A and
EDTA, the percentages of works discharging at greater than the EQS were 40% and 80%,
respectively; with a maximum dilution required to meet the EQS of 12 and 17 times. This therefore
suggests that compliance may be of concern for some WwTWs where only low dilution is available.
For triclosan, which has been proposed as a new Specific Pollutant with an EQS value of 0.1 µg/L in
Australia (NICNAS, 2012), 60% of UK WwTWs will require a dilution of up to 10 times to ensure
In relation to estimated compliance risk at 50% or more of the WwTWs the chemicals of concern are
the high molecular weight PAHs (fluoranthene, benzo(a)pyrene, benzo(b)fluoranthene,
benzo(k)fluoranthene, benzo(g,h,i)perylene and indeno(1,2,3-cd)pyrene); the BDEs 47 and 99; TBT
and triclosan. The herbicides are not of concern and EDTA, although present, is not regulated.
Implications of the occurrence of pharmaceuticals in final effluents
For pharmaceuticals, UK PNECs are currently estimated at typically 0.01 µg/L but the 2012 proposals
for new EQSs for EE2, E2 and diclofenac involve considerably lower limits (EE2, 3.5 x 10-5 µg/L) (EC,
2012). In many cases, there will be insufficient dilution available to meet this criterion, leading to a risk
of EQS/PNEC exceedance in the receiving water. For example, final effluents from all WwTWs would
require significant dilution (1:100 to 1:1000 times) to achieve proposed EE2 limits. For diclofenac and
E2 50% of the final effluents from the WwTWs would require dilution at 10 to 100 times to achieve
proposed limits. Currently, there is little available monitoring information for these pharmaceuticals in
UK river waters to confirm this assessment. For other substances (erythromycin, oxytetracycline,
ibuprofen, ofloxacin, fluoxetine and propanolol, and estrone - E1) there are no current plans for EQS
to be set at an EU level. However, even in relation to existing estimated PNECs the concentrations of
ibuprofen, propranolol, erythromycin and oxytetracycline in the final effluents are above 0.01 µg/L in
95% of the WwTWs.
In relation to exceedance of EQS or PNEC at 95% or more of the WwTWs the synthetic hormone and
pharmaceutical EE2 is of concern as well as the further pharmaceuticals erythromycin,
oxytetracycline, ibuprofen and propranolol. At 50% of the works fluoxetine and diclofenac are of
concern as is the natural steroid estrogen hormone E2. Ofloxacin is not of concern.
4. Discussion
Correlation analysis
Correlations (Spearman rank order rho (ρ) correlation, Kendall and Gibbons, 1990) were calculated
for all combinations of substances. Having such a large dataset meant that ρ values of greater than
0.23 achieved statistical significance (p=0.05). However, apart from the obvious and established links
between substances (eg total and dissolved metal, BOD and COD), there were few practically
important associations that might be used accurately to predict the concentrations of one substance
from that of another. Such relationships require correlation coefficients approaching 0.9 or greater that
were not evident in the data. The use of correlation to explore less predictive associations is also of
value in explaining the nature of contaminant behaviour and sources.
In order to be able readily to appreciate the large data array comprising the correlation matrix,
software (R development core team, 2008) was employed to produce the visualisation shown in
Figure 3. This portrays the associations between different substances as a family tree. It appears
there are four main groupings. On the far left of the diagram are substances that have little
relationship with the rest of the substances, or indeed with sewage or sewage treatment - calcium,
pH, sodium, chloride, potassium and sulphate as well as nickel which is largely unaffected by the
treatment process. Moving right, the next grouping includes the main sanitary parameters and the
substances that tend to be associated with them. Such association is defined as a tendency to follow
to some extent the sanitary parameters such that good effluent quality – eg low BOD and TSS – is
associated with low concentrations of ammonia ibuprofen, E1, E2 and, a little more distantly, with low
concentrations of TBT triclosan and nonylphenol. These are the trace contaminants for which effluent
concentrations might be likely to respond to (ie reduce as a result of) improvements in conventional
measures of treatment. The remaining two groups are more or less a miscellany of the remaining 48
substances including metals, pharmaceuticals and other trace organic substances. Particularly
interesting is the rightmost group in which the BDEs and PAHs all appear in the same grouping –
together with DOC and TOC. This is possibly important; it suggests that these substances might be
associated with organic carbon, rather than, as might have been expected, with suspended solids.
Figure 3 Dendrogram of associations between C1 substances
Prioritisation of the chemicals of concern in relation to EQS exceedance at over 50% of
Chemicals have been prioritised for further consideration on the basis of their concentrations in
effluent exceeding their EQS or PNEC values in over 50% of the WwTWs. These were:
2. PAHs - fluoranthene, benzo(a)pyrene, benzo(b)fluoranthene, benzo(k)fluoranthene,
benzo(g,h,i)perylene and indeno(1,2,3-cd)pyrene)
4. Organics - TBT
5. Emerging contaminants - triclosan
6. Pharmaceuticals - erythromycin, oxytetracycline, ibuprofen, propranolol, fluoxetine and
7. Steroids - EE2, E2.
It is stressed that this prioritisation is generic accounts only for the extent to which different
substances were found to be present at over 50% of the WwTWs effluents in relation to current or
proposed limit values; local issues will need to be considered separately.
In comparison with other countries where recent national/regional surveys have been completed
(Hope et al. 2012; Martin Ruel et al. 2012) common hazardous chemicals of concern are: PAHs,
BDEs, TBT, emerging chemicals such as triclosan and pharmaceuticals such as diclofenac. Martin
Ruel et al. (2012) prioritised their chemicals based on dividing the final effluent concentration by the
EQS. Values > 1 at a frequency of > 70% were classed as high frequency chemicals of concern. This
is similar to this study where values >1 at a frequency of > 50% were applied. In contrast, Hope et al.
(2012) in the US focussed more on persistent organic pollutants. Chemicals in common with this
study and that of Martin Ruel et al (2012) were the BDEs 47 and 99. In terms of the PAHs comparable
concentrations with those in the US were observed in the region of 0.01 µg/L. However, proposed EU
standards of 1.7x10-4 µg/L are more stringent than the US planned initiation level (PIL) applied by
Hope et al. (2012) of 0.02 - 0.5 µg/L. Therefore the occurrence of PAHs was prioritised in the EU
studies but not by the US study (Hope et al. 2012). The metals of concern detected in the final
effluents in France, at frequencies of >70%, were Ni, Pb, Cd, Hg. In this UK study these metals were
not observed at concentrations above the EQSs at those frequencies.
The chemicals occurring widely in final effluents throughout the UK, frequently above standards,
provide a focus for control measures that may need to be applied at a large number of locations. An
initial expectation may be that dilution of effluents in receiving waters will mean that exceedances of
EQS or PNEC values are limited. Historically, wastewater treatment design has been based on the
Royal Commission criteria which afforded a minimum dilution of 1:8 between the final effluent and
river water (Royal Commission 1898-1914). This dilution is now commonly interpreted as 1:10. The
availability of a 1:10 dilution would clearly increase the probability of downstream compliance. For
example, for Zn, PAHs, triclosan, fluoxetine and EE2 a 1:10 dilution would reduce the number of
potential exceedances from 50% of the WwTWs to below 10%.However, this dilution is not always
realistic at all sites. The fact that the "upstream" flow might already contain the contaminants of
interest further undermines the principle of reliance on dilution. For the PAHs, benzo(b)fluoranthene,
and the pharmaceutical erythromycin a dilution ratio of 1:50 would be required to reduce the number
of WwTWs effected (Table 5). Recent estimates have estimated that for 3,704 WwTW for which
estimated river flow data was available within 1 km of the discharge point, that 28% of the works had
a dilution of less than 1:10 compared with measured WwTW flows or consented dry weather flows
(Comber et al, 2011).
Dilution requirements for the BDEs are over one hundredfold - many times that available (Table 5).
Hence other options for control to ensure compliance with WFD requirements must be considered.
For instance, these might involve source control measures, enhanced treatment options or alternative
approaches to assessing compliance with standards such as taking into account bioavailability for
organic chemicals as currently utilised for metals. In a study by Eriksson et al. (2011) concentrations
would only be reduced for chemicals including Cd, hexachlorobenzene (HCB), nonylphenol and BDE
by fully implementing restrictions on use as part of an emission control strategy. In addition, the
scenarios studied illustrated other opportunities for managing hazardous chemicals before they
become part of the urban water cycle along with managing historic sinks such as sediments (Eriksson
et al. 2011). Source control measures are already widely applied to priority hazardous substances
owing to the need for cessation of discharge by 2020. OctaBDE and Penta-BDE (e.g. including
BDE47 and BDE99) flame retardants have been banned under the 24th amendment to the marketing
and use Directive 76/769/EEC since 15th August 2004. Their presence in wastewaters is therefore a
result of residual use as flame retardants in furniture in domestic properties. The breakdown of foams
leads to accumulation in materials such as clothes, curtains and fabrics, which when washed leads to
an input to sewer. These inputs to sewer would therefore be expected to decrease with time owing to
replacement of furniture and degradation. However, like the reductions in concentrations observed for
TBT and PCB any decline in concentration is likely to be greater than 30 years and will also still be
unlikely to reduce concentrations to below the EQS values in the short to medium term.
Concentrations, however, are only one approach, albeit the principal one used by regulatory
authorities, for measuring inputs of hazardous chemicals. There is an increasing focus on the loads of
chemicals input to the environment as a means of assessing possible impacts and of understanding
the relative significance of sources within catchments (Musoleff et al. 2010). The loads discharged
from the WwTWs included in this study are tabulated in the supplementary data. It is noteworthy that
loads of hydrophobic, recalcitrant chemicals that are likely to persist in the environment for some time.
This emphasises the importance of understanding loads for longer term impacts on the quality of
sediments (Yen et al., 2009) and groundwater (Musoleff et al., 2010).
Table 5 the potential dilution required for chemicals which are present in over 50% of UK WwTWs above the EQSs or PNEC concentrations
Dilution required
>1:100
benzo(b)fluoranthene
benzo(k)fluoranthene
benzo(ghi)perylene
indeno123(cd)pyrene
ethinylestradiol
Reliance on existing available tertiary treatment to meet EQS and reduce loads to the environment is
also not necessarily advisable as these data indicate a wide range of effluent quality for advanced or
tertiary wastewater treatment processes. Hence such processes do not represent a guaranteed
solution and could involve disproportionate costs if applied at all works. This emphasises the need for
a robust economic evaluation as part of any mitigation strategy (Eriksson et al. 2011; Jones et al.
2007), with careful consideration of the time required for marketing initiatives to take effect.
Nevertheless, concomitant improvements in the removal of hazardous chemicals can be achieved by
the optimisation of existing process (McAdam et al. 2010, 2011) and upgrading solutions for nutrient
removal and sanitary determinands. For example, the upgrading of Beckton WwTWs in London in the
mid 1960's with the introduction of activated sludge treatment to reduce BOD and SS discharges to
the River Thames was subsequently found by examination of the sediment record 30 years later to
have significantly improved the removal of heavy metals, polychlorinated biphenyls and
organochlorine insecticides (O'Reilly-Wiese et al. 1997a,b; Scrimshaw and Lester, 1997). If source
control cannot be utilised, for example for certain pharmaceuticals and natural hormones, advanced
tertiary wastewater treatment options may be an alternative to achieve compliance. A large number of
tertiary / "end of pipe" treatment options are available. However, some of these processes, notably
advanced oxidation or membrane filtration can be costly and may result in increased chemical use
(Jones et al. 2007).
Further data from this ongoing programme will explore topics including process performance and
contaminant sources.
5. Conclusions
1. This extensive monitoring programme has demonstrated that trace contaminant
concentrations in wastewater treatment works' effluents can exceed existing or proposed
EQS values. In over 50% of the WwTWs monitored, effluent concentrations of the
following substances exceed the relevant EQS Zn, PAHs - fluoranthene, benzo(a)pyrene,
benzo(b)fluoranthene, benzo(k)fluoranthene, benzo(g,h,i)perylene and indeno(1,2,3-
cd)pyrene), BDEs - 47 and 99, TBT, triclosan, erythromycin, oxytetracycline, ibuprofen,
propranolol, fluoxetine, diclofenac and EE2 and E2.
2. A nominal tenfold dilution in the receiving water will ensure compliance with EQSs for the
majority hazardous chemicals, apart from the BDEs and to a lesser extent the steroids
and (when / if regulated) some pharmaceuticals.
3. In some cases there will be insufficient dilution to guarantee compliance with downstream
EQSs. Here additional management options will have to be considered, taking account of
the need for proportionality between costs and benefits. Measures to be considered will
include: source control, substance substitution, tertiary treatment, and optimisation of
existing processes.
Acknowledgement - The authors wish to thank the co-ordinator of the CIP programme – UK Water
Industry Research (UKWIR) for authorising the use of the information reported here, and the UK
Water Utility companies Anglian, Dwr Cymru, Northumbrian, Scottish, Severn Trent, Southern, South
West, Thames, United Utilities, Wessex and Yorkshire Water for their considerable efforts in
6. References
Aquacheck Bury UK http://www.lgcpt.com/productviewnarrow.aspx?SchemeID=77
Bedding ND, McIntyre AE, Perry R and Lester JN. Organic contaminants in the aquatic environment.
I. sources and occurrence. Sci. Total Environ., 1982, 25, 143-167.
Bedding ND, McIntyre AE, Perry R and Lester JN. Organic contaminants in the aquatic environment.
II. behaviour and fate in the hydrological cycle. Sci. Total Environ., 1983, 26, 255-312.
Behera SK, Kim HW, Oh J-E, Park H-S. Occurrence and removal of antibiotics, hormones and several
other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Science of
the Total Environment 2011; 409, 4351-4360.
Buisson RSK, Kirk PWW and Lester JN. Determination of chlorinated phenols in water, wastewater
and wastewater sludge. Chromatogr. Sci., 1984, 22, 339-342.
Comber SDW, Merrington G, Sturdy L, Delbeke K and van Assche F. Copper and zinc water quality
standards under the EU Water Framework Directive: The use of a tiered approach to estimate levels
of failure. The Science of the Total Environment 2008; 403, 12-22.
Comber S, Daldorph P, Delaney P, Gardner M, Murrell K, Smith R and Constantino, C. Chemical
source apportionment under the WFD. Final Report for UK Water Industry Research (UKWIR), 1
Queen Anne's Gate, London, UK, 2011.
De Schamphelaere KAC, Janssen CR. Development and field validation of a BLM predicting chronic
copper toxicity to Daphnia magna. Environ Toxicol Chem 2004;23:1365–75.
Dodds EC, Goldberg L, Lawson W and Robinson R Oestrogenic activity of certain synthetic
compounds. Nature; 1938, 141, 247.
EC European Commission (2000). Directive 2000/60/EC of the European Parliament and of the
Council of 23 October 2000 establishing a framework for Community action in the field of water policy.
Accessed 23/05/2012 at http://eur-
lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32000L0060:EN:NOT
EC European Commission (2008). Priority Substances Daughter Directive - Directive 2008/105/EC of
the European Parliament and of the Council of 16 December 2008 on environmental quality standards
in the field of water policy Accessed 23/05/2012 at
EC European Commission (2009). Council of European Communities, ‘QA/QC directive' technical
specifications for chemical analysis and monitoring of water status. Commission Directive 2009/90/EC
of 31 July 2009
EC European Commission (2012). Proposal for a Directive of the European Parliament and of the
Council amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field
of water policy Brussels, 31.1.2012 COM(2011) 876 final 2011/0429 (COD)
Eriksson E, Revitt DM, Ledin A, Lundy L, Lützhøft H, Wickman T, Mikkelsen PS. Water management in cities of the future using emission control strategies for priority hazardous substances. Water Science and Technology 2011, 64(10), 2109-2117. Feldman., C., Preservation of dilute mercury solutions. Anal. Chem., (1974). 46 (1), pp 99–102. Gabriel O, Ruzicka K, Kreuzinger N. Upgrading Vienna's wastewater treatment plant – linking point source emissions to Environmental Quality standards. Water Science and Technology 2012; 65(7), 1290-1297. Gardner, M.J., Brown, B., Whitehouse, P. and Birch, M. Towards the Establishment of an Environmental Quality Standard for Aluminium in Surface Waters. Journal of Environmental Monitoring, (2008), 10, 877–882. Gardner MJ, Comber SDW, Leverett D and Gravell A. Sample Stability of Trace Priority Substances in Wastewater, Anal. Lett. 2012; in press, DOI 10.1080/00032719.2012.677789 González S, López-Roldán R, Cortina J-L. Presence and biological effects of emerging contaminants in Llobregat River basin: A review. Environmental Pollution 2012;161, 83-92. Hope BK, Pillsbury L, Boling B. A state-wide survey in Oregan (USA) of trace metals and organic chemicals in municipal effluent. Science of the Total Environment 2012;417-418, 263-272. Koh YKK, Lester JN and Scrimshaw MD. The Fate and Behaviour of Alkylphenols and their Poly-ethoxylates in an Activated Sludge Plant. Bull. Environ. Contam. Toxicol., 2005, 75, 1098-1106. Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999-2000: a national reconnaissance. Environmental Science and Technology 2002; 36(5), 1202-1211. Lester JN. Significance and behaviour of heavy metals in waste water treatment processes. I. sewage treatment and effluent discharge. Sci. Total Environ.; 1983, 30, 1-44. Lester JN, Sterritt RM and Kirk PWW. Significance and behaviour of heavy metals in waste water treatment processes. II. sludge treatment and disposal. Sci. Total Environ.; 1983, 30, 45-83. Martínez Bueno MJ, Gomez MJ, Herrera S, Hernando MD and Agüera A. Occurrence and persistence of organic emerging contaminants and priority pollutants in five sewage treatment plants of Spain: Two year pilot survey monitoring. Environmental Pollution 2012; 164, 267-273. Martin Ruel M, Choubert J-M, Budzinski H, Miége C, Esperanza M and Coquery M. Occurrence and fate of relevant substances in wastewater treatment plants regarding Water Framework Directive and future legislations. Water Science and Technology 2012; 65(7), 1179-1189. Musoleff A, Leschik S, Reinstorf F, Strauch G, Schirmer M. Micropollutant loads in the urban water cycle. Environmental Science and Technology 2010, 44, 4877-4883. NICNAS (2012) Priority Existing Chemical Assessment Report No. 30 Triclosan January 2009 NATIONAL Industrial Chemicals Notification and Assessment Scheme GPO Box 58, Sydney NSW 2001, Australi R Development Core Team (2008). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, UR Robertson AM and Lester JN Supercritical fluid extraction of s-triazines and phenylurea herbicides from sediment. Environ. Sci. and Technol. 1994; 28, 346-351.
Rodil R, Quintana JB, Concha-Graňa E, López-Mahía P, Muniategui-Lorenzo S, Prada-Rodríguez D. Emerging pollutants in sewage, surface and drinking water in Galicia (NW Spain). Chemosphere 2012; 86, 1040-1049. Soares A, Guieysse B, Jefferson B, Cartmell E and Lester JL.Nonylphenol in the environment: A critical review on occurrence, fate, toxicity and treatment in wastewaters. Environment International 2008; 34(7), 1033-1049. Sumpter J. Protecting aquatic organisms from chemicals: the harsh realities. Philosophical Transactions of the Royal Society 2009; 367, 3877-3894. Yen JH, Liao WC, Chen, WC Wang, YS. Interaction of polybrominated diphenyl ethers (PBDEs) with anaerobic mixed bacterial cultures isolated from river sediment. Journal of Hazardous Materials 2009, 165,518-524.
Source: http://v-scheiner.brunel.ac.uk/bitstream/2438/8867/5/Fulltext.pdf
Effects of Olanzapine and Haloperidol on theMetabolic Status of Healthy Men Solrun Vidarsdottir, Judith E. de Leeuw van Weenen, Marijke Fro¨lich,Ferdinand Roelfsema, Johannes A. Romijn, and Hanno Pijl Department of Endocrinology and Metabolism, Leiden University Medical Center, 2300 RC Leiden,The Netherlands Background: A large body of evidence suggests that antipsychotic drugs cause body weight gainand type 2 diabetes mellitus, and atypical (new generation) drugs appear to be most harmful. Theaim of this study was to determine the effect of short-term olanzapine (atypical antipsychotic drug)and haloperidol (conventional antipsychotic drug) treatment on glucose and lipid metabolism.
MATERIAL SAFETY DATA SHEET Resene True Prime Arch Vacsol Azure MATERIAL SAFETY DATA SHEET Product Name: TRUE PRIMEOther Names:Description: PAINTRecommended use: Solvent Alkyd Paint Address: 32-50 Vogel Street Phone: (04) 577 0500 Resene Paints Limited Naenae Wel ington NEW ZEALAND Fax: (04) 577 0600 P O Box 38242 Wel ington Mail Centre Emergency Telephone: 0800 737 363 Available Monday - Friday 8.00am - 4.30pm
