Doi:10.1016/j.jsbmb.2005.02.018
Journal of Steroid Biochemistry & Molecular Biology 96 (2005) 201–215
Effects of dehydroepiandrosterone, Premarin and Acolbifene on
histomorphology and sex steroid receptors in the rat vagina
L. Berger, M. El-Alfy, C. Martel, F. Labrie
Oncology and Molecular Endocrinology Research Center, Laval University Medical Center (CHUL),
2705 Laurier Boulevard, Quebec City, Que., Canada G1V 4G2
Received 14 June 2004; accepted 4 February 2005
To assess the specific estrogenic and/or androgenic effects of a potential novel hormone replacement therapy, we have examined the
morphology of the rat vagina 9 months after ovariectomy (OVX) and treatment of OVX animals with dehydroepiandrosterone (DHEA),conjugated estrogens Premarin and the selective estrogen receptor modulator Acolbifene. OVX led to atrophy and inflammatory changes whileAcolbifene reduced the inflammation incidence and induced mucification of the vaginal epithelium. Premarin induced a typical keratinizedstratified squamous epithelium while DHEA induced stimulation of the vaginal epithelium, with mucous cells typical of an androgenic effect,combined with increased collagen fiber compactness of the lamina propria. On the other hand, after OVX, the vaginal muscle layer decreasedby 46%, an effect which was 41 and 100% reversed by DHEA and Premarin, respectively. The present data show particularly interestingeffects of DHEA on the three layers of the vaginal wall, namely a highly mucified epithelium, an increased muscularis thickness and increasedcollagen fiber compactness in the lamina propria. DHEA exerts both androgenic and estrogenic effects on the vaginal mucosa, thus providinga more physiological replacement therapy.
2005 Elsevier Ltd. All rights reserved.
Androgen receptor; DHEA; Antiestrogen; SERM; Acolbifene; EM-652; Estrogen receptor; Hormone replacement therapy; Intracrinology;
Menopause; Progesterone receptor; Vagina; Sexual dysfunction
There is an increasing interest in the potential of combined
estrogen–androgen replacement therapy although the
Vaginal dryness affects about 50% of postmenopausal
use of the estrogenic component is limited by the potential
women at the age of 50–60 years and 72% after 70 years
negative impact on breast cancer and cardiovascular events
Of these women, about 80% experience urogenital disorders,
upon recent advances in our understanding of
especially vaginitis and dyspareunia Since these prob-
human sex steroid physiology, especially in postmenopausal
lems are believed to be at least partially related to the depri-
women use of DHEA becomes, in this case, a pos-
vation of sex steroids, appropriate local hormonal replace-
sibility to provide with the appropriate levels of androgens
ment therapy should be considered. In fact, postmenopausal
and estrogens synthesized in specific tissues by intracrine
women do not only lack all ovarian estrogens, but they are also
mechanisms, while avoiding systemic effects
progressively deprived of the androgens originating from the
The selective estrogen receptor modulator (SERM) Acolb-
peripheral intracrine transformation of dehydroepiandros-
ifene (EM-652) is a benzopyran derivative originally devel-
terone (DHEA) into both androgens and estrogens In
oped for the prevention and treatment of breast cancer
fact, serum DHEA and DHEA-S progressively decrease from
Acolbifene is the compound having the highest affinity of all
the age of 30–50 years
known compounds for the estrogen receptor (ER) This compound displays a pure and highly potent antie-
∗ Corresponding author. Tel.: +1 418 654 2704; fax: +1 418 654 2735.
strogenic activity in the mammary gland and endometrium
E-mail address: [email protected] (F. Labrie).
while decreasing serum cholesterol and triglycerides and
0960-0760/$ – see front matter 2005 Elsevier Ltd. All rights reserved.
doi:10.1016/j.jsbmb.2005.02.018
L. Berger et al. / Journal of Steroid Biochemistry & Molecular Biology 96 (2005) 201–215
preventing bone loss, at least in the rat On the other
(9) OVX + DHEA + Acolbifene + Premarin. On the first day
hand, the inhibitory effect of DHEA on the growth of human
of the study, the animals of all groups (except group one) were
breast cancer xenografts in nude mice provides further sup-
bilaterally ovariectomized under isoflurane-induced anesthe-
port for its use in hormone replacement therapy In
sia. Premarin and Acolbifene were administered by oral
fact, combined treatment of DHEA and Acolbifene has been
gavage (0.5 mL/rat) as suspensions in 0.4% methylcellulose
proposed as a beneficial chemopreventive and therapeutic
while DHEA was solubilized in 50% ethanol–50% propylene
approach in breast cancer It is possible that the combi-
glycol and was topically applied (0.5 mL/rat) on a shaved area
nation of DHEA, Acolbifene and possibly an estrogen could
of 2 cm × 2 cm of the dorsal skin. Acolbifene was synthe-
provide optimal benefits for women at menopause. Recently,
sized in the medicinal chemistry division of our laboratory,
we found that the high potency of Acolbifene completely
as described The compound had a purity of 99.2%.
blocks the stimulatory effect of estradiol (E2) on the mam-
DHEA was obtained from Scheweizerhall Inc. at a purity
mary gland and uterus in the rat and could thus avoid the risk
of 100% while Premarin was a product of Wyeth Pharma-
of breast and uterine cancer
ceuticals for intravenous human use containing 24.8 mg/vial
In the present study, the ovariectomized (OVX) rat model
(claimed for 25 mg) of total conjugated estrogens of which
was used to examine the effects of DHEA, Premarin and
54.4% were sodium estrone sulfate and 28.7% sodium equi-
Acolbifene, alone or in combination, on the morphology and
lin sulfate. At the DHEA administered dose, the DHEA blood
on the distribution of ER␣, PR and AR after 9 months of
level ranged between 70 and 100 nmol/L
treatment. Since the rat adrenal does not secrete DHEA or
selection for Premarin corresponds to the min-
DHEA-S the percutaneous administration of DHEA
imal dose sufficient to reverse OVX-induced uterine atrophy,
in OVX animals, while preventing first pass of the orally
while Acolbifene was administered at a dose sufficient to
administered steroid through the liver, is the only source of
cause uterine atrophy similar to OVX after its administration
sex steroids in the model used, thus facilitating the interpre-
to Premarin-treated OVX animals. Treatments were initiated
tation of the data. As an estrogen, Premarin was chosen in
on day 2 of the study and the compounds were administered
this protocol because it is the most commonly used estro-
once daily for 36 weeks. Animals from the intact and OVX
gen preparation in North America as hormone replacement
control groups received the vehicle alone by oral gavage and
therapy. In a previous study performed in our laboratory, the
morphology observed following treatment of OVX rats with
Twenty-four hours after the last dosing, overnight fasted
17-estradiol was identical to that seen with the use of Pre-
animals were sacrificed under isoflurane anesthesia by exsan-
guination at the abdominal aorta (nine animals per group) orby intracardiac perfusion with 10% neutral buffered formalin(five animals per group). Vaginae from non-perfused animals
2. Materials and methods
were collected and weighed, while the vaginae collected fromperfused animals were marked with black ink on the ventral
2.1. Animals and treatments
side and then trimmed as described below.
Ten to 12-week-old female Sprague–Dawley rats (Crl:
2.2. Histological procedures
CD®(SD)Br VAF/PlusTM) (Charles River Laboratory, St-Constant, Canada) weighing approximately 220–270 g at
The entire vagina of each perfused animal was postfixed in
start of the experiment were used. The animals were
10% neutral buffered formalin. Each vagina was then divided
acclimatized to the environmental conditions (temperature:
into seven equal cross-segments as illustrated in rou-
22 ± 3 ◦C; humidity: 50 ± 20%; 12-h light/12-h dark cycles,
tinely processed and embedded all together in the same paraf-
lights on at 07:15 h) for at least 1 week before starting the
fin block. Within the paraffin block, the seven vaginal cylin-
experiment. The animals were housed individually and were
drical segments were positioned in a sequence corresponding
allowed free access to water and rodent food (Lab Diet 5002,
to their original anatomical position and oriented perpendic-
Ralston Purina, St. Louis, MO). The experiment was con-
ular to the surface of the block, thus allowing the segments to
ducted in accordance with the CCAC Guide for Care and
be cut in cross-sections. For each animal, a 4 m-thick paraf-
Use of Experimental Animals in an animal facility approved
fin section was cut and stained with haematoxylin–eosin for
by the Canadian Council on Animal Care (CCAC) and the
Association for Assessment and Accreditation of LaboratoryAnimal Care (AAALAC).
A total of 126 female rats were randomly distributed
into 9 groups of 14 animals each as follows: (1) intact
Measurements of the different vaginal layers were per-
control; (2) ovariectomized control; (3) OVX + Acolbifene
formed on the fifth segment (which is approximately
(2.5 mg/kg); (4) OVX + Premarin (0.5 mg/kg); (5) OVX +
halfway between the middle region and the portio vaginalis
Premarin + Acolbifene; (6) OVX + DHEA (80 mg/kg); (7)
uteri (segment 7). This fifth segment was found to display
OVX + DHEA + Acolbifene; (8) OVX + DHEA + Premarin;
a representative epithelial surface and a sufficient thickness
L. Berger et al. / Journal of Steroid Biochemistry & Molecular Biology 96 (2005) 201–215
ies, at 1:200, 1:250 and 1:250, respectively. After washingin PBS buffer, sections were incubated with biotinylatedantirabbit secondary antibody for 10 min and thereafter withstreptavidin–peroxidase for another 10 min (Zymed, CA).
Diaminobenzidine was used as the chromogen to visual-ize the biotin/streptavidin–peroxidase complex, under micro-scope monitoring. Counterstaining was performed using #2Gill's hematoxylin for 30 s. For controls, immunoabsorptionwith an excess of the peptide used to raise the antibody, orsubstitution with non-immune rabbit IgG, was performed.
Semi-quantitative evaluation of the number and intensityof immunostained nuclei was performed as indicated in
2.5. Statistical analysis
Data are presented as means ± S.E.M. of eight to nine ani-
mals per group for vaginal weight or five animals per groupfor vaginal layer thickness determinations. Statistical signif-icance was determined according to the multiple-range testof Duncan–Kramer
3. Results
3.1. Morphology and thickness of the different layers of
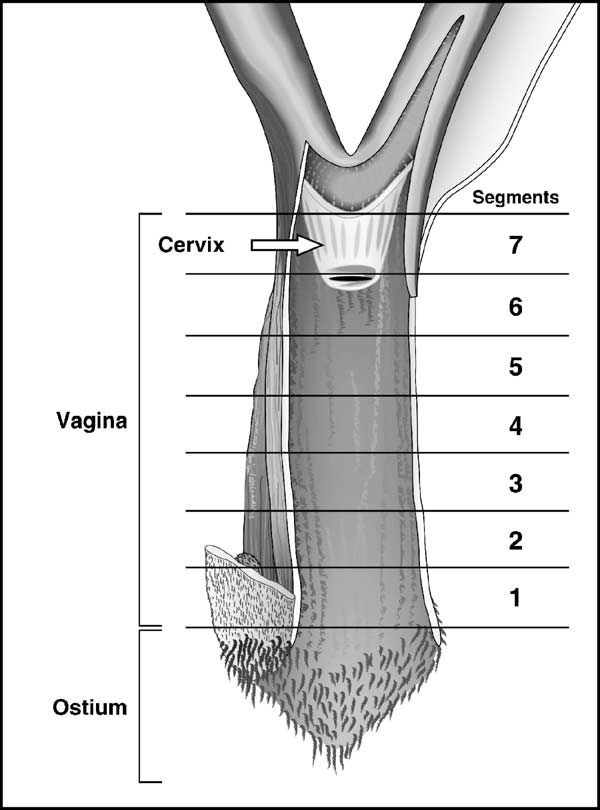
Fig. 1. Division along the longitudinal axis of the rat vagina into seven cross-
rat vagina
segments, from the external orifice (ostium) (segment 1) to the cervix level(segment 7) (portio vaginalis uteri) (modified from Popesko et al. Eachsegment is about 3–4 mm long.
To examine with precision the three layers of the rat
vaginal wall, namely the epithelium, lamina propria and mus-cularis, seven segments obtained along the longitudinal axis
of smooth muscle. Images were captured with a DC-330
(were first examined. While important morphologi-
3CCD color camera (Dage-MTI, Michigan City, IN, USA)
cal differences were observed between the different groups,
and quantified using Image-Pro Plus 3.0 software (Media
in general, morphology was uniform in all animals of the
Cybernetics, Silver Spring, MD, USA). Thus, using a 5×
same group and segments 2–7 have shown similar epithelial
objective (Leica Microsystems, Willowdale, Ont., Canada),
histological features. The few exceptions observed will be
three to four thickness measurements per layer were obtained
mentioned later. Segment 5 was thus used to illustrate the
from representative artifact free areas of the epithelium and
effect of the various treatments on the vaginal epithelium.
muscularis, as well as for the three vaginal layers together.
The thickness of the lamina propria was obtained by subtract-
3.1.1. Epithelium
ing the thickness of the epithelium and muscularis from total
In intact animals at estrus, which represents the typ-
vaginal thickness.
ical estrogenic pattern, a keratinized stratified squamousepithelium was observed in all segments
A similar epithelium characterized all segments of theOVX + Premarin group (with the exception of some
Immunostaining was performed using Zymed SP kits
areas of large mucous cells in segments 2–5 (a reac-
(San Francisco, CA). Paraffin sections (4 m) were deparaf-
tion seen following long-term administration of an estrogenic
finized in toluene and rehydrated through ethanol. Endoge-
compound n the other hand, cycling rats at proestrus,
nous peroxidase activity was eliminated by preincubation
which are under a mixed estrogenic-progestational influ-
with 3% H2O2 in methanol for 30 min. A microwave
ence, show the presence of epithelial mucification (
retrieval technique using 0.01 M citrate buffer for 15 min
This effect is illustrated by a stratified squamous epithe-
was applied. After cooling the slides, non-specific bind-
lium covered by layers of mucified cells lining segments 2–7
ing was blocked using 10% goat serum for 20 min. Sec-
tions were then incubated for 1.5 h at room temperature
In the OVX group, the absence of ovarian stimulation led
with ER␣ (AB-1, Calbiochem, CA), AR (N-20, Santa Cruz
to an atrophy of the vaginal epithelium, which characterized
Biotechnology, CA) or PR (Ab-4, NeoMarkers, CA) antibod-
all segments Thus, this poorly stratified
L. Berger et al. / Journal of Steroid Biochemistry & Molecular Biology 96 (2005) 201–215
Table 1Histological evaluation of the epithelium, lamina propria and muscularis in the seven rat vaginal segments
Premarin + Acolbifene
DHEA + Acolbifene
DHEA + Premarin + Acolbifene
E = epithelium morphology: KS (keratinized stratified squamous), LHM (large hypertrophied mucous cells), SCM (small aligned columnar or cuboidal mucouscells), MSM (mixed stratified squamous overlaid by mucous cells), A (atrophy: small cuboidal cells).
Two different abbreviations for a given segment indicate two different patterns and that the first one is the predominant.
a L = lamina propria thickness: T (thick), MT (moderately thick), t (thin).
b Compactness of collagen fibers: H (high), M (moderate), L (low), M (muscularis thickness), T (thick), MT (moderately thick), t (thin), s (scarce).
epithelium consisted of one to two atrophied cuboidal or flat-
1 was thus higher than that of OVX rats, which comprised
tened squamous cell layer(s) with some small mucous cell
only four to six cell layers. In the other segments (the
areas, overlying a basal cell layer. Most importantly, moderate
basal layer was covered by a layer of low columnar mucous
inflammation with many foci of intraepithelial microabcesses
cells (which were more developed than in OVX ani-
(small areas of agglomerated leucocytes within the epithe-
mals. In the OVX + Acolbifene group, only three out of five
lium) was a frequent finding. These changes were often
animals showed signs of minimal inflammation while mod-
accompanied by focal erosion (zones where the epithelial
erate inflammatory changes were general findings in all OVX
layer partially disappears) and ulceration (complete disap-
animals. When the combination of Premarin and Acolbifene
pearance of the epithelial layer) (
was used, the morphology was superimposable to that found
In OVX animals treated with Acolbifene, segment 1
in the OVX + Acolbifene group, namely the typical pattern of
showed a keratinized stratified squamous epithelium simi-
cylindrical mucous cells, more rarely seen in OVX animals
lar to that of the intact group, except that it included 9–11
(No inflammation occurred in the group of
cell layers compared to the 10–15 layers found in animals at
animals receiving both Premarin and Acolbifene.
estrus or when OVX animals received Premarin (The
In all groups, segment 1 showed a comparable strat-
epithelial thickness of Acolbifene-treated animals in segment
ified squamous epithelium, except in the animals of the
L. Berger et al. / Journal of Steroid Biochemistry & Molecular Biology 96 (2005) 201–215
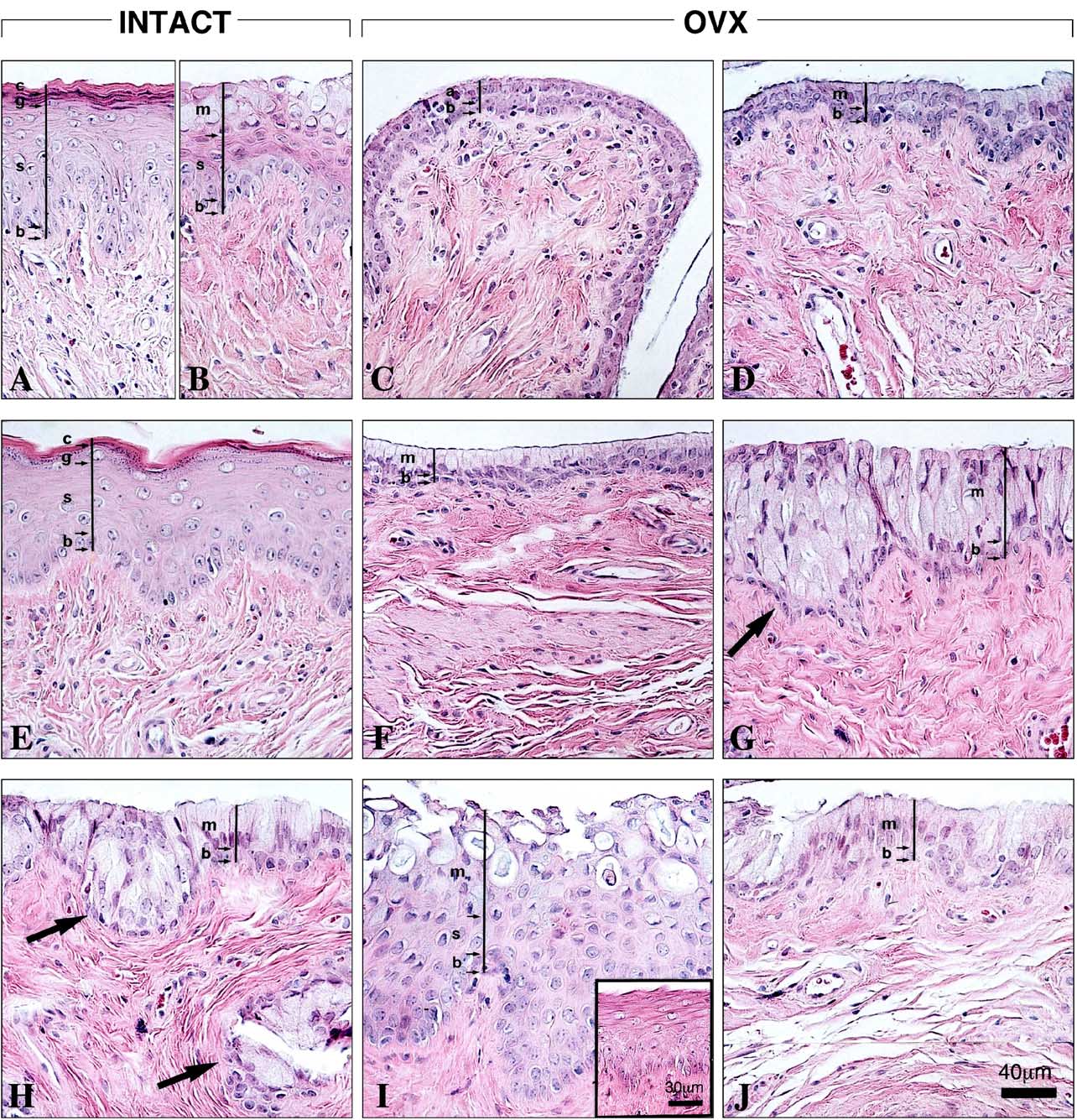
Fig. 2. Vaginal epithelium histomorphology of segment 5 in the nine groups of rats. Bar in (J) 40 m. (A) As representative of the estrogenic effect, thestratified squamous epithelium of cycling rats at estrus consists of four main layers: one cell layer of stratum basale (b), six to seven cell layers of stratumspinosum (s) and a stratum granulosum of five to six layers (g) overlaid by the tightly packed flattened cornified cells that form the stratum corneum (c). (B)Cycling rats at proestrus, which are under an estrogenic–progestational influence, are used to illustrate mucification. In most segments 2–7, one basal celllayer (b) was overlaid by five to six cell layers of stratum spinosum (s) and a stratum mucification (m) consisting of three to four layers of mucous cells.
(C) In the OVX control, a basal cell layer (b) was overlaid by one to two layer(s) of atrophic cuboidal or flattened cells (a). (D) The vaginal epithelium ofOVX rats treated with a daily oral dose of Acolbifene (2.5 mg/kg) shows atrophy, but with an outer layer of low columnar mucous cells (m) overlying thebasal cell layer (b). (E) Vaginal epithelium of OVX rats treated with a daily oral dose of Premarin (0.5 mg/kg). The OVX-induced atrophy was replaced byan estrogenic pattern similar to that found at estrus. (F) In OVX animals, which received Premarin + Acolbifene, atrophy predominated with a morphologysimilar to that of Acolbifene-treated animals, although larger mucous cells were seen. (G) Following treatment of OVX animals with a once daily cutaneousapplication of DHEA (80 mg/kg) on an area of 2 cm × 2 cm of the dorsal skin, an hypertrophic epithelium which consisted of three to five layers of mucouscells (m) was seen overlying a basal layer (b). Several invaginations characterized this epithelium (arrow). (H) In most areas of the vaginal epithelium ofDHEA + Acolbifene-treated animals, a layer of mucous cells (m) rested on a basal cell layer (b), while in some areas, many layers of mucous cells overlaid thebasal cell layer. Several invaginations characterized this epithelium (arrows). (I) Treatment with DHEA + Premarin led in three animals to a mixed epitheliumcomposed of three to seven cell layer-thick stratified squamous epithelium (s) overlaid by three to five layers of mucous cells (m). In other two animals, areasof stratified squamous epithelium were predominant (insert). Bar (in insert) 30 m. (J) When DHEA, Premarin and Acolbifene were combined, the epitheliumwas similar to that of the DHEA + Acolbifene group.
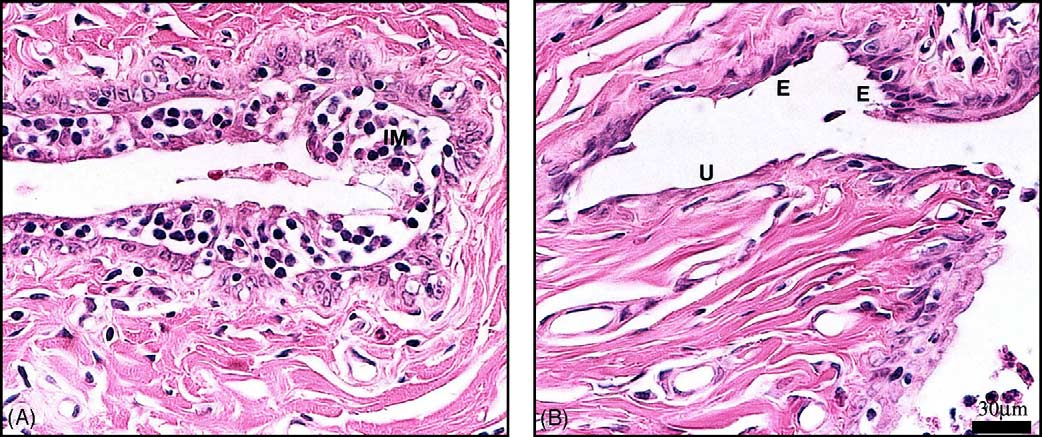
L. Berger et al. / Journal of Steroid Biochemistry & Molecular Biology 96 (2005) 201–215
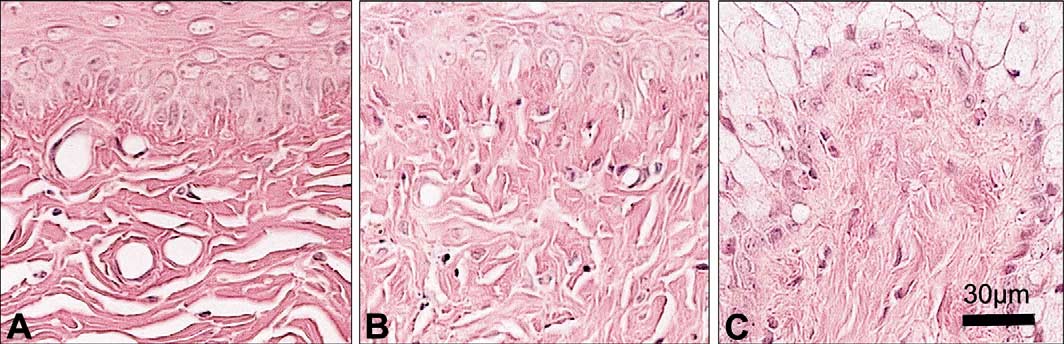
Fig. 3. Vaginal mucosa of OVX animals showing: (A) moderate inflammatory changes characterized by focal leukocyte infiltration with intraepithelialmicroabscess (IM) and (B) focal erosion (E) characterized by reduced epithelial thickness and ulceration (U) visualized as a complete disappearance of theepithelium. Bar in (B) 30 m.
OVX group, where atrophy was a predominant char-
the DHEA + Acolbifene group, thus indicating a blockade of
acteristic (Moreover, in the OVX + DHEA and
the estrogen-induced squamous cell proliferation by Acolb-
OVX + DHEA + Acolbifene groups, mucous cells frequently
accompanied the dominant stratified squamous epithelium of
As illustrated in OVX markedly reduced the
segment 1 (not shown).
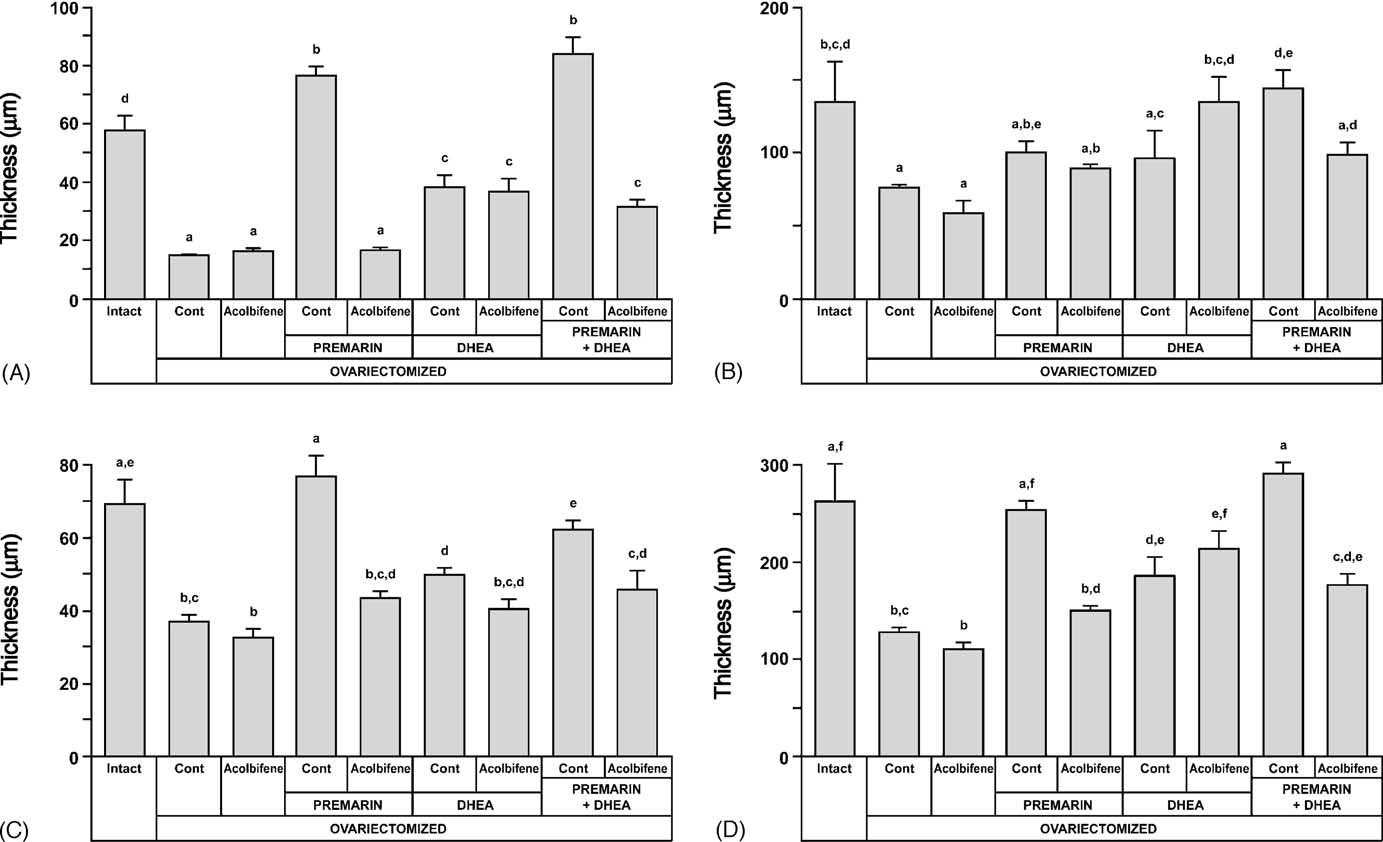
thickness of the vaginal epithelium (15 ± 1 m) in compari-
The seven segments of the vaginal epithelium of OVX
son with that of the intact group (58 ± 5 m). When animals
animals which received DHEA were composed of large
of the OVX group received Acolbifene, a similar low value
multilayered columnar mucous cells with distended cyto-
of 16 ± 1 m was obtained. The epithelium thickness was
plasmic vacuoles, a feature typical of an androgenic effect
restored to 76 ± 4 m in the group of animals which received
Several large invaginations char-
Premarin, a value significantly higher than that of the intact
acterized the epithelium after DHEA treatment. A simi-
group, which included animals at different stages of the estrus
lar epithelial morphology was found in all segments of
cycle. When Acolbifene was added to Premarin, the epithelial
the OVX + DHEA + Acolbifene group, except that the num-
thickness was reduced to 17 ± 1 m, thus showing a complete
ber of cell layers decreased (In the
blockade of the effect of the estrogen.
DHEA + Premarin-treated animals, a thick stratified epithe-
The administration of DHEA to OVX animals increased
lium of a "mixed" type composed of different ratios of squa-
thickness of the vaginal epithelium to 38 ± 4 m, while the
mous epithelium covered by layers of mucous cells
addition of Acolbifene to DHEA did not change the epithe-
was observed from the second to the fifth segment,
lial thickness which remained at 37 ± 5 m. When Premarin
thus revealing combined estrogenic and androgenic effects.
and DHEA were co-administered to OVX animals, a high
In this group, three animals displayed mucification over the
thickness of 84 ± 6 m was observed, a value not signif-
above-mentioned segments, while in the other two animals,
icantly different from that of the group of animals which
a stratified squamous epithelium was observed in all seg-
received Premarin alone. Finally, the combination of Pre-
ments (insert When Premarin was combined with
marin, DHEA and Acolbifene resulted in an epithelium
DHEA and Acolbifene, the epithelium was similar to that of
of 32 ± 3 m-thick, a value similar to that of the groups,
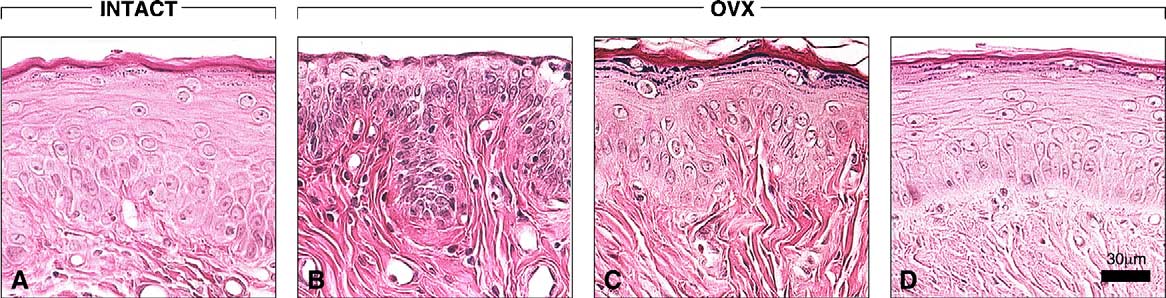
Fig. 4. To indicate the estrogen-like effect of Acolbifene on the vaginal epithelium lining segment 1 (external opening), comparisons with relevant groupsare illustrated. In intact rats at estrus (A) and in Premarin-treated (D) animals, the epithelium is 10–15 layer-thick and keratinized. In OVX controls (B), theepithelium is keratinized and its thickness is reduced to four to six layers and is generally not keratinized (see the absence of stratum granulosum). Meanwhile,in Acolbifene-treated animals (C), the thickness is restored to 9–11 layers and keratinized. Bar in (D) 30 m.
L. Berger et al. / Journal of Steroid Biochemistry & Molecular Biology 96 (2005) 201–215
Fig. 5. Thickness (m) of the three different vaginal compartments, as well as of the total rat vaginal wall, at the level of the fifth segment: (A) epithelium; (B)lamina propria; (C) muscularis; (D) total thickness, after 36 week-treatment of OVX animals with DHEA, Premarin and Acolbifene, alone or in combination.
Measurements in intact animals at estrus, proestrus and diestrus are averaged and presented as reference controls. Groups sharing the same letter are notstatistically different at p < 0.05. Dosages are described in legend of
which received DHEA alone or DHEA combined with
in rats at estrus and in Premarin-treated OVX animals or
high in OVX and DHEA-treated OVX rats), while increas-ing in compactness in segments 2 and 3 to reach a plateau
3.1.2. Lamina propria
that generally remains constant until segment 7. Thus, along
The degree of compactness of collagen fibers in the vagina
the longitudinal axis of the vagina in intact rats at estrus,
was categorized as low, moderate and high In all the
compactness of the collagen fibers was low in segments 1–3
animals, there were relatively few fibrocytes in proportion
and moderate in segments 4–7. Atrophy was often associ-
to the amount of collagen. Careful examination of each ani-
ated with increased compactness of collagen fibers in the
mal (reveals that, in general, the compactness of
OVX and OVX + Acolbifene groups In Premarin-
the collagen fibers in segment 1 is moderate (except low
treated OVX animals, compactness in segment 1 was low
Fig. 6. The compactness of collagen fibers of the lamina propria is illustrated in segment 5 as low (A), moderate (B) or high (C), as observed in the sub-epitheliallamina propria. The two former categories were associated with the presence of coarse collagen fibers loosely or less loosely aggregated together, respectively,and the latter was used to describe tightly packed fine collagen fibers, displaying a smooth, textured appearance. Bar in (C) 30 m.
L. Berger et al. / Journal of Steroid Biochemistry & Molecular Biology 96 (2005) 201–215
and moderate in segments 2 and 3, while in the other seg-
Premarin and DHEA resulted in a notable thickness increase
ments, compactness of the collagen fibers increased gradually
(62 ± 3 m), when compared to animals treated with DHEA
to become high in segments 6 and 7.
alone. When Acolbifene was added to Premarin and DHEA,
In all segments, except the orifice area of the two estro-
muscularis thickness decreased (46 ± 5 g) to a value com-
gen predominant groups as described earlier, compactness
parable to DHEA and Acolbifene.
of the collagen fibers could be described as moderate
When total vaginal wall thickness was measured (
in intact rats at proestrus, in OVX animals treated with
the outer layer of connective tissue composing the adven-
Premarin + Acolbifene or with Premarin + DHEA. On the
titia was not included. OVX led to a marked (51%) vagi-
other hand, most of the segments displayed highly com-
nal wall atrophy (128 ± 3 m versus 262 ± 39 m in the
pact collagen fibers in OVX animals treated with DHEA,
intact) and the addition of Acolbifene to OVX had no effect
DHEA + Acolbifene or DHEA + Premarin + Acolbifene.
(108 ± 8 m) Premarin treatment maintained
Light microscope examination of the lamina propria thick-
the total thickness to a value (253 ± 10 m) similar to that of
ness along the vagina generally revealed that it was mod-
intact animals while the addition of Acolbifene to Premarin
erately thick in segment 1 while thinner in segments 2–4.
reversed the effect of Premarin (150 ± 4 m) (and
In segments 5–7, the thickness increased progressively to a
E, respectively). Total vaginal thickness achieved by DHEA
level similar to segment 1. The corresponding mean values
treatment (184 ± 21 m) was about 25% lower than that of
of lamina propria thickness measured in segment 5 (
the Premarin alone-treated group On the other
indicate that OVX led to a significant decrease in lamina pro-
hand, the addition of Acolbifene to DHEA led to a non-
pria thickness (76 ± 2 m versus 135 ± 28 m in the intact
significant thickness increase (213 ± 20 m), which became
group) with a non-significant decrease induced by Acolbifene
non-significantly different from the intact group (
(60 ± 8 m). The increase observed after Premarin or Pre-
Finally, co-administration of Premarin and DHEA to OVX
marin + Acolbifene administration in OVX animals remained
animals markedly increased the thickness (290 ± 13 m) to
below the intact group (100 ± 9 and 90 ± 3 m, respectively,
a value similar to that of intact animals (The addition
versus 135 ± 28 m). The administration of DHEA led also
of Acolbifene to DHEA and Premarin reversed the effect to a
to a statistically non-significant increase in lamina propria
value (176 ± 11 m) not significatively different from DHEA
thickness (96 ± 20 m), and the addition of Acolbifene led
to further increased thickness (136 ± 17 m), a value sim-ilar to that of intact animals. Treatment of OVX animals
3.2. Vaginal weight
with Premarin + DHEA significantly increased the thickness(144 ± 14 m) when compared to DHEA alone. Finally,
After 9 months of treatment, the changes observed in
the animals treated with Premarin + DHEA + Acolbifene dis-
vaginal weight between the different groups (gener-
played a thickness (99 ± 9 m) similar to that of the group
ally follow the above-described morphological observations.
treated with DHEA alone and lower than that of the Pre-
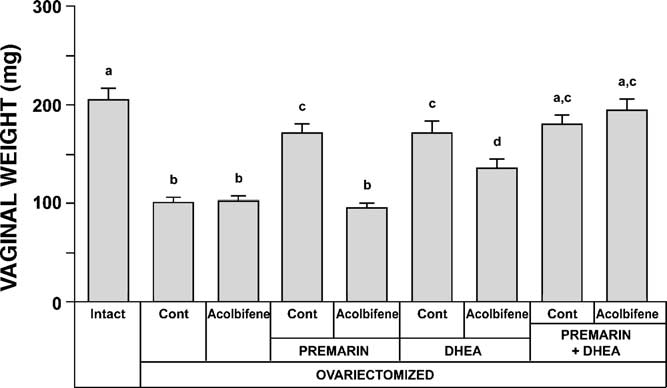
Indeed, vaginal weight after OVX decreased by about 50%
marin + DHEA group, although the difference was not statis-
(101 ± 5 mg) compared to the intact group (205 ± 11 mg)
while treatment of OVX animals with Acolbifene alone hadno effect on vaginal weight. On the other hand, adminis-
3.1.3. Muscularis
tration of Premarin led to a vaginal weight increase that
In all groups, only a few smooth muscle fibers of the
did not reach the value of intact animals (170 ± 9 mg)
muscularis were found in segment 1, along with few irregu-
while the addition of Acolbifene to Premarin reversed the
larly arranged striated muscle fibers. Gradually, the thickness
estrogen-induced weight gain to a value similar to that of
of the muscularis increased, remaining thin until segment
OVX animals (96 ± 4 mg). Conversely, when DHEA was
5, where an oblique smooth muscle layer was seen above
given to OVX animals, vaginal weight increased to a value
the longitudinal fibers, thus resulting in a further thickness
(171 ± 12 mg), similar to that of the Premarin-treated group,
increase in segments 6 and 7 (Measurement of mus-
and the combination of Acolbifene and DHEA resulted in
cularis thickness in segment 5 (revealed a value of
a decrease in weight (135 ± 9 mg), which remained above
69 ± 7 m in the intact group at estrus while a 46% signifi-
that of the OVX group. Finally, co-administration of Pre-
cant decrease was observed 9 months after OVX (37 ± 2 m).
marin and DHEA in OVX animals resulted in a vaginal
Acolbifene treatment did not change the muscularis thick-
weight gain (179 ± 10 mg) reaching a value similar to those of
ness, which remained at 33 ± 3 m.
the OVX + DHEA and OVX + Premarin groups. The addition
On the other hand, Premarin treatment of OVX animals
of Acolbifene to this combination had no significant effect
restored a thickness of 77 ± 5 m, a value similar to that
(194 ± 12 mg).
of the intact group while Acolbifene added to Premarinreversed this effect, thus resulting in a thickness of 43 ± 2 m.
3.3. Immunohistochemistry of steroid receptors
DHEA induced a moderate increase in muscularis thickness(50 ± 2 m), which was slightly decreased by the addition of
Examination of the seven vaginal cross-segments from
Acolbifene (41 ± 3 m). Finally, the combined treatment of
individual animals revealed that, in most groups, the intensity
L. Berger et al. / Journal of Steroid Biochemistry & Molecular Biology 96 (2005) 201–215
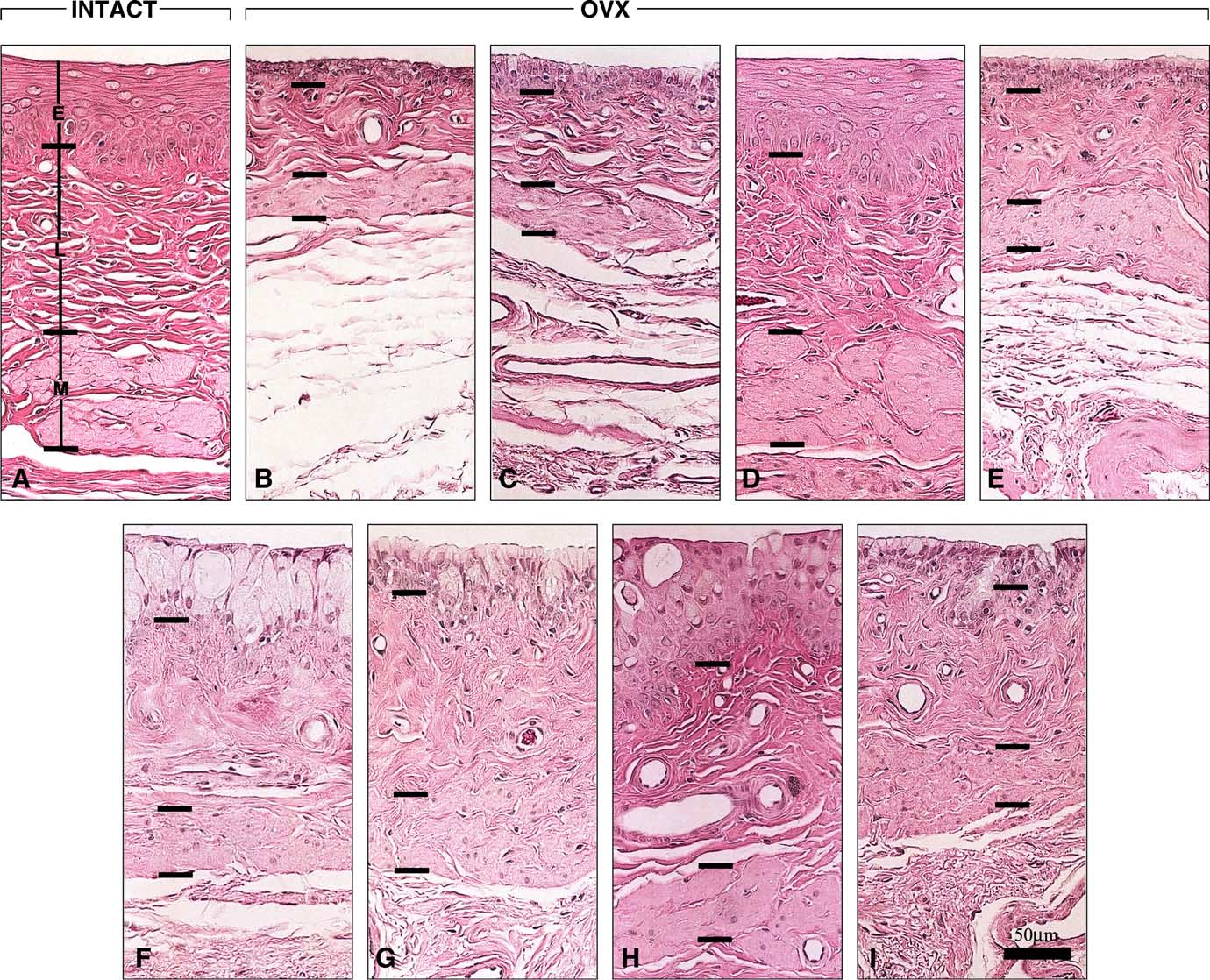
Fig. 7. Microphotographs of the three vaginal compartments: (E) epithelium, (L) lamina propria and (M) muscularis, at the level of the fifth segment of the ratvagina, with emphasis on the relative muscularis thickness in the different groups. Separation of the three vaginal wall layers with bars is indicated to best estimatethe thickness distribution between the different groups: (A) intact; (B) OVX and OVX treated with (C) Acolbifene; (D) Premarin; (E) Premarin + Acolbifene;(F) DHEA; (G) DHEA + Acolbifene; (H) DHEA + Premarin; (I) DHEA + Premarin + Acolbifene. Bar in (I) 50 m.
of AR, ER␣ and PR immunolabeling in the lamina propriaand muscularis generally increased from segment 1 to theplateau of segments 5–7. Since segment 5 was previouslychosen to compare morphology, the immunostaining resultswere semi-quantitatively evaluated in this segment, as shownin summarized in
3.3.1. Androgen receptor
As seen in the upper row of summarized in
the vaginal wall of intact rats at estrus showed amoderate nuclear labeling in the epithelium, with the basaland the three suprabasal layers stained. A moderate numberof strongly stained nuclei were seen in the lamina propria
Fig. 8. Vaginal weight measured 36 weeks after OVX and treatment of OVX
while a few weakly stained nuclei were observed in the mus-
animals with Acolbifene, Premarin and DHEA alone or in combination.
cularis. At diestrus, the basal and one suprabasal epithelial
Intact animals are added as controls.
layer intensely stained for AR and a large number of nuclei in
L. Berger et al. / Journal of Steroid Biochemistry & Molecular Biology 96 (2005) 201–215
Fig. 9. Immunohistochemical localization of AR, ER␣ and PR in the (E) epithelium, (L) lamina propria and (M) muscularis at the level of the fifth vaginalsegment of the different groups. The thin bars indicate the approximate separation between the three vaginal compartments. Bar in lower row (right) 30 m.
L. Berger et al. / Journal of Steroid Biochemistry & Molecular Biology 96 (2005) 201–215
Table 2Semi-quantitative evaluation of the number and intensity of immunostained nuclei for AR, ER alpha and PR in the epithelium, lamina propria and muscularisin the fifth segment of the rat vagina
Estrus reference of low ER␣ and high PR
Diestrus reference of high ER␣ and low PR
Premarin + Acolbifene
DHEA + Acolbifene
DHEA + Premarin + Acolbifene
Sex steroid receptors: AR, androgen receptor; ER␣, estrogen receptor alpha; PR, progesterone receptor. Vaginal layers: E, epithelium; L, lamina propria; M,muscularis. Numbers represent the semi-quantitative evaluation of the number of labeled nuclei: 0, none, 1, low; 2, moderate; 3, high. Labeling intensity isindicated as (+) low; (++) moderate; (+++) high.
the lamina propria and muscularis were also strongly stained.
few nuclei were moderately stained in the lamina propria and
In the OVX group, the basal epithelial layer showed few mod-
weakly labeled in the muscularis.
erately stained nuclei, while a few strongly stained nuclei
The epithelium of OVX animals revealed a moderate num-
were seen in the lamina propria and muscularis. On the other
ber of variably stained nuclei, with weakly to strongly labeled
hand, in the Acolbifene-treated group, the basal and super-
areas and few strongly labeled nuclei in the lamina propria
ficial epithelial layers were moderately labeled while few
and muscularis. Treatment of OVX animals with Acolbifene
nuclei were strongly stained in the lamina propria and mus-
eliminated ER␣ labeling from the three tissue layers. Mean-
while, treatment with Premarin led to strong to moderate
With Premarin, the epithelial basal and the three to four
staining of the basal cells, while the three suprabasal epithe-
suprabasal cell layers were moderately stained, while a mod-
lial layers were weakly stained. Many moderately stained
erate number of strongly labeled nuclei were found in the
nuclei were found in the lamina propria and muscularis fol-
lamina propria and a few weakly labeled nuclei were seen
lowing Premarin. No staining could be detected when the
in the muscularis. After treatment with Premarin and Acolb-
combination of Premarin and Acolbifene was used.
ifene, a moderate to strong, but scattered labeling was found
The vaginal epithelium of DHEA-treated animals showed
in the basal and in the superficial epithelial layers, while the
many strongly stained nuclei for ER␣ in the basal layer
lamina propria and muscularis displayed few weakly labeled
and a moderate scattered labeling in the superficial layers.
Following DHEA treatment, the lamina propria and muscu-
DHEA treatment of OVX animals led to the strong
laris displayed a moderate number and many strongly stained
labeling of many nuclei in the three vaginal layers.
nuclei, respectively. No staining was detected when the com-
The same pattern was found when the combinations of
bination of DHEA and Acolbifene was used. In animals
DHEA + Acolbifene and DHEA + Premarin were used, with
treated with DHEA and Premarin, the epithelial basal layer
the exception of a smaller number of labeled nuclei in
was moderately stained while a moderate number of nuclei
the superficial layers of the epithelium in the latter group.
in the suprabasal layers and some mucous cell nuclei were
The combination of DHEA, Premarin and Acolbifene also
weakly labeled. Following this treatment, the lamina propria
resulted in a strong staining of the majority of the nuclei in
and muscularis showed a moderate and a high number of
the three vaginal wall layers.
strongly stained nuclei, respectively. Combining Acolbifeneto DHEA and Premarin led to the complete disappearance of
3.3.2. Estrogen receptor alpha
ER␣ staining.
As presented in and the middle row of
in intact rats at estrus, the epithelium revealed few weakly
3.3.3. Progesterone receptor
labeled nuclei in the basal cell layer while some weakly
Immunolabeling for PR lower row of
stained nuclei were detected in the lamina propria and many
showed many strongly stained nuclei in all tissue compart-
weakly stained nuclei were observed in the muscularis. On
ments of the vagina in intact rats at estrus while such labeling
the other hand, at diestrus, basal and two suprabasal epithe-
was absent in the epithelium at diestrus, with the exception of
lial layers showed many strongly stained nuclei while very
cytoplasmic background. A moderate number of moderately
L. Berger et al. / Journal of Steroid Biochemistry & Molecular Biology 96 (2005) 201–215
stained nuclei were seen in the other two vaginal compart-
ment has been reported to lead to a significant decrease in
ments in rats at diestrus. In the vagina of OVX animals and all
total vaginal collagen
groups of animals which received Acolbifene, there was no
In the potential triple combination, the equine estrogen
detectable PR labeling. Premarin-treated animals displayed a
Premarin is aimed at acting in the brain to relieve the vaso-
variable degree of staining with a moderate number of mod-
motor symptoms. In fact, the benefits of co-administration
erately to strongly stained nuclei in the epithelium and lamina
of the pure selective antiestrogen Acolbifene with an estro-
propria and a high number of strongly labeled nuclei in the
gen in order to neutralize the unwanted systemic effects of
muscularis. The DHEA + Premarin group showed a staining
the estrogen have been well described by Labrie et al.
pattern similar to that of Premarin alone, while DHEA-treated
In the present study, when OVX animals were treated with
animals revealed few moderately stained nuclei only in the
Acolbifene, the most typical morphological feature induced
by the SERM was the appearance of a superficial layerof well-aligned small mucous cells overlying a basal celllayer. This pattern was slightly more pronounced in the Pre-
marin + Acolbifene group and remained preponderant in allgroups treated with Acolbifene, including when the SERM
Vaginal dryness or atrophic vaginitis, also referred to as
was combined with DHEA. Vaginal epithelium mucifica-
urogenital atrophy, with sexual dysfunction is a common
tion has been reported in immature adult
problem in postmenopausal women most common
rats under antiestrogen treatment. Although this morpholog-
symptoms are dryness, burning, pruritus, irritation and dys-
ical pattern has been compared to the progesterone-induced
pareunia, thus leading to decreased libido and quality of life
mucification the molecular mechanism by which
an antiestrogen induces epithelial mucification remains
The rat vaginal wall provides an excellent model to study
the inductive/suppressive effects of estrogens, androgens and
The most spectacular effects of Acolbifene in OVX +
estrogen antagonists. The overall hormonal antihor-
Premarin rat vagina reside in a weight decrease to the level
monal fects on the human vagina are usually deter-
seen in OVX animals and an important thickness reduction
mined by vaginal cytology, although the observations are lim-
of the epithelial and muscularis layers, as well as decreased
ited to the external layers of the epithelium n rodents,
total vaginal wall thickness, when compared to intact and
specific histological patterns of keratinization and mucifica-
Premarin-treated groups (Such effects support
tion of the vaginal epithelium provide the hallmarks of overall
the presence of a pure antiestrogenic action of Acolbifene on
estrogenic ve androgenic fects,
these parameters.
respectively. To the best of our knowledge, this is the first
Contrary to its antiestrogenic actions mentioned above, in
study that examines morphology over the entire longitudinal
the rat vaginal orifice (segment 1), Acolbifene surprisingly
axis of the vagina, especially in the OVX rat treated with
displays an estrogen-like effect of epithelial proliferation and
DHEA, an estrogen and an antiestrogen.
keratinization. Compared to the thin 4–6 layer-thick epithe-
In the present study, a typical estrogenic effect reflected
lium lining the same segment in OVX animals, the 9–11 lay-
by a keratinized stratified squamous epithelium was observed
ers of the cornified Acolbifene-induced epithelium is likely
in the rat vagina following treatment with the conjugated
to provide a better resistance, as well as reduction in inflam-
estrogen Premarin. In a previous study performed in our
mation incidence. The latter is supported by the observation
laboratory, the same morphology was observed following
of a reduced incidence of inflammatory changes, not only in
treatment with 17-estradiol instead of Premarin. Moreover,
the first segment but also throughout the vaginal mucosa of
the increased thickness and PR expression in the muscu-
Acolbifene-treated OVX rats. At the internal vaginal level,
laris observed after Premarin treatment represent estrogenic
the mucification induced by the SERM is likely to account
effects, similar to the findings in intact animals at estrus.
for the reduced inflammation incidence.
Interestingly, in the uterus of OVX mice treated with the
Many beneficial effects of DHEA have been reported in
antiestrogen Acolbifene, the atrophic changes were more pro-
postmenopausal women morphological changes
nounced in the myometrium supporting that, in the
observed in the rat vagina after DHEA treatment reflect its
absence of estrogen, cell proliferation in the smooth muscle
intracrine conversion into active sex steroids having andro-
is inhibited in the female rat reproductive tract.
genic and/or estrogenic action through intracrine mecha-
In the vaginal lamina propria of intact rats at estrus,
nisms Those changes comprise marked epithelial muci-
Premarin-treated rats and, to a lesser extent, Premarin +
fication, high compactness of delicate, finely woven lamina
DHEA-treated animals, low compactness of collagen fibers
propria collagen fibers and moderate muscularis thickness
in the first segments could also be ascribed to an estrogenic
increase when compared to OVX animals. The first and
effect. These data are in agreement with previous observa-
the second morphological changes are typical of androgenic
tions in rats and rabbits at estrus and after estradiol treatment
effects while the third shows an estrogen-like activity, which
following OVX Moreover, in postmenopausal
is further supported by a concomitant increase in proges-
women with stress incontinence, a 6-month estrogen treat-
terone receptor expression in the muscularis layer.
L. Berger et al. / Journal of Steroid Biochemistry & Molecular Biology 96 (2005) 201–215
In addition to the well-described epithelial squamous pro-
relaxation (reviewed in It is noteworthy that Acolbifene
liferation and maturation observed under estrogen treatment,
and DHEA have been found to induce eNOS in human and
it is well known that vaginal epithelial mucification occurs
rat endothelial cells
in the OVX rat in response to treatment with testosterone
Finally, the combination of Acolbifene, DHEA and Pre-
(Testo) or 5␣-dihydrotestosterone (DHT) The vagi-
marin induced mucification of the vaginal epithelium through
nal morphology under Testo treatment has been described
androgenic and antiestrogenic effects. These effects were
as "cuboidal to columnar mucified cells exhibiting a pseu-
reflected by invaginations of multilayered hypertrophied
dostratified appearance at places, superficial vacuolation and
mucous cells in alternance with well-aligned mucous cells.
occasional cornification in the top layer, with compact lam-
The high compactness of fine collagen fibers found in the lam-
ina propria and atrophic changes in the muscular layer and
ina propria is indicative of the androgenic action of DHEA,
weight similar to that of intact rats" Since DHEA is
while the significant thickness reduction of the epithelial and
transformed into either or both androgens and estrogens in
muscularis layers, when compared to the Premarin + DHEA
peripheral tissues, the thick mucified multilayered epithelium
group, is indicative of the reversal by Acolbifene of the major
observed in the present study after treatment of OVX ani-
and minor estrogenic actions of both Premarin and DHEA in
mals with DHEA, suggests a predominant androgenic effect
these two vaginal layers, respectively.
of mucification in the rat vagina, an effect which could mask
While the antiestrogenic action of Acolbifene should pro-
any potential co-existing minor estrogenic effect at the epithe-
tect the uterus and mammary gland against estrogen-induced
lial level. A previous study has shown the same mucification
cancer, and leads, in this study, to the formation of a con-
effect in the rat vagina and the intravaginal application of
sistent superficial mucous cell layer lining the rat vaginal
DHEA achieved a significant effect at a dose 10× lower than
lumen, the agonist-like action of Acolbifene gives rise to
that found to be active following application of DHEA on the
the inexpected formation of a keratinized squamous stratified
epithelium at the vaginal ostium (estrogen-like effect). These
The present data indicate co-existing major androgenic
two actions of Acolbifene were likely to bring protection
and minor estrogenic actions of DHEA in the rat vagina.
against the observed inflammation in the OVX group. The
Other studies using co-administration of DHEA and the antie-
mucifying action of Acolbifene is further increased by the
strogen Acolbifene or the antiandrogen FLU, respectively,
mucification effect of the androgenic component of DHEA,
have demonstrated that the action of DHEA in the rat mam-
thus potentially leading to an improved vaginal function,
mary gland sebaceous glands bone mineral
which is supported by the stimulatory effect of DHEA on
density almost exclusively androgenic. Nevertheless,
the lamina propria.
the presence of an estrogenic action of DHEA in the rat vagina
The beneficial morphological changes, observed con-
has been previously demonstrated, either by the formation
comitantly with the strong modulation of rat vaginal AR by
of a stratified epithelium in the vagina of rats treated with
androgens, suggest that decreased serum levels of DHEA-
FLU and DHEA (unpublished data), or through induction of
derived androgens in postmenopausal women could con-
vaginal opening and precocious ovulation in immature rats
tribute to the decreased vaginal health and eventually to the
treated with this compound, while DHT, an androgen not
loss of libido and sexual enjoyment observed in this age
aromatizable to estrogens, did not produce such effects
group. Decreased serum total Testo, free Testo and DHEA-S
The capacity of rat vaginal tissue to aromatize androgens,
were indeed found in women who consulted for decreased
especially, Testo, is likely to account for the major part of
the estrogenic effect of DHEA in this organ Androst-
The present data show low levels of ER␣ in the vaginal
5-ene-3, 17-diol (5-diol), a DHEA metabolite known to
epithelium and lamina propria of rats at estrus, which are
bind the estrogen receptor also contribute to
under high estrogen influence, and increased ER␣ levels in
the estrogenic effect The proposed combination of the
the epithelium of intact rats at diestrus, when estrogen lev-
antiestrogen Acolbifene with DHEA would thus prevent any
els are low. In addition, during the estrus cycle, ER␣ levels
unwanted stimulatory effect of 5-diol. In addition, preven-
fluctuated more in the epithelium than in the lamina propria.
tion of bone loss would represent an additional benefit of
These results are in agreement with those of other investi-
gators except that, in the lamina propria, they detected
To the best of our knowledge, no previous study has shown
more (nearly 60%) ER␣-immunolabeled nuclei throughout
the stimulatory effects of DHEA on the compactness and mor-
the rat estrus cycle than in the present study. Moreover, under
phology of the lamina propria's collagen fibers and, to a lesser
the different hormonal treatments, ER␣ labeling displayed
extent, on the muscularis. Such actions of DHEA-derived
more variations in the number and intensity of stained nuclei
androgens and estrogens could have beneficial effects on
in the epithelium than in the other two tissue layers. While in
vaginal function in postmenopausal women and possibly pro-
the Premarin, and DHEA + Premarin groups, the presence of
vide the substrate required for the action of inhibitors of type 5
estrogens appeared to up-regulate ER␣ expression, as already
phosphodiesterase, such as sildenafil or tadalafil, possibly via
demonstrated in the mouse uterus the existence of an
androgen- or estrogen-induced endothelial nitric oxide syn-
ER␣ up-regulation in the absence or presence of low levels of
thase (eNOS)-mediated facilitation of vaginal smooth muscle
estrogens was observed in OVX- and DHEA-treated animals,
L. Berger et al. / Journal of Steroid Biochemistry & Molecular Biology 96 (2005) 201–215
respectively. As expected, treatment with Acolbifene com-
[11] R.K. Ross, A. Paganini-Hill, P.C. Wan, M.C. Pike, Effect of hormone
pletely eliminated ER␣ immunostaining in the whole vaginal
replacement therapy on breast cancer risk: estrogen versus estrogen
wall and co-treatment with Premarin and/or DHEA did not
plus progestin, J. Natl. Cancer Inst. 92 (2000) 328–332.
[12] W.G.f.t.W.s.H.I. Investigators, Risks and benefits of estrogen plus
change this response. As discussed earlier, a potential bene-
progestin in healthy postmenopausal women: principal results from
ficial effect of the combination of Acolbifene and DHEA for
the Women's Health Initiative randomized controlled trial, JAMA
postmenopausal women is that the binding of the antiestrogen
288 (2002) 321–333.
to ER␣ would prevent estrogens produced from aromatiza-
[13] F. Labrie, P. Diamond, L. Cusan, J.L. Gomez, A. B´elanger, Effect
tion of DHEA-derived androgens and the metabolite 5-DIOL
of 12-month DHEA replacement therapy on bone, vagina, andendometrium in postmenopausal women, J. Clin. Endocrinol. Metab.
to bind ER␣, thus potentially preventing proliferation of
82 (1997) 3498–3505.
ER␣-sensitive breast cancers cell
[14] F. Labrie, Dehydroepiandrosterone (DHEA) as potential hormone
Various attempts have been made to solve the problem
replacement therapy, R´ef´erence en Gyn´ecologie Obst´etrique 8 (2001)
of vaginal dryness, often linked to dyspareunia and loss of
sexual enjoyment. As an example, local vaginal estrogen
[15] A. Lasco, N. Frisina, N. Morabito, A. Gaudio, E. Morini, A. Tri-
filetti, G. Basile, V. Nicita-Mauro, D. Cucinotta, Metabolic effects
preparations are often prescribed to provide relief but the
of dehydroepiandrosterone replacement therapy in postmenopausal
endometrium may be stimulated by the unopposed estrogen
women, Eur. J. Endocrinol. 145 (2002) 457–461.
In order to achieve a more physiological and tissue-
[16] S. Gauthier, B. Caron, J. Cloutier, Y.L. Dory, A. Favre, D.
specific HRT, DHEA combined with an estrogen and with
Larouche, J. Mailhot, C. Ouellet, A. Schwerdtfeger, G. Leblanc,
a SERM, such as Acolbifene, which displays antiestrogenic
C. Martel, J. Simard, Y. M´erand, A. B´elanger, C. Labrie, F.
Labrie, (S)-(+)-4-[7-(2,2-dimethyl-1-oxopropoxy)-4-methyl-2-[4-[2-
activity in the mammary gland and uterus, could potentially
meet the important needs of postmenopausal women. In fact,
dimethylpropanoate (EM-800): a highly potent, specific, and
control of vasomotor symptoms, relief of vaginal dryness
orally active nonsteroidal antiestrogen, J. Med. Chem. 40 (1997)
with potential benefits on sexual function could be achieved,
concomitantly with prevention of breast, uterine and ovarian
[17] F. Labrie, C. Labrie, A. B´elanger, J. Simard, S. Gauthier, V. Luu-The,
Y. M´erand, V. Gigu ere, B. Candas, S. Luo, C. Martel, S.M. Singh,
cancer as well as osteoporosis and potential improvement
M. Fournier, A. Coquet, V. Richard, R. Charbonneau, G. Charpenet,
of cognitive functions and memory as well as prevention of
A. Tremblay, G. Tremblay, L. Cusan, R. Veilleux, EM-652 (SCH
Alzheimer's disease
57068): a third generation SERM acting as pure antiestrogen in themammary gland and endometrium, J. Steroid Biochem. Mol. Biol.
69 (1999) 51–84.
[18] G.B. Tremblay, A. Tremblay, N.G. Copeland, D.J. Gilbert, N.A.
Jenkins, F. Labrie, V. Giguere, Cloning, chromosomal localizationand functional analysis of the murine estrogen receptor , Mol.
[1] B. Rossin-Amar, Vaginal dryness in the menopausal woman (physio-
Endocrinol. 11 (1997) 353–365.
logical and psychological aspects), Gynecol. Obstet. Fertil. 28 (2000)
[19] S. Dauvois, C.S. Geng, C. L´evesque, Y. M´erand, F. Labrie, Addi-
tive inhibitory effects of an androgen and the antiestrogen EM-170
[2] L. Pandit, J.G. Ouslander, Postmenopausal vaginal atrophy and
on estradiol-stimulated growth of human ZR-75-1 breast tumors in
atrophic vaginitis, Am. J. Med. Sci. 314 (1997) 228–231.
athymic mice, Cancer Res. 51 (1991) 3131–3135.
[3] F. Labrie, Intracrinology, Mol. Cell. Endocrinol. 78 (1991)
[20] S. Couillard, C. Labrie, A. B´elanger, B. Candas, F. Pouliot, F. Labrie,
Effect of dehydroepiandrosterone and the antiestrogen EM-800 on
[4] F. Labrie, A. B´elanger, J. Simard, V. Luu-The, C. Labrie, DHEA and
the growth of human ZR-75-1 breast cancer xenografts, J. Natl.
peripheral androgen and estrogen formation: intracrinology, Ann. N.
Cancer Inst. 90 (1998) 772–778.
Y. Acad. Sci. 774 (1995) 16–28.
[21] F. Labrie, M. El-Alfy, L. Berger, C. Labrie, C. Martel, A. B´elanger,
[5] F. Labrie, V. Luu-The, C. Labrie, A. B´elanger, J. Simard, S.-X.
B. Candas, G. Pelletier, The combination of a novel SERM with an
Lin, G. Pelletier, Endocrine and intracrine sources of androgens in
estrogen protects the mammary gland and uterus in a rodent model:
women: inhibition of breast cancer and other roles of androgens
the future of postmenopausal women's health? Endocrinology 144
and their precursor dehydroepiandrosterone, Endocr. Rev. 24 (2003)
(2003) 4700–4706.
[22] W.M. van Weerden, H.G. Bierings, G.J. van Steenbrugge, F.H. de
[6] N. Orentreich, J.L. Brind, R.L. Rizer, J.H. Vogelman, Age changes
Jong, F.H. Schroder, Adrenal glands of mouse and rat do not syn-
and sex differences in serum dehydroepiandrosterone sulfate concen-
thesize androgens, Life Sci. 50 (1992) 857–861.
trations throughout adulthood, J. Clin. Endocrinol. Metab. 59 (1984)
[23] C.Y. Kramer, Extension of multiple range tests to group means
with unique numbers of replications, Biometrics 12 (1956) 307–
[7] F. Labrie, A. B´elanger, L. Cusan, J.L. Gomez, B. Candas, Marked
decline in serum concentrations of adrenal C19 sex steroid pre-
[24] Y.D. Yuan, G.L. Foley, Female reproductive system, in: M.A. Wal-
cursors and conjugated androgen metabolites during aging, J. Clin.
lig (Ed.), Handbook of Toxicologic Pathology, second ed., Elsevier
Endocrinol. Metab. 82 (1997) 2396–2402.
Science, USA, 2002.
[8] M.J. Rosenberg, T.D. King, M.C. Timmons, Estrogen–androgen for
[25] J.L. Ladinsky, H.W. Gruchow, B.M. Peckham, Cellular behaviour
hormone replacement: a review, J. Reprod. Med. 42 (1997) 394–404.
of the vaginal epithelium treated with testosterone propionate alone
[9] I.D. Burd, G.A. Bachmann, Androgen replacement in menopause,
and in combination with diethylstilboestrol, J. Endocrinol. 41 (1968)
Curr. Womens Health Rep. 1 (2001) 202–205.
[10] G.A. Colditz, S.E. Hankinson, D.J. Hunter, W.C. Willett, J.E. Man-
[26] P.K. Mehrotra, J.N. Karkun, Studies on physiology and biochemistry
son, M.J. Stampfer, C. Hennekens, B. Rosner, F.E. Speizer, The use
of female genital tract: response of cervix and vagina of albino rats
of estrogens and progestins and the risk of breast cancer in post-
to estrogen, progesterone and testosterone, Indian J. Exp. Biol. 11
menopausal women, N. Engl. J. Med. 332 (1995) 1589–1593.
(1973) 1–6.
L. Berger et al. / Journal of Steroid Biochemistry & Molecular Biology 96 (2005) 201–215
[27] M. Notelovitz, Urogenital atrophy and low-dose vaginal estrogen
[46] E.D. Lephart, D. Mathews, J.F. Noble, S.R. Ojeda, The vagi-
therapy, Menopause 7 (2000) 140–142.
nal epithelium of immature rats metabolizes androgens through an
[28] J.R. Berman, L.A. Berman, T.J. Werbin, I. Goldstein, Female sexual
aromatase-like reaction: changes during the time of puberty, Biol.
dysfunction: anatomy, physiology, evaluation and treatment options,
Reprod. 40 (1989) 259–267.
Curr. Opin. Urol. 9 (1999) 563–568.
[47] T.C. Shao, E. Castaneda, R.L. Rosenfield, S. Liao, Selective reten-
[29] G.N. Papanicolaou, E. Shorr, The action of ovarian follicular hor-
tion and formation of a delta5-androstenediol–receptor complex in
mones in the menopause, as indicated by vaginal smears, Am. J.
cell nuclei of the rat vagina, J. Biol. Chem. 250 (1950) 3095–
Obstet. Gynecol. 31 (1936) 806–831.
[30] M. Friedrich, D. Mink, C. Villena-Heinsen, A. Woll-Hermann, S.
[48] J. Poortman, J.A. Prenen, F. Schwarz, J.H. Thijssen, Interaction
Wagner, W. Schmidt, The influence of tamoxifen on the maturation
of delta-5-androstene-3beta, 17beta-diol with estradiol and dihy-
index of vaginal epithelium, Clin. Exp. Obstet. Gynecol. 25 (1998)
drotestosterone receptors in human myometrial and mammary cancer
tissue, J. Clin. Endocrinol. Metab. 40 (1975) 373–379.
[31] B. Peckham, W. Kiekhofer, Cellular behavior in the vaginal epithe-
[49] L.G. Van Doorn, J. Poortman, J.H. Thijssen, F. Schwarz, Actions
lium of estrogen-treated rats, Am. J. Obstet. Gynecol. 83 (1962)
and interactions of 5-androstene-3, 17-diol and estradiol 17
in the immature rat uterus, Endocrinology 108 (1981) 1587–
[32] H. Selye, J.S.L. Browne, J.B. Collip, Effect of combined administra-
tion of oestrone and progesterone in adult ovariectomized rats, Proc.
[50] J. Adams, M. Garcia, H. Rochefort, Estrogenic effects of physio-
Soc. Exp. Biol. Med. 34 (1936) 198–200.
logical concentrations of 5-androstene-3 beta, 17 beta-diol and its
[33] S. Luo, A. Sourla, C. Labrie, S. Gauthier, Y. Merand, A. Belanger,
metabolism in MCF7 human breast cancer cells, Cancer Res. 41
F. Labrie, Effect of twenty-four-week treatment with the antiestro-
(1981) 4720–4726.
gen EM-800 on estrogen-sensitive parameters in intact and ovariec-
[51] R. Poulin, F. Labrie, Stimulation of cell proliferation and estrogenic
tomized mice, Endocrinology 139 (1998) 2645–2656.
response by adrenal C19-delta 5-steroids in the ZR-75-1 human
[34] M.G. Sartori, M.J. Girao, M. de Jesus Simoes, J.P. Sartori, E.C.
breast cancer cell line, Cancer Res. 46 (1986) 4933–4937.
Baracat, G. Rodrigues de Lima, Quantitative evaluation of collagen
[52] C. Martel, S. Picard, V. Richard, A. Belanger, C. Labrie, F.
and muscle fibers in the lower urinary tract of castrated and under-
Labrie, Prevention of bone loss by EM-800 and raloxifene in the
hormone replacement female rats, Clin. Exp. Obstet. Gynecol. 28
ovariectomized rat, J. Steroid Biochem. Mol. Biol. 74 (2000) 45–
(2001) 92–96.
[35] N. Fleischmann, G. Christ, T. Sclafani, A. Melman, The effect of
[53] R. Munarriz, S.W. Kim, N.N. Kim, A. Traish, I. Goldstein, A review
ovariectomy and long-term estrogen replacement on bladder structure
of the physiology and pharmacology of peripheral (vaginal and cli-
and function in the rat, J. Urol. 168 (2002) 1265–1268.
toral) female genital arousal in the animal model, J. Urol. 170 (2003)
[36] H.N. Yoon, W.S. Chung, Y.Y. Park, B.S. Shim, W.S. Han, S.W.
S40–S44 (discussion S44–S45).
Kwon, Effects of estrogen on nitric oxide synthase and histological
[54] T. Simoncini, G. Varone, L. Fornari, P. Mannella, M. Luisi, F. Labrie,
composition in the rabbit clitoris and vagina, Int. J. Impot. Res. 13
A.R. Genazzani, Genomic and nongenomic mechanisms of nitric
(2001) 205–211.
oxide synthesis induction in human endothelial cells by a fourth-
[37] S. Jackson, M. James, P. Abrams, The effect of oestradiol on vaginal
generation selective estrogen receptor modulator, Endocrinology 143
collagen metabolism in postmenopausal women with genuine stress
(2002) 2052–2061.
incontinence, BJOG 109 (2002) 339–344.
[55] T. Simoncini, P. Mannella, L. Fornari, G. Varone, A. Caruso, A.R.
[38] W.A. Anderson, Y.H. Kang, Estrogen and antagonist-induced struc-
Genazzani, Dehydroepiandrosterone modulates endothelial nitric
tural changes in the cervico-vaginal epithelium of immature rats,
oxide synthesis via direct genomic and nongenomic mechanisms,
Am. J. Anat. 144 (1975) 197–207.
Endocrinology 144 (2003) 3449–3455.
[39] M. Yoshida, K. Kudoh, S. Katsuda, M. Takahashi, J. Ando, A.
[56] A.T. Guay, J. Jacobson, Decreased free testosterone and dehy-
Maekawa, Inhibitory effects of uterine endometrial carcinogenesis
droepiandrosterone (DHEA-S) levels in women with decreased
in Donryu rats by tamoxifen, Cancer Lett. 134 (1998) 43–51.
libido, J. Sex Marit. Ther. 28 (2002) 129–142.
[40] T.G. Kennedy, D.T. Armstrong, Induction of vaginal mucification in
[57] H. Wang, H. Eriksson, L. Sahlin, Estrogen receptors alpha and beta
rats with testosterone and 17beta-hydroxy-5alpha-androstan-3-one,
in the female reproductive tract of the rat during the estrous cycle,
Steroids 27 (1976) 423–430.
Biol. Reprod. 63 (2000) 1331–1340.
[41] A. Sourla, M. Flamand, A. Belanger, F. Labrie, Effect of dehy-
[58] M.D. Mergman, B.S. Schachter, K. Karelus, E.P. Combatsiaris, T.
droepiandrosterone on vaginal and uterine histomorphology in the
Garcia, J.F. Nelson, Up-regulation of the uterine estrogen receptor
rat, J. Steroid Biochem. Mol. Biol. 66 (1998) 137–149.
and its messager ribonucleic acid during the mouse estrous cycle:
[42] A. Sourla, C. Martel, C. Labrie, F. Labrie, Almost exclusive andro-
the role of estradiol, Endocrinology 130 (1992) 1923–1930.
genic action of dehydroepiandrosterone in the rat mammary gland,
[59] M. Maggiolini, O. Donz´e, E. Jeannin, S. Ando, D. Picard, Adrenal
Endocrinology 139 (1998) 753–764.
androgens stimulate the proliferation of breast cancer cells as direct
[43] A. Sourla, V. Richard, F. Labrie, C. Labrie, Exclusive androgenic
activators of estrogen receptor a, Cancer Res. 59 (1999) 4864–
effect of dehydroepiandrosterone in sebaceous glands of rat skin, J.
Endocrinol. 166 (2000) 455–462.
[60] L.A. Mattson, G. Culberg, O. Eriksson, F. Kruitsson, Vaginal admin-
[44] C. Martel, A. Sourla, G. Pelletier, C. Labrie, M. Fournier, S. Picard,
istration of low dose estradiol: effects on endometrium and vaginal
S. Li, M. Stojanovic, F. Labrie, Predominant androgenic component
cytology, Maturitas (1989) 217–222.
in the stimulatory effect of dehydroepiandrosterone on bone mineral
[61] F. Labrie, Extragonadal synthesis of sex steroids: intracrinology,
density in the rat, J. Endocrinol. 157 (1998) 433–442.
Ann. Endocrinol. (Paris) 64 (2003) 95–107.
[45] J.F. Knudsen, V.B. Mahesh, Initiation of precocious sexual mat-
[62] P. Popesko, V. Rajtova, J. Horak, Female reproductive organs—a
uration in the immature rat treated with dehydroepiandrosterone,
colour atlas of anatomy of small laboratory animals, volume two:
Endocrinology 97 (1975) 458–468.
rat-mouse-hamster, Elsevier Sci. (2003) 85.
Source: http://www.health-mall.in/files_hl/Effects_dehydroepiandrosterone.pdf
[LEGAL NOTICE NO. 92] MARITIME TRANSPORT DECREE 2013 (DECREE NO. 20 OF 2013) Maritime (Ships Medical Requirements) IN exercise of the powers conferred upon me by section 240(1)(o) of the Maritime Transport Decree 2013, I hereby make these Regulations— PART 1—PRELIMINARY Short title and commencement 1. These Regulations may be cited as the Maritime (Ships Medical Requirements)
DATE: 16/03/2015 INVITATION TO BID: No. ITB/ UNHCR/PMCS/2015/GOODS/MI ITB/007 FOR THE ESTABLISHMENT OF A FRAME AGREEMENT FOR THE SUPPLY AND DELIVERY DAP GENEVA OF MEDICAL KITS CLOSING DATE AND TIME: 17/04/2015 – 23:59 hrs CET INTRODUCTION TO UNHCR







