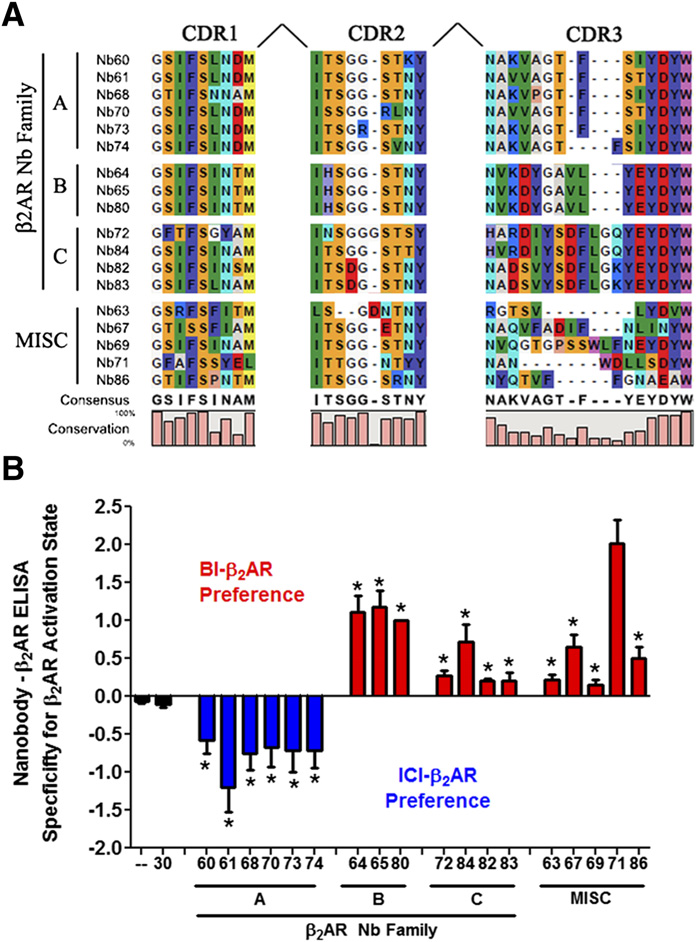
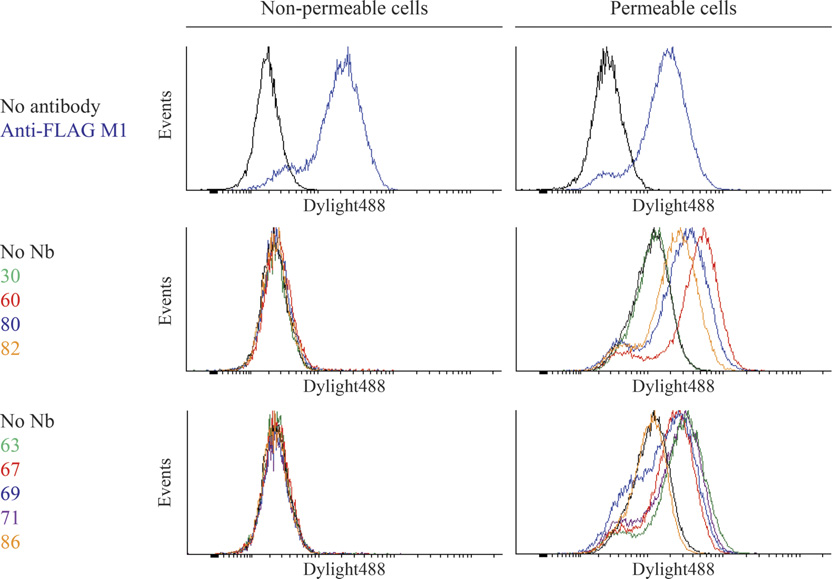
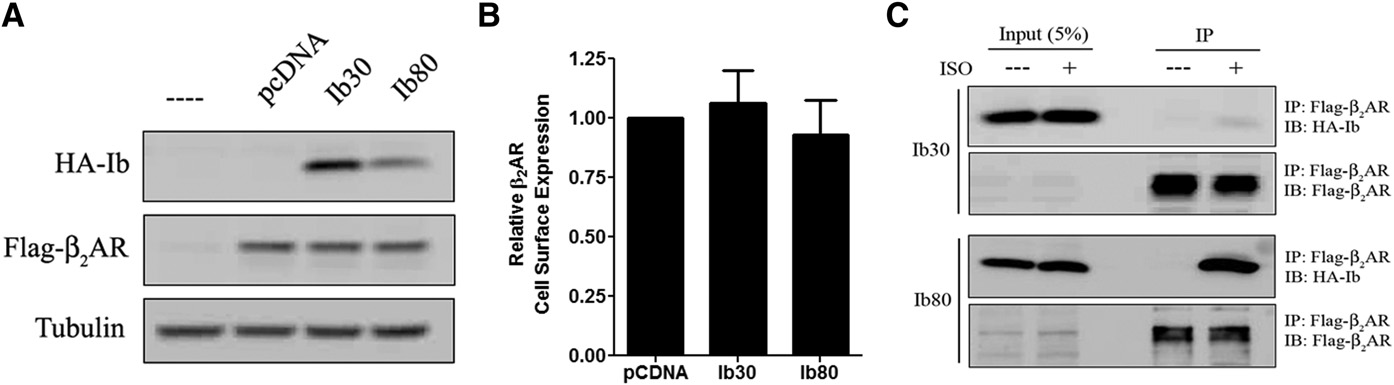
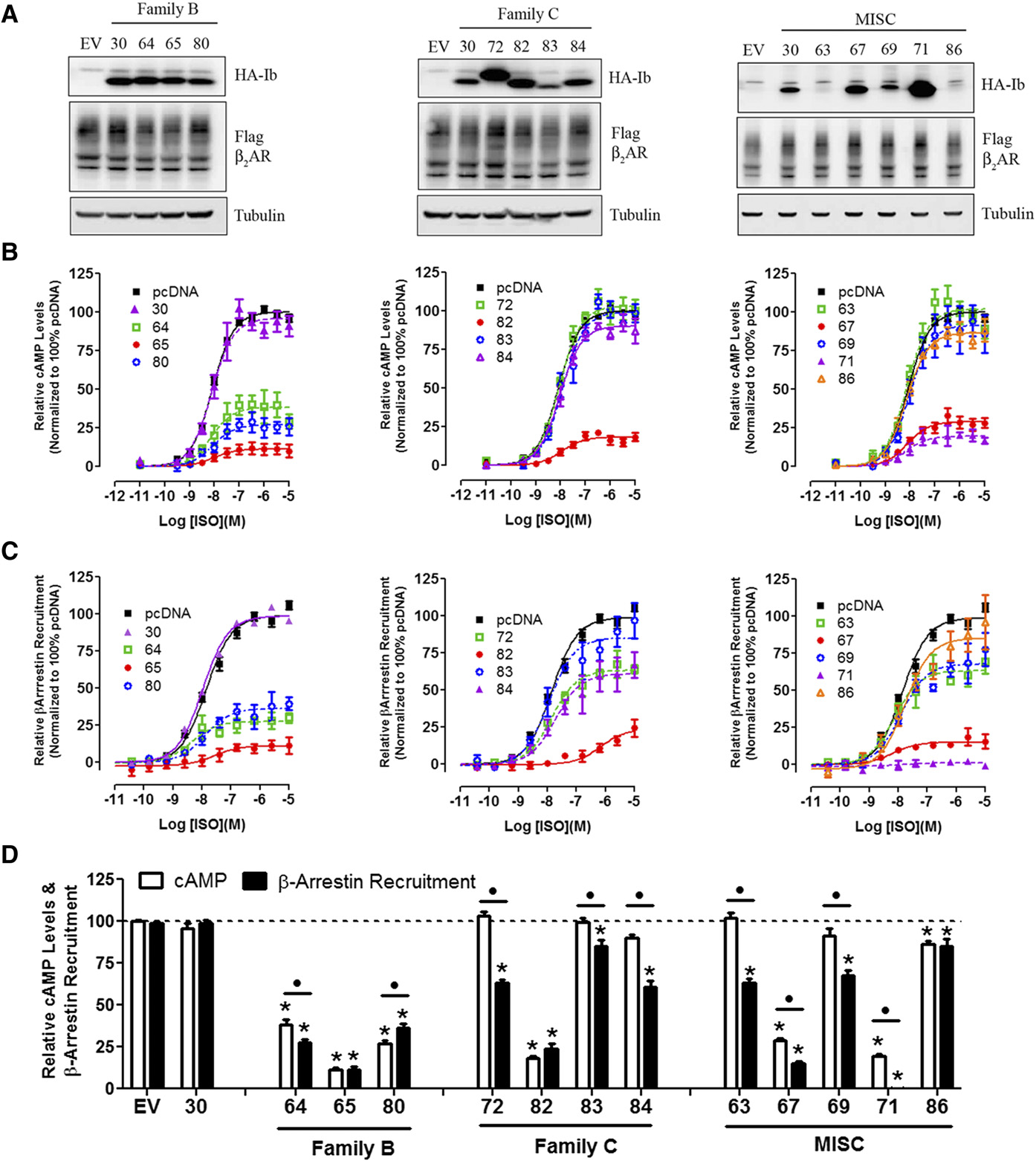
M300358
Cross-inhibition of SR-BI- and ABCA1-mediatedcholesterol transport by the small molecules BLT-4and glyburide Thomas J. F. Nieland,*,†,§ Angeliki Chroni,** Michael L. Fitzgerald,†† Zoltan Maliga,†,§Vassilis I. Zannis,** Tomas Kirchhausen,† and Monty Krieger1,* Department of Biology,* Massachusetts Institute of Technology, Cambridge, MA 02139; Department of Cell Biology,† Harvard Medical School, and The CBR Institute for Biomedical Research, Inc., Boston, MA02115-5701; Molecular Genetics, Whitaker Cardiovascular Institute, Department of Medicine and Biochemistry,** Boston University School of Medicine, Boston, MA 02118; Lipid Metabolism Unit,†† Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114; and Harvard Institute for Chemistry and Cell Biology,§ Seeley G. Mudd 604, Boston, MA 02115