Www2.nobicon.se

ISSN nr: 1830-6993
Catalogue nr: ND-AG-12-002 EN-N
of the European Commission Scientific Committees
Review of events .1
The Scientific Committees .2
Opinions Adopted .2-4
Ongoing work .5-7
Public Consultation .7
Inter CommIttee CoordIna-
tIon Group meetInG,
8 oCtober 2012
The chairs and vice chairs of the Scientific Committees met on 8 October 2012 in or-der to discuss general issues related to the Committees. Topics discussed at the meet-ing were: collaboration with other community bodies, joint opinions, horizontal activities and other general matters. Agreement was reached on the way forward on documents on Future Challenges in Risk Assessment and Making Risk Assessments more Relevant for Risk Managers. Both will be further developed as discussion documents and not as opinions. The outcome of the meeting was found to be very positive by the participants.
SCIentIfIC CommIttee expertS partICIpated In the efSa workInG Group on
Following the submission of a mandate
the Commission's Scientific Commit-
EFSA Working Group. The first meeting
to EFSA for the elaboration of an opin-
tees and other Agencies, experts from the
of the EFSA Working Group took place on
ion on Endocrine Disrupting Chemicals
three Scientific Committees have been
18-19 October 2012 in Brussels.
(EDCs) in collaboration with experts from
identified to participate in the work of the
workShop on dermal Safety aSSeSSment for SubStanCeS wIth low oral bIoavaIlabIlIty
This workshop took place on 12 November
an assessment, do not deliver reliable data
assessment strategies for such substances
2012. The safety assessment of cosmetic
in this case. This scientific workshop com-
were discussed. The outcome of the work-
ingredients with very low oral bioavailabil-
prised members of the Scientific Commit-
shop will serve as a basis for the develop-
ity (e.g. UV-filters) is challenging. Oral toxic-
tees, industry toxicologists as well as exter-
ment of SCCS guidance on this issue.
ity studies, which are usually the basis for
nal experts. Suitable safety testing and risk
Health and Consumers

the Scientific Committees
SCIentIfIC CommIttee on ConSumer Safety (SCCS)
Cosmetic ingredients
librium with formaldehyde in aqueous solu-
tions. Hair straightening products containing
The Annexes to Council Directive 76/768/EEC
this ingredient have been suspected to re-
Under the safety evaluation of hair dyes, the
on cosmetic products do list substances which
lease formaldehyde under the conditions of
SCCS has recently adopted a number of opin-
are banned or restricted for use in cosmetic
use, which involve i.a. heating the hair with a
ions of which the outcome is as follows:
products as well as authorised colorants, UV-
hot straightening iron. The SCCS was asked
filters and preservatives. For updates of these
to assess whether methylene glycol should be
Hair dye found to be safe under the intended
annexes, the SCCS has to be consulted to car-
considered equivalent to formaldehyde, which
ry out risk assessments based on safety data
is restricted to a maximum concentration of
• A5, Toluene-2,5-diamine
provided by industry and/or data available in
0.2% in cosmetic products, and to evaluate
• A159, 2,3-Diaminodihydroxypyrazolopyra-
the public domain.
the safety of use of this ingredient.
zolone dimethosulfonate
• B28, Picramic acid and sodium picramate
The following risk assessments have recently
The SCCS concluded that from a chemical
• B50, HC Red n° 3
point of view, there is a close interrelation-
• B60, 2-Nitro-5-glyceryl methylaniline
ship of formaldehyde and methyleneglycol in
• B119, HC Blue 16
aqueous solution. Therefore, the SCCS, in line
Acetaldehyde is used as a fragrance/flavour in-
with the position of other bodies and panels,
gredient in cosmetic products and, as a metab-
considered methylene glycol as a formalde-
olite of ethanol, can also be present due to the
hyde equivalent. Moreover, when methylene
use of ethanol or plant extracts. It is classified
glycol/formaldehyde is used in hair straight-
as carcinogen cat. 2 under the EU chemicals
ening products at the maximum authorised
legislation. The SCCS was asked to evaluate
concentration of 0.2%, the amount of gase-
the safety of Acetaldehyde when present up to
ous formaldehyde released may exceed the
100 ppm in cosmetic product.
WHO indoor air quality guideline for short term exposure. Therefore the SCCS does con-
The SCCS concluded that acetaldehyde, based
sider the use of methylene glycol/formalde-
on life time cancer risk, is not safe at 100 ppm.
hyde at 0.2% formaldehyde equivalent not
However, as exposure estimates were based on
safe in hair straighteners.
a number of worse case considerations, the risk may be overestimated. The SCCS further con-
The opinion is available at:
cluded that acetaldehyde should not be used
as an intended ingredient in cosmetic products
except used as a fragrance/flavour ingredient
The following hair dyes were found not safe
at a maximum concentration of 0.0025% (25
Kojic acid
or not possible to assess due to missing data:
ppm) in the fragrance compound (as assessed
Kojic Acid is used in cosmetic products as a
• A75, 6-Amino-m-cresol
in the SCCNFP opinion SCCNFP/0821/04), re-
skin lightening agent. In a previous opinion
• B34, N,N'-bis-(2-hydroxyethylamino)
sulting in approximately 2 ppm in the finished
in 2008 the SCCP had stated some concerns
cosmetic product or 4 ppm in fine fragrances.
in relation to the ingredient. On the basis of
• C170, Indigofera tinctoria
As acetaldehyde has increased the risk of al-
additional data the SCCS now re-assessed
• C183, Tetrabromophenol Blue
cohol-related cancer in particular of the upper
the safety of kojic acid and concluded that
aero digestive tract, the SCCS concluded that
its use at a concentration of 1.0% in leave-
The following hair dye was considered to be
acetaldehyde should not be intentionally used
on creams applied to the face and/or hands
safe from the point of view of general toxi-
in mouth-washing products.
is safe for the consumers. Concerns remain
ity, however concerns were raised due to its
The opinion is available at:
in situations where the human skin barrier is
high allergenic potential:
weakened or Kojic Acid is applied on larger
• A7, p-Phenylenediamine
skin surfaces.
The hair dye opinions are available at:
Methylene Glycol
The opinion is available at:
Methylene glycol is formed upon dissolution
of formaldehyde in water and exists in equi-
Health and Consumers


the Scientific Committees
The Guidance is available at:
Benzisothiazolinone is a preservative intend-ed to be used in cosmetic products in a con-
Opinion on Zinc oxide, nano-form
centration up to 0.01%. The SCCS considered
Zinc oxide (CAS No 1314-13-2; EC No 215-
benzisothiazolinone safe for use as a preserv-
222-5) has a widespread use in cosmetic
ative in cosmetics products up to 0.01% with
products and has been previously assessed
respect to systemic toxicity, but expressed
by the SCCS on its non-nano form. A complete
concerns about its sensitising potential.
safety dossier on micronsized and nanosized ZnO was requested for the assessment of its
The opinion is available at:
use in the nano form. The SCCS concluded
that based on the available evidence, the use
of ZnO nanoparticles with the characteristics as indicated in the submitted dossier, at a
Nanomaterials in Cosmetics
concentration up to 25% as UV- filter in sun-
and cosmetics. Some of these nitrosamines,
screens, can be considered not to pose a risk
such as N-nitrosodiethanolamine (NDELA)
Guidance on the Safety Assessment of Nano-
of adverse effects in humans after dermal
and N-nitrosodimethylamine (NDMA) are clas-
materials in Cosmetics
application. This assessment does not apply
sified as category 1B carcinogens. Cosmetic
The Scientific Committee on Consumers Safety
to applications that might lead to inhalation
products containing nitrosamines including
(SCCS) has produced a Guidance document in
exposure to ZnO nanoparticles.
NDELA are banned under the Cosmetics Direc-
order to help the cosmetics industry comply
tive1 and its Annex III refers to the limit of 50
with the strict conditions and deadlines of
The opinion is available at:
μg/kg for nitrosamines. When limit values are
Article 16 of the Cosmetics Regulation (EC)
exceeded, cosmetics containing NDELA and
1223/2009, which will enter into force on 11
balloons containing nitrosamines or nitrosat-
July 2013. From then on, the cosmetics indus-
able substances are notified by Member State
try will have to notify the Commission of all
Nitrosamines in Cosmetics
authorities to RAPEX. Due to the discrepancies
cosmetic products containing nanomaterials,
amongst the authorities to classify the risk as
six months prior to placing them on the market.
Opinion on NDELA in Cosmetic Products and
"serious" or "less than serious" the SCCS was
The guidance document is specific for nano-
Nitrosamines in Balloons
consulted in order to provide their view.
materials. It adds to the existing SCCS Notes of
Nitrosamines are chemical compounds that
Guidance for the testing and safety evaluation
may be present as contaminants in a number
The opinion is available at:
of cosmetic ingredients that already provide
of products including food (such as certain
general guidance to the cosmetics industry.
beverages), tobacco products, rubber products
SCIentIfIC CommIttee on health and envIronmental rISkS (SCher)
Cadmium in Fertilizers – Additional Request for
adoption of the harmonised measure in 2003?
a SCHER opinion on the Risk Assessment report
The SCHER stated in its conclusion that the
from the Kingdom of Sweden (human health)
report does not provide convincing arguments
This request was based on a report sub-
to show that the Swedish case is a unique one
mitted by Sweden. The Kingdom of Sweden
or that data which appeared after the adop-
has notified the Commission on 17 October
tion of Regulation (EC) No 2003/2003 sug-
2011, of its intention to reduce its national
gest specific reasons for additional concern.
provision on the cadmium content of mineral
Overall, the SCHER concluded that it does not
phosphate fertilisers from 100 mg Cd/kg P to
consider the assumptions made in the Swed-
46 mg Cd/kg P. SCHER issued an opinion on 5
ish report as appropriate for calculating risk in
March 2012. Now SCHER was asked the fol-
the Swedish environment.
lowing question: Have the Swedish authorities demonstrated that there is a risk to human
The opinion is available at:
health from Cd in phosphate fertilisers that
is specific to Sweden and has arisen after the
1 OJ L 768, 14.10.2008, p.1
http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1976L0768:20080424:EN:PDF Entries 410 and 704 in Annex II of the Directive.
Health and Consumers


the Scientific Committees
Opinion on tris(2-chloroethyl)phosphate (TCEP)
The opinion is available at:
recommends the amount allocated to the toy
to be limited to 10% of the migration limits,
The substance tris(2-chloroethyl)phosphate
the Committee concluded that the portion of
(TCEP) is an alkyl phosphate ester used as a
the TDI for barium allocated to toy exposure
flame-retardant plasticiser and viscosity
should not exceed 0.02 mg barium/kg bw/day.
regulator in polyurethanes, polyester resins,
Mercury in compact fluorescent lamps (CFLs)
polyacrylates and other polymers. The Com-
In follow-up to the SCHER's opinion on mer-
The opinion is available at:
mittee concluded that (i) there is no reason
cury in lamps and at the request of DG ENER
to set any limit for TCEP in toys, since no safe
for defensive communication support on the
limit could be identified based on the exposure
issues, the Chair and rapporteur evaluated
from other sources; (ii) the limit should be set
a media report in Austria titled ‘Bulb Fiction'
Chemicals and the Water Framework Direc-
at the detection limit of a sufficiently sensi-
which claimed that mercury released when
tive: Draft Environmental Quality Standards:
tive analytical test method; and (ii ) since the
CFLs are accidentally broken result in adverse
The Water Framework Directive (WFD,
estimated exposure from all possible sources
health effects including hair loss and increas-
2000/60/EC) Article 16 requires the Commis-
of TCEP, excluding the toy scenario, is close
ing a person's susceptibility to other stres-
sion to review periodically the list of priority
to the provisional TDI value, the use of TCEP
sors. In a statement issued the two members
substances. Article 8 of the EQSD required
should be avoided also in toys intended for
of the SCHER provided a scientific rationale
the Commission to finalise its next review by
use by children older than 3 years of age.
which dispelled the Austrian film allegations.
January 2011, accompanying its conclusion,
The statement is available at: http://ec.europa.
where appropriate, with proposals to identify
The opinion is available at:
new priority substances and to set EQSs for
them in water, sediment and/or biota.
Assessment of the Tolerable Daily Intake of
A shortlist of 19 possible new priority sub-
Barium in Toys
stances was identified in June 2010. A group
The new Toys Safety Directive (TSD) estab-
of experts from Member States, EFTA coun-
lishes migration limits of 19 elements from
tries, candidate countries and more than 25
toys or components of toys. The migration
European umbrella organisations represent-
limits shall not exceed the listed limits, de-
ing a wide range of interests (industry, agri-
pending on the toy material used. However,
culture, water, environment, etc.) was estab-
the elements can be used if the toy or com-
lished. This group has been deriving EQS for
ponents of the toy exclude any hazard due
these substances and has produced draft EQS
to sucking, licking, swallowing or prolonged
for most of them. In some cases, a consensus
contact with the skin when used as intended
has been reached, but in some others there
or in a foreseeable way, bearing in mind the
is disagreement about one or other compo-
Mercury in Energy-saving Light Bulbs –
behavior of children.
nent of the draft dossier. Directorate-General
Exposure of children
Environment seeks the opinion of the SCHER
In particular the potential risk for children is
The migration limits are based on a study by
on these draft EQS for the proposed priority
still causing public concern, resulting in ques-
the Dutch National Institute for Public Health
substances and the revised EQS for a number
tions from members of the European Parlia-
and the Environment (RIVM) and opinions
of existing priority substances. The SCHER
ment and media reports.
of the Scientific Committee. In view of the
has already published its opinions on aclon-
RIVM report and other documents on barium,
ifen, anthracene, beta-estradiol, bifenox, cy-
This concern has been raised in relation to the
SCHER has been asked to deliver an additional
butryne, cypermethrin, dichlorvos, diclofenac,
issue of mercury emissions from certain en-
opinion on the evaluation of the migration
dicofol, dioxins, ethinylestradiol, fluoranthene,
ergy-saving light bulbs (compact fluorescent
limits for barium. Considering that the SCHER
HBCDD, heptachlor, ibuprofen, lead, naphtha-
lamps, CFLs) upon breakage. In May 2010
lene, nickel, PBDE, polyaromatic hydrocarbons,
the SCHER provided an opinion on mercury
quinoxyfen, and terbutryne. The work on cya-
released from breaking CFLs but at the time
nides and nickel continues and is expected to
could not conclude on the potential risk of
be completed by the end of 2013.
children. Now SCHER has come to an opinion on the potential mercury exposure to children,
The following opinion is on Zinc.
and thus the risk. The SCHER considers that short peak inhalation exposures to peak Hg-
The opinion is available at:
concentrations in air occurring as a result of
accidental breakage of CFLs and intakes of
Hg above the TDI for a very limited time are unlikely to pose a health risk.
Health and

the Scientific Committees
SCIentIfIC CommIttee on emerGInG and newly IdentIfIed health rISkS (SCenIhr)
New Challenges to Risk Assessment
The preliminary opinion can be found at:
A preliminary opinion was adopted for New
Challenges to Risk Assessment. Information about the related public consultation is avail-able on the last page.
opInIon of SCenIhr, SCCS and SCher
the opinion on threshold of toxicological
pared with the EFSA opinion on TTC which
All opinions delivered by the Scientific Com-
was adopted earlier, on 22 May 2012.
mittees are without prejudice to personal ethi-cal considerations of the experts.
The opinion on TTC was adopted by the three
The opinion is available at:
Scientific Committees on 8 June 2012. The
final draft was carefully examined and com-
SCIentIfIC CommIttee on ConSumer Safety - SCCS
The following mandates are currently under
Other cosmetic ingredients
the skin. It has now been asked to evaluate
− Dichloromethane
the safety of use of DEGEE in cosmetic prod-ucts which are sprayed and lead to inhalation
Hair dyes
exposure (e.g. perfumes).
To ensure the safety of hair dye products, a
− Update to the Notes of Guidance (8th revi-
complete review of all hair dye substances on
All mandates for the SCCS are available at:
the European market has been initiated by the
European Commission. Under this framework,
NEw MANDAtES foR SCCS
full safety evaluations of 3 hair dyes remain to be performed. In addition, supplementary
Propyl and butyl paraben
data on 114 substances that have been ini-
The SCCS has on several occasions performed
tially evaluated have been received and are
safety assessments on parabens used as cos-
under assessment and further data on 12
metic preservatives. It has been asked to eval-
substances have been requested.
uate a new developmental study performed with propylparaben and to revise, if neces-
Preservatives
sary, its earlier assessments in the light of this
study. In this assessment also the exposure to
− Zinc pyrithione
sunscreens, especially of children below three years should be taken into account.
UV-filters− HAA299 / C-1332
Diethylene glycol monoethyl ether (DEGEE)
− Titanium dioxide (nano-sized)
The SCCS has previously assessed this sub-stance taking into account only exposure via
Health and Consumers
the Scientific Committees
SCIentIfIC CommIttee on health and envIronmental rISkS – SCher
The following mandates are currently under
ceramics and gold alloys, is increasing either
SCHER to review and update, if appropriate,
due to their aesthetic properties or alleged
the scientific opinion adopted in May 2008 on
health concerns in relation to the use of den-
"The environmental risks and indirect health
the environmental risks and indirect health
effects of mercury in dental amalgam". This
effects of mercury in dental amalgam
2008 opinion is available at:
In the past the Commission services con-
Dental amalgam has been used for over 150
sulted two Scientific Committees on the use
years for the treatment of dental cavities and
of dental amalgam, the Committee for Envi-
is still used, in particular in large cavities, due
ronmental and Health Risks (SCHER) and the
The new mandate is available at:
to its excellent mechanical properties and
Committee for Emerging and Newly Identified
durability. Dental amalgam is a combination
Health Risks (SCENIHR). The opinions of both
of alloy particles and mercury that contains
Committees were not conclusive regarding
about 50% of mercury in the elemental form.
the appropriateness of additional regula-
The opinion is expected by the end of June
Overall, the use of alternative materials, such
tory measures to restrict the use of dental
as composite resins, glass ionomer cements,
amalgam. The Commission now requested
SCIentIfIC CommIttee on emerGInG and newly IdentIfIed health rISkS (SCenIhr)
The following mandates are currently under
arthroplasty, paying special attention to de-
which the European Commission is funding
sign and patient groups.
through the LIFE+ programme, has recently issued a press release asserting that "blood
New Mandates for SCENIHR
The mandate is available at:
bags made of DEHP-plasticised PVC pose a
significant risk to human health, due to both
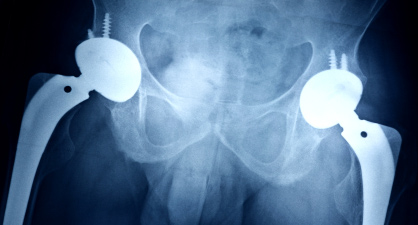
Safety of metal-on-metal joint replacements
DEHP and PVC". In the light of this new study,
with a particular focus on hip implants.
the Scientific Committee on Emerging and
The Scientific Committee on Emerging and
Safety of medical devices containing DEHP (di
Newly Identified Health Risks (SCENIHR) was
Newly Identified Health Risks is requested
(2-ethylhexyl) phthalate) plasticized PVC on
asked to review and, if appropriate, update
to provide a scientific opinion on the safety
groups possibly at risk.
the opinion adopted in 2008.
of metal-on-metal joint replacements with
Various substances are used to ensure flex-
a particular focus on hip implants. The Com-
ibility of medical devices, among which DEHP
The mandate is available at:
mittee is asked, in particular, to determine
[Di-(2- EthylHexyl) Phthalate] is the most
the health effects resulting from metals (in
frequently used plasticizer in PVC. DEHP may
any form) released by the implanted medical
migrate from the device to the human body,
device and to identify criteria regarding the
resulting in a certain degree of patient ex-
The safety of dental amalgam and alternative
safety of metal-on-metal implants used in
posure. A project entitled PVCfreeBloodBag,
dental restoration materials for patients and usersDental amalgam and its substitutes are regu-lated under Council Directive 93/42/EEC con-cerning medical devices, according to which they must comply with the essential require-ments laid out in the directive, in particular in relation to the health and safety of the patients. According to the SCENIHR opinion adopted in May 2008, dental amalgam is a safe material to use in restorative dentistry for patients. No health risk other than an al-lergic reaction in certain individuals can be associated with the use of dental amalgam. The alternatives are not without clinical limi-tations and toxicological hazards and less is known about these alternatives for which available scientific data are more limited.
Health and Consumers
the Scientific Committees
In the light of recent developments and stud-
The safety of PIP silicone breast implants
The second opinion has been rescheduled to
ies on dental amalgam the Commission has
asked the Scientific Committee on Emerging
The SCENIHR was asked to contribute to the
and Newly Identified Health Risks (SCENIHR)
creation of an EU questionnaire to collect data
The PIP2 questionnaire is available at:
to update, if appropriate, the opinion adopted
on implanted patients, to provide guidance on
in 2008. This opinion is available at:
the testing undertaken by the member states,
and to update its scientific opinion on the
safety of the PIP silicone breast implants on
Mandates for SCENIHR are available at:
the basis of the new data collected.
The new mandate is available at:
Following the rapid scientific opinion adopted
by SCENIHR on 1 February 2012 covering "The
You may find all information about the work
Safety of PIP Silicone Breast Implants", the
of the Committees, on the opinions and the
The adoption of the opinion is expected by the
Commission recognised that an update of this
mandates (requests) via the following web-
end of June 2013.
opinion would be necessary, mainly because
the data available on PIP silicone breast im-
plants at the time of the opinion was limited.
publIC ConSultatIon for SCenIhr, SCCS and SCher
Public consultation on the Discussion Paper
for human and environmental risk assess-
addressing the New Challenges for Risk As-
ment will change substantially over the next
few decades. Those changes will result from advances in scientific knowledge as well as in
The European Commission and its 3 inde-
modelling and measuring techniques. In ad-
pendent, non-food Scientific Committees
dition, as regards to human-health risk as-
have launched a consultation on the following
sessment, a paradigm shift is likely to occur
discussion paper: Addressing the New Chal-
from a hazard-driven process to one that is
lenges for Risk Assessment. The consultation
was launched on 22 October 2012 and ran till 30 November 2012.
More information can be found at:
The motivation for this opinion has been the
perception that the procedures currently used
Contact details:
Health and Consumers
Source: http://www2.nobicon.se/nobicon/func/click.php?docID=920371&UID=2275&delivery=rss&noblink=http://ec.europa.eu/health/scientific_committees/docs/december2012_en.pdf
Chem. Mater. 2001, 13, 565-574 Luminescence Properties of Structurally Modified PPVs: PPV Derivatives Bearing Dong Won Lee,† Ki-Young Kwon,† Jung-Il Jin,*,† Yongsup Park,‡ Yong-Rok Kim,§ and In-Wook Hwang§ Division of Chemistry and Molecular Engineering and the Center for Electro- and Photo-Responsive Molecules, Korea University, Seoul 136-701, Korea, Surface Analysis Group,
Associate Degree Registered Nursing Program Pediatric Nursing Maricela Arnaud, RN, MSN, FNP Brenda Harrell, RN, MSN, EdD Ronda Wood, RN-BC, MN, EdD ©2013 Long Beach City College Associate Degree Nursing Program ADN 35B: PEDIATRIC NURSING SYLLABUS TABLE OF CONTENTS INTRODUCTION TO 35B Course Information . 1.0 Course Outline . 1.2 Orem's Conceptual Framework . 1.5 Course Requirements . 1.6 Course Student Learning Outcomes/Objectives . 1.7 Challenge Policy (General and 235B) . 1.8 Course Schedule . 1.10 Grades/ Assignments . 1.11