Celentyx.co.uk
Invest New DrugsDOI 10.1007/s10637-011-9730-5
PRECLINICAL STUDIES
Enhancing the anti-lymphoma potential of 3,4-methylenedioxymethamphetamine (‘ecstasy')through iterative chemical redesign: mechanismsand pathways to cell death
Agata M. Wasik & Michael N. Gandy & Matthew McIldowie & Michelle J. Holder &Anita Chamba & Anita Challa & Katie D. Lewis & Stephen P. Young &Dagmar Scheel-Toellner & Martin J. Dyer & Nicholas M. Barnes & Matthew J. Piggott &John Gordon
Received: 8 June 2011 / Accepted: 1 August 2011
# Springer Science+Business Media, LLC 2011
Summary While 3,4-methylenedioxymethamphetamine
‘best' compounds (containing 1- and 2-naphthyl and para-
(MDMA/‘ecstasy') is cytostatic towards lymphoma cells
biphenyl substituents) some 100-fold more potent than
in vitro, the concentrations required militate against its
MDMA versus the BL target. When assessed against
translation directly to a therapeutic in vivo. The possibility
derived lines from a diversity of B-cell tumors MDMA
of ‘redesigning the designer drug', separating desired anti-
analogues were seen to impact the broad spectrum of
lymphoma activity from unwanted psychoactivity and
malignancy. Expressing a BCL2 transgene in BL cells
neurotoxicity, was therefore mooted. From an initial
afforded only scant protection against the analogues and
analysis of MDMA analogues synthesized with a modified
across the malignancies no significant correlation between
α-substituent, it was found that incorporating a phenyl
constitutive Bcl-2 levels and sensitivity to compounds was
group increased potency against sensitive, Bcl-2-deplete,
observed. Bcl-2-deplete cells displayed hallmarks of apo-
Burkitt's lymphoma (BL) cells 10-fold relative to MDMA.
ptotic death in response to the analogues while BCL2
From this lead, related analogs were synthesized with the
overexpressing equivalents died in a caspase-3-independentmanner. Despite lymphoma cells expressing monoamine
Matthew J. Piggott, Nicholas M. Barnes, and John Gordon are joint
transporters, their pharmacological blockade failed to
reverse the anti-lymphoma actions of the analogues studied.
A. M. Wasik : M. J. Holder : A. Chamba : A. Challa :
Neither did reactive oxygen species account for ensuing
S. P. Young : D. Scheel-Toellner : J. Gordon (*)
cell death. Enhanced cytotoxic performance did however
School of Immunity & Infection, The Medical School,
track with predicted lipophilicity amongst the designed
Birmingham, University of Birmingham,
compounds. In conclusion, MDMA analogues have been
Edgbaston,Birmingham B15 2TT, UK
discovered with enhanced cytotoxic efficacy against lym-
phoma subtypes amongst which high-level Bcl-2—often abarrier to drug performance for this indication—fails to
M. N. Gandy : M. McIldowie : K. D. Lewis : M. J. Piggott
School of Biomedical, Biomolecular and Chemical Sciences,The University of Western Australia,Crawley, Australia
Keywords Apoptosis . Bcl-2 . Cytotoxicity. Lymphoma .
MDMA
M. J. DyerMedical Research Council Toxicology Unit,Leicester, UK
Activated B-Cell-like
Burkitt's lymphoma
Cellular and Molecular Neuropharmacology Research Group,
Dopamine transporter
Clinical and Experimental Medicine, The Medical School,Birmingham, UK
Diffuse large B-cell lymphoma
Epstein-Barr virus
background the impact of its dysregulated, high level
Follicular lymphoma
expression on the efficacy of promising new drug
Germinal B-Cell-like
candidates. Within this context we have been investigating
compounds which target components of neurotransmitter
Non-Hodgkin lymphomas
pathways that can be found in immune cells and their
Poly (ADP-ribose) polymerase
cancers: most notably the transporters for serotonin and
dopamine (SERT and DAT, respectively), each expressed in
Post-transplant lymphoproliferative disease
a broad range of the NHL subtypes and other B-cell
Serotonin transporter
Amongst such compounds, the amphetamine derivatives
fenfluramine and 3,4-methylenedioxymethamphetamine(MDMA, ‘Ecstasy') were found to be anti-proliferative
against B-cell lines of diverse malignant B-cell origin. Itwas shown (at least with fenfluramine) that in Bcl-2-deplete
The incidence of B-cell lymphomas, constituting around
BL cells, growth arrest was accompanied by apoptotic cell
95% of all the non-Hodgkin lymphomas (NHL), is
death following activation of caspase-3: these latter features
increasing steadily year-on-year. NHL is a heterogeneous
being reversed on introducing BCL2 as a transgene
group of neoplasia ranging from indolent examples like
Unfortunately the concentrations of the amphetamine
slow growing follicular lymphoma (FL) to highly aggres-
derivatives required to elicit anti-proliferative/pro-apoptotic
sive, rapidly proliferating entities exemplified by diffuse
activity in vitro were too high for safe translation to a
large B-cell lymphoma (DLBCL) - the most common of the
cancer therapeutic in vivo. Therefore we mooted for
NHL in Europe, Australasia and the US—and Burkitt's
MDMA the potential of "redesigning the designer drug"
lymphoma (BL): rare in the West but endemic in the
to enhance lymphoma killing while reducing neurotoxicity
World's malarial belt. The diversity of tumors reflects a
and psychoactivity.
composite of factors including the differentiation stage of
Research by Shulgin and co-workers suggests that
the target B-cell and the mutations/translocations arising
extending the α- or N-substituent of MDMA to anything
therein. Multiple profiling platforms such as gene array are
larger than an ethyl group abolishes the drug's psycho-
disclosing additional heterogeneity within previously con-
activity. Nash and Nichols, studying acute effects in rats,
sidered single clinical entities which can be manifested
showed that a simple substitution of the methyl group at the
molecularly, cellularly and prognostically. DLBCL for
α-C of MDMA with an ethyl substituent, creating MBDB,
example is now considered a composite of disease subtypes
significantly diminishes the amount of dopamine released
comprising primarily ‘Activated B-Cell-like' (ABC) cases
in the striatum . The α-substituent was therefore
and those that are ‘Germinal B-Cell-like' (GCB): survival
deemed a rational plinth for redesign. We now describe
rates among the former being substantially worse than the
improved cytotoxic performance of MDMA analogues with
latter. Moreover, within ABC DLBCL constitutive expres-
modified α-substituents against a spectrum of B-cell
sion of the pro-survival gene BCL2 further discriminates a
malignancies giving attention to the mechanisms and
substantially inferior subgroup with regards overall survival
pathways to cell death, including the impact of anti-
even in the face of intense therapy.
apoptotic Bcl-2. A companion study details in full the
Anti-apoptotic BCL2, originally identified as the gene
chemistry and synthesis of the analogues while providing
translocating to the IGH locus on chromosome 14 in the
evidence for diminished neurotoxicity and psychoactivity
hallmark t(14;18) of FL, offers a considerable barrier to
of selected compounds, together with a brief description of
drug efficacy in lymphoma treatment. BL, while extremely
their rank potency in targeting a BL cell line ].
aggressive, lacks genetic alterations in BCL2, is deplete inBcl-2 protein and has a high cure rate using combinationchemotherapy. Over the past decade we have adopted BL
Materials and methods
as a template on which to explore novel therapeuticopportunities for lymphoma: BL offering a sensitive
monitor of pro-apoptotic/anti-proliferative activities andat the same time being a tumor that is readily adaptable to
MDMA and analogues with modified α-substituents were
tissue culture with derived lines remaining ‘biopsy-like'
synthesized by reductive amination of the corresponding
when maintained in early passage. Transfection of BCL2
piperonyl ketones as described recently All target
on a constitutive promoter into these cells allows the
amines were converted to their hydrochlorides and were
opportunity to model directly on an otherwise isogenic
tested as such.
2.1 available online . A plot of average log P versuspIC50 showing the SEM in each variable was constructed
Cell lines deriving from different B-cell malignancies and
and a curve was fitted by weighted linear regression using
variants of the L3055 BL cell line were as described
Grafit 4, where the weighting of each point was inversely
previously []. EBV-transformed lymphoblastoid cell lines
proportional to the respective error in pIC50. Due to the
were from the School of Cancer Sciences, University of
uniformity in the magnitude of errors of average log P
Birmingham U.K. All cell lines were cultured in RPMI 1640
across the dataset, these were ignored when the weighted
medium supplemented with 2 mM glutamine, 10% v/v FCS,
curve was fitted.
100 U/ml penicillin, 100 U/ml streptomycin under 5% CO2 at37°C and passaged three times weekly.
Pharmacological interpretation and statistics
Cellular cytotoxicity
Pharmacological interpretation of cytotoxicty assays togenerate the pIC50 and Hill coefficient of a compound's
Cellular cytotoxicity/viability was assessed by staining
activity against L3055 cells was performed using a four
treated cells with propidium iodide (PI, a DNA binding
parameter logistic equation with iterative fitting using
dye incapable of penetrating intact cell membranes, (Sigma
Kaleida Graph []. Regression analysis for cytotoxic
Aldrich, Dorset, UK)) at a final concentration of 0.85 μg/ml
response vs Bcl-2 expression was calculated as a ratio
or 1.15 μg/ml prior to flow cytometric analysis (FACS
between remaining cell viability (assessed as in 2.3 above)
Calibur BD) of PI+ve versus PI-ve cells. Results were
following treatment with MDMA and analogues and the
analysed using FlowJo 8 software for Macintosh.
optical density (computed using ImageJ for Macintosh) ofBcl-2 vs calnexin protein bands as determined by Western
blot. Graphs were created in OriginPro 8 (OriginLab,Northampton, MA).
Apoptosis was assessed by dual staining of cells with PIand PhiPhiLux (Oncoimmunin, Gaithersburg, MD, USA)an indicator of active caspase-3 followed by analysis on
FACS exactly as described previously []. Activation ofcaspase-3 was additionally assessed by staining cells with a
Substitutions at the α-carbon in MDMA can augment
rabbit antibody specific for the active form of caspase-3
cytotoxic performance against L3055 Burkitt's lymphoma
(BD Pharmigen, Oxford, UK), followed by FACS analysis;
non-immune rabbit IgG (control) was from Sigma Aldrich.
Cells were pre-treated using the FIX and PERM kit for
The first generation of α-substituted MDMA analogues
intracellular staining (Caltag, Invitrogen, Paisley, UK)
synthesized contain either novel alkyl/cycloalkyl groups
according to the manufacturer's instructions. Cleavage of
(compounds 1–5) or, in the case of compound 6, a phenyl
poly(ADP-ribose) polymerase-1 (PARP-1) as determined by
substituent. When assessed for anti-lymphoma potential
Western blot and mitochondrial membrane permeability as
against L3055, a prototype early-passage BL cell line,
assessed by JC-1 staining were performed as detailed
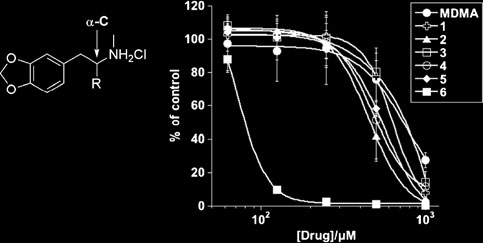
compound 6 was the most potent (approximately 10-fold >
elsewhere Bcl-2 protein content of cells was deter-
MDMA) both in inhibiting 3H-thymidine incorporation
mined by Western blot as described previously [].
(data not shown) and in its cytotoxic efficacy (Fig. ):pIC50=4.12±0.03 versus pIC50=3.39±0.09 for MDMA.
Treatment with antioxidants
Compound 6 therefore formed the template on which todesign the next generation of compounds in the quest for a
Cells were pre-treated with catalase (Sigma Aldrich, Dorset,
lymphoma therapeutic based on MDMA.
UK) for 1 h or PEG-catalase (Sigma Aldrich, Dorset, UK)for 1.5 h before seeding cells onto 96-well plates containing
Larger aromatic α-substituents enhance cytotoxic potential
MDMA/MDMA analogue. Cells at final density at 105/ml
towards L3055 cells
were incubated with drug for 24 h and cell viability wasassessed by PI uptake analysed by flow cytometry.
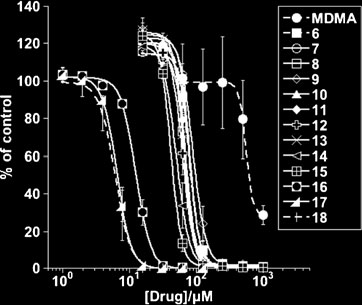
The second generation of α-modified MDMA analogues allcontain aromatic rings (two in the case of compounds 16,
17, 18), apart from compound 7, which possesses acyclohexyl group (Fig. ). The substituents in this series
Estimates of lipophilicity were obtained from the
of MDMA analogues differ from each other with respect to
"average log P" value output by the applet ALOGPs
three-dimensional structure, rigidity, and electron density:
Hill coefficient ± SEM
3.39 ± 0 09
3.89 ± 1 12
3.10 ± 0.02
3.36 ± 0 06
4.56 ± 0 98
3.15 ± 0 01
3.4 ± 0 93
3.42 ± 0.03
4.79 ± 0.77
3.20 ± 0.07
3.40 ± 0.81
4.12 ± 0.03
4.65 ± 0.31
Fig. 1 Cytotoxic efficacy of MDMA and Series 1 (first generation)
b typical concentration-response curves showing cytotoxic perfor-
MDMA analogues versus L3055 Burkitt's lymphoma cells.
mance of MDMA and Series 1 analogues against the L3055 BL cell
a Chemical structure of MDMA with α-carbon (α-C) indicated (and
line. Cells were cultured at 5 x 105/ml with MDMA or indicated
in tabulated right hand panel) MDMA analogues with the first
analogue at concentrations shown for 48 h prior to measuring
iteration of α-C substituents constituting Series 1 compounds 1–6 as
cytotoxicity by PI uptake using flow cytometry. Results are repre-
shown, together with calculated pIC50 ± SEM and Hill coefficients ±
sented as the mean of three independent experiments ± SEM in terms
SEM from response curves as generated in (b) with number of
of the percentage of cells remaining viable with respect to vehicle (no
separate experiments performed with each compound given as ‘n';
the benzene ring possessing all six carbons within one
acceptor and therefore adds to the hydrophobicity of the
plane (sp2-hybridized) by contrast to the cyclohexyl group,
molecule [Metabolic stability is also increased by
where the carbon atoms are sp3-hybridized and therefore
the inclusion of fluorine.
non-planar and conformationally flexible. Compound 8 has
Compounds 16, 17 and 18 have much larger hydrophobic
an α-benzyl group and thus an additional sp3-hybridized
substituents at the α-position of MDMA, and therefore
carbon between the main carbon chain and the aromatic α-
increased lipophilicity. The naphthyl group (compounds 16
substituent. This provides additional flexibility compared to
and 17) is highly rigid as all the carbon atoms are positioned
phenyl substituents, and extends the aromatic ring from the
in one plane, whereas the biphenyl group differs from
main chain, exploring the depth of a putative hydrophobic
compound 6 by the addition of a para-phenyl group and
pocket in the target receptor(s).
therefore both of the benzene rings are able to rotate around
Compounds 9, 10 and 11 are more polar than their parent
the axis of the bond between them.
(6) due to the addition of a methoxy group. The lone pairs
From results presented in Fig. it can be noted that from
of electrons make the methoxy oxygens hydrogen bond
the second generation of MDMA analogues modified at the
acceptors, and also increase the electron density in the
α-carbon, compounds 16–18 were by far the most potent
aromatic ring. Compounds 9–11 differ only in the position
regards cytotoxicity towards L3055 cells; compounds 17
of the methoxy group. Similarly, compounds 13, 14 and 15
and 18 being the most efficacious and equipotent with a
possess ortho-, meta-, and para-methyl groups, respectively,
pIC50=5.18±0.03 and 5.22±0.08; representing a 10-fold
exploring steric tolerance within the binding site(s). The
and 100-fold improvement over compound 6 and MDMA
ortho-substituents in 9 and 13 are also likely to reduce the
respectively. Similar rank potency of these analogues was
range of low energy conformations available to the side
observed when assessed for their capacity to inhibit 3H-
thymidine incorporation into L3055 cells (data not shown).
Compound 12 contains fluorine in the para-position
It should be noted that all compounds tested for
which reduces electron density in the aromatic ring but
concentration-dependent cytotoxicity generated steep
otherwise is very similar to a hydrogen atom (i.e. an
response curves yielding relatively high Hill coefficients
isosteric replacement). Although the fluorine atom has three
(Figs. and ) suggesting deviation from simple mass
lone pairs of electrons, it is a very poor hydrogen bond
interaction ].
Hill coefficient ± SEM
4.10 ± 0.04
6.56 ± 0.43
4.16 ± 0 03
5.92 ± 0 58
4.04 ± 0.04
4.82 ± 0.38
4.10 ± 0.01
6.05 ± 0.16
4.16 ± 0.02
5.60 ± 0.73
4.20 ± 0.02
5.89 ± 1.12
4.20 ± 0.05
6.68 ± 073
4.29 ± 0.05
5.05 ± 0.17
4.37 ± 0.03
5.69 ± 0.27
4.90 ± 0.02
4.04 ± 0.74
5.18 ± 0.03
4.38 ± 0.73
5.22 ± 0.08
5.05 ± 0.97
Fig. 2 Cytotoxic efficacy of Series 2 (second generation) analogues versus L3055 Burkitt's lymphoma cells. As in Fig. but here with Series 2compounds 7–18; MDMA again included for comparison
Cytotoxic efficacy of selected α-substituted MDMA
(primarily detecting active caspase-3) and propidium iodide
analogues towards B-cell lines of different malignant
(plasma membrane permeability) staining, L3055 BL cells
transfected with empty vector showed classic progressionfrom early to late apoptosis over the course of the 6 h
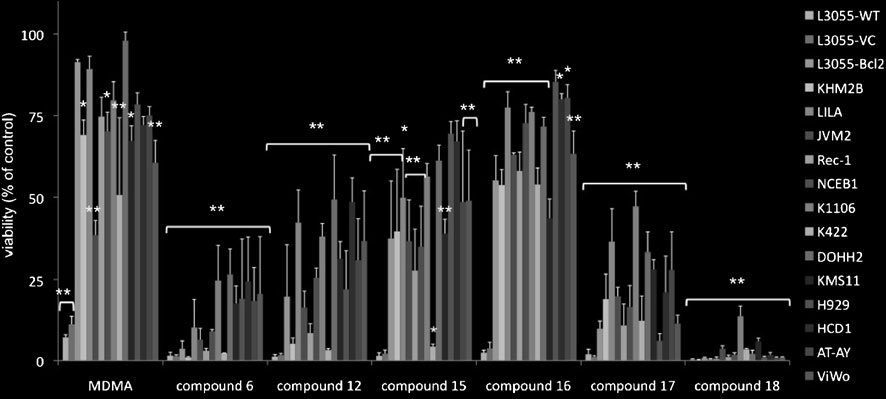
The constituent cells of B-cell lines from a diverse
monitored (Fig. ). While at the fixed concentration of the
range of malignancies were treated with MDMA and six
analogues used L3055-Bcl-2 cells again showed a degree of
of the α-substituted MDMA analogues (selected accord-
resistance to their cytotoxic actions, nevertheless the death
ing to their activity versus sensitive L3055 cells and
that occurred failed to register an ‘early apoptosis' stage at
applied at a concentration at or close to their maximal
any time point as indicated by cells staining as PhiPhiLux+/
cytotoxic performance against this cell line) then
PI-. While no PhiPhiLux positivity was developed with
analysed for remaining viability (Fig. Given the
compound 6, compound 18 progressively moved a portion
resistance often afforded to therapeutic regimens by
of cells to what is conventionally considered a ‘late
dysregulated/overexpressed BCL2 in B-cell lymphoma,
apoptotic' stage: PhiPhiLux+/PI+. However, assessing
cells were simultaneously assessed for Bcl-2 protein
engagement of the apoptotic machinery by alternative more
content (vs calnexin standard) by Western blotting. The
direct methods gave no evidence for compound 18
origin of cells spanned patients additional to those
provoking this pathway in L3055-Bcl-2 cells. Thus the
diagnosed with BL (L3055 series; KHM2B): precursor
specific detection of active caspase-3 by antibody revealed
acute lymphoblastic leukemia (LILA), pro-lymphocytic leu-
its appearance in response to compounds 6 and 18 in
kemia (JVM2), mantle cell lymphoma (Rec-1; NCEB-1),
L3055-VC but not in L3055-Bcl-2 cells (Fig. Like-
primary mediastinal B-cell lymphoma (K1106), diffuse large
wise, the cleavage of poly (ADP-ribose) polymerase
B-cell lymphoma (K422; DoHH2), multiple myeloma
(PARP) ], as shown occurring in L3055-VC cells with
(KMS11; H929). Immortalized B-cell lines generated from
the well characterized apoptosis-inducing agent anti-IgM,
the peripheral blood of three donors by transformation with
was also seen on application of MDMA and here more
Epstein-Barr virus (EBV) were also included (HCD1; AT-AY;
potently with compounds 16, 17, and 18 whereas L3055-
ViWo)—EBV being invariably linked to endemic BL, PTLD
Bcl-2 cells revealed little if any PARP cleavage in response
(post-transplant lymphoproliferative disease) and a high
to any of the agents applied (Fig. JC-1 staining to
proportion of HIV-associated lymphoma.
indicate collapse of mitochondrial potential similarly
MDMA and its analogues were set at concentrations
supported the different routes to cell death by analogs
displaying maximal/near-maximal impact on L3055 cell
depending on the expression of Bcl-2 in L3055 BL cells
viability to serve as a reference. At these concentrations
(data not shown).
each of the analogues tested displayed (albeit a varyingdegree of) cytotoxicity against the spectrum of malignan-
Mechanisms and pathways to lymphoma cell killing
cies included. The best compound (18) showed a consis-
by selected α-substituted MDMA analogues
tently substantive impact against each of the subtypes. Asreported previously Bcl-2 content showed some degree
Depending upon cell type and system studied, MDMA has
of correlation with a cell's ability to resist killing from
been purported to provoke toxicity via a diverse array of
MDMA. With each of the analogues, however, there wasscant correlation between Bcl-2 protein level and extent of
Fig. 3 Cytotoxic performance of MDMA and selected analogues b
against B-cell lines of different malignant derivation and relation-
cytotoxicity observed (Fig. ). To assess the influence of
ship to Bcl-2 expression. a Cells from lines as shown plated at 5 x
Bcl-2 directly, a detailed concentration-dependent response
105/ml and cultured for 24 h with compounds indicated prior to
was established for the cytotoxic efficacy of the analogues
assessing (absolute) % viability of population as in Fig.
against L3055 cells transfected with empty vector versus
Concentration of drug applied as follows: MDMA, 2000 μM;compound 6, 500 μM; compound 12, 250 μM; compound 15,
cells expressing the BCL2 transgene. The latter were only
125 μM; compound 16, 31.25 μM; compound 17, 31.25 μM;
marginally more resistant (approximately a single log2
compound 18, 31.25 μM. Below is shown representative western
difference) to each of the analogues than cells negative for
blot analysis of Bcl-2 protein levels amongst the lines together with
Bcl-2 expression (Fig. ). This was consistent for cells
calnexin blotting control. Next are shown regression plots with Rvalues generated from remaining % viability in response to
plated at relatively low or high starting density.
compound versus relative Bcl-2 content amongst the lines tested; bconcentration-response curves to compounds of L3055 cells carrying
Mode of cell death induced by selected first and second
empty vector (L3055-VC) or a Bcl-2 transgene (L3055-Bcl2). Cells were
generation α-substituted MDMA analogues
plated at two different starting densities, 105 cells/ml and 5x105 cells/mlas indicated, and incubated with compound for 24 h prior toassessing viability/cytotoxicity as in Fig. Results represent the
When assessing cell integrity in response to compound 6 at
mean of three independent experiments ± SEM given as % viability
500 μM and compound 18 at 31.25 μM by dual PhiPhiLux
relative to vehicle control
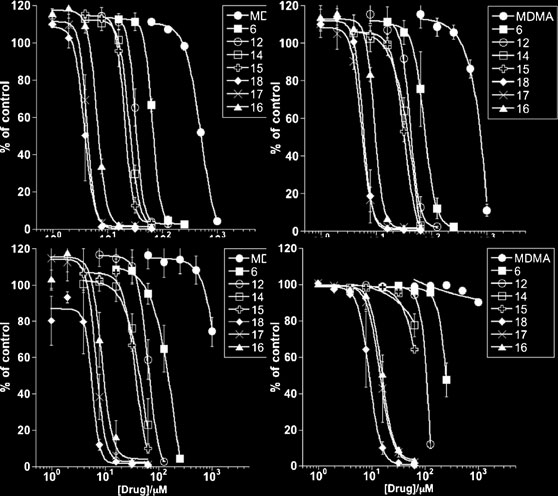

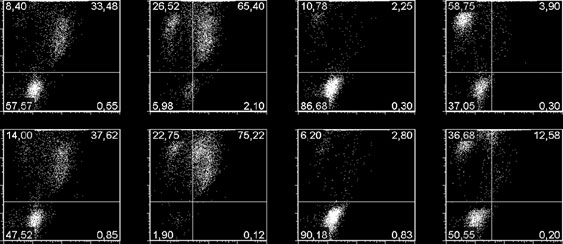

compound 6
compound 18
f respo
o
% o
time of incubation [hours]
compound 6
compound 18
100 101 102 103 104
100 101 102 103 104
100 101 102 103 104
Fig. 4 Mode of cell death in L3055-VC and L3055/Bcl2 cells in
generate an estimate of lipophilicity where the output value
response to MDMA analogues 6 and 18. a Cells from L3055 variant
is known as "average log P" [In brief, the average log
lines indicated were cultured with compound 6 (500 μM) or
P value is the simple average of log P estimates determined
compound 18 (31.25 μM) for 6 h before dual staining with PhiPhiLux(PPL) and PI (upper graph; dot plots) revealing four subpopulations of
using eight different models. A plot of average log P versus
cells: PIlo/PPLlo = viable (bottom left quadrant), PIlo/PPLhi = early
the pIC50 value of cytotoxic performance (including SEM
apoptotic (bottom right), PIhi/PPLhi=late apoptotic (top right), and
values for both variables) was constructed and a curve was
PIhi/PPLhi=necrotic (top left). The lower set of graphs illustrate similar
fitted by weighted linear regression as detailed in Fig.
analyses arising from exposing cells to the compounds over 1-6 h withthe data represented as the % of cells arising in each quadrant at the
When operating at pH values that favour ionisation of the
different times of harvest: viable marked in white, early apoptotic
compounds under consideration, as in this case (pH=7.4),
marked light grey, late apoptotic marked dark grey, necrotic marked
log P values should be corrected using the pKa to account
black. Data are the mean of three independent experiments (n=3) ±
for the increase in aqueous solubility of the ionised form.
SEM with the values shown obtained after subtracting vehicle control;b L3055-VC and L3055-Bcl2 cells at 5x105/ml treated for 2 h with
However, uncorrected log P values were used here as the
compound 6 at 500 μM and compound 18 at 31.25 μM (black line) or
pKa values (dictated by the shared amino group), and
vehicle control (shaded) then stained with antibody to active caspase-3
therefore the correction factors, were expected to be very
with intensity of staining analysed by FACS. A representative example
similar for all compounds, thus, not affecting the rank order
of two independent experiments is shown. c Western blot analysis ofPARP cleavage in L3055-VC and L3055-Bcl2 cells plated at 106/ml
obtained. Furthermore, the use of uncorrected log P
and treated for 6 h with MDMA at 2000 μM or compounds 16, 17 or
predictions to estimate lipophilicity has been shown to be
18 at 31.25 μM; upper 117 kDa band = intact PARP, lower 97 kDa
more reliable, as it avoids the introduction of a second
band = cleaved PARP; anti-IgM (25 μg/ml) is a positive control
source of error associated with the calculation of pK
treatment known to signal PARP cleavage in L3055-VC cells via cell
surface BCR. This experiment was performed twice with a represen-
Bearing these considerations in mind, a persuasive correla-
tative example shown
tion emerges with r2=0.88 as seen in Fig.
not necessarily mutually exclusive pathways as reviewedfor example in ]. Here, the possible involvement of
monoamine transporters in delivering MDMA and itsanalogues to engage intracellular pathways for lymphoma
Analogues of MDMA with modified α-substituents were
B-cell killing was first investigated. For this, L3055-VC cells
iteratively designed and synthesised, and found to be up to
were pre-treated with a range of monoamine transporter
10-fold (first generation) and 100-fold (second generation)
(MAT) inhibitors targeting: SERT (fluoxetine), SERTand NET
more potent than the parent amphetamine derivative at
(clomipramine and imipramine) or DAT (GBR12909); and
promoting lymphoma cell death: the goal and driver to this
also with cocaine, which blocks all three MATs. From results
study. Impressively, forced over-expression or high consti-
detailed in Fig. a it can be seen that none of the MAT
tutive levels of anti-apoptotic Bcl-2 failed to protect, to any
inhibitors afforded protection against lymphoma cell toxicity
significant degree, the anti-lymphoma actions of the
induced by MDMA or two of its more potent analogues
analogues; this despite their ability to promote apoptotic
indicating that they are unlikely to be serving as conduits to
cell death in Bcl-2-deplete cells. Thus, in the face of high-
the compounds' actions in this regard.
level Bcl-2, death still occurred but in a caspase-3-, PARP-
Since MDMA has been widely reported to mediate
independent fashion that was similarly independent from a
toxicity via direct or indirect production of reactive oxygen
collapse in mitochondrial membrane potential. It should be
species (ROS), L3055-WT and -VC cells were pre-treated
noted, however, that while analogues of MDMA efficiently
with enzymes that either degrade superoxide (O •–
generated active caspase-3 within 4–6 h of exposure in the
oxide dismutase, SOD) or H2O2 (catalase). However,
bulk of native L3055 BL cells, a majority of their Bcl-2-
neither SOD (data not detailed) nor catalase were seen to
overexpressing counterparts were still alive at 6 h. Thus, at
protect tumor B cells from the detrimental effect of the
least for BL, if translated to an in vivo therapeutic, these
MDMA analogues studied, whereas catalase efficiently
compounds show potential to reduce tumor burden through
reversed cell killing provoked by H2O2 (Fig. ). Cells
efficient apoptotic clearance without the attendant inflam-
pre-treated with these enzymes but now conjugated to
matory side effects of necrotic death.
polyethylene glycol (PEG) to facilitate cell uptake ,
Importantly, improved cytotoxic performance against
similarly failed to protect (Fig. and data not detailed).
lymphoma cells does not simply reflect a generally
Finally, we examined the possibility that increased
enhanced, non-specific toxicity profile of the compounds.
lipophilicity may be associated with the enhanced anti-
A companion study shows that the most active compound
lymphoma performance of the more potent analogues in
versus lymphoma cells from Series 1 (compound 6) and
this study. To explore this, we used the online program,
two of the even more active ones from Series 2 (compounds
ALOGPs 2.1, which accepts a structural formula to
16 and 17) are in fact less toxic than MDMA to SH-SY5Y:
compound 15
compound 16
at 10µMa
catalase at 1500U/ml
catalase at 1000U/ml
PEG-catalase
at 100U/ml
catalase at 500U/ml
ability %
at 62.5µ
Fig. 5 Investigation of potential pathways through which MDMA
H2O2 or compounds indicated (compound 6, 500 μM; compound 12,
analogues elicit cytotoxicity in L3055 cells. a Impact of monoamine
250 μM; compound 15, 125 μM; compounds 16, 17 and 18,
transporter (MAT) inhibitors. L3055 cells at 105/ml were pre-incubated
31.25 μM) and then culturing for 20 h prior to assessing viability as
with MAT inhibitors for 1 h before adding MDMA or compounds 15
above; c Influence of scavenging intracellular ROS with PEG-
and 16 at 125 μM and 31.25 μM respectively then culturing for 20 h
catalase. L3055-VC cells at 105/ml were pre-treated with PEG-
prior to assessing cell viability as in Fig. b Influence of scavenging
catalase for 1.5 h before adding H2O2, MDMA, or compound 6 at
extracellular ROS with catalase. L3055 cells at 105/ml were pre-
concentrations indicated and then culturing for 24 h prior to assessing
treated with catalase at concentrations shown for 1 h before adding
a catecholaminergic neuroblastoma cell line that is used to
The literature around MDMA and the mechanisms
model MDMA neurotoxicity. The same study also shows
underlying its toxicity is large, varied and occasionally
compound 6 having diminished psychoactivity when
contradictory –the cell system, cellular origin,
compared with MDMA in the prepulse inhibition of the
animal species, drug concentration and other elements all
acoustic startle reflex test in Wistar rats []. Further-
contributing confounding factors. Here we scrutinized
more, in the present study, while constituent cells of
several of the major candidate pathways proposed for
derived lines from all B-cell malignancies proved suscep-
MDMA for their potential contribution to the toxic action
tible to one or more of the analogues tested, the relative
of the analogues versus B-lymphoma cells. The current
level of sensitivity to a given compound could be quite
study was predicated on the discovery that B lymphoma
different depending upon the cell line targeted indicating a
cells express both SERT and DAT, the transporters for
degree of selectivity in the compounds' actions against
serotonin and dopamine, respectively, and to which MDMA
lymphoma cell subtypes.
binds in the human with high affinity as it also does to NET,
MDMA and its analogues in this respect. Similar failure ofPEGylated SOD and catalase to inhibit death deliveredfrom the compounds under study equally argued againstintracellular ROS formation contributing to the lymphomacell killing observed.
If not through ROS generation or from entering via
monoamine transporters, how are MDMA and its rede-signed analogues attacking the lymphoma cells? Screeningagainst the sensitive L3055 cell line revealed no significantdifference in the cells' response to compounds containing
α-subsituents with either different steric (13–15, 16–17) orstereoelectronic (9–12) properties. Instead, the addition offurther aromatic rings, thereby increasing the size ofsubstituents at the α-carbon of MDMA, appeared aunifying factor to increasing potency: i.e. compound 6 inSeries 1 with a single aromatic ring and compounds 18, 17and 16 in Series 2 with two aromatic rings being the mostpotent from each iteration. That said, the non-aromaticcyclohexyl substituent confers equipotency to phenyl. Size
Fig. 6 Calculated liphophilicities of MDMA and analogues versus
of the α-subsituent and overall lipophilicity of the com-
cytotoxic performance. Relationship between average log P ± SEM
pound may therefore be primary determinants of potency. In
and pIC50 ± SEM for MDMA (□), alkyl α-substituted analogues 1–5
an earlier study we noted from a seemingly otherwise
(◊), monocyclic aromatic α-substituted analogues 6–15 (Δ) and
disparate set of compounds capable of killing lymphoma
polycyclic aromatic α-substituted analogues 16–18 (○). The curvewas fitted to all data points shown using weighted linear regression
cells the shared feature of being cationic amphiphiles
that gave an r2 value of 0.88
This class of compounds has the capacity to disrupt cellularmembranes, as do amphiphilic molecules generally. Greater
the norepinephrine transporter [Against serotoner-
lipophilicity also enhances entry into cells, thereby increasing
gic JAR cells for example, MDMA's cytotoxicity is
the effective intracellular concentration, and entropically
delivered via SERT: being inhibited by imipramine, a
favours complex formation (the hydrophobic effect) and thus,
monoamine transporter blocker with highest affinity for
potentially, affinity of drug for intracellular receptors/targets.
SERT [The capacity of serotonin to drive apoptosis in
Numerous studies indicate a selectivity of lipophilic com-
BL cells is reversed by SERT blockade with e.g. the
pounds for impacting rapidly proliferating cancer cells over
selective serotonin reuptake inhibitor, fluoxetine []. How-
normal cells ] and others show, amongst related series
ever, adopting the approach of pharmacological transporter
of compounds, a clear correlation between anti-proliferative
blockade in this work, neither MDMA nor two of its more
activity/cytotoxicity and degree of lipophilicity
potent redesigned analogues were seen to be delivering
When this relationship was examined for the newly
their toxic hit to lymphoma cells via any of the three
synthesized analogues of MDMA, a strong correlation was
monoamine transporters probed. Moreover, Montgomery
indeed observed with anti-lymphoma potency closely track-
and colleagues [examining the action of MDMA and
ing calculated lipophilicity, at least for those compounds with
several MDMA analogues on 5-HT and NA uptake in cells
aromatic α-substituents. We are currently exploring pre-
transfected with SERT or NET reported a Hill coefficient for
cisely how this physiochemical property of the compounds
inhibition by MBDB (our compound 3) of 1 for both
translates mechanistically to improved lymphoma killing
HEK-SERT and PC12-NET compared to that generated
in order to assist further rational design of MDMA
from its anti-lymphoma action in this study of >3. Others
analogues as anti-neoplastics.
have shown that MDMA is capable of promoting cell death
Irrespective of relative anti-lymphoma potency all com-
independently of SERT expression or activity [A
pounds including MDMA generated steep inhibition curves
second major mechanism for MDMA's cellular toxicity in
with Hill coefficients >3 indicating a high degree of
other systems was similarly ruled out here for both the lead
cooperativity in their action. Similar behaviour has been
compound and the more (anti-lymphoma) potent synthe-
observed from SSRIs and tricyclic antidepressants (Sera-
sized analogues: namely the, direct or indirect, production
feim Blood 2003; Meredith FASEB J 2005) and at least
of reactive oxygen species. Inhibitors of extracellular ROS
with the former class of compound we know that cell death
which have previously been shown to reverse the anti-
is preceded by the stimulation of Ca2+ entry. Preliminary
lymphoma actions of dopamine ] did not protect against
data (unpublished) indicate similarly altered Ca2+ flux in
L3055 BL cells on exposure to MDMA and analogues
8. Nichols DE, Hoffman AJ, Oberlender RA, Jacob P 3rd, Shulgin
AT (1986) Derivatives of 1-(1,3-benzodioxol-5-yl)-2-butanamine:
studied here. As an alternative to cooperative binding at a
representatives of a novel therapeutic class. J Med Chem
defined molecular target, a possibility under consideration
is that the lipophilic compounds undergo aggregate forma-
9. Shulgin A, Shulgin A (1991) Phenethylamines I have known and
tion dependent upon a critical association concentration—
loved: A chemical love story transform Pr Berkeley, California
10. Nash JF, Nichols DE (1991) Microdialysis studies on 3,4-
perhaps established in situ within the lipid bilayer of the
methylenedioxyamphetamine and structurally related analogues.
cell membrane ]—and that it is these higher order
Eur J Pharmacol 200:53–58
complexes that cause cell death conceivably analogous to—
11. Gandy MN, McIldowie M, Lewis K, Wasik AM, Salomonczyk D,
or directly behaving as—ionophores ].
Wagg K, Millar ZA, Tindiglia D, Huot P, Johnston T, Thiele S,Nguyen B, Barnes NM, Brotchie JM, Martin-Inverson MT, Nash
In conclusion, a series of iterations positioned on a
J, Gordon J, Piggott MJ (2010) Redesigning the designer drug
modified α-substituent of MDMA resulted in a number of
ecstasy: non-psychoactive MDMA analogues exhibiting Burkitt's
lead compounds with respect to prospective novel thera-
lymphoma cytotoxicity. Med Chem Comm 1:287–293
peutics for non-Hodgkin lymphomas. Insight into the
12. Tetko IV, Gasteiger J, Todeschini R, Mauri A, Livingstone D, Ertl
P, Palyulin VA, Radchenko EV, Zefirov NS, Makarenko AS,
mechanism of their actions and the pathways by which
Tanchuk VY, Prokopenko VV (2005) Virtual computational
they promote cell death opens a door to further rational
chemistry laboratory–design and description. J Comput Aided
modifications that hold the promise of accelerating transla-
Mol Des 19:453–463
tion of a redesigned MDMA to the clinic for this important
13. Steward LJ, Ge J, Bentley KR, Barber PC, Hope AG, Lambert JJ,
Peters JA, Blackburn TP, Barnes NM (1995) Evidence that the
cancer indication.
atypical 5-HT3 receptor ligand, [3H]-BRL46470, labels additional5-HT3 binding sites compared to [3H]-granisetron. Br J Pharmacol
This work was supported in part by Leukaemia
and Lymphoma Research, UK, and the Ada Bartholomew Medical
14. Bohm HJ, Banner D, Bendels S, Kansy M, Kuhn B, Muller K,
Research Trust, W.A. MNG and KDL were recipients of a UWA
Obst-Sander U, Stahl M (2004) Fluorine in medicinal chemistry.
postgraduate scholarship and Australian Postgraduate Award, respec-
Chembiochem 5:637–643
tively. DS-T was supported by an Arthritis Research UK Career
15. Smart BE (2001) Fluorine substutuent effects (on bioactivity). J
Progression Fellowship. JG was in receipt of a Raine Visiting
Fluorine Chem 109:3–11
Professorship at the University of Western Australia while writing
16. Cornish-Bowden A, Koshland DE Jr (1975) Diagnostic uses of
the paper. The authors declare that they have no conflict of interest.
the Hill (Logit and Nernst) plots. J Mol Biol 95:201–212
17. Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer
S, Smulson M (1999) Role of poly(ADP-ribose) polymerase
(PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutantincreases rates of apoptosis in transfected cells. J Biol Chem274:22932–22940
1. Serafeim A, Grafton G, Chamba A, Gregory CD, Blakely RD,
18. Cadet JL, Krasnova IN, Jayanthi S, Lyles J (2007) Neurotoxicity
Bowery NG, Barnes NM, Gordon J (2002) 5-Hydroxytryptamine
of substituted amphetamines: molecular and cellular mechanisms.
drives apoptosis in biopsylike Burkitt lymphoma cells: reversal by
Neurotox Res 11:183–202
selective serotonin reuptake inhibitors. Blood 99:2545–2553
19. Beckman JW, Wang Q, Guengerich FP (2008) Kinetic analysis of
2. Meredith EJ, Chamba A, Holder MJ, Barnes NM, Gordon J
correct nucleotide insertion by a Y-family DNA polymerase
(2005) Close encounters of the monoamine kind: immune cells
reveals conformational changes both prior to and following
betray their nervous disposition. Immunology 115:289–295
phosphodiester bond formation as detected by tryptophan fluo-
3. Serafeim A, Holder MJ, Grafton G, Chamba A, Drayson MT,
rescence. J Biol Chem 283:36711–36723
Luong QT, Bunce CM, Gregory CD, Barnes NM, Gordon J (2003)
20. Blatt NB, Boitano AE, Lyssiotis CA, Opipari AW Jr, Glick GD
Selective serotonin reuptake inhibitors directly signal for apoptosis in
(2009) Bz-423 superoxide signals B cell apoptosis via Mcl-1,
biopsy-like Burkitt lymphoma cells. Blood 101:3212–3219
Bak, and Bax. Biochem Pharmacol 78:966–973
4. Meredith EJ, Holder MJ, Chamba A, Challa A, Drake-Lee A,
21. Tetko IV, Tanchuk VY (2002) Application of associative neural
Bunce CM, Drayson MT, Pilkington G, Blakely RD, Dyer MJ,
networks for prediction of lipophilicity in ALOGPS 2.1 program.
Barnes NM, Gordon J (2005) The serotonin transporter (SLC6A4)
J Chem Inf Comput Sci 42:1136–1145
is present in B-cell clones of diverse malignant origin: probing a
22. Tetko IV, Bruneau P (2004) Application of ALOGPS to predict 1-
potential anti-tumor target for psychotropics. FASEB J 19:1187–
octanol/water distribution coefficients, logP, and logD, of Astra-
Zeneca in-house database. J Pharm Sci 93:3103–3110
5. Chamba A, Holder MJ, Jarrett RF, Shield L, Toellner KM,
23. Yamamoto B, Zhu W (1998) The effects of methamphetamine on
Drayson MT, Barnes NM, Gordon J. SLC6A4 expression and
the production of free radicals and oxidative stress. J Pharmacol
anti-proliferative responses to serotonin transporter ligands chlo-
Exp Ther 287:107–114
mipramine and fluoxetine in primary B-cell malignancies. Leuk
24. Davidson C, Gow A, Lee T, Ellinwood E (2001) Methamphet-
Res 34:1103–1106
amine neurotoxicity: necrotic and apoptotic mechanisms and
6. Braun U, Shulgin AT, Braun G (1980) Centrally active N-substituted
relevance to human abuse and treatment. Brain Res Brain Res
analogs of 3,4-methylenedioxyphenylisopropylamine (3,4-methyle-
nedioxyamphetamine). J Pharm Sci 69:192–195
25. Yamamoto BK, Bankson MG (2005) Amphetamine neurotoxicity:
7. Braun U, Shulgin AT, Braun G (1980) Research on the central
cause and consequence of oxidative stress. Crit Rev Neurobiol
activity and analgesia of N-substituted analogs of the amphet-
amine derivative 3,4-methylenedioxyphenylisopropylamine. Arz-
26. Montiel-Duarte C, Ansorena E, Lopez-Zabalza M, Cenarruzabeitia
E, Iraburu M (2004) Role of reactive oxygen species, glutathione and
NF-kappaB in apoptosis induced by 3,4-methylenedioxymetham-
35. Hayat S, Williams RJ, Rattray M (2006) Serotonin transporter
phetamine ("ecstasy") on hepatic stellate cells. Biochem Pharmacol
expression is not sufficient to confer cytotoxicity to 3,4-methyl-
enedioxymethamphetamine (MDMA) in vitro. J Psychopharmacol
27. Monks TJ, Jones DC, Bai F, Lau SS (2004) The role of
metabolism in 3,4-(+)-methylenedioxyamphetamine and 3,4-(+)-
36. Meredith EJ, Holder MJ, Rosen A, Lee AD, Dyer MJ, Barnes
methylenedioxymethamphetamine (ecstasy) toxicity. Ther Drug
NM, Gordon J (2006) Dopamine targets cycling B cells
Monit 26:132–136
independent of receptors/transporter for oxidative attack: Implica-
28. Milhazes N, Cunha-Oliveira T, Martins P, Garrido J, Oliveira C,
tions for non-Hodgkin's lymphoma. Proc Natl Acad Sci USA 103
Rego AC, Borges F (2006) Synthesis and cytotoxic profile of 3,4-
methylenedioxymethamphetamine ("ecstasy") and its metabolites
37. Biasutto L, Dong LF, Zoratti M, Neuzil J. Mitochondrially
on undifferentiated PC12 cells: A putative structure-toxicity
targeted anti-cancer agents. Mitochondrion 10:670–681
relationship. Chem Res Toxicol 19:1294–1304
38. Christman JE, Miller DS, Coward P, Smith LH, Teng NN (1990)
29. Keizers PH, de Graaf C, de Kanter FJ, Oostenbrink C, Feenstra KA,
Study of the selective cytotoxic properties of cationic, lipophilic
Commandeur JN, Vermeulen NP (2005) Metabolic regio- and stereo-
mitochondrial-specific compounds in gynecologic malignancies.
selectivity of cytochrome P450 2D6 towards 3,4-methylenedioxy-N-
Gynecol Oncol 39:72–79
alkylamphetamines: in silico predictions and experimental validation. J
39. Huszar M, Varga A, Horvath A, Lorand T, Agocs A, Idei M,
Med Chem 48:6117–6127
Mandl J, Vantus T, Keri G. Comparative characterization of
30. Capela JP, Carmo H, Remiao F, Bastos ML, Meisel A, Carvalho F
experimental and calculated lipophilicity and anti-tumour activity
(2009) Molecular and cellular mechanisms of ecstasy-induced
of isochromanone derivatives. Curr Med Chem 17:321–333
neurotoxicity: an overview. Mol Neurobiol 39:210–271
40. Adams DJ, da Silva MW, Flowers JL, Kohlhagen G, Pommier Y,
31. Callahan BT, Cord BJ, Yuan J, McCann UD, Ricaurte GA (2001)
Colvin OM, Manikumar G, Wani MC (2006) Camptothecin
Inhibitors of Na(+)/H(+) and Na(+)/Ca(2+) exchange potentiate
analogs with enhanced activity against human breast cancer
methamphetamine-induced dopamine neurotoxicity: possible role
cells. I. Correlation of potency with lipophilicity and persis-
of ionic dysregulation in methamphetamine neurotoxicity. J
tence in the cleavage complex. Cancer Chemother Pharmacol
Neurochem 77:1348–1362
32. Verrico CD, Miller GM, Madras BK (2007) MDMA (Ecstasy) and
41. Maliepaard M, de Mol NJ, Janssen LH, van der Neut W, Verboom
human dopamine, norepinephrine, and serotonin transporters:
W, Reinhoudt DN (1992) Role of lipophilicity in the in vitro
implications for MDMA-induced neurotoxicity and treatment.
antitumour activity of a series of new mitosene compounds.
Psychopharmacology (Berl) 189:489–503
Anticancer Drug Des 7(5):415–425
33. Simantov R, Tauber M (1997) The abused drug MDMA (Ecstasy)
42. Soderberg L, Haag L, Hoglund P, Roth B, Stenberg P, Wahlgren M
induces programmed death of human serotonergic cells. FASEB J
(2009) The effects of lipophilic substances on the shape of
erythrocytes demonstrated by a new in vitro-method. Eur J Pharm
34. Montgomery T, Buon C, Eibauer S, Guiry PJ, Keenan AK,
Sci 36(4–5):458–464
McBean GJ (2007) Comparative potencies of 3,4-methylenediox-
43. Sanderson KL, Butler L, Ingram VM (1997) Aggregates of a beta-
ymethamphetamine (MDMA) analogues as inhibitors of [3H]
amyloid peptide are required to induce calcium currents in
noradrenaline and [3H]5-HT transport in mammalian cell lines. Br
neuron-like human teratocarcinoma cells: relation to Alzheimer's
J Pharmacol 152:1121–1130
disease. Brain Res 744(1):7–14
Source: http://www.celentyx.co.uk/Wasik%20et%20al.%202011%20Investigational%20New%20Drugs.pdf
Toxicology 192 (2003) 249–261 Reducing acute poisoning in developing countries—options for restricting the availability of pesticides Flemming Konradsen , Wim van der Hoek , Donald C. Cole , Gerard Hutchinson , Hubert Daisley , Surjit Singh , Michael Eddleston a Department of International Health, Institute of Public Health, University of Copenhagen, Panum, Blegdamsvej 3,
North Manchester CCG Board Meeting – 11 February 2015 Dr Martin Whiting Paper prepared by: Dr Martin Whiting Dr Martin Whiting Sub-Committee consideration Chief Clinical Officer's Report Background papers and links to priorities/objectives: To provide an update to the board on strategic Purpose of the paper: developments within Greater Manchester.







