Characterisation of salmonella


Characterisation of Salmonella
(09 11406)
MAF Technical Paper No: 2011/67
Prepared for MAF Biosecurity Operational Research
By Senior Lecturer Daniel Petkov, IVABS, Massey University, Dr. Julie Collins-Emerson, mEpiLab, IVABS, Massey University and
Prof. Nigel French, Food Safety &Veterinary Public Health, mEpiLab, IVABS, Massey University
ISBN 978-0-478-38701-8 (online) ISSN 2230-2794 (online)
November 2010
Disclaimer
While every effort has been made to ensure the information in this publication is accurate, the
Ministry of Agriculture and Forestry does not accept any responsibility or liability for error or
fact omission, interpretation or opinion which may be present, nor for the consequences of
any decisions based on this information.
Any view or opinions expressed do not necessarily represent the official view of the Ministry
of Agriculture and Forestry.
The information in this report and any accompanying documentation is accurate to the best of
the knowledge and belief of Massey University acting on behalf of the Ministry of
Agriculture and Forestry. While Massey University has exercised all reasonable skill and care
in preparation of information in this report, neither Massey University nor the Ministry of
Agriculture and Forestry accept any liability in contract, tort or otherwise for any loss,
damage, injury, or expense, whether direct, indirect or consequential, arising out of the
provision of information in this report.
Requests for further copies should be directed to:
Strategic Science Team
Strategy Directorate
MAF
P O Box 2526
WELLINGTON
Telephone: 0800 00 83 33
Facsimile: 04-894 0300
This publication is also available on the MAF website at
Crown Copyright - Ministry of Agriculture and Forestry
Contents
Abstract
Materials and methods
3.1. Colony morphology, serology and antibiotic sensitivity Testing
3.2. Virulotyping
3.3. Pulsed Field gel electrophoresis (PFGE)
4.1. Colony morphology and serology
4.2. Antimicrobial sensitivity testing
4.3. Virulotyping and PFGE
Discussion
Conclusion
References
Appendices
10. Supplementary Information
1. Abstract
Objective:
The objectives of this study were to investigate the molecular epidemiology of Salmonella
Typhimurium DT160 between 1999 and 2009 and examine the relatedness between isolates
obtained from avian species, the environment associated with poultry, and humans.
Methods:
Ninety New Zealand (NZ) Salmonella enterica subsp. enterica serotype Typhimurium DT160
(DT160) isolates obtained in the period between 1999 and 2009 were assessed for colony
morphology and serotype, and four isolates were further evaluated with Triple Sugar Agar,
biochemical and motility tests. The susceptibility of the isolates to 11 antimicrobials was also
determined. In an attempt to discriminate between potentially pathogenic and pathogenic
Salmonella isolates, virulotyping was performed based on 12 potential virulence genes.
Pulsed Field Gel Electrophoresis (PFGE) patterns were determined using restriction enzymes
XbaI and SpeI.
Results:
All 90 isolates were confirmed as Salmonella spp. All isolates were susceptible to the
antimicrobials used in this study, with the exception of susceptibility to tetracycline and
oxytetracycline, for which 26 and 8 isolates, respectively, fell within the intermediate range.
However, all isolates were positive for at least 10 of the 12 virulence genes. Two of the six
isolates PCR negative for one of the virulence genes (invA, iroN, pefA or sifA) were of human,
and the remaining four were of sparrow origin. PFGE combined with virulotyping
demonstrated that the genotype profile AA1 accounted for 78/90 (86.7%) of the isolates and
persisted as the dominant genotype throughout the time period, whilst the second most
common profile, AA2, was found in only three isolates (3.3%), comprising two isolates from
sparrows and one from a human. The remaining nine profiles were found in single isolates.
The AA2 isolates were PCR negative for sifA.
Conclusion:
There was no indication of multi-drug resistance within the isolates examined in this study
and no obvious correlation was found between intermediate susceptibility to tetracycline or
oxytetracycline, and virulotype, PFGE profile, origin of the isolate, or seasonality. There was
little overall variation between the isolates with the majority demonstrating a single combined
virulence gene and PFGE genotype profile. The similarity between the isolates that originated
from avian, human and poultry environments over this time period suggests that a dominant
epidemiologically-important genotype of DT160 was transmitted freely between humans,
wildlife and farmed animals over an extended period. Further, the genotyping data provide
evidence that a relatively small number of variants arose in the population between 1999 and
2009, but these did not become prevalent or replace the dominant genotype.
Key words: avian, human salmonellosis, S. Typhimurium DT160, PFGE, virulotyping,
zoonosis
Ministry of Agriculture and Forestry
Characterisation of Salmonella 1
2. Introduction
2.1. BACKGROUND
Salmonellosis is a zoonotic bacterial disease of national and international importance. The
worldwide distribution of salmonellosis often parallels the patterns of trade of animal
products and food, and the migration patterns of humans and animals (Callaghan & Simmons
2001; ESR 2010; Gilbert et al. 2010; Penfold et al. 1979; Wong et al. 2007). In common with
other countries New Zealand (NZ) has experienced the repeated introduction and
dissemination of a wide range of Salmonella serotypes that have become a major concern for
both human and animal health. The rate of human salmonellosis notifications in NZ ranged
between 26.2 to 64.7 cases per 100,000 population between 2000 and 2009 (Lim et al 2010)
and currently salmonellosis is the second most commonly notified enteric disease in NZ, with
campylobacteriosis being the most common [Institute of Environmental Science and Research
(ESR), NZ, 2010)]
Taxonomically, the genus Salmonella which belongs to the Enterobacteriaceae family is
divided into two species: Salmonella bongori and Salmonella enterica. Using the Kauffmann-
White Scheme, members of this genus are classified into more than 2500 serotypes based on
differences between the O (cell wall) and H (flagella) antigens (Luderitz et al. 1966; Grimont
& Weill 2007). Lipopolysaccharide is a major cell wall component comprising lipid A, core
oligosaccharide and O antigenic polysaccharide chain (Luk & Lindberg 1991). While some
Salmonella serotypes such as S. enterica Typhimurium and S. Enteritidis can infect humans as
well as several animal species, others such as S. Cholerasuis, S. Dublin, and S. Typhi are
restricted to fewer host species, primarily infecting pigs, cattle and humans, respectively
(Kingsley & Baumler 2000; Parry et al. 2002).
The spread of salmonellosis is associated with a wide range of sources and transmission
pathways including: the consumption of contaminated food products from pigs, poultry and
ruminants; contaminated drinking water; overseas travel; and direct contact with domestic and
wild animal faeces via environmental and occupational exposure (Mullner et al 2009, Wilson
and Baker 2009). The spatial and temporal epidemiology of different serotypes of Salmonella
in humans often reflect the patterns of infection in animal reservoirs, and this is evident in NZ
where some serotypes have been predominant in either South or North Island, whereas others
have been widespread across the whole country (ESR 2010).
Salmonella enterica subspecies enterica serotype Typhimurium Definitive Type DT160
(DT160) was isolated from sparrows in Canada (Tizzard et al. 1979) and in England sparrows
infected with DT160 contaminated food for human consumption in a hospital that resulted in
gastroenteritis in the human patients (Penfold et al. 1979). In vivo experiments have shown
that the ability of Salmonella strains to invade and survive within the intestine may correlate
with their ability to cause systemic infections (Connolly et al. 2006). In 1998, S.
Typhimurium DT160 was identified as a human pathogen in NZ (Alley et al. 2002; ESR
2010) and since then has become widespread, and a common epidemic strain in NZ (ESR
2010). Various transmission pathways have been identified including: the handling of birds,
contact with patients with diarrhoea, the consumption of contaminated food and drinking roof
collected rainwater (Thornley et al. 2003). However, elsewhere in the world DT160 seems to
be uncommon and a rare cause of illnesses (Fisher 2004). Although salmonellosis can be a
self-limiting disease, the host may become a persistently infected asymptomatic carrier (Todd
et al. 2008) with intermittent shedding of bacteria. Epidemics affecting birds have caused
high mortality mostly in passerines and, to a lesser extent, in psittacines (Alley et al. 2002).
Since 2000, the incidence as well as the geographical distribution of DT160 in NZ has
2 Characterisation of Salmonella
Ministry of Agriculture and Forestry
increased significantly, progressing from the South to the North Island (Callaghan & Simmons 2001), although the reason for this has not been identified (Alley et al. 2002). Of growing concern is the use of antimicrobials that may enhance the severity of the disease and select for resistance (Poppe et al. 1998; WHO 2002 and 2005). Sub-inhibitory treatment with tetracycline was associated with the emergence of multidrug resistant S. Typhimurium DT104 (Weir et al. 2008). In addition, diminished sensitivity within the clinically susceptible range may evolve to clinically resistant bacteria (Phillips et al. 2004). While numerous outbreaks globally have been caused by multi-drug resistant strains of Salmonella Typhi (Rowe et al. 1997), in Denmark a high correlation was identified between the mortality rate in humans and infections with S. Typhimurium resistant to ampicillin, chloramphenicol, streptomycin, sulphonamide and tetracycline (Helms et al. 2002).
2.2. PROJECT OBJECTIVES
The objectives of this study were to investigate the molecular epidemiology of Salmonella
Typhimurium DT160 in New Zealand between 1999 and 2009, and to examine the genetic
relatedness of isolates obtained from three sources: avian species, poultry environments and
humans over this time period.
Ministry of Agriculture and Forestry
Characterisation of Salmonella 3
3. Materials and methods
3.1. COLONY MORPHOLOGY, SEROLOGY AND ANTIBIOTIC SENSITIVITY
TESTING
Bacterial strains
Ninety isolates of S. Typhimurium DT160 were obtained on Dorset egg slopes from ESR Ltd, NZ. The sources of these isolates were avian species, poultry-related environmental samples, and human clinical isolates that had been obtained between the years 1999 and 2009. Thirty isolates were of human origin and 60 were isolated either from avian species or from environments related to poultry. These were selected using a stratified random process whereby isolates in the sampling frame were grouped according to year and source, and the appropriate number randomly selected from each group. Freeze-dried S. Menston [National Collection of Type Cultures (NCTC7836)] and Escherichia coli (E. coli) [America Type Cell Cultures (ATCC 25922)] were purchased from the New Zealand Reference Culture Collection, ESR. Salmonella Typhimurium F98 phage type 14 (NCTC12190) and S. Typhimurium SL1344 with unknown phage type (NCTC 13347) cultures were purchased in freeze-dried form from the Health Protection Agency, United Kingdom. Prior to further testing, all Salmonella strains were sub-cultured on Columbia Horse Blood agar (Fort Richard Laboratories Ltd., Auckland, NZ) and nutrient agar (Merck, Germany), incubated at 37oC for 24 hours.
Colony morphology
All Salmonella isolates were streaked onto nutrient agar and onto blood agar plates. Their size and morphological characteristics were observed and recorded.
Serotyping
All 90 S. Typhimurium DT160 isolates and the S. Menston positive control were serotyped by a slide agglutination serological test using a polyclonal mixture of monoclonal mouse antibodies [Enteroclon Anti-Salmonella A-67, omnivalent, specific for O and Vi antigens and Enteroclon Anti-Salmonella poly-H phase 1 & 2 sera (SIFIN, Berlin, Germany)] as recommended by the manufacturer. Briefly, using a wire loop, a small amount of bacteria was picked from a well-isolated colony, transferred onto a glass slide and mixed with a drop of either poly-H or poly-O antiserum. The homogenous, slightly milky suspension was tilted back and forth for less than 20 times on the glass slide and the reaction read on a dark surface by naked eye within 1 minute (min) from the time of mixing. A drop of sterile physiological saline (0.85% NaCl) instead of the serum was used as a negative control and the test performed simultaneously with the test sample. The Salmonella isolates that were negative for either poly-H or poly-O anti-Salmonella serum from SIFIN were retested using Salmonella H antiserum polyclonal rabbit a-z and Salmonella O antiserum polyA-I and Vi from BD (Becton Dickinson, NZ) as recommended by the manufacturer. The biochemical profiles of the same isolates were also tested with the API20E bacterial identification test kit (Biomerieux, I'Etoile, France), Triple Sugar Iron (TSI) agar and motility confirmed using the motility test. A well-isolated colony was used for inoculation of the TSI agar taking care to ensure that the bottom portion of the agar had also been inoculated. The cap of the tube was placed on loosely, and the tubes were incubated for 18 to 24 hours (hrs) at 37oC. The motility test was conducted by observing the growth of the bacteria in a semi-solid Bacto motility test medium (Becton Dickinson, United States of America, USA). The centre of the medium was inoculated with well-isolated colony and the
4 Characterisation of Salmonella
Ministry of Agriculture and Forestry
medium incubated for 24 hrs at 37oC. The API20E test consisting of 20 separate biochemical reactions and an additional oxidase test were used as per manufacturer's instructions. Briefly, inocula from the isolates were prepared by mixing a single bacterial colony with 5 millilitres (ml) of sterile distilled water until homogenous suspensions were formed. The biochemical reactions were inoculated with 200 µl suspensions, incubated at for 18-24 hrs at 37ºC and the results read based on colour changes for each reaction. The tests were separated into 3 groups and on each positive reaction a specific numerical value was assigned "1" for a positive reaction in the first test of the group "2" for a positive reaction in the second test of the group and "4" for a positive reaction in the third test of the group. A 7 digit number (e.g. 4305542) was obtained after the values corresponding to positive reactions in each group were added and the analytical profile index was analysed using APILab Plus software (Biomerieux, I'Etoile, France).
Antimicrobial sensitivity testing
All 90 Salmonella isolates and the control E. coli ATCC25922 were tested using the Kirby Bauer Disc Diffusion method following the "M31-A3 Performance Standards For Antimicrobial Disk And Dilution Susceptibility Tests For Bacteria Isolated From Animals; Approved Standards" (Clinical and Laboratory Standards Institute (CLSI), 2008) guidelines. Isolates were tested for susceptibility to the following antibiotics with the respective disc concentrations: 10 µg ampicillin (AMP), 30 µg amikacin (AK), 30 µg amoxicillin-clavulanic acid (AMC), 30 µg chloramphenicol (C), 30 µg cefoxitin (FOX), 10 µg cefpodoxime (CPD), 5 µg ciprofloxacin (CIP), 30 µg nalidixic acid (NA), 25 µg trimethoprim-sulfamethoxazole (SXT), 30 µg oxytetracycline (OXT) and 30 µg tetracycline (TET) antibiotic discs (Oxoid, NZ Ltd.). It should be noted that, although not included in the initial panel of antimicrobials, isolates were later tested against tetracycline as there were no guidelines available for oxytetracycline. Briefly, a sterile, non-toxic swab was used to collect between 4 to 5 well-separated colonies from an overnight culture streaked on a nutrient agar plate. The bacteria were suspended into 4 to 5 ml of sterile, normal saline and the turbidity of the suspension was adjusted with sterile saline to obtain a suspension visually similar to that of a 0.5 McFarland standard. The turbidity was read against a standard card with black lines on a white background. A new swab was dipped into the suspension and, following removal of excess inoculum by pressing the swab gently against the wall of the tube, bacteria were spread evenly on the Mueller Hinton agar plate (Fort Richard Laboratory Ltd., Auckland, NZ). No more than 6 drug-impregnated 6 mm discs were applied to the agar surface. An automatic dispenser was employed to ensure discs were no closer than 24 mm from centre to centre. Each disc was pressed gently with sterile forceps to ensure complete contact with the agar and the inoculated plates were incubated for 16 to 18 hrs at 37oC. The zones of inhibition were measured using a digital calliper and end points determined based on the areas showing no bacterial growth visible to the naked eye. The interpretation of the inhibition zones for AK, AMC, AMP, C, FOX, CPD, SXT, OXT and TET and E. coli ATCC25922 was based on M31-A3, CLSI 2008 and for NA and CIP on those described in M100-S20, CLSI 2010.
Statistical analysis
To assess concordance, an inter-rater agreement Kappa quadratic weighted analysis was performed for repeat antimicrobial susceptibility using MedCalc Software bvba version 11.4.20 (www.medcalc.be/manual/kappa.php, Belgium). Kappa analysis evaluates the agreement between two classifications on ordinal or nominal scales (Fleiss et al. 2003).
Ministry of Agriculture and Forestry
Characterisation of Salmonella 5
3.2. VIRULOTYPING
DNA extraction
DNA of S. Typhimurium was extracted by boiling bacteria in water as previously described by Hughes et al., 2008, with some modifications. Briefly, genomic DNA was extracted from an overnight culture grown on a nutrient agar plate. Using a disposable wire loop, three to four colonies were picked up and suspended in 1 ml of 2% Chelex (BioRad Laboratories, USA) and mixed by vortexing. The negatively-charged Chelex resin binds divalent ions in the samples and may improve the efficiency of the PCR (Walsh et al. 1991). The mixture was heated at 95oC for 10 min using a heating block and the tube was cooled at room temperature and centrifuged at 12,470 g for 3 min. Approximately 800 µl of the supernatant containing extracted DNA was aliquoted and stored at -20oC until further used.
Polymerase chain reaction (PCR)
The PCR reaction was synthesised with primers, the sequences of which were previously published (Table 1), in a 25 µl mixture containing 5 µl crude DNA, 1X PCR reaction buffer, 0.2 mM of each dNTP, 1.5 mM MgCl2, and 0.2 µM of each of the primers and, 0.5 U Platinum Taq polymerase (Invitrogen NZ Ltd., NZ). The PCR cycle parameters were as follows: 96oC for 2 min followed by 32 cycles at 94oC for 30 sec for denaturation, 55oC for 30 sec (annealing) and at 72oC for 30 sec (extension) applied for the orfL, pefA, pipD, sopB, spiC, and sifA genes and 1 min (extention) for the invA, iroN, misL, prgH, sitC, and sopE genes. A final extension at 72oC for 2 min was used for all genes.
Gel electrophoresis and documentation
Three microlitres of PCR products from misL, orfL, pipD, pefA, sopB, spiC, and sifA were electrophoresed on a 1.3% agarose gel (AppliChem GmbH, Germany) and run at a constant 70 V for 105 min. The PCR products for the remaining genes were electrophoresed on a 1.8% agarose gel and run at constant 70 V for 120 min. A 1 kb plus DNA ladder (Invitrogen NZ Ltd., NZ) was used as the molecular marker. The PCR products were visualised with ethidium bromide as per the manufacturer's recommendations and documented using Gel Doc with Quantity One 4.6.2 (Bio-Rad, Segrate, Milan, Italy) software. (Figure 1)
PCR sequence analysis
To confirm that the PCR products were the targeted sequences, PCR products obtained from the two control strains, S. Typhimurium SL1344 and F98, using the primers described in Table 1 were sequenced. The PCR products were purified using an ethanol precipitation technique and products quantified using a NanoDrop Spectrophotometer ND-1000 (Biolab, USA). Separate stocks were prepared for forward and reverse primers at a concentration of 3.2 pmol/15 µl. The templates were prepared to a concentration of 2 ng/100 bp/15 µl then premixed with the forward primer in a total reaction volume of 15 µl by adding sterile distilled water. The same method was applied for the reverse primer mixture. Sequencing was performed by capillary separation on an ABI3730 DNA Analyzer at the Allan Wilson Centre Genome Services, Massey University, Palmerston North, NZ. The nucleotide sequences from all targeted genes were aligned with the previously published sequences of S. Typhimurium SL1344 (Wellcome Trust Sanger Institute, UK) using the BLAST nucleotide algorithm at the National Center for Biotechnology Information, United States National Library of Medicine.
6 Characterisation of Salmonella
Ministry of Agriculture and Forestry
3.3. PULSED FIELD GEL ELECTROPHORESIS (PFGE)
Preparation of plugs
All 90 DT160 isolates and the 3 control strains, S. Braenderup H9812, S. Typhimurium SL1344 and F98, were placed on Columbia Horse Blood agar (Fort Richard) and incubated for 24 hrs at 37ºC. Two millilitres of a cell suspension buffer (CSB) (100 mM Tris, 100 mM EDTA, pH 8.0) was transferred into a 12 mm x 75 mm Falcon tube and a small portion of a bacterial colony from an agar plate was transferred to the tube with a cotton swab moistened with CSB and then mixed until an even suspension was formed. The concentration of the cells was adjusted to between 0.40 and 0.45 optical density using a Dade Microscan Turbidity Meter and read at 610 nanometres. Four hundred microlitres of the adjusted suspension was transferred into a 1.5 ml microcentrifuge Eppendorf tube and gently mixed with 20 mg/ml Proteinase K (Bioline GmbH, Germany). One percent SeaKem Gold (SKG) agarose (Cambrex Bio Science, Inc. Rockland, USA) in 0.5 X Tris-Borate EDTA (TBE) buffer was melted in a microwave oven on a low-medium power for 20-30 sec and 400 µl of the molten agarose mixed with the cell suspension in a microcentrifuge tube. The mixture was dispensed into the reusable plug mould where it was solidified at room temperature for 10-15 min.
Lysis and washing of cells in agarose plugs
Five millilitres of cell lysis buffer (CLB) (50 mM Tris, 50 mM EDTA, pH 8.0, 1% Sarcosyl), 0.5 mg/µl of Proteinase K, and solidified plugs were mixed into a 50 ml tube and incubated in a water bath for 120 min between 54 and 55ºC with constant agitation at 150-175 revolutions per minute (rpm). Following incubation, the supernatant was decanted, between 10 and 15 ml of pre-heated sterile water was added and the mixture incubated at the above conditions. This washing step was repeated one more time. Preheated TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0) was added to the tube and the mixture incubated at the above conditions. This step was repeated three times and the plugs were transferred into a microcentrifuge tube and stored at 4ºC in 5-10 ml of TE buffer until further use.
Restriction digestion of DNA in agarose plugs with XbaI and SpeI
Plugs were removed from the TE buffer, placed on a glass slide and cut into 2.0 to 2.5 mm lengths. Each plug was transferred to a 1.5 ml Eppendorf tube and 175 µl of sterile water, 20 µl of restriction buffer, and 50 U of XbaI restriction enzyme (Hoffmann-La Roche Ltd., Germany) were added as recommended by the PulseNet PFGE manual (Centers for Disease Control and Prevention (CDC) 2009)). The above mixture was incubated in a water bath for 120 min at 37ºC. The same procedure was applied for SpeI 50 U (New England Biolabs Inc.) restriction enzyme.
PFGE electrophoresis and gel documentation
Electrophoresis was performed on a contour-clamped, homogenous electric field (CHEF) Mapper electrophoresis system (BioRad, USA), the cooling module of which was set at 14ºC with the pump turned on 30 min prior to the beginning of a gel run. The plugs, digested with either XbaI or SpeI, were loaded into the wells in an agarose gel (1% SKG agarose in 0.5 X TBE), ensuring an absence of air bubbles, and run for 18 hrs at electrophoresis conditions recommended for Salmonella restriction with XbaI by the PulseNet PFGE manual. Finally, the gel was stained with ethidium bromide for approximately 10 min followed by de-staining with distilled water for approximately 30 min. The DNA banding patterns were visualised with a Gel Doc and analysis performed with BioNumerics Version 2.0 software (Applied Maths NV, USA). PFGE analysis is based on a comparison between selected isolates and the percentage similarity between banding patterns. The percentage similarity was calculated using the Dice similarity coefficient method with a 1.5% optimisation and 2% band filtering
Ministry of Agriculture and Forestry
Characterisation of Salmonella 7
tolerance window. A dendrogram was constructed using the Unweighted Pair Group Method
with Arithmetic Mean (UPGMA).
4. Results
4.1. COLONY MORPHOLOGY AND SEROLOGY
The colonies of all Salmonella isolates were approximately 2-3 mm in size and appeared
moist and raised on nutrient agar, and grey, round and non-haemolytic on blood agar
regardless of their species of origin.
At the time of initial isolation, all isolates were serotyped by ESR using the Kauffmann-
White Scheme. Serological confirmation was undertaken in this laboratory using poly-O and
poly-H sera. Eighty six isolates showed agglutination with poly-O and poly-H sera from
SIFIN. Three of the isolates, when tested with sera from SIFIN were negative for
agglutination when tested with poly-H but positive for poly-O, and one isolate was negative
when tested with poly-O but positive with poly-H. These four isolates agglutinated with poly-
H and poly-O sera from BD and were also confirmed to be Salmonella spp. with the API20E
biochemical, TSI and motility test (Table 2). The API20E profile index for the four isolates
indicated 76% probability that the isolates are indeed Salmonella spp. On TSI agar the four
isolates produced red (alkaline) slant, yellow (acid) butt with blackening of the medium as a
result of hydrogen sulphide production which is indicative of members of Enterobacteriaceae
family.
4.2. ANTIMICROBIAL SENSITIVITY TESTING
Due to the growing concerns related to antimicrobial susceptibility of S. Typhimurium
DT160, all isolates were tested against 11 antimicrobials that are often used for treatment of
human patients or animals (Tables 3 and 4). Except for tetracycline and oxytetracycline, all
isolates were susceptible to these antimicrobials. Based on the reference standards for
tetracycline described in CLSI M31-A3, 26 isolates fell within the intermediate range of
susceptibility to tetracycline and 8 isolates within the same range for susceptibility to
oxytetracycline (Table 5).
Kappa analysis for the antimicrobial susceptibility test indicated good agreement between the
two technicians who each performed the tests for all antimicrobials, with the exception of
ciprofloxacin for which the borders of the inhibition zones were not clear and therefore not
easily measured (Table 6).
4.3. VIRULOTYPING AND PFGE
S. Typhimurium SL1344 was chosen as one of the two control strains as it is well
characterised, and the genome is completely sequenced and available for analysis. As
expected, SL1344 was positive for the sopE gene whilst F98 was PCR negative. Sequence
analysis of our 12 PCR products confirmed that the primers amplified the desired target genes
and demonstrated that they shared a high degree of similarity to the published gene sequences
for S. Typhimurium SL1344 and, that the products were of the expected length. The reference
strain SL1344 was the only strain to carry sopE. Most of the isolates carried the 11 virulence
genes. The genes misL, orfL, pipD, prgH, sitC, spiC and sopB were identified in every isolate.
In addition to the sopE gene, six isolates did not have one of the following genes; invA, iroN,
pefA and sifA (Figure 2) and of these isolates, four originated from sparrows and two from
humans.
8 Characterisation of Salmonella
Ministry of Agriculture and Forestry
The pulsed field profiles for all 90 isolates using both XbaI and SpeI restriction enzymes were analysed using Bionumerics. Visual inspection of the PFGE gels revealed at least five different SpeI and six different XbaI patterns, but there was evidence of a single common two-enzyme profile that accounted for the majority of isolates. Cluster analysis, using a cut-off value of 95% similarity based on the Dice index, identified five SpeI and five XbaI clusters. When combined with the virulence gene PCR assay there were a total of 11 different genotype profiles. However, genotype profile AA1 accounted for 78/90 (86.7%) of isolates. The second most common was genotype profile AA2 which accounted for 3/90 (3.3%). These isolates were PCR negative for sifA and comprised two sparrow isolates and one human isolate. The remaining nine profiles were represented by single isolates (Figure 2).
Ministry of Agriculture and Forestry
Characterisation of Salmonella 9
5. Discussion
Ninety isolates were compared in an attempt to identify possible clonal relatedness between
isolates obtained from avian species, from environments associated with poultry, and from
humans. The relatedness was determined through a combination of techniques including
virulotyping, PFGE patterns and antibiotic susceptibility.
Proper characterisation of Salmonella isolates is essential for investigations of Salmonella
outbreaks as non-typhoidal Salmonella strains have reached epidemic proportions in many
countries despite improvements in sanitation and hygiene. Precise strain identification is a
prerequisite for implementing efficient surveillance and preventative measures aimed to
reduce the spread and eradicate the source of infection (Threlfall & Frost 1990).
While four Salmonella isolates tested negative for either for poly-H or poly-O sera from
SIFIN, all of them were positive to poly-H and poly-O sera from BD. The conventional
serotyping method is based on bacterial surface antigens and has a few limitations, such as
subjective interpretation of the results based on the level of experience of the individual
performing the test and quality of the antisera. The ambiguity of the results could also be due
to the different preparations of the antisera. The polyclonal anti-Salmonella rabbit sera from
BD may have broader antigen coverage than the mouse serum from SIFIN which is a mixture
of monoclonal antibodies. Also, some Salmonella isolates may lack the antigen specificity
needed for this product resulting in a negative reaction. In addition, the nature of the
antibodies might have been affected by the different animal species in which the antibodies
were generated.
Results from the antimicrobial tests provided no indication of multidrug resistance among the
DT160 isolates tested. However, a total of 34 DT160 isolates characterised in this study fell
within the intermediate range of susceptibility to either tetracycline or oxytetracycline. This
result is slightly different to the one reported in 2003 for the same definitive type in NZ
(Thornley et al. 2003) where the isolates were found to be susceptible to all antimicrobials
tested, including tetracycline. This may be a concern since resistance towards the tetracycline
group has already been reported in Taiwan for both human and animal isolates (Tsen et al.
2002). Due to the rapid development of multi-drug resistance (MDR) among Salmonella
strains, it is possible that DT160 may acquire resistance to a number of antibiotics used
frequently to treat gastrointestinal infections both in humans and in farm animals. The
reported rates of drug-resistant Salmonella, including MDR strains, have increased
considerably. There is a considerable concern that once MDR develops in some of the
variants the resistance genes are retained in the population even when antimicrobial drugs are
withdrawn (WHO 2005). The use of chloramphenicol as an antibiotic growth promoter was
banned in the US and European Union partly because of its overuse in animal production and
the likely contribution to increased resistance in Salmonella Typhi (Hughes & Heritage 2004).
To establish infection, Salmonella spp. may require up to 200 pathogenic genes (Bowe et al.
1998). A panel of 12 potential virulence genes were targeted for PCR-based virulotyping. One
human and two sparrow isolates were PCR negative for the sifA gene that is responsible for
invasion and the survival of the Salmonella in macrophages (Brumell et al. 2001; Galan et al.
1992). However, the apparent absence (or sequence divergence) of either invA or sifA genes
does not necessarily mean that Salmonella would be unable to either invade or survive
respectively, as other genes may also encode for these functions. The iroN gene is associated
with iron acquisition and, while present in all phylogenetic lineages of S. enterica, it is absent
in other closely related species like S. bongori and E. coli (Baumler et al. 1998). Interestingly
in this study, only one isolate was PCR negative for the pefA gene. This plasmid-encoded
gene is essential for Salmonella attachment to intestinal epithelial surfaces (Baumler et al.
10 Characterisation of Salmonella
Ministry of Agriculture and Forestry
1997). The absence of targeted genes has to be interpreted with caution as a nucleotide mismatch between the primers and the target genomic sequence may lead to a false negative result. The sopE gene coding for translocator effector protein can be present in Salmonella isolates related to clinical disease, particularly in humans (Hopkins & Threlfall 2004). This gene, however, appeared to be absent from all tested isolates except the control SL1344 strain. PFGE sub-typing can be used to trace the clonal relationship between isolates. Interpretation of PFGE data can be highly subjective so, to minimise potential inter-laboratory variation, certification from the PulseNet Aotearoa NZ, which is recognised by the CDC, USA, was obtained. The enzyme initially selected for PFGE was XbaI as this infrequently-cutting enzyme produces well defined and reliable restriction DNA banding patterns of the Salmonella genomes. Due to the expected high genetic similarity among the isolates, a second restriction digestion was performed with SpeI enzyme to increase the overall discriminatory power of PFGE (Ribot et al. 2006). Analyses of the isolates digested with either XbaI or SpeI enzymes revealed five SpeI and five XbaI clusters that, when combined with the virulence gene PCR assays, resulted in 11 different genotype profiles. However, as 86.7% of isolates grouped to the AA1 genotype profile, it suggests that the majority of isolates were genetically very similar and that this has remained relatively stable over the time period sampled. Our findings are consistent with a recent report (Dyet et al.2010) using multilocus variable-number tandem-repeat analysis (MLVA). There was no obvious correlation between genotype profile, virulotype, source or date of isolation (Figure2), although most of the uncommon genotypes were found in human and wild bird sources.
Ministry of Agriculture and Forestry
Characterisation of Salmonella 11
6. Conclusion
Our data suggest that there are no indications for multi-drug resistance among the 90 DT160
isolates examined in this study. PFGE profiles, virulence gene PCR assay results and
sensitivity to the various antimicrobials tested indicate the majority of the Salmonella DT160
isolates were very closely related and that isolates grouping in the genotype profile AA1 were
predominant (87% of isolates), representing a dominant epidemiologically-important clone
that has persisted in multiple hosts, including avian wildlife and poultry. The small proportion
of isolates that were distinguishable from genotype AA1 each occurred at low prevalence and
in multiple hosts, indicating that genomic variants arose in the population, but this did not
result in the evolution and emergence of a new dominant, host-associated clone. No obvious
correlation was found between the source and the date of isolation and any of the other
characteristics examined. Finally, the overall genetic similarity between the majority of
isolates from avian, human and poultry environments in NZ in the 1999 and 2009 time period
suggests that either the same or, very closely related isolates, circulated freely between a
range of animal species.
7. Acknowledgements
We would like to thank Dr Phil Carter and Dr Muriel Dufour from ESR Ltd for supplying the
isolates used in this study. We thank Sharina Omar for her contribution to report writing as
well as laboratory work and Errol Kwan, Lynn Rogers, Rebecca Pattison and Rukhshana
Akhter for their excellent technical assistance, and for Dr Eve Pleydell for technical advice
and proof reading. This work was supported by grant 11406 from the Ministry of Agriculture
and Forestry, Biosecurity, NZ.
12 Characterisation of Salmonella
Ministry of Agriculture and Forestry
8. References
Alley MR, Connolly JH, Fenwick SG, Mackereth GF, Leyland MJ, Rogers LE, Haycock
M, Nicol C, Reed CEM. An epidemic of Salmonellosis caused by Salmonella Typhimurium
DT160 in wild birds and humans in New Zealand. New Zealand Veterinary Journal 50, 170-6,
2002
Baumler A, Norris T, Lasco T, Voigt W, Reissbrodt R, Rabsch W, Heffron F. IroN, a
novel outer membrane siderophore receptor characteristic of Salmonella enterica. Journal of
Bacteriology 180, 1446, 1998
Baumler AJ, Tsolis RM, Heffron F. Fimbrial adhesins of Salmonella typhimurium. Role in
bacterial interactions with epithelial cells. Advances in Experimental Medicine and Biology
412, 149-58, 1997
Bowe F, Lipps CJ, Tsolis R, Groisman E, Heffron F, Kusters JG. At least four percent of
the Salmonella typhimurium genome is required for fatal infection of mice. Infection and
Immunity 66, 856-61, 1998
Brumell JH, Rosenberger CM, Gotto GT, Marcus SL, Finlay BB. SifA permits survival
and replication of Salmonella typhimurium in murine macrophages. Cellular Microbiology.
3(2), 75-4, 2001
Callaghan M, Simmons G. Outbreak of Salmonella Typhimurium type 160 in Auckland
linked to an umu function. New Zealand Public Health Report 8, 44-45, 2001
Centers for Disease Control and Prevention. PulseNetUSA. The national molecular
subtyping network for foodborne disease surveillance. In: One-day (24-28 hour) standardized
laboratory protocol for molecular subtyping of Escherichia coli O157:H7, Salmonella
serotypes, and Shigella sonnei by Pulsed-field Gel Electrophoresis (PFGE), 2009
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial
Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard
3rd edition. M31-A3 2008
Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial
Susceptibility Testing; Twentieth Information Supplement. M31-S20, 2010
Connolly JH, Alley MR, Dutton GJ, Rogers LE. Infectivity and persistence of an outbreak
strain of Salmonella enterica serotype Typhimurium DT160 for house sparrows (Passer
domesticus) in New Zealand. New Zealand Veterinary Journal 54, 329-32, 2006
Dyet KH, Turbitt E, Carter PE. Multiple-locus variable-number tandem-repeat analysis for
discriminating within Salmonella enterica serovar Typhimurium definitive types and
investigation of outbreaks. Epidemiology and Infection 2010, epub ahead of print.
Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions, 3rd edition. Wiley,
2003
Fisher IS. Dramatic shift in the epidemiology of Salmonella enterica serotype Enteritidis
phage types in western Europe, 1998-2003 results from the Enter-net international Salmonella
database. Euro Surveillance 9(11):43-5, 2004
Ministry of Agriculture and Forestry
Characterisation of Salmonella 13
Galan J, Ginocchio C, Costeas P. Molecular and functional characterization of the
Salmonella invasion gene invA: homology of InvA to members of a new protein family.
Journal of Bacteriology 174, 4338, 1992
Gassama-Sow A, Wane AA, Canu NA, Uzzau S, Aidara-Kane A, Rubino S.
Characterization of virulence factors in the newly described Salmonella enterica serotype
Keurmassar emerging in Senegal (sub-Saharan Africa). Epidemiology and Infection 134:741–
743, 2006
Gilbert S, Lake R, Cressey P, Hudson A, King N. Risk Profile: salmonella (Non Typoidal)
in Pork and Pork Products, Institute of Environmental Science and Research Limited. 2010
Grimont PAD, Weill FX. Antigenic Formulae of the Salmonella serovars 9th edition. WHO
Collaborating Centre for Reference and Research on Salmonella Institut Pasteur & World
Health Organization, 2007
Helms M, Vastrup P, Gerner-Smidt P, Molbak K. Excess mortality associated with
antimicrobial drug-resistant Salmonella Typhimurium. Emerging Infectious Diseases 8, 490-5,
2002
Hopkins KL, Threlfall EJ. Frequency and polymorphism of sopE in isolates of Salmonella
enterica belonging to the ten most prevalent serotypes in England and Wales. Journal of
Medical Microbiology 53, 539-43, 2004
Hughes LA, Shopland S, Wigley P, Bradon H, Leatherbarrow AH, Williams NJ, Bennett
M, De Pinna E, Lawson B, Cunningham AA, Chantrey J. Characterisation of Salmonella
enterica serotype Typhimurium isolates from wild birds in northern England from 2005-2006.
BMC Veterinary Research 4, 4-13, 2008
Hughes P, Heritage J. Antibiotic growth-promoters in food animals. Assessing quality and
safety of animal feeds; FAO Animal Production and Health Paper (FAO), No. 160, Italy,
Animal Production and Health Division 129-152, 2004
Institute of Environmental Science and Research Limited. Notifiable and other diseases in
New Zealand 2009 annual surveillance report. Population and Environmental Health Group,
2010
Kingsley RA, Bäumler AJ. Host adaptation and the emergence of infectious disease: the
Salmonella paradigm. Molecular Microbiology 36 (5),1006-4, 2000
Lim E, Tisch C, Cressey P, Pirie R. Annual report concerning food-borne disease in New
Zealand 2009. Prepared by the Institiute of Environmental Science and Research Ltd for the
New Zealand Food Safety Authority under project MRP/09/04 – Systematic eporting of
epidemiology of potentially foodborne disease in New Zealand for year 2009. 2010.
Luderitz O, Staub AM, Westphal O. Immunochemistry of O and R antigens of Salmonella
and related Enterobacteriaceae. Bacteriological Reviews 30, 193-258, 1966
Luk JMC, Lindberg AA. Anti-Salmonella lipopolysaccharide monoclonal antibodies:
Characterization of Salmonella BO-, CO-, DO-, and EO-specific clones and their diagnostic
usefulness. Journal of Clinical Microbiology 29, 2424-33, 1991
14 Characterisation of Salmonella
Ministry of Agriculture and Forestry
Mullner P, Jones G, Noble A, Spencer SE, Hathaway S, French NP. Source attribution of
food-borne zoonoses in New Zealand: a modified Hald model. Risk Analysis. 29(7), 970-84,
2009
Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid fever. The New England
Journal of Medicine 347(22), 1770-2, 2002
Penfold JB, Amery HCC, Peet PJM. Gastroenteritis associated with wild birds in a hospital
kitchen. British Medical Journal 2, 802-3, 1979
Phillips I, M. C, Cox T, De Groot B, Friis C, Jones R, Nightingale C, Preston R, Waddell
J. Does the use of antibiotics in food animals pose a risk to human health? A critical review of
published data. Journal of Antimicrobial Chemotheraphy 53, 28-52, 2004
Poppe C, Smart N, Khakhria R, Johnson W, Spika J, Prescott J. Salmonella typhimurium
DT104: A virulent and drug-resistant pathogen. Canadian Veterinary Journal 39, 559-5, 1998
Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, Barrett TJ.
Standardization of Pulsed-Field Gel Electrophoresis Protocols for the Subtyping of
Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathogens and
Disease 3(1), 59-7. 2006
Rowe B, Ward LR, Threlfall EJ. Multidrug-resistant Salmonella typhi: A worldwide
epidemic. Clinical Infectious Diseases 24, S106-S9, 1997
Skyberg JA, Logue CM, Nolan LK. Virulence Genotyping of Salmonella spp. with
Multiplex PCR. Avian Diseases 50, (1), 77-81, 2006
Thornley CN, Simmons GC, Callaghan ML, Nicol CM, Baker MG, Garrett KG. First
incursion of Salmonella enterica serotype Typhimurium DT160 into New Zealand. Emerging
Infectious Diseases 9, 493-5, 2003
Threlfall EJ, Frost JA. The identification, typing and fingerprinting of Salmonella:
Laboratory aspects and epidemiological applications. Journal of Applied Bacteriology 68, 5-
16, 1990
Tizzard IR, Fish NA, Harmenson J. Free-flying sparrows as carriers of salmonellosis.
Canadian Veterinary Journal 20, 143-4, 1979
Todd EC, Greig JD, Bartleson CA, Michaels BS. Outbreaks where food workers have been
implicated in the spread of foodborne disease. Part 4. Infective doses and pathogen carriage.
Journal of Food Protection 71(11), 2339-73, 2008
Tsen H-Y, Lin J-S, Hsih H-Y. Pulsed field gel electrophoresis for animal Salmonella
enterica serovar Typhimurium isolates in Taiwan. Veterinary Microbiology 87, 73-80, 2002
Walsh PS, Metzger DA, Higuchi R. Chelex 100 as a medium for simple extraction of DNA
for PCR-based typing from forensic material. Biotechniques 10 (4), 506-13, 1991
Ministry of Agriculture and Forestry
Characterisation of Salmonella 15
Weir EK, Martin LC, Poppe C, Coombes BK, Boerlin P. Subinhibitory concentrations of
tetracycline affect virulence gene expression in a multi-resistant Salmonella enterica subsp.
enterica serovar Typhimurium DT104. Microbes and Infection 10, 901-7, 2008
Wilson N, M Baker. A systematic review of the aetiology of salmonellosis in New Zealand.
Accessed from the New Zealand Fo
Wong TL, Nicol C, Cook R, MacDiarmid S. Salmonella in Uncooked Retail Meats in New
Zealand. Journal of Food Protection 70 (6) 1360-65, 2007
World Health Organisation. Antimicrobial resistance. Geneva, Fact sheet N 194, 2002
World Health Organisation. Drug-resistant Salmonella. Food safety Department Geneva,
Fact sheet N 139, 2005
16 Characterisation of Salmonella
Ministry of Agriculture and Forestry
9. Appendices
Table 1. Virulence genes with the expected PCR product sizes used to virulotype Salmonella
isolates.
Table 2. Results of the four isolates tested negative for either poly-H or poly-O sera from
SIFIN and further evaluated with sera from BD, TSI and motility tests.
Table 3. Results from the assessment of antibiotic susceptibility of the Salmonella isolates.
Table 4. Zone diameter interpretive standard and our quality control ranges of antimicrobial
disc susceptibility test for the reference strain Escherichia coli ATCC25922 on Mueller
Hinton Agar.
Table 5. Summary of the 26 isolates that were within the intermediate range for sensitivity to
tetracycline and 8 isolates within the same range for sensitivity to oxytetracycline.
Table 6. Twenty five percent randomly selected Salmonella isolates were retested for
antibiotic sensitivity against all antibiotics used in the first experiment. Measurements were
taken from the same two technicians independently from each other.
Figure 1. Virulotyping and representative gels for the twelve virulent genes and the two
Salmonella control strains SL1344 and F989 used in this study.
Figure 2. Genotypes of Salmonella DT160 based on two-enzyme PFGE and virulence gene
PCR.
Ministry of Agriculture and Forestry
Characterisation of Salmonella 17
Table 1. Virulence genes with the expected PCR product sizes used to virulotype
Salmonella isolates.
Gene Salmonella Gene
Primers (5' to 3')
Predicted Reference
Pathogenicity function
Island (SPI)
size (bp)
F: CTGGCGGTGGGTTTTGTTGTCTTCTCTATT 1070
R: AGTTTCTCCCCCTCTTCATGCGTTACCC
R: CGGGGCGAAAATAAAGGCTGTGATGAAC
prgH SPI-1 Type
F: GCCCGAGCAGCCTGAGAAGTTAGAAA
R: TGAAATGAGCGCCCCTTGAGCCAGTC
sopE SPI-1 Type
F:TCCAAAAACAGGAAACCACAC
R:TCAGTTGGAATTGCTGTGGA
F:GTCGGCGAATGCCGCGAATA
macrophages R:GCGCTGTTAACGCTAATAGT
N.B.: PCR product size is approximate and based on SL1344 genome; may vary between strains and forward and reverse primer designation is based on orientation within the SL1344 published genome.
18 Characterisation of Salmonella
Ministry of Agriculture and Forestry
Table 1. (cont.) Virulence genes with the expected PCR product sizes used to virulotype
Salmonella isolates.
Gene Salmonella
Primers (5' to 3')
Predicted Reference
function
Island (SPI)
size (bp)
F:CGGCGATTCATGACTTTGAT
R:CGTTATCATTCGGATCGTAA
et al. 2006
associated with SPI-1
F:GGAGTATCGATAAAGATGTT
R:GCGCGTAACGTCAGAATCAA
et al. 2006
F:CCTGGATAATGACTATTGAT
R:AGTTTATGGTGATTGCGTAT
et al. 2006
R: TAGTGATGCCCGTTATGCGTGAGTGTATT
F:GCGCCGCTCAGCCGAACCAG
N.B.: PCR product size is approximate and based on SL1344 genome; may vary between strains and forward and reverse primer designation is based on orientation within the SL1344 published genome.
Ministry of Agriculture and Forestry
Characterisation of Salmonella 19
Table 2. Results of the four isolates tested negative for either poly-H or poly-O sera from SIFIN
and further evaluated with sera from BD, TSI and motility tests.
Internal
Serotyping
API20E test
Motility
laboratory
*4305542=SSP **R/Y/H2S+
White eye
Sparrow N
*4305542=SSP is an API20E analytical profile index
** R, Alkaline, Y, Acid, H2S hydrogen sulphide production
NM, Non-motile
P, positive; N, negative
TSI, Triple Sugar Iron agar
20 Characterisation of Salmonella
Ministry of Agriculture and Forestry
Table 3. Results from the assessment of antibiotic susceptibility of the Salmonella isolates.
Internal lab.
Antibiotics
AK AMC AMP C FOX CIP CPD NA OT SXT TET
Ministry of Agriculture and Forestry
Characterisation of Salmonella 21
Table 3. (cont.) Results from the assessment of antibiotic susceptibility of the Salmonella
isolates.
Internal lab.
Antibiotics
AK AMC AMP C FOX CIP CPD NA OT SXT TET
22 Characterisation of Salmonella
Ministry of Agriculture and Forestry
Table 3. (cont.) Results from the assessment of antibiotic susceptibility of the Salmonella
isolates.
Internal lab.
Antibiotics
AK AMC AMP C FOX CIP CPD NA OT SXT TET
E. coli**
23-25 20-23 16-21 23-25 26-28 33-39 25-28 23-27 22-25 24-29 20-23
AK, Amikacin; AMC, Amoxicillin-clavulanic acid; AMP, Ampicillin; C, Chloramphenicol; FOX, Cefoxitin; CIP, Ciprofloxacin; CPD, Cefpodoxime; OT, Oxytetracycline; NA, Nalidixin acid; SXT, Trimethoprim-sulfamethazole; TET, Tetracycline *zone diameter in millimetres **E. coli = ATCC 25922
Ministry of Agriculture and Forestry
Characterisation of Salmonella 23
Table 4. Zone diameter interpretive standard for Enterobacteriaceae and our quality control
ranges of antimicrobial disc susceptibility test for the reference strain E. coli ATCC25922 on
Mueller Hinton Agar.
Antimicrobial agent
E. coli
content (µg)
Zone diameter (mm)
Amoxicillin-clavulanic acid, AMC
Cefoxitin, FOX**
Cefpodoxime, CPD
Chloramphenicol, C
Ciprofloxacin, CIP**
Nalidixic acid, NA**
Trimethoprim-sulfamethoxazole, SXT
S, susceptible; I, Intermediate; R, resistant *CLSI M31-A3; **CLSI M100-S20 µg, microgram; mm, millimetres na, not available
24 Characterisation of Salmonella
Ministry of Agriculture and Forestry
Table 5. Summary of the 26 isolates that were within the intermediate range for sensitivity to
tetracycline and 8 isolates within the same range for sensitivity to oxytetracycline.
Internal
Internal
Internal
laboratory
laboratory
laboratory
*zone in millimetres
**CLSI M31- A3
OT, Oxytetracycline disc content 30µg; TET, Tetracycline disc content 30µg.
Ministry of Agriculture and Forestry
Characterisation of Salmonella 25
Table 6. Twenty five percent randomly selected Salmonella isolates were retested for antibiotic
sensitivity against all antibiotics used in the original panel of antimicrobials. Measurements were
taken from the same two technicians independently from each other.
Internal Antibiotics
laboratory
*1, Technician; **2, Technician; ***millimetres
AK, Amikacin; AMC, Amoxicillin-clavulanic acid; AMP, Ampicillin; C, Chloramphenicol; FOX,
Cefoxitin; CIP, Ciprofloxacin; CPD, Cefpodoxime; OT, Oxytetracycline; NA, Nalidixin acid; SXT,
Trimethoprim-sulfamethazole.
26 Characterisation of Salmonella
Ministry of Agriculture and Forestry
100 1 2 3 4 5 6 7 8 9 10
Salmonella enterica Typhimurium F98
1 2 3 4 5 6 7 8 9 10 11 12 13 14
Salmonella enterica
Typhimurium SL1344
Lane Gene
Results (-/
Lane Gene Size Results
F98 SL1344
marker + +
sifA 449 + +
misL 561 + +
sopE 642 - +
sopB 220 + +
prgH 756 + +
sitC 768 + +
invA 1070 + +
iroN 1205 + +
Figure 1. Virulotyping and representative gels for the 12 virulence genes and the 2 Salmonella
control strains SL1344 and F989 used in this study.
Ministry of Agriculture and Forestry
Characterisation of Salmonella 27
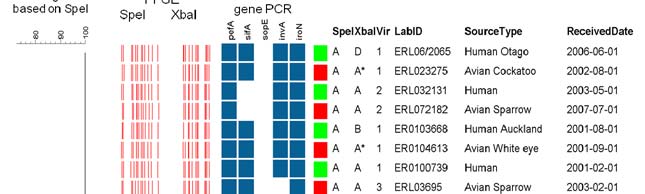

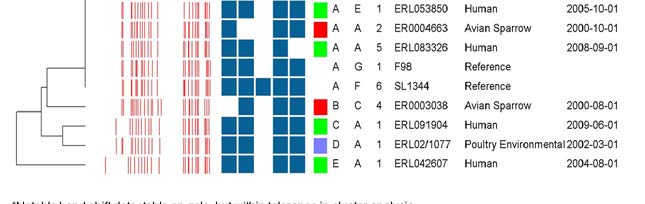
Figure 2. Genotypes of Salmonella DT160 based on two-enzyme PFGE and virulence gene PCR.
The UPGMA dendrogram was based on the SpeI band pattern, using a Dice similarity coefficient
calculated using 1.5% optimisation and 2% band filtering tolerance. Combined profile AA1 was
the most common, accounting for 78/90 (87%) of isolates (only four representatives of this
profile are provided in the diagram).
28 Characterisation of Salmonella
Ministry of Agriculture and Forestry
10. Supplementary Information
Table 7. Key table showing corresponding Environmental Science and Research (ESR) isolate
number from the ERL, Enteric Reference Laboratory (ERL) to our internal laboratory identifier
number (DT).
Internal
ESR isolate
Received date
Source type
laboratory
Poultry Environmental
Poultry Environmental
Poultry Environmental
Poultry Environmental
Poultry Environmental
Poultry Environmental
Poultry Environmental
Poultry Environmental
Poultry Environmental
Poultry Environmental
Poultry Environmental
Poultry Environmental
Poultry Environmental
Ministry of Agriculture and Forestry
Characterisation of Salmonella 29
Table 7. (cont.) Key table showing corresponding Environmental Science and Research (ESR)
isolate number from the ERL, Enteric Reference Laboratory (ERL) to our internal laboratory
identifier number (DT).
Internal
ESR isolate
Received date
Source type
laboratory
Poultry Environmental
Poultry Environmental
Poultry Environmental
Poultry Environmental
Poulty Environmental
Poultry Environmental
Poultry Environmental
Poultry Environmental
Poultry Environmental
30 Characterisation of Salmonella
Ministry of Agriculture and Forestry
Table 7. (cont.) Key table showing corresponding Environmental Science and Research (ESR)
isolate number from the ERL, Enteric Reference Laboratory (ERL) to our internal laboratory
identifier number (DT).
Internal
ESR isolate
Received date
Source type
laboratory
Poultry Environmental
Poultry Environmental
Poultry Environmental
Poultry Environmental
Poultry Environmental
Poultry Environmental
Poultry Environmental
Poultry Environmental
Note: There is some inconsistency in the numbering format in the ESR database. Some numbers have a forward slash separating the year from the isolate number for that year, others have a varying number of zeros before the allocated isolate number. The reference numbers should therefore be interpreted as the ER or ERL prefix followed by two digits representing the year of isolation, followed by the isolate number for that year.
Ministry of Agriculture and Forestry
Characterisation of Salmonella 31
Source: http://www.biosecurity.govt.nz/files/publications/technical-papers/characterisation-of-salmonella.pdf
Capítulo 8 Intoxicación por paracetamol C. Luaces i Cubells, A. Noguera Julian El paracetamol (acetaminofén) es el analgésico-antipirético más uti- lizado en el mundo. Su fácil accesibilidad y su presencia en la mayoríade hogares, lo convierten también en la primera causa de intoxicaciónmedicamentosa (accidental y voluntaria) y de insuficiencia hepáticaaguda. Datos recientes aportados por el Grupo de Trabajo de Intoxica-ciones de la SEUP, lo sitúan como la primera causa de intoxicación far-macológica en menores de 5 años, sobre todo por ingesta de prepara-ciones líquidas sin tapones de seguridad. Así, y según comunica dichoGrupo de Trabajo, la ingesta accidental de paracetamol resultó ser el 16%del total de intoxicaciones, el 25% de las medicamentosas y el 88.5% delas intoxicaciones por antitérmicos entre un grupo de 1700 pacientes aten-didos en 18 Hospitales desde Enero de 2001 hasta Diciembre de 2002. Enuna revisión efectuada por el Servicio de Información Toxicológica entreenero de 1998 y diciembre de 2000, de 13.044 intoxicaciones registradas,el 11% estuvieron causadas por paracetamol.
NEUROBIOLOGY, COGNITIVE AND EMOTION ALTERATIONS NEUROBIOLÓGICAS, AND PREDICTORS OF RESPONSE PHARMACOLOGICAL EMOCIONALES E ÍNDICES TREATMENT IN MAJOR DEPRESSION DISORDER PREDICTIVOS DE LARESPUESTA AL GABRIELA CASTILLO-PARRA Universidad Camilo José Cela FEGGY OSTROSKY-SOLÍS Universidad Nacional Autónoma de México FARMACOLÓGICO EN EL HUMBERTO NICOLINI




