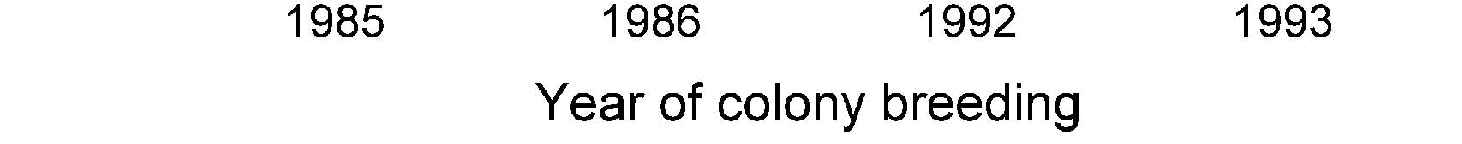What drives the 10-year cycle of snowshoe hares?
What Drives the 10-year
Cycle of Snowshoe Hares?
CHARLES J. KREBS, RUDY BOONSTRA, STAN BOUTIN AND A.R.E. SINCLAIR
In 1831 the manager of a Hudson's Bay Company post
in northern Ontario wrote to the head office in London.
THE TEN-YEAR CYCLE OF SNOWSHOE
The local Ojibway Indians were starving, he reported, becauseof a scarcity of "rabbits," and they were unable to trap for furs
HARES—ONE OF THE MOST STRIKING
because they spent all their time fishing for food (Winter-halder 1980). These shortages of so-called rabbits, which ap-
FEATURES OF THE BOREAL FOREST—
parently occurred approximately every 10 years, are regularlymentioned in Canadian historical documents from the 18th
IS A PRODUCT OF THE INTERACTION
and 19th centuries. Those rabbits were in fact snowshoe hares
BETWEEN PREDATION AND FOOD
(
Lepus americanus), and their 10-year cycle is one of the mostintriguing features of the ecology of the boreal forest.
SUPPLIES, AS LARGE-SCALE
Ten-year cycles were first analyzed quantitatively when
wildlife biologists began to plot the fur trading records of
EXPERIMENTS IN THE YUKON HAVE
Hudson's Bay Company during the early 1900s. The Hud-son's Bay Company, established in 1671, kept meticulous
records of the numbers of furs traded from different postsspread across Canada. The most famous time series drawntogether from those records was that of Canada lynx (Eltonand Nicholson 1942; Figure 1). The lynx is a specialist preda-
over the last 40 years ecologists working in Alberta, the
tor of snowshoe hares, and the rise and fall in lynx numbers
Yukon Territory, and Alaska have put together an array of
mirrors, with a slight time lag, the rise and fall of snowshoe
studies that have resolved most, but not all, of the enigmas
hare populations across the boreal region.
behind these cycles (Keith 1990, Boutin et al. 1995).
The spectacular cycles of snowshoe hares and their preda-
To understand any fluctuating population, one must first
tors have captured the attention of biologists as well as his-
know in detail the mechanisms of changes in births, deaths,
torians. These cycles are highlighted in virtually all ecology
and movements that are the proximate causes of the changes
texts and are often cited as one of the few examples of
in numbers. Before we describe these details, we should
Lotka-Volterra predator–prey equations, a simple model
note that these 10-year hare cycles tend to occur in synchrony
which shows never-ending oscillations in the numbers of
across broad regions. Indeed, hares across most of Canada
predators and their prey. Cycles seem to violate the implicit
and Alaska reached a peak in 1997–1999 during the most re-
assumption of many ecologists that there is a balance in na-
cent cycle. We explain the reasons behind this synchrony be-
ture, and anyone living in the boreal forest would be hard
low, but let us note here that movements of hares cannot
pressed to recognize a balance among the boom and bust in
explain these population changes via immigration or emi-
nature's economy. The challenge to biologists has been to un-
gration. Movements on a local level might be important, but
derstand the mechanisms behind these cycles, which has not
at the regional level all populations rise and fall in unison.
been easy. One cycle lasts 10 years, and few PhD students or
Population changes must be driven by changes in births
researchers wish to take 10 years to obtain
n = 1. Fortunately,
Charles J. Krebs (e-mail:
[email protected]), an ecologist in the Department of Zoology at the University of British Columbia, Vancouver, B.C.
V6T 1Z4, has been studying population cycles for 41 years; A. R. E. Sinclair, also with the Department of Zoology at the University of BritishColumbia, is a population and community ecologist who works extensively in East Africa on ungulate and bird populations and in the Yukon onhare population cycles. Rudy Boonstra, a population biologist in the Division of Life Sciences, University of Toronto, Scarborough, Ontario M1C1A4, is interested in the role of stress in population dynamics of mammals. Stan Boutin is an ecologist in the Department of Biological Sciences,University of Alberta, Edmonton, Alberta T6G 2E9; he has been studying northern mammal populations for 21 years. 2001 American Instituteof Biological Sciences.
January 2001 / Vol. 51 No. 1 BioScience 25



Figure 1. Canada lynx fur returns from the Northern Department of the Hudson's Bay Company from 1821 to 1910. The
Northern Department occupied most of western Canada. The cycle for these data averages 9.6 years. Data are from Elton
and Nicholson (1942). Photo: Mark O'Donoghue.
Reproductive and survival changes
ductive output occurred in 1973, 2 years after the density
Snowshoe hares can have three or four litters over a summer,
peak of 1971. We found a similar but not identical pattern
with five leverets on average in each litter. All hares begin to
of response in hares in the southwestern Yukon. The re-
breed in spring when they are 1 year old, so age at sexual ma-
productive rate at the cyclic peak in the Yukon was about two-
turity is a constant. If reproductive rates are to vary, only lit-
thirds of the maximum, compared with one-half of the
ter size or the number of litters can change. Lloyd Keith and
maximum in the Alberta peak. But reproduction in both ar-
his students at the University of Wisconsin, working in cen-
eas was minimal in the decline phase (about one-third of
tral Alberta, supplied the first detailed description of the way
maximum reproduction) and highest in the early increase
in which hares change their reproductive rate over a 10-year
phase of the 10-year cycle (Stefan 1998, Hodges 2000).
cycle (Cary and Keith 1979). Reproductive output reaches
Changes in mortality rates are the other driver of changes
its peak very early in the phase of population increase, when
in hare numbers over the cycle. At Kluane Lake in the Yukon
females are producing 16–18 young per summer. It then be-
we measured survival rates of hares with radio collars dur-
gins to fall rapidly while numbers are still rising; it reaches
ing two population cycles. Additional data on survival of
a nadir in the year of the density peak or 1–2 years thereafter,
hares over two cycles in central Alberta was obtained through
during the decline phase of the cycle. The response of re-
mark-and-recapture methods and radiotelemetry (Keith
productive output to hare density seems to lag 1–2 years, so
1990). The pattern of change for adult hare survival is
that, as shown in Figure 2, for example, the lowest repro-
shown in Figure 3. Adult survival rates begin to drop slowly
Figure 2. Changes in the annual reproductive output of female snowshoe hares in the Rochester area of central Alberta,
1962–1976. Reproductive output was measured in autopsy samples. Data from Cary and Keith (1979). Photo: Alice Kenney.
26 BioScience January 2001 / Vol. 51 No. 1
Figure 3. Changes in adult hare survival rates over the 10-year cycle at Kluane Lake, Yukon, from 1977 to 1996. Hare density
(histogram) in spring of year t is plotted along with survival rates averaged from spring of year t to t+1 for radio-collared
hares in control areas. Too few hares were captured in 1985–1987 to estimate survival accurately.
as the population increases to a peak but then drops dra-
consistent. Lloyd Keith and colleagues found the same
matically for 1 to 2 years, part of the cause of the collapse
changes in central Alberta cycles that we found in south-
in population numbers (Krebs et al. 1986, Hodges 2000a).
western Yukon cycles (Keith and Windberg 1978). The key
Once low numbers are reached, adult survival rates im-
finding is that both reproduction and survival rates begin
prove slowly but do not reach the maximum until 4 to 5 years
to decay in the increase phase of the cycle, 2 years before peak
after the peak.
densities are reached. Maximal reproduction and highest sur-
Juvenile survival can be broken down into preweaning sur-
vival rates occur early in the increase phase of the cycle. By
vival for the first 30 days of life, and postweaning survival
the late increase phase, reproduction has already slowed
from 30 days to 1 year of age the following April. Post-
and survival rates of both adults and juveniles are falling.
weaning survival follows the pat-tern already illustrated in Figure 3for adult hares. Preweaning survivalis more difficult to measure, and wehave data only from the Yukon forthis stage of the life cycle. We cagedpregnant hares caught in the wildwhen they were near term and thenradio-tagged the leverets immedi-ately after birth when the cage wasremoved. Figure 4 shows that sur-vival is very poor in this early stage.
Relatively high survival occurs onlyin the first 2 to 3 years of the in-crease phase, and survival is alreadylow before peak hare density isreached. Preweaning survival re-mains low in the peak and at least 2years into the decline phase, and thelowest survival occurs near the endof the decline when hare density is
Figure 4. Preweaning survival of snowshoe hares over a population cycle at Kluane
already very low (Stefan 1998).
Lake, Yukon Territority. Leverets were radio-tagged at birth and followed for 30
The demographic pattern of the
days. It was impossible to obtain any juveniles in the summer of 1993. Hare density
hare cycle is remarkably clear and
in spring is shown by the histogram.
January 2001 / Vol. 51 No. 1 BioScience 27
Both reproduction and survival rates continue to fall for 2
Alternatively, food quality could change over the cycle.
to 3 years after the peak of the cycle, and over the low phase
John Bryant at the University of Alaska suggested one at-
they start to recover to high values.
tractive qualitative food hypothesis based on secondarychemicals: Shrubs and small trees can fight back against
Causes of the cycle
browsers by increasing their content of secondary chemicals
What causes these changes in reproduction and survival?
such as tannins and resins, which deter digestion in herbi-
There are three main factors that seem most likely to cause
vores (Bryant 1981). Indeed, experimental browsing of
hare cycles: food, predation, and social interactions. In ad-
shrubs in Alaska has shown that the plants can respond to
dition to these single-factor explanations, two multifactor
damage by increasing their secondary chemical defenses
explanations have been suggested, one involving food and
(Bryant et al. 1985). The key question is whether these plant
predation, and the other—the most complex hypothesis—
changes can influence the hare cycle.
involving all three factors.
To answer this question, we conducted five food-addition
Many other factors might affect snowshoe hare cycles, but
experiments during two hare cycles in the southwestern
these seem more likely to be modifying influences than pri-
Yukon. In four we provided high-quality rabbit chow with-
mary causes. Disease and parasitism are two ecological fac-
out limit to hares; in the fifth experiment we added high-
tors that might affect hare populations but do not seem to
quality natural food to a declining hare population (Krebs
be an essential cause of cycles. Parasite loads, for example,
et al. 1985, 1995, Sinclair et al. 1988). The response of hares
might cause hares to be in poor condition and therefore more
to rabbit chow is classic: Hares move into the food-addition
susceptible to predators. Lloyd Keith and his students sur-
areas and their density increases approximately two- to
veyed hare parasite loads for many years in Alberta and
threefold in comparison with control areas. But once the den-
concluded that none of the many parasites of the hares
sity increases on the food-addition areas, the hare cycle
caused much direct mortality (Keith et al. 1985). Experi-
continues unchanged. Hares decline in number at the same
mental work with antihelminthics in field populations of
time and at the same rate on the food areas as on unma-
hares either had no measurable impact on reproduction
nipulated controls.
and survival (Sovell and Holmes 1996) or produced mini-
Artificial food-addition experiments have been criticized
mal effects (Murray et al. 1997). The conclusion is that dis-
because the added food is high quality and not natural. We
ease and parasites may affect some hare populations
tried to address this criticism by supplying natural food to
sporadically (see reports in Chitty 1948, 1950, for example),
one declining hare population. Tony Sinclair and Jamie
but they cannot be an essential cause of cycles.
Smith had shown that snowshoe hares largely avoided small
The food hypothesis is attractive because it can explain
white spruce trees because the needles contain camphor
both why reproduction changes over the cycle and why
(Sinclair and Smith 1984, Rodgers and Sinclair 1997). Con-
survival might change as well. There are two variants of
sequently, small spruce seedlings were the least preferred food
the food hypothesis. First, hares may run out of food and
in cafeteria trials with hares. But foliage from large white
starve, or, second, the quality of the food may decline. Be-
spruce trees with branches beyond the reach of hares con-
cause hares eat a variety of green plants in the summer, no
tain no camphor, and these branches become highly pre-
one has considered food shortage in summer to be an im-
ferred food when supplied in a cafeteria trial. This
portant factor. Winter food plants are the small terminal
observation was dramatically verified when a large white
twigs of willow, birch, and small trees, as well as other
spruce tree was blown over by a windstorm: Hares devoured
shrubs; most studies have concentrated on the possibility that
the fallen branches. We therefore decided to feed a popula-
winter foods are limiting to hares (Keith et al. 1984). Like all
tion of hares through a decline by cutting down white
herbivores, snowshoe hares have preferred winter foods
spruce trees and thus providing natural, highly preferred food
and may browse a large fraction of these preferred plants at
to a collapsing hare population.
the peak of the cycle.
Stan Boutin and Scott Gilbert did this experiment over
There is little evidence from our Yukon studies that over-
three winters, with the results shown in Figure 5. The extra
all food quantity is limiting at any time. We measured food
natural food produced no detectable effect on the rate of
abundance over the cycle by quantifying edible forage and
population collapse. The failure of this extra food to affect
we assessed consumption rates of marked twigs. Con-
the hare population decline was shown clearly on five areas
sumption increased markedly during the peak (Smith et al.
in two cycles in the southwest Yukon. Such results imply that
1988, Hik 1995, Krebs et al. 2001), with 80% to 90% of the
food shortage by itself is not the explanation for the hare cy-
available bog birch being consumed. However, only 20% to
cle. Whatever secondary chemical changes occur in winter
40% of the grey willow—the dominant shrub in the area—
food plants, they are at most a contributing factor, not the
was consumed, thus indicating that food was not limiting
primary cause of the hare collapse.
at any time in the cycle. If the absolute abundance of food
Another way of approaching the possible role of food in
were limiting, we should have found hares that had starved,
hare cycles is to improve the quality of the vegetation by
but only about 3% of the hare mortalities could be directly
adding nutrients in fertilizer. We ran this experiment from
attributed to starvation.
1987 to 1996 on two areas in the southwestern Yukon, each
28 BioScience January 2001 / Vol. 51 No. 1
Figure 5. Changes in snowshoe hare numbers on control (1050, red) and food-supplemented (blue) areas during the
population decline of 1981–1983 at Kluane, Yukon. The natural feeding experiment was begun in October 1981 (blue
triangle). Summer months are shaded yellow. Data are from Krebs et al. (1985).
1 km2. On each area we added NPK fertilizer each spring to
rels and ground squirrels. Of the leverets with radio tags, 81%
increase the availability of soil nutrients. Boreal forest soils
died because of predation (O'Donoghue 1994). These ob-
are typically impoverished in nutrients, particularly nitro-
servations of natural history support the contention that pre-
gen, and nutrients added through fertilizer are immedi-
dation by a variety of birds and mammals plays an important
ately taken up by plants. We measured plant responses to
role in the hare cycle.
nutrient additions and found large increases in individual
For mammalian predators in winter, we used snow track-
plant growth in grasses, shrubs, and trees (Turkington et al.
ing to monitor density changes and kill rates of lynx and coy-
1998). None of this plant improvement resulted in more
otes. All hare predators showed strong numerical changes that
snowshoe hares on the fertilized areas, relative to the con-
lagged behind the hare cycle 1–2 years (Boutin et al. 1995).
trols; we therefore concluded that the dynamics of the hare
In addition, both lynx and coyotes killed more hares per day
cycle cannot be changed by nutrient additions to the ecosys-
in the peak and decline phases than during the increase.
tem. The message seemed to be repeated: The hare cycle is
These kill rates were well above previous estimates and well
not driven primarily by plant–herbivore interactions.
in excess of energy demands. Surplus killing seems to be a
The predation hypothesis is the next most likely expla-
characteristic feature of these predators.
nation for the hare cycle. In our studies with radio-collared
To test the predation hypothesis, we excluded mammalian
hares, the immediate cause of death of 95% of the hares was
predators from two areas by constructing electric fences in
predation by a variety of predators, the main ones in the
the Yukon, each 1 km2. We also attempted to exclude avian
Yukon being lynx, coyotes, goshawks, and great horned owls
predators from smaller areas inside the electric fence by
(Rohner and Krebs 1996, O'Donoghue et al. 1997). Few
means of fish netting and monofilament fishing line strung
hares died with signs of malnutrition, and those that did
in trees, but these proved impractical and ineffective. The
starve were found more often in the increase and peak
fence was permeable to hares and other small mammals,
phases rather than in the decline (Hodges 2000a). In con-
which could move in and out at will. In one fenced area we
trast to adult hares, leverets are killed by a variety of small
also added food, so we had a combined treatment manip-
raptors, such as boreal owls, red-tailed hawks, kestrels, and
ulating predation pressure and food supplies (Krebs et al.
hawk owls, and by small mammals, particularly red squir-
1995). Because of the size of the exclosures and the main-
January 2001 / Vol. 51 No. 1 BioScience 29
Figure 6. Changes in survival rates of
snowshoe hare numbers on control
and fenced areas during the
population cycle of 1988–1996 at
Kluane, Yukon. The arrows show the
mean survival rate for each
treatment. The survival rate per 30
days is averaged over each year, with
90% confidence limits, for radio-
collared hares.
tenance they required, we were unable to replicate these
food during the peak of the cycle in 1989 and 1990 had no
fence treatments.
impact on reproductive output (O'Donoghue 1994). How-
The main impact of the electric-fence predator exclo-
ever, during the decline phase in 1991 and 1992, the preda-
sures was to increase the survival rate of radio-collared
tor exclosure plus food treatment caused a dramatic increase
hares. Figure 6 shows that the collapse in survival that nor-
in reproductive output over that found in control hares
mally occurs in the peak and decline phases of the cycle was
(Hik 1995, Stefan 1998). Because we were unable to obtain
nearly eliminated by the exclusion of mammalian predators.
these reproductive data on food addition sites in 1991 and
There is a slight decrease in survival during the decline
1992, we do not know if it is the additional food or the ex-
phase with the combined predator exclosure and food treat-
clusion of mammalian predators, or a combination of these
ment, but the impact of additional food on survival during
factors, that produced the results shown in Figure 7.
the decline is small. The mortality rate is almost entirely dri-ven by predation.
Two further conclusions follow from these experiments.
How might predator exclusion affect reproductive output?
Inasmuch as avian predators had access to the two preda-
There has been much interest in the indirect effects of preda-
tor exclosures, avian predation by itself is not sufficient to
tors on prey (Lima 1998). One way in which predators
explain the changes in survival rates. There is a slight re-
might cause a decline in reproductive output is by stressing
duction in survival inside the predator exclosures during the
hares through high rates of encounter and repeated unsuc-
decline (Figure 6), and this is a measure of the impact of bird
cessful attacks during the peak and decline phases (Boon-
predation by itself on hare survival. Since many species of
stra et al. 1998a). Chronic stress has many direct detrimental
predators are involved in causing the collapse in prewean-
effects on mammals, including reduction in reproductive
ing, juvenile, and adult hare survival, we cannot pinpoint the
rate, mobilization of energy reserves, and increased sus-
role of any individual predator species. In particular, the
ceptibility to diseases and parasites. Stress effects may also
snowshoe hare cycle is not strictly a lynx–hare cycle, as
be indirect and long term, affecting adult brain function
many textbooks claim, and if the lynx is removed from the
(Lupien and McEwen 1997) and offspring viability and be-
predator community—as it is on Anticosti Island in the St.
havior (Matthews 2000). Figure 8 illustrates these ideas for
Lawrence River in eastern Canada—the hare cycle contin-
a hypothetical hare cycle in which predation pressure causes
ues unchanged because of predator compensation (Stenseth
chronic stress. Unfortunately, we have not yet been able to
et al. 1998).
measure stress levels through an entire hare cycle, but the data
If we can explain the changes in mortality rates by
we have from declining and low populations is consistent
predation, how can we explain the changes in reproduction
with this hypothetical scheme.
that accompany the cycle? We have attacked this question
What is the role of high-quality food in the hare cycle?
experimentally by providing hares on two open areas with
Population density increased on areas provided with sup-
supplemental food year-round. We measured annual re-
plemental food. Hares on the areas provided with supple-
productive output for female hares by multiplying preg-
mental food did not lose weight over winter and in general
nancy rates and average litter sizes for each of the summer
were in better body condition than control hares. Food
litters and summing these for each breeding season. Figure
quality clearly limits body condition and population den-
7 summarizes our data. Reproductive output at Kluane
sity in hares. In addition to these direct effects, changes in
Lake was severely reduced in the decline phase, as Lloyd Keith
food quality could have subtle indirect effects on hares,
(Cary and Keith 1979) found in Alberta (Figure 2). Adding
contributing to chronic stress and making them more or less
30 BioScience January 2001 / Vol. 51 No. 1
Figure 7. Reproductive output of female snowshoe hares over a population cycle at Kluane Lake, Yukon. The blue line shows
hare density in control populations. Supplemental food (green bars) does not affect reproductive output at the peak of the
cycle, but in the decline phase of 1991 and 1992 high reproductive output was sustained on the experimental area fenced
from mammalian predators and provided with supplemental food (red bars). We were unable to measure reproductive
output in every year for all treatments, and thus we do not know for the critical decline years of 1991 and 1992 whether
reduced reproductive output is caused by food or predation or both.
able to avoid predators, parasites, and disease (Hik 1995,
(Krebs 1986). Hares are not territorial, and aggression seems
Boonstra et al. 1998a, Hodges et al. 1999). Poorer body con-
to be restricted to males competing in mating chases and to
dition in the peak of the cycle is one consequence when hares
females resisting attentive males. There are no records of in-
overgraze their preferred winterfoods and suffer from chronicstress, causing them to mobilizeenergy. This loss of condition maylead to reduced ability to avoidpredators and thus to an increase inhares' stress levels. The impact ofextra food would therefore not di-rectly affect reproduction and mor-tality rates, but it would actindirectly to make hares less sus-ceptible to stress. Indirect effectshave limited power to affect popu-lation dynamics, however, and allour feeding experiments show thatextra food does not make hares lesssusceptible to predation and cannotprevent the hare population de-cline.
Figure 8. The chronic stress hypothesis as an explanation of the changes in reproduction
Another possible source of stress
that accompany the snowshoe hare cycle. Chronic stress is postulated to arise from signs
for hares is social interactions.
of predator abundance (odor, visual sightings, tracks, unsuccessful chases), and is thus
Snowshoe hares are not particu-
related to predator densities. According to this hypothesis, any physiological measure of
larly good candidates for social reg-
chronic stress, such as cortisol levels, should be maximal late in the decline phase of the
ulation of population size, and
hare cycle. The immune system would also be compromised because of chronic stress.
most hypotheses about hare cycles
We do not yet know whether this hypothesis is the correct explanation for the reduced
have ignored the social dimension
reproduction that occurs during the hare cycle.
January 2001 / Vol. 51 No. 1 BioScience 31
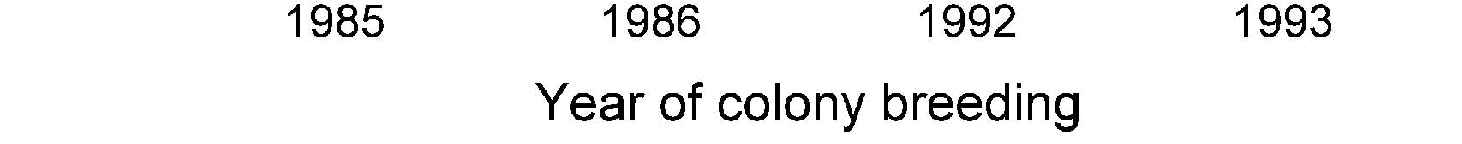
Figure 9. The annual reproductive
output of snowshoe hare females
maintained in a colony at
Vancouver. Individual females were
taken from field populations and
classified by the state of the field
population at the time of initial
capture. For 4 years there were
enough females to compare
simultaneously the population of
females taken from the peak phase
of the cycle with those taken from
the low phase. Low-phase females
maintain a lifetime reproductive
output much greater than peak-
phase females; these lab results
mimic the observed changes in field
populations. Numbers above bars
are numbers of females.
fanticide in hares, and without territoriality and infanti-
predation and food supplies, but of these two factors, pre-
cide there would seem to be little room for social interactions
dation is clearly the dominant process. The impact of food
to play a role in population dynamics.
is felt largely in winter and it is mostly indirect. Hares do not
Nevertheless, there remain some puzzles. One is the lack
usually die directly of starvation or malnutrition—the im-
of an explanation for the low phase, which follows the de-
mediate cause of death is virtually always predation. But food
cline and lasts 2–4 years (Boonstra et al. 1998b). A second
quality and quantity affect body condition and in this way
puzzle is evidence implicating the role of maternal effects in
may predispose hares to predation, increased parasite loads,
reproductive performance. Tony Sinclair maintained a lab-
and higher levels of chronic stress. These indirect effects of
oratory colony of hares for 14 years in Vancouver to test for
predation and food are the probable cause of reduced re-
impacts of secondary chemicals on food choice. Females were
productive output. Hares in peak and declining popula-
maintained in individual cages with high-quality food and
tions must trade off safety and food, and these behavioral
were treated for potential parasites. Hares in captivity reg-
tradeoffs define the dynamics of the decline (Hik 1995,
ularly live 5–7 years, much longer than they live in nature.
Hodges 2000b). The result is a time lag in both the indirect
Therefore the colony at times consisted of a mixture of fe-
effects and the direct effects of predation, which causes the
males trapped live in the Yukon from peak populations and
cyclicity. The low phase of the cycle is the combined result
from low populations. Figure 9 compares the reproductive
of continuing predation mortality and slowly recovering
output in captivity of these two groups of hares (A.R.E.
Sinclair, personal communication, 1999). Hares taken fromlow populations maintained a lifetime reproductive output
Synchrony in the hare cycle
more than double that of hares taken from peak populations,
Not only do snowshoe hare numbers fluctuate in 10-year cy-
suggesting that female hares are already programmed for
cles, but these cycles tend to occur in synchrony across
their reproductive success early in life, and no matter how
much of the boreal forests of Canada and Alaska. Synchrony
fine an environment is provided, they cannot change.
is not absolute, however; different regions within Canada can
These results are completely serendipitous: This was not
drift out of phase. Smith (1983) used questionnaire data on
a planned comparison, females could not be matched for age,
snowshoe hare abundance from 1931 to 1948 to measure
and this reproductive measurement was not the reason the
synchrony across Canada (Figure 10). He concluded that
colony was maintained. There was no correlation in these
there was a large area of cyclic synchrony in northern Man-
colony data between the reproductive output of mothers and
itoba and Saskatchewan that was 2 years ahead of "average,"
daughters, suggesting that this effect was not a genetic. A
and areas in southern Ontario and Quebec that were up to
planned comparison of colony hares should be carried out
2 years later than average for this time period. Populations
to see whether the effect can be verified in another popula-
in the Yukon were also up to 2 years later than average. In
tion. At present, these results must be considered unproven,
some sense, the peak of the hare cycle for this time period
but tantalizing.
was like a traveling wave in a pond, with the center in the
We are now close to understanding the snowshoe hare cy-
middle of the boreal forest. The result of this kind of trav-
cle. The 10-year cycle is a result of the interaction between
eling wave is that synchrony falls off with distance, as shown
32 BioScience January 2001 / Vol. 51 No. 1
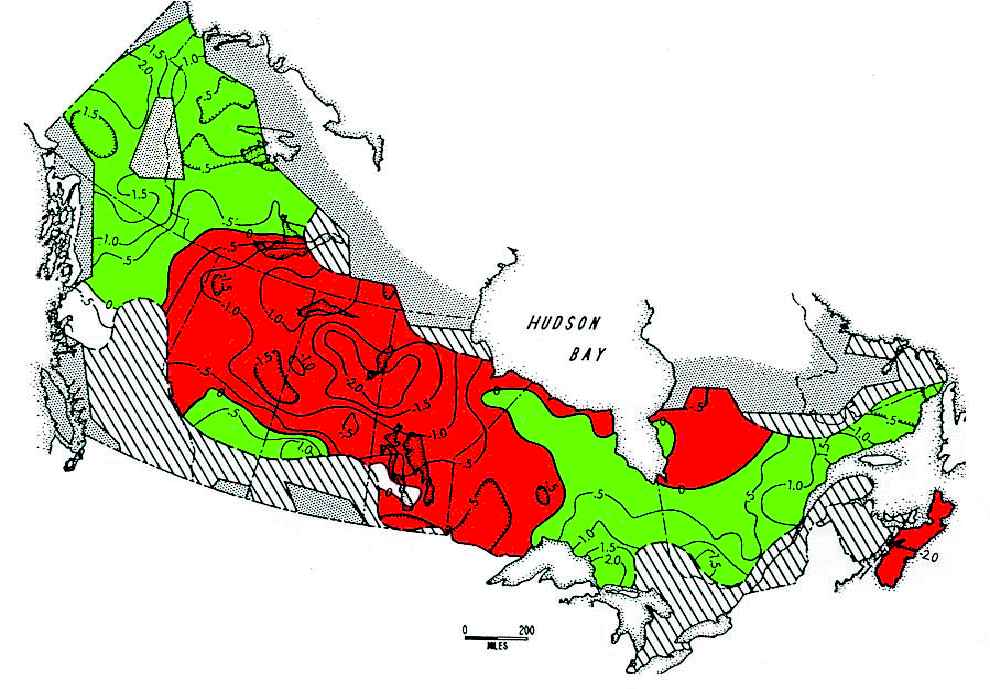
larly structured (Figure 12). These three regions coincidedwith the climatic zones defined independently from theNorth Atlantic Oscillation (NAO), a climatic oscillationsimilar to the El Niño in the Pacific Ocean. The reasons forthese correlations are not clear, though Stenseth et al. (1999)suggest that the changing snow levels associated with theNAO might affect the hunting efficiency of lynx.
What ecological factors might cause synchrony across
large geographic regions? Two general models for synchronycan be suggested, one driven by weather and one by dispersalmovements. The most famous model to synchronize the harecycle across North America is the weather model related tothe sunspot cycle (Sinclair et al. 1993). Sunspots and harenumbers are highly correlated for three time periods dur-ing the past 250 years: 1751–1787, 1838–1870, and1948–1986, which were all periods of high-amplitudesunspot fluctuations (Sinclair and Gosline 1997). Because
Figure 10. Synchrony in snowshoe hare cycles across
sunspots affect broad weather patterns, it is possible that
Canada, 1931–1948, as measured by questionnaires
sunspots might, through weather, entrain snowshoe hare cy-
(Chitty 1948, 1950). The average peak phase across
cles across the continent when solar activity is unusually high.
Canada was scaled as 0.0, and the contour lines indicate
The main problem with this explanation is that there is at
peaks occurring earlier than average (red, negative
present no mechanism for sunspot-affected weather patterns
contours) or later than average (green, positive contours).
to translate into demographic impacts on hares. All our
During this period, hare peaks were reached earliest in
studies in the Yukon suggest the predominant role of preda-
the central boreal region of northern Saskatchewan and
tors in driving the hare cycle. As mentioned above, Stenseth
Manitoba (Smith 1983).
et al. (1999) suggested an indirect impact of snow depth on
in Figure 11 for lynx fur return data from the Canadian
hunting success of mammalian predators as one way in
provinces for the period 1920–1987 (Ranta et al. 1997).
which synchrony might be coordinated, but there are no data
Because Canada lynx fur harvests are highly correlated
to test this suggestion. There is also a problem in reconcil-
with snowshoe hare numbers, the fur trading records of
ing the global nature of sunspot activity with the local vari-
the Hudson's Bay Company have been used extensively to
ations in synchrony shown in Figure 10.
analyze synchrony in the 10-year cycle (Stenseth et al. 1999,
Dispersal movements would appear to be the more likely
Haydon and Greenwood 2000). Regions across Canada
explanation for synchrony. Hares disperse on a local scale of
showed a higher degree of synchrony in the 19th century than
a few kilometers (Gillis 1997, Hodges 2000b), so hare move-
during the 20th century, for reasons that are not clear.
ments seem incapable of affecting synchrony except on a lo-
Within Canada, Stenseth et al. (1999) showed, there were
cal scale. Predator movements are more likely the key element
three broad regions within which the lynx cycle was simi-
causing synchrony within the boreal region. Long-range
Figure 11. Synchrony among pairs of
provinces in Canada for lynx fur
return data from 1920 to 1987. The
geographic midpoint of each province
was used to calculate the distance
between provinces. The synchrony
index is the simple correlation
coefficient between the pairs of
provinces. Synchrony falls off with
distance and then rises again because
British Columbia and the Yukon as
well as eastern Canada tend to peak
2–3 years later than the average of
central Canada. The dashed line is a
simple polynomial regression. Data
from Statistics Canada, as used by
Ranta et al. (1997).
January 2001 / Vol. 51 No. 1 BioScience 33
Lotka and Volterra were
partly correct when they
guessed that the snowshoe
by the North
hare cycle was a predator–
prey oscillation, but they
missed the critical point that
large circles define
the cycle can be understood
the three regions
only by analyzing three
within which the
trophic levels rather than
lynx cycle is most
two. The hare cycle is pro-
duced by an interaction
between predation and food
regions fit within
supplies, and its biological
impacts ripple across many
species of predators and prey
zones. Stenseth
in the boreal forest.
et al. (1999).
The boreal forest is one of
the great ecosystems of theearth, and the 10-year snow-
dispersal movements of radio-collared lynx have been de-
shoe hare cycle is one of the most striking features of this
scribed in several studies (Mowat et al. 2000). Documented
ecosystem. After 70 years of questionnaire research, time
dispersal movements up to 1100 km have been recorded, with
series analyses, and field experiments, we have a good
15 movements greater than 500 km. These dispersal move-
understanding of the dynamics behind the hare cycle and
ments were detected only because the dispersing lynx were
the importance of predation and food supplies in regulat-
caught by fur trappers, and thus constitute a biased (trun-
ing that cycle. The snowshoe hare is a critical species in the
cated) set of true dispersal movements. Houston (1978) re-
boreal forest: If it should disappear, many species of
ported similar data for movements of great horned owls, with
predators would go with it, and the structure of the plant
36 banded owls moving 265 to1415 km before being shot,
community would be altered substantially.
trapped, or found dead. These data for great horned owls and
The boreal forest ecosystem and the 10-year cycle in
lynx indicate the potential for predator dispersal to syn-
snowshoe hares are clearly resilient to a variety of natural
chronize hare cycles over large areas of the boreal forest re-
disturbances, from forest fires to short-term climatic fluc-
gion (Ydenberg 1987, Ims and Steen 1990). If predators
tuations, but it is unclear whether they can withstand the
move from food-poor to food-rich regions on a scale of 1000
changes that humans impose without collapsing. The
km, they could readily bring local regions into synchrony in
impact of directional climate change on the boreal forest
the general pattern shown in Figure 10. The predator-
community will probably be less in the short term than the
dispersal hypothesis for synchrony of hare cycles is attrac-
impact of forestry and other human activities such as oil
tive because it is parsimonious, since predation plays such
and gas exploration. We should explore the limits of
an important role in driving the hare cycle through its
resilience of the snowshoe hare cycle as forest harvesting
direct effects on hare mortality and its indirect effects on hare
extends north into the boreal forest, lest we compromise
this fascinating system.
Lynx, which have been declared a threatened species in the
continental United States, and the dynamics of its interac-
tion with snowshoe hares in states with southern borealforests, such as Montana, are in critical need of study (Rug-
Boonstra R, Hik D, Singleton GR, Tinnikov A. 1998a. The impact of
predator-induced stress on the snowshoe hare cycle.
giero et al. 2000). If hare populations are synchronized re-
gionally by predator movements, the fragmentation of hare
Boonstra R, Krebs CJ, Stenseth NC. 1998b. Population cycles in mammals:
and lynx habitat in the southern parts of Canada and in the
The problem of explaining the low phase.
northern United States could break the ebb and flow of
Boutin S, et al. 1995. Population changes of the vertebrate community dur-
these linkages. The present shortage of genetic informa-
ing a snowshoe hare cycle in Canada's boreal forest.
Bryant JP. 1981. Phytochemical deterrence of snowshoe hare browsing by ad-
tion on the geographic structure of both snowshoe hare
ventitious shoots of four Alaskan trees. Science 213: 889–890.
populations and lynx populations needs to be remedied to
Bryant JP,Wieland GD, Clausen T, Kuropat P. 1985. Interactions of snowshoe
define these spatial issues more clearly for conservation.
hare and feltleaf willow in Alaska.
34 BioScience January 2001 / Vol. 51 No. 1
Cary JR, Keith LB. 1979. Reproductive change in the 10-year cycle of snow-
SW, Koehler GM, Krebs CJ, McKelvey KS, Squires JR, eds. Ecology and
shoe hares. Canadian Journal of Zoology 57: 375–390.
Conservation of Lynx in the United States. Denver (CO): University
Chitty H. 1948. The snowshoe rabbit enquiry, 1943–46. Journal of Animal
Press of Colorado.
Ecology 17: 39–44.
Murray DL, Cary JR, Keith LB. 1997. Interactive effects of sublethal nema-
———. 1950. The snowshoe rabbit enquiry, 1946–48. Journal of Animal Ecol-
todes and nutritional status on snowshoe hare vulnerability to predation.
ogy 19: 15–20.
Elton C, Nicholson M. 1942. The ten-year cycle in numbers of the lynx in
O'Donoghue M. 1994. Early survival of juvenile snowshoe hares.
Canada. Journal of Animal Ecology 11: 215–244.
Gillis EA. 1997. Natal dispersal and post-weaning survival of juvenile snow-
O'Donoghue M, Boutin S, Krebs CJ, Hofer EJ. 1997. Numerical responses of
shoe hares during a cyclic population increase. Master's thesis. Univer-sity of British Columbia, Vancouver, BC.
coyotes and lynx to the snowshoe hare cycle.
Haydon DT, Greenwood PE. 2000. Spatial coupling in cyclic population dy-
Ranta E, Kaitala V, Lundberg P. 1997. The spatial dimension in population
namics: Models and data.
Hik DS. 1995. Does risk of predation influence population dynamics? Evi-
Rodgers AR, Sinclair ARE. 1997. Diet choice and nutrition of captive snow-
dence from the cyclic decline of snowshoe hares. Wildlife Research 22:
shoe hares (Lepus americanus): Interactions of energy, protein, and plant
secondary compounds.
Hodges KE. 2000a. The ecology of snowshoe hares in northern boreal forests.
Rohner C, Krebs CJ. 1996. Owl predation on snowshoe hares: Consequences
Pages 117–161 in Ruggiero LF, Aubry KB, Buskirk SW, Koehler GM, Krebs
of antipredator behaviour.
CJ, McKelvey KS, Squires JR, eds. Ecology and Conservation of Lynx in
Ruggiero LF, Aubry KB, Buskirk SW, Koehler GM, Krebs CJ, McKelvey KS,
the United States. Denver (CO): University Press of Colorado.
Squires JR, eds. 2000. Ecology and Conservation of Lynx in the United
———. 2000b. Proximate factors affecting snowshoe hare movements dur-
States. Boulder (CO): University Press of Colorado.
ing a cyclic population low phase.
Sinclair ARE, Gosline JM. 1997. Solar activity and mammal cycles in the
Hodges KE, Krebs CJ, Sinclair ARE. 1999. Snowshoe hare demography dur-
Northern Hemisphere.
ing a cyclic population low.
Houston CS. 1978. Recoveries of Saskatchewan-banded great horned owls.
Sinclair ARE, Smith JNM. 1984. Do plant secondary compounds determine
Canadian Field-Naturalist 92: 61–66.
feeding preferences of snowshoe hares?
Ims RA, Steen H. 1990. Geographical synchrony in microtine population cy-
Sinclair ARE, Krebs CJ, Smith JNM, Boutin S. 1988. Population biology of
cles: A theoretical evaluation of the role of nomadic avian predators.
snowshoe hares. III. Nutrition, plant secondary compounds and food lim-
Keith LB. 1990. Dynamics of snowshoe hare populations. Current Mam-
Sinclair ARE, Gosline JM, Holdsworth G, Krebs CJ, Boutin S, Smith JNM,
malogy 4: 119–195.
Boonstra R, Dale M. 1993. Can the solar cycle and climate synchronize
Keith LB, Windberg LA. 1978. A demographic analysis of the snowshoe
the snowshoe hare cycle in Canada? Evidence from tree rings and ice cores.
hare cycle. Wildlife Monographs 58: 1–70.
American Naturalist 141: 173–198.
Keith LB, Cary JR, Rongstad OJ, Brittingham MC. 1984. Demography and
Smith CH. 1983. Spatial trends in Canadian snowshoe hare, Lepus americanus,
ecology of a declining snowshoe hare population. Wildlife Monographs
population cycles.
Smith JNM, Krebs CJ, Sinclair ARE, Boonstra R. 1988. Population biology
Keith LB, Cary JR,Yuill TM, Keith IM. 1985. Prevalence of helminths in a cyclic
of snowshoe hares. II. Interactions with winter food plants.
snowshoe hare population.
Krebs CJ. 1986. Are lagomorphs similar to other small mammals in their pop-
ulation ecology? Mammal Review 16: 187–194.
Sovell JR, Holmes JC. 1996. Efficacy of ivermectin against nematodes infecting
Krebs CJ, Boutin S, Gilbert BS. 1985. A natural feeding experiment on a de-
field populations of snowshoe hares (Lepus americanus) in Yukon,
clining snowshoe hare population.
Krebs CJ, Gilbert BS, Boutin S, Sinclair ARE, Smith JNM. 1986. Population
Stefan CI. 1998. Reproduction and pre-weaning juvenile survival in a cyclic
biology of snowshoe hares. I. Demography of food-supplemented pop-
population of snowshoe hares. Master's thesis. University of British Co-
ulations in the southern Yukon, 1976–84.
lumbia, Vancouver, BC.
Stenseth NC, Falck W, Chan KS, Bjornstad ON, O'Donoghue M, Tong H,
Krebs CJ, Boutin S, Boonstra R, Sinclair ARE, Smith JNM, Dale MRT, Mar-
Boonstra R, Boutin S, Krebs CJ, Yoccoz NG. 1998. From patterns to
tin K, Turkington R. 1995. Impact of food and predation on the snow-
processes: Phase and density dependencies in the Canadian lynx cycle.
shoe hare cycle.
Krebs CJ, Boutin S, Boonstra R, eds. 2001. Ecosystem Dynamics of the Bo-
Stenseth NC, et al. 1999. Common dynamic structure of Canada lynx pop-
real Forest: The Kluane Project. New York: Oxford University Press.
ulations within three climatic regions.
Lima SL. 1998. Nonlethal effects in the ecology of predator–prey interactions.
Turkington R, John E, Krebs CJ, Dale MRT, Nams V, Boonstra R, Boutin S,
Martin K, Sinclair ARE, Smith JNM. 1998. The effects of fertilization on
Lupien SJ, McEwen BS. 1997. The acute effects of corticosteroids on cogni-
tion: Integration of animal and human-model studies.
the vegetation of the boreal forest. Journal of Vegetation Science 9:
Matthews SG. 2000. Antenatal glucocorticoids and programming of the de-
Winterhalder BP. 1980. Canadian fur bearer cycles and Cree-Ojibway hunt-
ing and trapping practices. American Naturalist 116: 870–879.
Mowat G, Poole KG, O'Donoghue M. 2000. Ecology of lynx in northern
Ydenberg RC. 1987. Nomadic predators and geographical synchrony in mi-
Canada and Alaska. Pages 265–306 in Ruggiero LF, Aubry KB, Buskirk
crotine population cycles.
January 2001 / Vol. 51 No. 1 BioScience 35
Source: http://fla.st/1m4r1NB
Catamaran Home Delivery 1 Member information: Please verify or provide member information below. Please send me e-mail notices about the status of the enclosed Member ID: prescription(s) and online ordering at: New Shipping Address: Catamaran Home Delivery will keep this address on file for all orders from City, State, Zip:
Utilization of Benzodiazepines and Barbiturates after Medicare Part D Coverage Van Doren Hsu, Pharm.D., Jean O'Donnell, M.S. Figure 2. Barbiturate PDEs by Pharmacy Dispensing Type and Prescriber Specialty Figure 5. Prevalence of Benzodiazepine by Drug • Overall BARB and BZD PDE use and by • A total of 65.8% and 72.9% of Benzodiazepines (BZD) and barbiturates (BARB) were excluded from