Xic.postech.ac.kr
Biophysical Journal
Imaging Cells and Tissues with Refractive Index Radiology
Y. Hwu,* W. L. Tsai,* H. M. Chang,y H. I. Yeh,z P. C. Hsu,* Y. C. Yang,* Y. T. Su,* H. L. Tsai,yG. M. Chow,§ P. C. Ho,{ S. C. Li,{ H. O. Moser,k P. Yang,k S. K. Seol,** C. C. Kim,**J. H. Je,** E. Stefanekova,yy A. Groso,yy and G. Margaritondoyy*Institute of Physics, Academia Sinica, Nankang, Taipei, Taiwan; yPandis Biomedical Research Association, Chupei, Taiwan;
zDepartments of Medical Research and Internal Medicine, Mackay Memorial Hospital, Mackay Medicine, Nursing andManagement College, and Taipei Medical University, Taipei, Taiwan; §Department of Materials Science andSingapore-Massachusetts Institute of Technology Alliance, National University of Singapore, Singapore; {Department of Pharmacy,National University of Singapore, Singapore; kSingapore Synchrotron Light Source, National University of Singapore, Singapore;**Department of Materials Science and Engineering, Pohang University of Science and Technology, Pohang, Korea; and
yyFaculte´ des Sciences de Base, Ecole Polytechnique Fe´de´rale, CH-1015 Lausanne, Switzerland
Can individual cells, including live cells, be imaged using hard x rays? Common wisdom until now required
sophisticated staining techniques for this task. We show instead that individual cells and cell details can be detected in culturesolution and tissues with no staining and no other contrast-enhancing preparation. The sample examined can be much thickerthan for many other microscopy techniques without sacrificing the capability to resolve cells. The key factor in our approach isthe use of a coherent synchrotron source and of contrast mechanisms based on the refractive index. The first successful testswere conducted on a variety of cell systems including skin and internal leaf cells, mouse neurons, rabbit fibroblast cells, andhuman tumor cells.
We present experimental evidence that synchrotron hard
(Hwu et al., 2002). This enhanced the visibility of the edges
x rays are suitable for radiological imaging of thick and live
between regions with different refractive index. As a result,
biological samples down to the cellular level. In recent years,
fine details could be observed at the cellular and subcel-
substantial research efforts drastically improved the lateral
lular level without any staining or contrast-enhancing cell
resolution and enhanced the contrast in synchrotron-based
radiology (Hwu et al., 2002). In principle, radiological
This is an interesting complementary approach to the
images based on hard x ray (wavelength ,1 A
standard optical microscopy of living cells. In fact, x-ray
sufficient lateral resolution (Yun et al., 1999) and can resolve
microradiology has the capability to examine thicker samples
structures of biological samples down to the cell level.
in a more ‘‘realistic'' environment and potentially even in
However, such performances have not been demonstrated
living bodies. We demonstrate in this work, for example, that
without sophisticated staining procedures excluding live
cellular detail within a sample as thick as 5 mm can still be
cells (Yun, 2004).
clearly identified at selective depth from the sample surface
Cells can be observed in soft-x-ray microscopy using
without sacrificing the lateral resolution. Furthermore, recent
x rays with wavelengths in the so-called ‘‘water window''
tests based on advances in detectors and in image reconstruc-
(284–543 eV) that maximizes the contrast between carbon-
tion demonstrated that the short wavelength of hard x rays
containing areas and water (Kirz et al., 1994; Jacobsen et al.,
can yield reconstructed images with 10–50-nm resolution—
2002). The development of advanced x-ray optics success-
well beyond the diffraction limit of optical microscopy
fully achieved the observation of subcell structures in
(Miao et al., 2002).
hydrated state (Larabell et al., 2000; Ford et al., 2000;
Edge enhancement cannot be easily observed with con-
Weiß et al., 2000). However, x rays in this photon energy
ventional x-ray sources due to their large size and angular
range cannot penetrate specimens thicker than a few tens of
spread (Hwu et al., 2002). In fact, the enhancement is due to
a micrometer, approximately the thickness of a single cell,
small deviations of the x rays propagating through the edge,
and this limits their potential application. Moreover, there is
which is a region with high refractive-index gradient. A large
no report that this type of approach can produce images of
source size and angular spread wash out the effect. To reduce
living cells due to the sample preparation requirements.
this problem, one can either use an aperture to limit the
The key to our success in using hard x rays to image
effective source size or increase the source-sample distance.
unstained cells was contrast based on the refractive index
However, this greatly reduces the portion of the emittedx rays that is actually used for radiological imaging.
Synchrotron sources remove the problem because of their
natural coherence (Hwu et al., 2002; Margaritondo, 2002).
Submitted October 2, 2003, and accepted for publication September 14,
The x rays are emitted from a small source area and are
strongly collimated. Edge enhancement thus becomes easily
Address reprint requests to J. H. Je, E-mail:
[email protected].
Ó 2004 by the Biophysical Society
Imaging Cells with Radiology
visible and—as we demonstrate here—microradiology of
vertebrates in real time, which is not possible with optical
live cells is feasible. Our experimental results show indeed
microscopy due to opacity.
that fine details can be imaged with high lateral and timeresolution in a variety of biology materials. These includedleaf skin cells, human tumor cells, mouse neurons, and rabbitbone cells.
MATERIALS AND METHODS
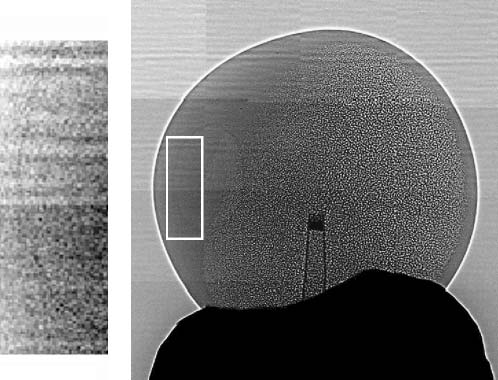
Fig. 1 shows a nice example of our results: the image of
a Xenopus oocyte. Note that one can clearly identify theanimal pole (left hemisphere with even gray level), the
The tests were performed with a microradiography system whose details aredescribed in Baik et al. (2004). Unmonochromatized x-ray beams from the
vegetal pole (right hemisphere with granular appearance),
7B2 beamline of the Pohang Light Source and the PCI beamline of the
and the nucleus within the animal pole (see the magnified
Singapore Synchrotron Light Source were used to illuminate the sample.
and filtered inset). The edge enhancement is extremely clear
The transmitted x rays were captured by a cleaved CdWO4 single-crystal
for the outer boundary of the oocyte and also visible for the
scintillator and converted to a visible image. This image was then magnified
boundary between the nucleus and the surrounding region
by an optical lens before being captured and stored by a charge-coupleddevice camera.
The samples were mounted on a translation/rotation stage that allowed
The different appearance of the left and right hemisphere
precise positioning. For biological samples with intrinsically limited x-ray
can be attributed to an inhomogeneous distribution of cyto-
absorption, a single image (typically 1280 3 1024 pixel, horizontal field of
skeletons creating an asymmetry between the two hemi-
view (FOV) 500 mm) could be taken within 100 ms. The corresponding
spheres. The animal pole has much more densely connected
radiation dose did not produce any detectable damage.
keratin than the vegetal pole (Gard et al., 1997). The meshsize is 10 mm in the vegetal pole, 10 times larger than those
Sample preparation
in the animal pole. The much smaller mesh size of the
The leaf cell samples were obtained from Hippeastrum epidermis. Tissue
cytoskeleton network and the presence of pigment granules
specimens were obtained by peeling off the bottom skin of the leaf and then
(;1 mm in diameter) blur the edges of the network and only
sandwiched between two moisturized Kapton foils to preserve water. The
an evenly distributed gray level is observed in the animal
thickness of the leaf skin is ;30–50 mm and the Kapton foil is 50 mm. Such
a procedure was not required to observe cells; it was possible to see
These arguments are consistent with the electron micros-
individual cells even in the leaf without peeling. The peeling andsandwiching procedure, however, eliminated a problem: the superposition
copy results (Gard et al., 1997). In addition, our technique
of many cell layers results in complicated images. An alternate solution for
leads to nondestructive and three-dimensional (3D) obser-
this problem is tomography. We demonstrated the tomography reconstruc-
vation of the oocyte interior. Also note that the observation
tion of plant cell by using thick samples (;5 mm) taken from the skin part of
of the nucleus within the animal pole is important because
an aloe leaf.
it could lead to the observation of the embryogenesis of
Oocytes were selected for our tests to assess the penetration and the
spatial resolution of our approach. Oocytes at stage 6 were surgically re-moved from the ovary of a Xenopus and incubated at 18°C in the standardND96 medium (in mM) (NaCl, 96; KCl, 2.0; CaCl2, 1.8; MgCl2, 1.0; andHEPES, 5, pH 7.6).
Cerebral neurons from rat brains were prepared from an 18-day-old rat
embryo (Lin et al., 2002). They were placed on a coverslip glass locallycoated with poly-D-lysine so that neurons accumulated over the poly-D-lysine patches. Cell cultures were incubated at 37°C with 95% humidity and5% CO2. The samples for the microradiology tests were prepared from theculture after 9–11 days of incubation. A medium with collagen was usedto seal the neurons on the coverslip and a gel-like layer was formed after;20 min in ambient air at room temperature. The average thickness of thesamples was ;200 mm.
Fibroblast cells were used to test the feasibility of detecting changes in
the cell morphology. The cell line (HIG-82) was obtained from the FoodScience Research Institute (Industrial Technology Research Institute,Taiwan). It was prepared from the fibroblast of rabbit joints. The cellswere cultured in 35-mm culture dishes without specific coating at the bottomand incubated at 37°C with 95% humidity and 5% CO2. The medium wasHam's F-12 medium (2 mM L-glutamine and Earle's buffered salt solutionadjusted to contain 1.5 g/L sodium bicarbonate, 0.1 mM nonessential aminoacids, and 1.0 mM sodium pyruvate 90%; fetal calf serum, 10%). Dishes
Phase-contrast micrograph showing the interiors of a Xenopus
with ;80% cell confluence were prepared for microradiology tests after
oocyte. A sharp-tipped glass capillary (visible in the middle of the image)
was used to fix the sample to the holder (the bottom dark region). The sample
All animal cell samples were fixed with 4% paraformaldehyde overnight
diameter was 1.5 mm. The inset shows a magnified version of the marked
before being mounted on the sample holder. The oocyte was placed in air.
left-hand portion of the image. This magnified picture was processed to
The coverslips with the neurons were placed in a sealed plastic bag to
emphasize the boundary of the nucleus.
preserve sufficient moisture. The HIG-82 fibroblast cells were observed with
Biophysical Journal 87(6) 4180–4187
the culture dish water-tight sealed and vertically placed in the path of thex-ray beam.
For preparation of mouse aorta, adult C57/BL6 mice were anesthetized
with ether inhalation and were perfusion fixed via direct intracardiacinjection, initially with heparinized PBS (10 units/ml) followed byphosphate-buffered 2% paraformaldehyde (pH 7.4) for 10 min (Yeh et al.,2003). The thoracic aortas were dissected and cut into transverse rings.
Thereafter the aortas were embedded in resin using standard preparationprocedures for thin-section electron microscopy (Blackburn et al., 1995).
The above-described preparation procedures are much less complicated
than the standard methods for optical microscopy and other microscopytechniques—and resulted in specimens very close to their natural state.
Specifically, no staining or other contrast-enhancing procedure was used.
The cultured cells were fixed to preserve their morphology because the x-raybeam propagates horizontally and therefore the specimens had to be placedvertically. The specimens were much thicker than for optical microscopy
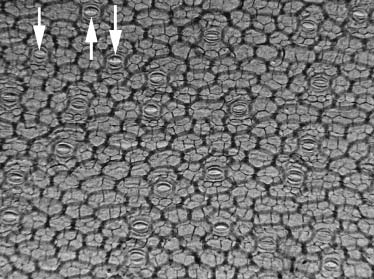
Microradiograph showing the entire leaf depth.
and an opaque medium could be used.
the gaps among the spongy parenchyma cells under thepalisade cells. Note that x ray can penetrate into the leaf and
RESULTS AND DISCUSSION
reveal the different morphologies of these cells and their
The specific objectives of our tests were the following. First,
packing. Fig. 3 stresses the difference with respect to optical
we wanted to demonstrate sufficient (submicron) lateral res-
microscopy: not only cells in the outermost layer are visible
olution and contrast with hard x rays (wavelength ,0.1 nm)
but also deeper cells.
to detect cellular details. Second, we wanted to show such
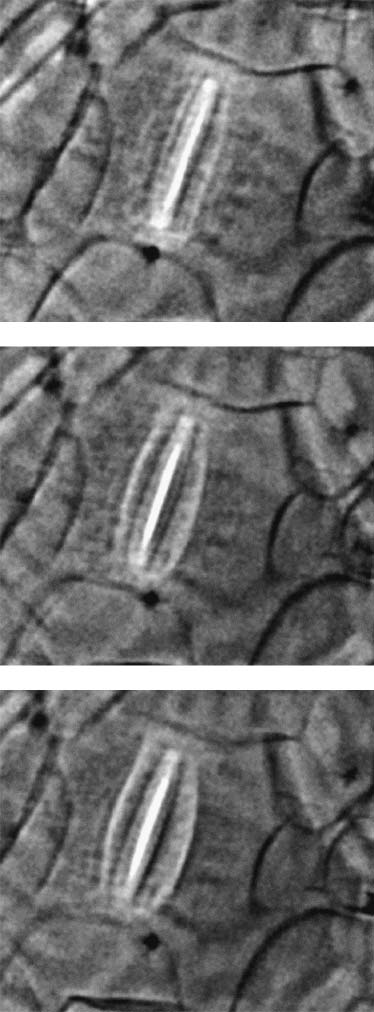
The arrows in Fig. 2 identify stomata in the epidermis as
a performance with thick samples and without complicated
they open up. Such cells enabled us to demonstrate real-time
sample processing. Third, we sought specific evidence of
imaging of live specimens. Fig. 4 shows in fact individual
cellular-level imaging of live specimens. The fourth
images captured from a movie taken with a video camera.
objective was detection in real time on a video (�33 ms)
The images clearly show the opening of a live stoma cell.
A careful examination of the sequence of images in Fig. 4
All such objectives were reached with tests on the already-
reveals features beyond the mere opening. These include
mentioned variety of specimens. Figs. 2 and 3 show micro-
subcellular features and the induced changes (such as com-
radiographs of a Hippeastrum leaf taken with broadband
pression and displacement) of the surrounding cells. We
(unmonochromatized) x rays. The (horizontal) FOV was
suspect, for example, that the darker lines on the guard cells
are those from the cellulose microfibrils. The live detection
These images constitute, to the best of our knowledge, the
of such minute movements is quite remarkable if one con-
very first example of cell radiographs obtained with hard
siders the complete absence of staining.
x rays without staining. Fig. 2 shows the leaf skin (thickness
We also tested a procedure to enhance the moving cell
;30–50 mm) whereas Fig. 3 shows the entire leaf (thickness
with respect to the nonmoving ones by subtraction. This is
;300 mm). The little triangle-shaped features in Fig. 3 are
quite desirable because the nonmoving cells inside the leaf(particularly the parenchyma cells) have much strongercontrast than the epidermal cells and outer cuticle and there-fore complicate the image when one tries to observe themovement in a natural, unpeeled leaf. The first tests areencouraging and may provide a simple and feasible alternateprocedure with respect to tomography.
In parallel, we performed successful tests of tomographic
reconstruction of microradiographs of plants cells. We useda standard filtered back projection algorithm from 1000projections. We found that the boundaries of individual cellcan be effectively reconstructed. Fig. 5 shows an example ofreconstruction performed for the skin of an aloe leaf ;5-mmthick. Note that only the area near the surface (shown in Fig.
5) is reconstructed due to the limited field of view whereas
Microradiograph of a Hippeastrum leaf taken with broadband
the entire thick sample was kept during the image acquisition
(unmonochromatized) x rays. The image size was 1.5 3 1 mm. The figure
for the required tomography reconstruction. The quality of
shows cells from the leaf skin membrane. The arrows identify three of the
the reconstruction was not optimized due to the slight move-
stoma cells. To the best of our knowledge, these results constitute the firstexample of cell radiographs without staining.
ment of the sample during the measurements. Moreover,
Biophysical Journal 87(6) 4180–4187
Imaging Cells with Radiology
Tomographic reconstruction of an aloe leaf sample. The re-
construction is based on 1000 projections taken within 2 min. Scale bar is50 mm.
show that tomographic reconstruction is feasible for thicksamples.
To better demonstrate this high ‘‘selectivity'' resulting
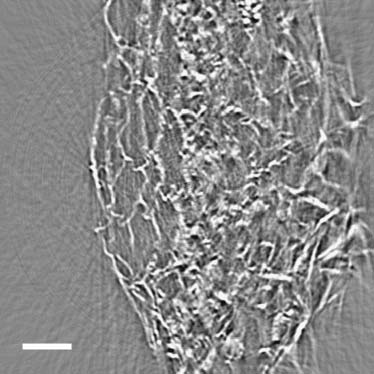
from the enhanced contrast due to phase effect and from thehigh-penetration nature of x rays, we show in Fig. 6 a similarlocal tomography reconstruction of a toothpick. The di-ameter of the toothpick is ;3 mm and the reconstructed areais 240 3 240 mm. The quality of the reconstructed image isimproved with respect to the previous case by the eliminationof the artifacts due to dehydration-induced sample move-ment as seen for the aloe leaf. In the inset, it is clear that smallfeatures can still be easily detected despite the rather largesample examined. Fig. 6 b shows the 3D volume-generatedsurface contour image constructed from the tomography re-constructed data as exampled in Fig. 6 a. Note that not onlyeach channel is clear imaged, but also the texture of the cellwalls along the direction parallel to the rotation axis.
The added phase effect, however, complicated the tomog-
raphy reconstruction based on the filtered back projectionalgorithm. One can use a more rigorous approach to extractthe embedded phase information (Bronnikov, 2002; Barty
Images captured from a movie taken with a video camera
et al., 2000; Cloetens et al., 2002) and obtain the real-space
showing the opening movement of a live stoma cell.
image before initiating the normal tomography reconstruc-tion. Another simpler approach is to consider the phase
although the image sequence was taken within 2 min,
effects, in this case the dark and bright fringes on the edge,
notable dehydration was observed. The reconstruction was
simply as a region of light adsorption. By adjusting the
also blurred by the ‘‘local tomography'' effects (Faridani
sample to detector distance, such phase effect can be opti-
et al., 1997; Anastasio et al., 2003) due to the large dimen-
mized for best visual clarity without adding unwanted effect
sion of the sample, which is larger than the field of view of
to the tomography reconstruction as demonstrated in the
each individual projection. In this specific case, the di-
previous examples.
mension of the sample was 5 3 5 3 5 mm but only ;400 3
Note that in conventional tomography the reconstruction
400 3 300 mm was reconstructed. The corresponding arti-
based on absorption works because absorption follows a
facts are most noticeable in the area away from the center of
linear dependence along the line of projection. This is in
rotation. Such problems notwithstanding, the tests clearly
fact a consequence of the small magnitude of the imaginary
Biophysical Journal 87(6) 4180–4187
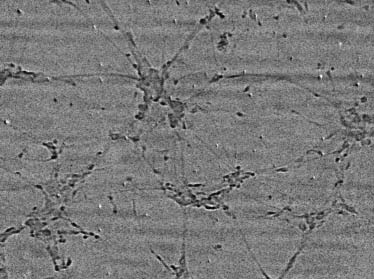

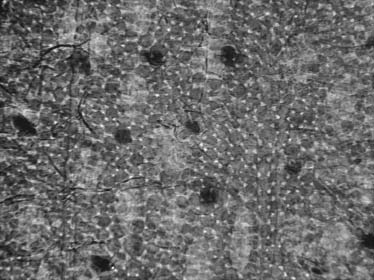
Radiograph of cultured and fixed neuron cells from mouse
brain. The field of view (horizontal) was 500 mm.
Note that the neuron specimens were not even optimized
for x-ray imaging. In fact, the collagen used for sealing wasobtained from three rat tails and dissolved in 0.1% acetate atthe concentration of 2 mg/ml, which is not specially designedfor microradiology and therefore leads to weak contrast. Thefixation collagen are also quite thick, ;300 mm, much morethan normally required for optical microscopy. The slightblurring in the microradiographs is likely due to collagen-induced diffuse scattering and can be improved by furtheroptimization of the collagen materials.
Fig. 8 shows cultured fibroblast cells from rabbit joints.
The cells can be fixed with paraformaldehyde beforemounting the specimen in a vertical position. The fixed cellswere then tightly packed and all with a fibril or spindle shape.
In contrast, Fig. 8 shows the same type of cells imagedwithout being fixed: the cells are not close packed and
(a) Tomographic reconstruction of a toothpick, used to assess
morphological modifications can be seen. Nevertheless, the
the resolution performances of our apparatus and procedure. The diameter of
image quality is still quite reasonable, indicating that our
the sample is ;3 mm but only a central area of diameter ;240 mm isreconstructed. Features of mm size can be observed. (b) The volume-
approach can be used for mammalian cells in their natural
rendered 3D structure of the same toothpick sample.
With suitable fixation procedures, we could even obtain
high-resolution tomography and 3D reconstructed images of
part of the refractive index. Similarly, the phase shift due to
mammalian cells. Fig. 9 a shows an example. The sample is
the real part of the refractive index is approximately linear,because the real part is very close to unity and the imaginaryexponential in the wave function leads to a constant-phasefactor multiplied by a linearly changing phase factor.
Plant cells are quite easy to image with our approach. Figs.
7 and 8, however, show that animal cells can be seen as well.
Fig. 7 shows cultured and fixed neuron cells from mousebrain. Not only are the neuron bodies clearly visible but alsothe interconnected axons.
Specifically, it is possible to observe the region under the
ganglion neurons so that the attachment of the neuronswith the extracellular matrix and with one another can berevealed. The x-ray penetration to the region under theganglion surface could be exploited to analyze neurons-glia
Radiograph of cultured fibroblast cells extracted from a rabbit
interactions and even the interaction between cells and
bone. The cell samples were again vertically mounted but not fixed. The field
of view (horizontal) was 500 mm.
Biophysical Journal 87(6) 4180–4187
Imaging Cells with Radiology
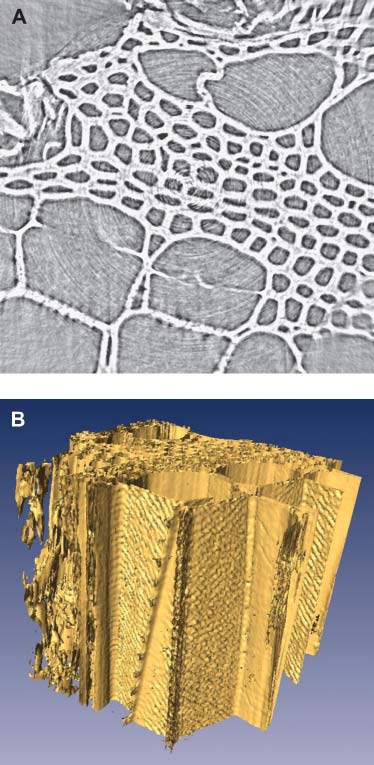
a piece of aorta fixed in resin; the overall sample dimension,including the resin, is ;5 3 7 3 10 mm. Fig. 9 b showsone of the tomography reconstructed slices. In this cross-sectional view of mouse aorta, ;240- 3 240-mm field ofview, the detailed structure of the vessel wall is clearlyobserved after tomography reconstruction despite the factthat the sample is larger than the imaged area. The aorta wallis layered by the waving elastic laminae, the innermost ofwhich, the internal elastic lamina (marked by black arrows),is isolated from the lumen (L) by a monolayer of endothelialcells (white arrows). Fig. 10 is the volume-rendered 3Dstructure of this sample. The white arrows point to bordersbetween neighboring cells, whereas the black arrow pointsto a cell nucleus that extrudes from the surface and whoseoutline is well preserved in the 3D reconstruction analysis. Inthis case, the imaging capability goes beyond the mereimaging of the outline and shape of the individual cell andprovides subcell information.
Our results, thus, show that refractive index imaging based
on coherent x rays can produce clear images at the cell andsometimes subcell level without staining. It is quite im-portant to compare this approach to other techniques andassess its advantages, limitations, and possible future im-provements. The resolution obtained so far is similar tooptical microscopy and can be substantially improved. Softx-ray microscopy has better resolution but has not beenimplemented on samples thicker than a single layer of cells.
Transmission electron microscopy and scanning electronmicroscopy require specific sample preparation proceduresand are limited in the sample thickness. Overall the biggestadvantage of our approach is offered by the high penetrationof hard x rays, easily the whole body of an animal, and theconsequent potential to examine systems close to naturalliving conditions. No microscopy in fact matches refractiveindex microradiology in examining thick and opaque spec-imens with high resolution (W. Yun, private communica-tion).
This approach is certainly not without limitations. The
possible radiation damage is certainly less severe than inabsorption-contrast radiology but it cannot be neglected. Inmost of the two-dimensional measurements, where exposureto synchrotron x rays can be limited, radiation damage ordehydration is not observed. Although not evident in ourtests, this issue must nevertheless be carefully explored toassess its impact. The longer data-taking time required forthe tomography (sometimes .1000 snapshots at differentangles is necessary to preserve the resolution) could ag-gravate the problem. On the other hand, the current time perframe (1 ms) (Hwu et al., 2004) and the corresponding dose
(a) X-ray micrographs of a mouse aorta sample fixed in resin;
than the imaged area. The aorta wall is layered by the waving elastic
the overall sample dimension, including the resin, is ;5 3 7 3 10 mm.
laminae, the innermost of which, internal elastic lamina (marked by black
Scale bar is 400 mm. (b) One of the tomography reconstructed slices. The
arrows), is isolated from the lumen (L) by a monolayer of endothelial cells
detailed structure of the vessel wall is clearly seen even if the sample is larger
(white arrows). Scale bar is 15 mm.
Biophysical Journal 87(6) 4180–4187
tests, should be systematically explored. Although ourtechnique can already clearly detect different cell morphol-ogies and cell details, even finer internal structure detailsshould become visible at higher magnification. Furthermore,future x-ray lasers will provide ultraintense and ultrashortpulses to perform one-shot imaging before damage. Evenwithout waiting for these future developments, our testsclearly show that coherent microradiology is a very helpfulinstrument in cell biology, largely complementary to opticalmicroscopy.
We are grateful to W. Y. Chow and Y. C. Chang (Department of LifeScience, National Tsing Hua University, Taiwan) for providing oocytes andneurons for our experiments.
This work was supported by the National Science Council (Taiwan), theAcademia Sinica (Taiwan), the BK21 Project, the Korea Institute of Scienceand Technology Evaluation and Planning through the National ResearchLaboratory and SKORE-A projects, the Fonds National Suisse de laRecherche Scientifique, the Ecole Polytechnique Fe´de´rale de Lausanne, and
The volume-rendered 3D structure of the same aorta sample
the National University of Singapore.
of Fig. 9. The white arrows point to borders between neighboring cells,whereas black arrow points a cell nucleus that extrudes from the surface andwhose outline is well preserved in the 3D reconstruction. Scale bar is 50 mm.
Anastasio, M. A., F. De Carlo, and X. Pan. 2003. Phase-contrast to-
can be reduced by 1–2 orders of magnitude with advanced
mography and the local tomography problem. Proc. SPIE. 5030:
As to other future improvements, the full development
Baik, S., H. S. Kim, M. H. Jeong, C. L. Lee, J. H. Je, Y. Hwu, and G.
of tomographic reconstruction will further exploit the key
Margaritondo. 2004. International consortium on phase contrast imagingand radiology beamline at the Pohang Light Source. Rev. Sci. Instrum.
advantage of our approach in exploring thick living samples
up to a few mm with minimal sample preparation. The
Barty, A., K. A. Nugent, A. Roberts, and D. Paganin. 2000. Quantitative
technique will be further enhanced by the future ultrabright
phase tomography. Opt. Commun. 175:329–36
x-ray laser and by the potential to implement new image
Blackburn, J. P., N. S. Peters, H. I. Yeh, S. Rothery, C. R. Green, and N. J.
detection strategies with their ultrashort pulses.
Severs. 1995. Upregulation of connexin43 gap junctions during earlystages of human coronary atherosclerosis. Arterioscler. Thromb. Vasc.
Biol. 15:1219–1228.
Bronnikov, A. 2002. Theory of quantitative phase-contrast computed
tomography. J. Opt. Soc. Am. A. 19:472–480.
The limited absorption by biological tissues has so far
Cloetens, P., W. Ludwig, E. Boller, L. Helfen, L. Salvo, R. Mache, and M.
prevented x rays from producing radiological images of
Schlenker. 2002. Quantitative phase-contrast tomography using coherentsynchrotron radiation. Proc. SPIE. 4503:82–91.
individual cells without staining. We demonstrated that this
Faridani, A., D. Finch, E. L. Ritman, and K. T. Smith. 1997. Local
limitation can be overcome. We specifically presented hard-
tomography II. SIAM J. Appl. Math. 57:1095–1127.
x-ray radiographs of different types of living and nonliving
Ford, T., W. Meyer-Ilse, and A. D. Stead. 2000. Development and
cells with no staining at all.
evaluation of cryo-imaging of unicellular algae using soft X-ray
The result was achieved by using coherent x rays from
transmission microscopy: ultrastructure and elemental analysis. W.
Meyer-Ilse, T. Warwick, and D. Attwood, editors. Proc. of the 6th
a synchrotron source with no monochromatization (because
X-Ray Microscopy Conference, American Institute of Physics, College
very limited longitudinal coherence was required) (Hwu
Park, MD. 507:119–122.
et al., 2002; Margaritondo, 2002), x-ray detection with
Gard, D. L., B. J. Cha, and E. King. 1997. The organization and animal-
high lateral resolution, and a detection geometry that en-
vegetal asymmetry of cytokeratin filaments in stage VI Xenopus oocytesis dependent upon F-actin and microtubules. Dev. Biol. 184:95–114.
hances the contrast due to variations in the x-ray refractiveindex. The image quality in these first tests is not yet at
Hwu, Y., W.-L. Tsai, A. Groso, G. Margaritondo, and J. H. Je. 2002.
Coherence-enhanced synchrotron radiology: simple theory and practical
the same level as state-of-the-art optical microscopy. How-
applications. J. Appl. D: Appl. Phys. 35:R105–R120.
ever, there is substantial potential for quality improvement,
Hwu, Y., W. L. Tsai, J. H. Je, S. K. Seol, B. Kim, A. Groso, G.
and, of course, x-ray images have the strong advantage of
Margaritondo, K.-H. Lee, and J.-K. Seong. 2004. Synchrotron micro-
revealing internal features of a large specimen in a rather
angiography with no contrast agent. Phys. Med. Biol. 49:501–508.
Kirz, J., H. Ade, E. Anderson, C. Buckley, H. Chapman, M. Howells,
C. Jacobsen, C. H. Ko, S. Lindaas, D. Sayre, S. Williams, S. Wirick, and
Several aspects of our tests should be further explored. For
X. Zhang. 1994. New results in soft X-ray microscopy. Nucl. Instrum.
example, possible radiation damage, not observed in our first
Biophysical Journal 87(6) 4180–4187
Imaging Cells with Radiology
Jacobsen, C., T. Beetz, M. Feser, A. Osanna, A. Stein, and S. Wirick. 2002.
Weiß, D., G. Schneider, B. Niemann, P. Guttmann, D. Rudolph, and G.
Spectromicroscopy of biological and environmental systems at Stony
Schmahl. 2000. Tomographic imaging of cryogenic biological specimens
Brook: instrumentation and analysis. Surf. Rev. Lett. 9:185–191.
with the X-ray microscope at BESSY I. W. Meyer-Ilse, T. Warwick, and
Larabell, C. A., D. Yager, and W. Meyer-Ilse. 2000. Localization of
D. Attwood, editors. Proc. of the 6th X-Ray Microscopy Conference,
proteins and nucleic acids using soft X-ray microscopy. W. Meyer-Ilse,
American Institute of Physics, College Park, MD. 507:123–128.
T. Warwick, and D. Attwood, editors. Proc. of the 6th X-Ray MicroscopyConference, American Institute of Physics, College Park, MD. 507:107–
Yeh, H.-I., C.-S. Lu, Y.-J. Wu, C.-C. Chen, R.-C. Hong, Y.-S. Ko,
M.-S. Shiao, N. J. Severs, and C.-H. Tsai. 2003. Reduced expression ofendothelial connexin37 and connexin40 in hyperlipidemic mice: re-
Lin, Y. C., Z. H. Huang, I. S. Jan, C. C. Yeh, H. J. Wu, Y. C. Chou, and
covery of connexin37 after 7-day simvastatin treatment. Arterioscler.
Y. C. Chang. 2002. Development of excitatory synapses in cultured
Thromb. Vasc. Biol. 23:1391–1397.
neurons dissociated from the cortices of rat embryos and rat pups at birth.
J. Neurosci. Res. 67:484–493.
Yun, W. 2004. [online]. http://www.xradia.com.
Margaritondo, G. 2002. Elements of Synchrotron Light for Biology,
Chemistry, and Medical Research. Oxford University Press, New York.
Yun, W., B. Lai, A. Krasnoperova, E. Di Fabrizio, Z. Cai, F. Cerrina, Z.
Miao, J. W., T. Ishikawa, B. Johnson, E. H. Anderson, B. Lai, and K. O.
Chen, M. Gentili, and E. Gluskin. 1999. Development of zone plates
Hodgson. 2002. High resolution 3D X-ray diffraction microscopy. Phys.
with a blazed profile for hard x-ray applications. Rev. Sci. Instrum. 70:
Rev. Lett. 89:088303.
Biophysical Journal 87(6) 4180–4187
Source: http://xic.postech.ac.kr/?page=msgs/download&print=1&blankPage=1&filename=files%2F988fbf8ef38719217a60bf928ee4e151%2F0%2F147%2F0402.pdf
ודי לע רשואו קדבנ ונכותו תואירבה דרשמ י"ע עבקנ הז ןולע טמרופ PRESCRIBING INFORMATION NAME OF THE MEDICIAL PRODUCT Vimpat 50 mg film-coated tablets Vimpat 100 mg film-coated tablets Vimpat 150 mg film-coated tablets Vimpat 200 mg film-coated tablets QUALITATIVE AND QUANTITATIVE COMPOSITION
ARTICLE IN PRESS Medical Engineering & Physics xxx (2006) xxx–xxx Photoacoustic monitoring of the absorption of isotonic saline solution by human mucus F.L. Dumas , F.R. Marciano , L.V.F. Oliveira , P.R. Barja , D. Acosta-Avalos a Instituto de Pesquisa e Desenvolvimento (IP&D), Universidade do Vale do Paraiba, Av. Shishima Hifumi 2911, CEP 12244-000, S˜ao Jos´e dos Campos, SP, Brazil







