Theoldexchangesurgery.co.uk
Vitamin D accelerates resolution of inflammatory
responses during tuberculosis treatmentAnna K. Coussensa, Robert J. Wilkinsona,b, Yasmeen Hanifac, Vladyslav Nikolayevskyyd, Paul T. Elkingtone,Kamrul Islamc, Peter M. Timmsf, Timothy R. Ventonf, Graham H. Bothamleyf, Geoffrey E. Packeg,Mathina Darmalingamh, Robert N. Davidsoni, Heather J. Milburnj, Lucy V. Bakerk, Richard D. Barkerl,Charles A. Meinm, Leena Bhaw-Rosunm, Rosamond Nuamahm, Douglas B. Younga, Francis A. Drobniewskid,Christopher J. Griffithsc, and Adrian R. Martineauc,a,b,1
aDivision of Mycobacterial Research, Medical Research Council National Institute for Medical Research, London NW7 1AA, United Kingdom; bDivision ofMedicine, Imperial College London, London W2 1PG, United Kingdom; cBlizard Institute, Barts and The London School of Medicine and Dentistry, Queen MaryUniversity of London, London E1 2AB, United Kingdom; dHealth Protection Agency National Mycobacterium Reference Laboratory, Barts and The LondonSchool of Medicine and Dentistry, London E1 2AT, United Kingdom; eDepartment of Infectious Diseases and Immunity, Imperial College London, London W120NN, United Kingdom; fHomerton University Hospital, London E9 6SR, United Kingdom; gNewham Chest Clinic, Forest Gate, London E7 8QP, United Kingdom;
hDepartment of Respiratory Medicine, Whipps Cross University Hospital, London E11 1NR, United Kingdom; iTuberculosis Clinic, Northwick Park Hospital,Harrow HA1 3UJ, United Kingdom; jDepartment of Respiratory Medicine, Guy's and St Thomas' Hospitals, London SE1 9RT, United Kingdom; kDepartment ofRespiratory Medicine, Lewisham Hospital, London SE13 6LH, United Kingdom; lDepartment of Respiratory Medicine, Kings College Hospital, London SE5 9RS,United Kingdom; and mGenome Centre, Barts and The London School of Medicine, Queen Mary University of London, London EC1M 6BQ, United Kingdom
Edited* by Barry R. Bloom, Harvard School of Public Health, Boston, MA, and approved July 25, 2012 (received for review January 18, 2012)
Calcidiol, the major circulating metabolite of vitamin D, supports
pathogen-associated ligands (11, 12). Extrarenal generation of
induction of pleiotropic antimicrobial responses in vitro. Vitamin D
calcitriol is dependent on the availability of its precursor calcidiol,
supplementation elevates circulating calcidiol concentrations, and
the major circulating vitamin D metabolite that supports in-
thus has a potential role in the prevention and treatment of in-
duction of antimicrobial responses in vitro (11, 13) and the con-
fection. The immunomodulatory effects of administering vitamin D
centrations of which are often low in patients with pulmonary
to humans with an infectious disease have not previously been re-
infection (14–16). Vitamin D supplementation elevates circulat-
ported. To characterize these effects, we conducted a detailed
ing calcidiol concentrations, and may therefore enhance response
longitudinal study of circulating and antigen-stimulated immune
to antimicrobial therapy for respiratory infections. However,
responses in ninety-five patients receiving antimicrobial therapy for
the effects of in vivo vitamin D supplementation on immune re-sponses in humans with an infectious disease have not previously
pulmonary tuberculosis who were randomized to receive adjunctive
been described.
high-dose vitamin D or placebo in a clinical trial, and who fulfilled
Vitamin D was used to treat tuberculosis in the preantibiotic
criteria for per-protocol analysis. Vitamin D supplementation accel-
era (17), and vitamin D supplementation has been shown to
erated sputum smear conversion and enhanced treatment-induced
enhance healthy tuberculosis contacts' immunity to mycobacteria
resolution of lymphopaenia, monocytosis, hypercytokinaemia, and
(18). These observations prompted us to conduct a randomized
hyperchemokinaemia. Administration of vitamin D also suppressed
controlled trial evaluating the influence of adjunctive high-dose
antigen-stimulated proinflammatory cytokine responses, but atten-
vitamin D on time to bacterial clearance in patients receiving
uated the suppressive effect of antimicrobial therapy on antigen-
antimicrobial therapy for smear-positive pulmonary tuberculosis
stimulated secretion of IL-4, CC chemokine ligand 5, and IFN-α. We
(19). We now present results of a detailed analysis of longitu-
demonstrate a previously unappreciated role for vitamin D supple-
dinal changes in the immune response in trial participants during
mentation in accelerating resolution of inflammatory responses dur-
the 8-wk course of intensive-phase antituberculous therapy.
ing tuberculosis treatment. Our findings suggest a potential role for
Initially we describe the effects of antituberculous therapy alone
adjunctive vitamin D supplementation in the treatment of pulmo-
on circulating and antigen-stimulated immune responses, using
nary infections to accelerate resolution of inflammatory responses
samples from patients randomized to the placebo arm of the
associated with increased risk of mortality.
trial. Subsequently we proceed to characterize the effects of vi-tamin D supplementation using samples from those randomized
adjunctive therapy immunomodulation antimicrobial peptides
to the intervention arm of the study, showing that administrationof adjunctive vitamin D exerts pleiotropic immunomodulatory
effects in patients with pulmonary tuberculosis.
espite the widespread availability of antimicrobials, bacterialrespiratory infections remain a major global cause of death
(1). Mortality is associated with infection with antibiotic-resistantorganisms (2, 3) and with failure to resolve immunopathological
Effect of Antimicrobials on Circulating Responses. Determination of
inflammatory responses (4–6). Immunomodulatory agents that
the immunomodulatory effects of vitamin D supplementationnecessitated an initial, comprehensive characterization of the
augment antimicrobial immune responses and accelerate reso-
changes in immune responses induced by antituberculous therapy
lution of pulmonary inflammation could be used as adjuncts to
alone. To this end, 42 soluble factors and 14 hematological
antimicrobial therapy to improve treatment outcomes (7).
Calcitriol, the active metabolite of vitamin D, induces innate
antimicrobial responses and suppresses proinflammatory cytokineresponses in vitro (8). Calcitriol's antimicrobial activity is mediated
Author contributions: C.J.G. and A.R.M. designed research; A.K.C., R.J.W., Y.H., V.N., P.T.E.,K.I., P.M.T., T.R.V., G.H.B., G.E.P., M.D., R.N.D., H.J.M., L.V.B., R.D.B., C.A.M., L.B.-R., R.N.,
via induction of reactive nitrogen intermediates, reactive oxygen
D.B.Y., F.A.D., C.J.G., and A.R.M. performed research; A.K.C. and A.R.M. analyzed data;
intermediates, antimicrobial peptides, and autophagy (9). Calci-
and A.K.C. and A.R.M. wrote the paper.
triol also modulates adaptive responses, both indirectly (by sup-
Conflict of interest statement: Merck Serono donated €7,000 to Queen Mary University of
pression of MHC class II expression and IL-12 secretion by
London in 2010 to support an academic meeting entitled "Vitamin D: Mechanisms of
antigen-presenting cells) and directly [by suppressing secretion of
Action in Health and Disease"; this meeting was convened by C.J.G. and A.R.M.
IFN-γ and IL-2 from CD4+ T-helper type 1 (Th1) cells] (10).
*This Direct Submission article had a prearranged editor.
Calcitriol is synthesized by the vitamin D 1-α hydroxylase enzyme,
1To whom correspondence should be addressed. E-mail:
the expression of which is up-regulated in leukocytes and pul-
This article contains supporting information online at
monary epithelium following ligation of Toll-like receptors by
PNAS Early Edition 1 of 6
parameters, detailed in Materials and Methods, were measured in
Effect of Antimicrobials on Antigen-Stimulated Responses. Whole-
samples of serum, plasma, and whole blood taken from 51
blood samples taken from 28 patients randomized to the placebo
patients randomized to the placebo arm of the trial at 0, 2, 4, 6,
arm of the trial were stimulated ex vivo with a panel of mycobac-
and 8 wk of treatment (for trial profile, see for baseline
terial antigens, and the concentration of IFN-γ in supernatants of
characteristics, see Parameters were selected on the
baseline samples was compared between stimuli. Of the MTB-
basis that they played a role in host defense against Mycobacte-
specific antigens tested, recombinant early-secreted antigenic tar-
rium tuberculosis (MTB) (20) or that they were biomarkers of
get 6 kDa (rESAT-6) and recombinant culture filtrate protein 10
treatment response (21). Median serum concentrations of seven
kDa (rCFP-10) induced the greatest IFN-γ responses ). We
soluble factors [IL-2, IL-5, IL-13, IL-17, TNF, FGF-β, and matrix
therefore proceeded to assay concentrations of the 39 soluble
metalloproteinase-7 (MMP-7)] were below the limit of detection
factors listed in Materials and Methods in supernatants of whole
at baseline, and these were excluded from statistical analyses. The
blood stimulated with these two antigens at 0, 2, 4, 6, and 8 wk of
remaining 49 parameters were assessed using principal compo-
treatment. Median concentrations of six soluble factors (IL-2, IL-
nent analysis (PCA), a well-established mathematical technique
5, IL-13, EGF, FGF-β, and MMP-7) were below the limit of de-
for reducing the dimensionality of complex datasets by trans-
tection in these samples, and these parameters were therefore
forming the data to a new coordinate system. The first three
excluded from statistical analyses. The remaining 33 parameters
coordinates (principal components) are represented as a 3D plot.
underwent PCA transformation and rank regression analysis. The
The first principal component accounts for as much of the vari-
resultant PCA plots for rESAT-6–and rCFP-10–stimulated
ability in the data as possible, and each succeeding component
responses were similar to each other: samples
has the highest variance possible under the constraint that it is
converged from a loosely clustered pattern at baseline toward
uncorrelated with preceding components. This method allows
a more tightly clustered pattern at 4 wk, and then back to a more
visualization of the differences between patient samples and
loosely clustered pattern at 8 wk, changes confirmed as being
analytes within complex datasets (22). The resultant PCA plot
statistically significant by analysis of the sums of Euclidean dis-
showed that baseline samples were less tightly
tances at these time points (P < 0.0001) (Twenty-
clustered than follow-up samples. Comparison of the median sum
seven antigen-stimulated parameters contributed to the pattern of
of Euclidean distances between points in the PCA plot at baseline
response to antituberculous therapy (). All analytes whose
vs. 8 wk confirmed that this convergence was statistically signifi-
concentration changed significantly during the course of intensive
cant (P < 0.0001) (), indicating that patients had a rela-
phase antituberculous therapy showed a decrease in secretion over
tively heterogeneous circulating immunological profile at baseline
time. Of note, IFN-γ was among the analytes whose antigen-
that became more homogenous as treatment progressed.
stimulated concentration did not change significantly during the
Rank-regression analysis (23) was applied to PCA-transformed
course of antituberculous therapy, even when corrected for
data to identify parameters whose concentration changed signifi-
changes in lymphocyte count ).
cantly over time. presents details of the 42 circulating
To determine whether changes in antigen-stimulated immune
immunological parameters so identified. Of the hematological
responses corresponded to changes in whole-blood cellular
parameters investigated, platelet count, neutrophil count, and
composition during antituberculous therapy, network PCA was
monocyte count decreased during the course of treatment (P ≤
applied to the antigen-stimulated analytes listed in
0.0018), but lymphocyte count and eosinophil count both in-
together with cell-count data obtained for the relevant samples
creased (P ≤ 0.0019). Increases were also seen in hemoglobin
before antigenic stimulation. Similar PCA networks were iden-
concentration and red blood cell parameters (P ≤ 0.015), reflect-
tified for rCFP-10– and rESAT-6–stimulated responses (
ing resolution of microcytic anemia as treatment progressed.
). Platelets, CCL5, IL-4, G-CSF, and CCL11 were
Decreases in erythrocyte sedimentation rate (ESR) and C-reactive
connected in both plots, replicating the platelet–CCL5 connec-
protein (CRP) (P ≤ 1.24 × 10−12) and an increase in serum al-
tion observed in the analysis of circulating parameters (
bumin concentration (P = 5.17 × 10−21) were also seen, indicating
). Neutrophils occupied a similar space to neutrophil gran-
resolution of the acute-phase response. These changes were ac-
ule-associated proteins MMP-9 and NGAL. IL-7 clustered with
companied by a decrease in circulating concentrations of all
another angiogenic factor, VEGF, and Th1 cytokines. Lympho-
cytokines, chemokines, antimicrobial peptides (AMP), MMP, and
cytes were not connected directly to any cytokines stimulated by
angiogenic factors identified (P ≤ 0.0240), except for CC chemo-
rESAT-6 or rCFP-10.
kine ligand 2 (CCL2) and MMP-2, the concentrations of whichincreased during the course of treatment (P ≤ 0.0035).
Effects of Vitamin D on Circulating Responses. We have previously
To investigate the relationship between changes in cell counts
reported results of the intention-to-treat analysis of study data,
and circulating concentrations of inflammatory mediators observed
indicating that administration of adjunctive vitamin D was asso-
during treatment, a PCA network was created for the parameters
ciated with a trend toward faster sputum culture conversion (P =
listed in . This network connected each analyte to one
0.14) (19). We repeated this analysis in the subgroup of 95 par-
other analyte with which it shared the most similar pattern of
ticipants fulfilling per-protocol analysis criteria, adjusting for fac-
change over time; the distance between analytes in the network
tors previously shown to influence time-to-sputum conversion in
represents their Pearson correlation coefficients. Seven distinct
this dataset (19) (age, ethnicity, baseline sputum smear, neutrophil
clusters were identified ). For parameters whose values
count, and presence or absence of cavitation on baseline chest
fell during treatment, the tightest cluster incorporated three MMP
radiograph). Median time to sputum culture conversion in this
(MMP-1, MMP-8, and MMP-9) with three AMP [human neutro-
subset of patients was 35 d in the intervention group and 46.5 d in
phil peptides (HNP) 1–3, neutrophil gelatinase-associated lipocalin
the control group (hazard ratio 1.27, 95% confidence interval
(NGAL), and cathelicidin (LL-37)]; this cluster was close to neu-
0.76–2.13, P = 0.36), and median time to sputum smear conver-
trophils, CXC chemokine ligand 8 (CXCL8), and prostaglandin E2
sion in the intervention arm was significantly shorter than that in
(PGE2). Neutrophils were linked to monocytes and CRP, which in
the control arm (23 vs. 36 d; hazard ratio 1.69, 95% confidence
turn was linked to IL-6 and ESR. Platelet count and CCL5 formed
interval 1.02–2.79, P = 0.04) (Fig. 1A).
a distinct grouping, and the other IFN-γ–stimulated chemokines,
To determine whether the effect of vitamin D on time to sputum
CXCL9 and CXCL10, were linked to each other and to IFN-γ,
clearance in the per-protocol subgroup was associated with im-
which was linked to IL-6. The angiogenic factors, EGF, hepatocyte
munomodulatory activity, we compared the effect of antituber-
growth factor (HGF), and VEGF were linked to each other and to
culous therapy on the circulating immunological parameters
IL-7, IL-10, IL-15, and soluble IL-2 receptor (IL-2R). For
investigated above in 51 patients randomized to the placebo arm
parameters increasing during treatment, one network incorporated
of the trial vs. 44 patients randomized to receive adjunctive vitamin
red blood cell parameters, albumin, and MMP-2, and another
D using PCA and rank regression on the interaction term "treat-
linked lymphocyte and eosinophil counts.
ment duration*allocation." The PCA plot is shown in .
Coussens et al.
Kinetics of circulating and antigen-stimulated immune responses during the course of antituberculous therapy in the presence vs. the absence of ad-
junctive vitamin D. Vitamin D accelerated sputum smear conversion in patients fulfilling per-protocol analysis criteria (A). Monocyte counts fell more quickly (B)and lymphocyte counts rose more quickly (C) among patients in the intervention arm of the trial. Vitamin D also accelerated treatment-induced decreases in ESR(D), circulating concentrations of CXCL9 (E), CXCL10 (F), MMP-9 (G), and LL-37 (H) and rCFP-10–stimulated supernatant concentrations of IL-1RA (I), IL-6 (J),IL-12p40/p70 (K), and TNF (L). Means ± SEM at 0, 2, 4, 6, and 8 wk of treatment are presented. Dotted lines, placebo arm; solid lines, vitamin D arm.
The 17 circulating parameters identified as being significantly
receptor (VDR), such that vitamin D hastened sputum culture
affected by vitamin D are detailed in All of these
conversion in patients with the tt genotype, but not in those with
parameters were also significantly affected by intensive-phase
Tt or TT genotypes (Fig. 2 A–C) (19). To investigate whether the
antituberculous therapy: in every case, vitamin D accelerated the
effects of vitamin D on circulating immune responses were also
effect of antituberculous therapy. The analyte most affected by
restricted to patients with this genotype, we stratified analysis of
vitamin D was the chemokine CXCL9, whose serum concentration
the effects of vitamin D on 8-wk values of analytes listed in
decreased significantly faster in patients randomized to receive
according to TaqI genotype; patients with the tt genotype
vitamin D vs. placebo (P = 5.92 × 10−12). Serum concentrations
were not included in this analysis because of small numbers with
of three other chemokines (CXCL10, CCL3, and CCL5) also fell
immunological data available (n = 7). In patients with the TT
more rapidly in patients randomized to vitamin D vs. placebo (P ≤
genotype, vitamin D supplementation significantly reduced 8-wk
0.0164), as did IFN-γ (P = 0.0012). Monocyte counts fell more
circulating concentrations of CRP, CXCL9, CXCL10, NGAL,
rapidly (P = 0.0003) and lymphocyte counts rose more rapidly
and LL-37, and in those with the Tt genotype, vitamin D sig-
(P = 0.0364) among patients receiving vitamin D. Neutrophil
nificantly reduced 8-wk circulating concentrations of CXCL9 and
counts were not significantly affected by allocation, but plasma
IL-10 (Fig. 2 D–I) (P ≤ 0.04). Our finding that vitamin D sup-
concentrations of neutrophil-associated AMP (LL-37, HNP1–3,
plementation modulated immune responses in patients with the
and NGAL) and MMP-9 fell more quickly in patients receiving
Tt and TT genotypes indicates that immunomodulatory effects of
adjunctive vitamin D (P ≤ 0.0112). Administration of vitamin D
vitamin D are not restricted to individuals with the tt genotype of
also induced a more rapid drop in ESR and serum CRP concen-
the TaqI VDR polymorphism.
tration (P ≤ 0.0072), indicating accelerated resolution of theacute-phase response.
Effects of Vitamin D on Antigen-Stimulated Responses. We next in-
Network PCA indicated that the accelerated fall in monocyte
vestigated the effect of vitamin D supplementation on antigen-
count in patients receiving vitamin D was linked to a rise in lym-
stimulated responses using PCA and rank regression interaction
phocyte count and a decrease in ESR and serum CRP concentration
analysis on 33 analytes detailed above for 19 patients allocated to
The IFN-γ–inducible chemokines CXCL9 and CXCL10
vitamin D vs. 28 patients allocated to placebo. The PCA plots
were linked to IFN-γ, IL-2R, IL-10, and CCL3. NGAL, HNP1–3,
generated for responses to rESAT-6 and rCFP-10 were similar to
and MMP-9 formed another cluster, which was linked to LL-37 and
each other, and samples from patients allocated to vitamin D vs.
PGE2. The kinetics of change in a representative group of circu-
placebo were clearly separated (). Vitamin D
lating immunological parameters over the course of intensive-phase
supplementation influenced supernatant concentrations of seven
therapy in vitamin D vs. placebo arms are presented in Fig. 1 B–H.
analytes in both rESAT-6– and rCFP-10–stimulated whole blood
We have previously reported that the effect of vitamin D on
); notably, IFN-γ was not among them (Vi-
time to sputum culture conversion in the intention-to-treat
tamin D enhanced the suppressive effect of antimicrobial ther-
analysis was modified by the TaqI genotype of the vitamin D
apy on secretion of IL-1 receptor antagonist (IL-1RA), IL-6, IL-
Coussens et al.
PNAS Early Edition 3 of 6
Immunomodulatory actions of vitamin D are not restricted to individuals with the tt genotype of the TaqI vitamin D receptor polymorphism. Vitamin
D supplementation accelerates sputum culture conversion in patients with the tt genotype of the TaqI vitamin D receptor polymorphism (A), but not in thosewith the Tt (B) or TT (C) genotypes (Pinteraction = 0.03). Vitamin D, solid line; placebo, dotted line. In contrast, the immunomodulatory actions of vitamin D arenot restricted to those with the tt genotype of the TaqI polymorphism. In patients with the TT genotype, vitamin D supplementation significantly reduced 8-wk circulating concentrations of CRP (D), CXCL9 (E), CXCL10 (F), NGAL (G), and LL-37 (H); in patients with the Tt genotype, statistically significant reductions in8-wk serum concentrations of CXCL9 (E) and IL-10 (I) were also seen in patients randomized to vitamin D (Vit D) vs. Placebo (Plac). Data for patients with the ttgenotype are not presented because of small numbers entering per-protocol analysis. Line at median; TT placebo (□) n = 23, TT vitamin D (■) n = 22, Ttplacebo (○) n = 21, Tt vitamin D () n = 19. LOD, limit of detection.
12, and TNF (P ≤ 0.0437), and attenuated treatment-induced
during treatment of an infectious disease. In patients taking
reductions in secretion of IL-4, CCL5, and IFN-α (P ≤ 0.0323).
antimicrobial therapy for smear-positive pulmonary tuberculo-
Network PCA showed that IL-12, TNF, and IL-1RA were linked,
sis, adjunctive vitamin D accelerated sputum smear conversion,
but IL-6 was connected to monocytes directly or via VEGF, and
augmented treatment-induced increases in lymphocyte count,
CCL5 and IL-4 were tightly clustered and linked to IFN-α for
and enhanced the suppressive effect of treatment on monocyte
both antigens (Vitamin D significantly reduced
count, inflammatory markers, and circulating concentrations of
antigen-stimulated secretion of IL-12, TNF, IL-1RA, IL-6,
chemokines, AMPs, and MMP-9. Administration of vitamin D
CXCL10, CCL3, CCL4, and VEGF (Fig. 1 I–L) and enhanced
also enhanced treatment-induced suppression of antigen-stimulated
CCL5, CCL11, IL-4, and IFN-α secretion.
Th1 cytokine responses, but attenuated treatment-induced sup-pression of antigen-stimulated IL-4, CCL5, and IFN-α secretion.
Among 51 patients randomized to receive antituberculous
Our study represents the most detailed characterization of the
therapy plus placebo, we observed an increase in circulating lym-
effects of antituberculous therapy on the immune response con-
phocyte counts and a reduction in circulating neutrophil counts,
ducted to date, and is unique in being a clinical investigation into the
monocyte counts, and concentrations of IFN-inducible parame-
immunomodulatory actions of in vivo vitamin D supplementation
ters following initiation of antituberculous therapy, consistent with
Coussens et al.
previous reports (24–26). These changes were associated with
Although many of the immunomodulatory effects of in vivo
decreases in circulating concentrations of lymphocyte chemo-
vitamin D supplementation that we observed were in keeping
attractants CXCL9 and CXCL10, and an increase in the monocyte
with the in vitro actions of calcitriol, there were two exceptions:
chemoattractant CCL2; they may therefore reflect reduced re-
calcitriol has been reported to induce IL-10 (36) and the anti-
cruitment of lymphocytes and increased recruitment of monocytes
microbial peptides LL-37 and NGAL (40, 41) in vitro, but we
to the lung. In keeping with this hypothesis, the proportion of
found that in vivo vitamin D supplementation suppressed cir-
macrophages in sputum of tuberculosis patients has been reported
culating concentrations of IL-10, LL-37, and NGAL. All three of
to increase as treatment progresses (27). Interestingly, increases in
these markers are suppressed by antituberculous therapy alone
circulating lymphocyte count following initiation of antituberculous
and the fact their concentration fell more quickly
therapy were not associated with any change in antigen-stimu-
among patients in the intervention arm of the study may arise as
lated production of IFN-γ as treatment progressed. In contrast,
an indirect consequence of enhanced microbial killing in patients
antigen-stimulated production of CCL5, IL-4, G-CSF, IFN-α, and
receiving vitamin D. Alternatively, this observation may repre-
CXCL10 were greatly decreased over the course of intensive-
sent a direct suppressive effect of vitamin D on release of these
phase therapy. Antigen-stimulated CXCL10 responses have been
mediators into the circulation from neutrophil granules.
reported to be more sensitive than IFN-γ for the diagnosis of
Interestingly, and in contrast to the effects of vitamin D sup-
active tuberculosis (28), and these data suggest that this panel of
plementation on sputum clearance that we have previously dem-
analytes may also hold promise as antigen-stimulated biomarkers
onstrated (19), we found that immunomodulatory effects of
of treatment response. Resolution of thrombocytosis is another
vitamin D were observed in patients having the TT and Tt geno-
well-recognized phenomenon associated with tuberculosis treat-
types of the TaqI VDR polymorphism. This observation suggests
ment (29), and network analysis revealed this to be linked to
that if these responses can be augmented—by administering vi-
a decrease in circulating CCL5 and antigen-stimulated CCL5 and
tamin D at higher doses, for example—then tuberculosis patients
IL-4 among patients in our study. Although best known for their
might derive a clinical benefit from vitamin D supplementation
role in hemostasis, platelets are also recognized to secrete CCL5
irrespective of TaqI genotype. More broadly, the ability of vitamin
(30), which can enhance production of IL-4 by CD4+ T cells (31).
D to accelerate resolution of potentially immunopathological in-
The role of platelets in the antimycobacterial response warrants
flammatory responses without compromising bacterial killing rai-
ses the possibility that supplementation might also have benefits in
Having characterized the immune response to antituberculous
patients receiving antimicrobial therapy for pneumonia and sepsis,
therapy, we proceeded to investigate how this was affected by
in whom failure to resolve hypercytokinaemia is associated with
administration of adjunctive vitamin D. In contrast to studies
increased mortality (5, 6).
investigating immunomodulatory actions of vitamin D supple-mentation in healthy people and in those with noncommunicable
Materials and Methods
diseases (32–35), we report pleiotropic immunomodulatory
Details of the trial protocol have previously been reported; participants were
actions of vitamin D in tuberculosis patients. This difference may
randomized to receive four fortnightly doses of 2.5 mg vitamin D3 vs. placebo
reflect the very high prevalence of profound deficiency at base-
in addition to standard antituberculous therapy (19). Antigen-stimulated
line among participants in our study; the relatively high dose of
whole-blood assays were performed as previously described (42). Concen-
vitamin D administered; or the fact that MTB can up-regulate
trations of IL-1β, IL-1RA, IL-2, IL-2R, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12 (p40/p70),
expression of the vitamin D 1-α hydroxylase CYP27B1 to gen-
IL-13, IL-15, IL-17, G-CSF, GM-CSF, IFN-α, IFN-γ, TNF, CXCL8, CXCL9, CXCL10,
erate immunomodulatory concentrations of calcitriol at sites of
CCL2, CCL3, CCL4, CCL5, CCL11, EGF, FGF-β, HGF, and VEGF were quantified
infection (11). Among patients fulfilling criteria for per-protocol
using a human 30-plex bead immunoassay panel (Invitrogen). Serum CRP
analysis (n = 96), vitamin D accelerated sputum smear conver-
and albumin concentrations were assayed using an Architect ci8200 analyzer(Abbott Diagnostics). Serum PGE2 concentration was analyzed by high-sensi-
sion (P = 0.04). This finding contrasts with results of our pre-
tivity competitive enzyme immunoassay (Assay Designs). Concentrations of
viously published intention-to-treat analysis (n = 126), in which
LL-37, HNP1-3, and NGAL were analyzed by ELISA (Hycult Biotechnology).
a trend toward faster conversion in vitamin D-supplemented
Concentrations of MMP-1, -2, -3, -7, and -8 were determined by Fluorokine
patients did not attain statistical significance (19). This differ-
MAP multianlalyte profiling (R&D Systems); concentration of MMP-9 was
ence may reflect the superior compliance of participants in-
determined by DuoSet ELISA (R&D Systems). Antigen-stimulated AMP and
cluded in the per-protocol analysis, which excluded patients who
MMP concentrations were corrected by subtraction of unstimulated values.
did not take a full course of study medication. Vitamin D also
Full blood counts were performed using a LH750 hematology analyzer
suppressed circulating concentrations of IFN-γ and IFN-γ-in-
(Beckman Coulter). ESR was measured by the Wintrobe method using
ducible chemokines CXCL9 and CXCL10, MMP-9, and antigen-
a s2000 analyzer (Desaga). DNA extraction and genotyping were performed
stimulated Th1 responses. These in vivo findings are consistent
as previously described (19).
with reported immunomodulatory actions of calcitriol in vitro (8,
PCA was conducted using Qlucore Omics Explorer 2.2 (Qlucore). Analyte
36, 37). In contrast to these suppressive actions, vitamin D also
concentrations were log2-converted and normalized to the mean for each
attenuated treatment-induced falls in antigen-stimulated CCL5,
analyte with variance −1 to +1. Rank-regression analysis was applied to PCA-
IL-4, and IFN-α. IL-4 has recently been reported to induce ex-
transformed data to identify parameters whose concentration was affected by
pression of CYP24A, the principal catabolic enzyme of both
antituberculous therapy (by making within-patient comparison of samples at
calcidiol and calcitriol (38); the increase in antigen-stimulated
different time points among patients allocated to placebo) and vitamin D (by
IL-4 secretion observed in the intervention arm of the study may
making between-patient comparison of samples from patients allocated to
therefore represent part of a negative-feedback loop via which
placebo vs. vitamin D at each time point). This analysis yielded t statistics
calcitriol regulates its own concentration at the site of disease.
(calculated as the regression coefficient for each parameter divided by its SD)representing the magnitude of difference in concentration of a given pa-
The finding that administration of vitamin D enhanced antigen-
rameter between groups being compared; P values, representing the proba-
stimulated IFN-α responses is of particular interest, given the
bility that such differences could have arisen by chance alone; and q values,
pivotal role of type 1 interferons in antiviral responses (39), and
representing the lowest false discovery rate for which differences would be
the clinical observation of a sixfold reduction in upper re-
accepted as statistically significant under the Benjamini–Hochberg procedure
spiratory tract infections among patients in the intervention arm
for multiple-testing correction (43). The effects of allocation on circulating
of the trial (19). Modulation of antigen-stimulated responses by
immune responses at 8 wk within genetically defined subgroups were ana-
vitamin D supplementation may represent changes in numbers of
lyzed using Mann–Whitney U tests. The effect of allocation on time to sputum
circulating lymphocyte subpopulations or direct effects of vita-
clearance was analyzed by Cox regression analysis, adjusting for age, ethnicity,
min D on lymphocyte function. More detailed characterization
baseline sputum smear, neutrophil count, and presence or absence of cavita-
of the effects of vitamin D supplementation on numbers and
tion on baseline chest radiograph as previously described (19).
cytokine profiles of lymphocyte subsets is warranted.
Further details are presented in .
Coussens et al.
PNAS Early Edition 5 of 6
ACKNOWLEDGMENTS. We thank Dr. Delmiro Fernandez-Reyes (Medical
acting as Principal Investigators; the members of our Data Monitoring
Research Council National Institute of Medical Research) for advice on
Committee, Dr. Guy E. Thwaites (Chair), Dr. Brenda E. Jones, and Dr. Tuan
statistical analysis; Dr. Alleyna Claxton (Homerton University National
Q. Phung; and all the tuberculosis nurses and administrative staff who
Health Service Foundation Trust) for assistance with development of
referred patients to the study and all patients who participated in the
microbiological outcome measures; Drs. John C. Moore-Gillon (Barts and
trial. The study was funded by the British Lung Foundation (TB05/11) and
The London National Health Service Trust), Thomas C. Stokes (Queen
the United Kingdom Medical Research Council (U1175 22141). Merck
Elizabeth Hospital), and Stefan Lozewicz (North Middlesex Hospital) for
Serono donated study medication.
1. WHO (2008) Global Burden of Disease: 2004 Update (WHO, Geneva).
24. Jones BE, et al. (1997) CD4 cell counts in human immunodeficiency virus-negative
2. Tleyjeh IM, Tlaygeh HM, Hejal R, Montori VM, Baddour LM (2006) The impact of
patients with tuberculosis. Clin Infect Dis 24:988–991.
penicillin resistance on short-term mortality in hospitalized adults with pneumococcal
25. Brahmbhatt S, et al. (2006) Immune markers measured before treatment predict
pneumonia: A systematic review and meta-analysis. Clin Infect Dis 42:788–797.
outcome of intensive phase tuberculosis therapy. Clin Exp Immunol 146:243–252.
3. Gandhi NR, et al. (2006) Extensively drug-resistant tuberculosis as a cause of death in
26. Berry MP, et al. (2010) An interferon-inducible neutrophil-driven blood transcrip-
patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet
tional signature in human tuberculosis. Nature 466:973–977.
27. Ribeiro-Rodrigues R, et al. (2002) Sputum cytokine levels in patients with pulmonary
4. Barnes PF, et al. (1988) Predictors of short-term prognosis in patients with pulmonary
tuberculosis as early markers of mycobacterial clearance. Clin Diagn Lab Immunol 9:
tuberculosis. J Infect Dis 158:366–371.
5. Kellum JA, et al.; GenIMS Investigators (2007) Understanding the inflammatory cy-
28. Ruhwald M, et al. (2007) CXCL10/IP-10 release is induced by incubation of whole
tokine response in pneumonia and sepsis: Results of the Genetic and Inflammatory
blood from tuberculosis patients with ESAT-6, CFP10 and TB7.7. Microbes Infect 9:
Markers of Sepsis (GenIMS) Study. Arch Intern Med 167:1655–1663.
6. Yende S, et al.; GenIMS Investigators (2008) Inflammatory markers at hospital dis-
29. Tozkoparan E, Deniz O, Ucar E, Bilgic H, Ekiz K (2007) Changes in platelet count and
charge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit
indices in pulmonary tuberculosis. Clin Chem Lab Med 45:1009–1013.
Care Med 177:1242–1247.
30. Schober A, et al. (2002) Deposition of platelet RANTES triggering monocyte re-
7. Siempos II, Vardakas KZ, Kopterides P, Falagas ME (2008) Adjunctive therapies for
cruitment requires P-selectin and is involved in neointima formation after arterial
community-acquired pneumonia: a systematic review. J Antimicrob Chemother 62:
injury. Circulation 106:1523–1529.
31. Saito S, Yamaguchi E, Nakayama H, Miyamoto K, Kawakami Y (2000) Modulatory
8. Martineau AR, et al. (2007) IFN-γ- and TNF-independent vitamin D-inducible human
roles of RANTES in IL-4 production by human blood CD4(+)T cells. Cytokine 12:
suppression of mycobacteria: The role of cathelicidin LL-37. J Immunol 178:7190–7198.
9. Hewison M (2011) Antibacterial effects of vitamin D. Nat Rev Endocrinol 7:337–345.
32. Mahon BD, Gordon SA, Cruz J, Cosman F, Cantorna MT (2003) Cytokine profile in
10. Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C (2010) Vitamin D: Modulator of
patients with multiple sclerosis following vitamin D supplementation. J Neuro-
the immune system. Curr Opin Pharmacol 10:482–496.
11. Liu PT, et al. (2006) Toll-like receptor triggering of a vitamin D-mediated human
33. Schleithoff SS, et al. (2006) Vitamin D supplementation improves cytokine profiles in
antimicrobial response. Science 311:1770–1773.
patients with congestive heart failure: A double-blind, randomized, placebo-con-
12. Hansdottir S, et al. (2008) Respiratory epithelial cells convert inactive vitamin D to its
trolled trial. Am J Clin Nutr 83:754–759.
active form: Potential effects on host defense. J Immunol 181:7090–7099.
34. Jorde R, et al. (2010) No effect of supplementation with cholecalciferol on cytokines
13. Fabri M, et al. (2011) Vitamin D is required for IFN-γ-mediated antimicrobial activity of
and markers of inflammation in overweight and obese subjects. Cytokine 50:175–180.
human macrophages. Sci Transl Med 3(104):104ra102.
35. Yusupov E, et al. (2010) Vitamin D and serum cytokines in a randomized clinical trial.
14. Wayse V, Yousafzai A, Mogale K, Filteau S (2004) Association of subclinical vitamin D
Int J Endocrinol 2010:2010.
deficiency with severe acute lower respiratory infection in Indian children under 5 y.
36. Coussens A, et al. (2009) 1alpha,25-dihydroxyvitamin D3 inhibits matrix metal-
Eur J Clin Nutr 58:563–567.
loproteinases induced by Mycobacterium tuberculosis infection. Immunology 127:
15. Martineau AR, et al. (2010) Association between Gc genotype and susceptibility to TB
is dependent on vitamin D status. Eur Respir J 35:1106–1112.
16. Martineau AR, et al. (2011) Reciprocal seasonal variation in vitamin D status and
37. Helming L, et al. (2005) 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of
tuberculosis notifications in Cape Town, South Africa. Proc Natl Acad Sci USA 108:
interferon gamma-mediated macrophage activation. Blood 106:4351–4358.
38. Edfeldt K, et al. (2010) T-cell cytokines differentially control human monocyte anti-
17. Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ (2007) Vitamin D in the
microbial responses by regulating vitamin D metabolism. Proc Natl Acad Sci USA 107:
treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol 103:793–798.
18. Martineau AR, et al. (2007) A single dose of vitamin D enhances immunity to myco-
39. Takaoka A, et al. (2003) Integration of interferon-alpha/beta signalling to p53 re-
bacteria. Am J Respir Crit Care Med 176:208–213.
sponses in tumour suppression and antiviral defence. Nature 424:516–523.
19. Martineau AR, et al. (2011) High-dose vitamin D
40. Wang TT, et al. (2004) Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of
3 during intensive-phase antimicro-
bial treatment of pulmonary tuberculosis: A double-blind randomised controlled trial.
antimicrobial peptide gene expression. J Immunol 173:2909–2912.
41. Gombart AF, Borregaard N, Koeffler HP (2005) Human cathelicidin antimicrobial
20. Cooper AM (2009) Cell-mediated immune responses in tuberculosis. Annu Rev Im-
peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-
munol 27:393–422.
regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 19:1067–1077.
21. Perrin FM, Lipman MC, McHugh TD, Gillespie SH (2007) Biomarkers of treatment re-
42. Schölvinck E, et al. (2004) Gamma interferon-based immunodiagnosis of tuberculosis:
sponse in clinical trials of novel antituberculosis agents. Lancet Infect Dis 7:481–490.
Comparison between whole-blood and enzyme-linked immunospot methods. J Clin
22. Jolliffe IT (2002) Principal Component Analysis (Springer, New York), 2nd Ed.
23. Cuzick J (2005) Rank Regression. Encyclopedia of Biostatistics, eds Armitage P,
43. Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate—A practical and
Colton T (John Wiley & Sons, New York), 2nd Ed.
powerful approach to multiple testing. J Roy Stat Soc B Met 57:289–300.
Coussens et al.
Supporting Information
Coussens et al. 10.1073/pnas.1200072109
SI Materials and Methods
IL-1β, IL-1RA, IL-2, IL-2R, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12
Participants. The patients included in this study were participants in
(p40/p70), IL-13, IL-15, IL-17, G-CSF, GM-CSF, IFN-α, IFN-γ,
the AdjuVIT study: a double-blind randomized placebo-controlled
TNF, CXC chemokine ligand 8 (CXCL8), CXCL9, CXCL10,
trial of high-dose vitamin D during intensive-phase antimicrobial
chemokine ligand 2 (CCL2), CCL3, CCL4, CCL5, CCL11, EGF,
treatment of pulmonary tuberculosis (TB), conducted in London,
FGF-β, hepatocyte growth factor (HGF), and VEGF were
United Kingdom. A detailed account of study design has previously
quantified using a human 30-plex bead immunoassay panel
been given (1). Briefly, 146 adults with smear-positive pulmonary
(sensitivity according to Lot #617361; Invitrogen). Serum sam-
TB were randomized to receive four doses of 2.5 mg vitamin D
ples required high dilution for accurate determination of CCL5
placebo in addition to standard intensive-phase antituberculous
concentration and all were reassayed using a single-plex bead
therapy. The first dose of study preparation was administered
assay (Invitrogen). Serum PGE2 concentration was analyzed by
within 7 d of the start of antituberculous therapy, and subsequent
high sensitivity competitive enzyme immunoassay (EIA; Assay
doses were administered 14, 28, and 42 d after the start of treat-
Designs; sensitivity 13.4 pg/mL). Plasma concentrations of cath-
ment. Baseline assessment included collection of a sputum sample
elicidin (LL-37; sensitivity 31 pg/mL), human neutrophil peptides
for microscopy and culture and a blood sample. Fresh whole blood
(HNP1-3; sensitivity 156 pg/mL) and neutrophil gelatinase-asso-
was sent for determination of full-blood count and erythrocyte
ciated lipocalin (NGAL; sensitivity 400 pg/mL) were analyzed by
sedimentation rate (ESR) and ex vivo stimulation with mycobac-
ELISA (Hycult Biotechnology). Plasma concentrations of MMP-
terial antigens as described below. Aliquots of serum, plasma, and
1, -2, -3, -7, and -8 were determine by Fluorokine Multianlalyte
whole blood were also stored at −80 °C until completion of the
Profiling (sensitivity according to Lot #273379; R&D Systems);
trial. Participants were reviewed at 14, 28, 42, and 56 d after starting
plasma concentration of MMP-9 was determined by DuoSet
antituberculous therapy to assess clinical status and to monitor for
ELISA (sensitivity 3 pg/mL; R&D Systems). Multiplex bead as-
adverse events. Blood and sputum samples were collected at each
says were performed on a Luminex 200 anlayzer (Luminex).
time point and processed as above. Full characterization of the
ELISA and EIA absorbances were measured using a Benchmark
immune response to antituberculous therapy and adjunctive vita-
Plus microplate spectrophotometer (Bio-Rad Laboratories). The
min D was performed in the subset of participants who fulfilled
concentrations of 39 of these analytes (all of the above except
predefined criteria for per-protocol analysis (i.e., those infected
PGE2, CRP, and albumin) were also determined in supernatants
with a rifampicin-sensitive isolate of Mycobacterium tuberculosis
of rESAT-6– and rCFP-10–stimulated whole blood, as above;
who received at least three doses of study preparation, who were
preliminary determination of IFN-γ concentration in supernatants
compliant with antituberculous therapy, who did not take second-
of baseline whole blood samples stimulated with the six-antigen
line antituberculous therapy or oral corticosteroids, who com-
panel was performed using ELISA (Cellestis). Antigen-stimulated
pleted all study visits and who were not HIV sero-positive). The
AMP and MMP concentrations were corrected by subtraction of
study was approved by East London and The City Research Ethics
unstimulated values. For MMP-2, -3, -8, and HNP and NGAL,
Committee (06/Q0605/83), and registered with
unstimulated values were generally greater than stimulated values,
(NCT00419068). Written informed consent was obtained from all
and this was the case sometimes for MMP-9 and LL-37. Cytokine/
participants before enrolment.
chemokine values were generally undetectable in unstimulatedsamples and a correction was not applied.
Antigen-Stimulated Whole-Blood Assay. Fresh whole blood was di-
Fourteen hematological parameters [lymphocyte, monocyte,
luted 1:10 in RPMI medium 1640 (Sigma-Aldrich) and duplicate
neutrophil, eosinophil, basophil, RBC and platelet counts, he-
180-μL aliquots were stimulated in 96-well plates at 37 °C in the
moglobin concentration (Hb), mean corpuscular hemoglobin
presence of 5% CO2 with the following: recombinant early secreted
(MCH), MCH concentration (MCHC), mean cell volume
antigenic target 6 kDa, (rESAT-6) (Rv3875; Proteix Biotechnolo-
(MCV), packed cell volume (PCV), red cell distribution width
gies; final concentration 2.5 μg/mL), recombinant culture filtrate
(RDW), and ESR] were also measured in fresh whole blood.
protein 10 kDa (rCFP-10) (Rv3874; Proteix Biotechnologies; final
Full blood counts were performed using a LH750 hematology
concentration 2.5 μg/mL), peptide pools (15-mers overlapping by
analyzer (Beckman Coulter). ESR was measured by the Wint-
10 residues) for ESAT-6, CFP-10, and TB7.7 (Rv2654; all from
robe method using an s2000 analyzer (Desaga).
Peptide and Protein Research; final concentration 5 μg/mL), tu-berculin-purified protein derivative (Statens Serum Institute; final
DNA Extraction and Genotyping. DNA was extracted from whole
concentration 20 μg/mL) and 2% (wt/vol) BSA in PBS (negative
blood using the Promega Wizard SV 96 Genomic DNA Purifi-
control). Plates were centrifuged after 72-h incubation, and cell-
cation System on the Biomek FX robot (Beckman Coulter),
free supernatants were aspirated and frozen at −80 °C pending
quantified using the Nanodrop spectrophotometer and normal-
immunological analysis. Recombinant antigens were tested for
ized to 5 ng/mL per 10 ng DNA was used as template for a 5 mL
presence of endotoxin: concentrations were found to be 1,723 IU
predeveloped TaqMan assay (Applied Biosystems) to type the
(EU)/mg for rESAT-6 and 260 IU (EU)/mg for rCFP-10. Control
TaqI polymorphism (rs731236) of the vitamin D receptor. This
experiments to determine the effects of adding these concen-
assay was performed on the ABI 7900HT platform in 384-well
trations of endotoxin to whole blood were not performed.
format, and data were analyzed with Autocaller software.
Immunological Analysis. Concentrations of 42 soluble factors [C
Statistical Analysis. Analyte concentrations were calculated from
reactive protein (CRP), albumin, and a panel of 18 cytokines, 8
raw ELISA data using Masterplex 2010 software (Hitachi Solutions
chemokines, 4 angiogenic factors, 3 antimicrobial peptides
America) and GraphPad Prism 5 software. Principal component
(AMP), 6 matrix metalloproteinases (MMP), and prostaglandin
analysis (PCA) was conducted using Qlucore Omics Explorer 2.2
E2 (PGE2)] were determined in serum/plasma as follows. Serum
(Qlucore). Analyte concentrations were log2-converted and nor-
CRP and albumin concentrations were assayed using an Architect
malized to the mean for each analyte with variance −1 to +1.
ci8200 analyzer (Abbott Diagnostics). Serum concentrations of
Missing values were imputed by K nearest neighbors (2); they
arose when a sample was not taken at a particular time point, or
nicity, and age, were adjusted for by using the eliminated factors
when a sample from a particular time point was of inadequate
approach applied to the sample annotation "patient ID," which
volume to allow determination of the concentration of a given
generates a general linear model to normalize baseline differences
analyte. For circulating parameters, 2.5% of data points were
between patients (3). Analytes significantly affected by the addi-
missing. For ESAT-6–stimulated parameters, 4% of datapoints
tion of vitamin D to antituberculous therapy were identified by
were missing. For CFP-10–stimulated parameters, 3% of data-
creating an interaction variable between week of treatment (weeks
points were missing. The degree of sample clustering in PCA plots
0, 2, 4, 6, 8) and allocation (vitamin D vs. placebo). The 10 in-
was quantified by calculating the sum of the Euclidean distances
teraction groups were analyzed by rank regression (ordered by
between each point in the PCA plot and all other points in the plot
week 0–8 in alternating groups, placebo vs. vitamin D), eliminating
at the same time point: high values of the sum of Euclidean dis-
factors "week" and "patient ID" and selecting P value <0.05 and q
tances represent a lower degree of "cluster tightness" at a given
value <0.1. PCA networks were created using one connection, i.e.,
time point, and vice versa. Median values of the sum of Euclidean
by connecting each analyte to one other analyte with which it
distances were compared between time points using the Kruskal–
shared the most similar pattern of change over time. The distance
Wallis test with Dunn's post hoc analysis.
between analytes in the network represents their Pearson corre-
Parameters whose concentration changed significantly during
lation coefficients. The effects of allocation on circulating immune
intensive phase antituberculous therapy were identified by
responses at 8 wk within genetically defined subgroups were ana-
analysis of samples from patients randomized to the placebo
lyzed using Mann–Whitney U tests. The effect of allocation on
control arm of the study, conducting a rank regression on week of
time to sputum clearance was analyzed by Cox regression analysis,
treatment with thresholds of P = 0.05 for statistical significance
adjusting for age, ethnicity, baseline sputum smear, neutrophil
and q = 0.1 for false discovery rate. Differences in baseline
count, and presence or absence of cavitation on baseline chest
characteristics between patients, such as extent of disease, eth-
radiograph, as previously described (1).
1. Martineau AR, et al. (2011) High-dose vitamin D(3) during intensive-phase antimicrobial
3. Wichura MJ (2006) The Coordinate-Free Approach to Linear Models (Cambridge Univ
treatment of pulmonary tuberculosis: A double-blind randomised controlled trial. Lancet
Press, Cambridge).
2. Troyanskaya O, et al. (2001) Missing value estimation methods for DNA microarrays.
464 assessed for eligibility
214 ineligible for randomisation104 declined to participate
146 enrolled and randomised
73 allocated to vitamin D
73 allocated to placebo
29 excluded from per-protocol
22 excluded from per-protocol
- 2 sputum culture negative
- 1 sputum culture negative
- 6 isolate other than
M. tuberculosis
- 3 isolate other than
M. tuberculosis
- 0 with rifampicin-resistant isolate
- 4 with rifampicin-resistant isolate
- 10 received < 3 doses of study
- 6 received < 3 doses of study
- 3 diagnosed with HIV infection
- 2 diagnosed with HIV infection
- 5 took oral corticosteroids
- 3 took oral corticosteroids
- 2 interrupted treatment
- 1 interrupted treatment
- 1 non-compliant with treatment
- 2 non-compliant with treatment
- 2 took second-line therapy
- 3 took second-line therapy
44 included in analysis of circulating
51 included in analysis of circulating
25 – antigen-stimulated whole blood
23 – antigen-stimulated whole blood
assays not performed
assays not performed
19 included in analysis of antigen-
28 included in analysis of antigen-
stimulated responses
stimulated responses
Trial profile. We assessed 464 patients for eligibility to participate in the trial between January 25, 2007 and July 3, 2009; 214 were ineligible, 104 were
eligible but declined randomization, and 146 were randomized. Of these subjects, 95 patients (44 allocated to vitamin D, 51 allocated to placebo) fulfilledpredefined criteria for per-protocol analysis and contributed samples to the analysis of circulating immunological parameters. Antigen-stimulated whole-bloodassays were performed on samples taken from all study participants who were recruited on or after May 15, 2008; 47 of these participants (19 allocated tovitamin D, 28 allocated to placebo) fulfilled per-protocol criteria and contributed samples to the analysis of antigen-stimulated responses. †Some participantswere ineligible to enter per-protocol analysis for more than one reason.
Sum of Euclidean distances for principal component analysis plots presented in Median values of the sums of the Euclidean distances
between each point in the PCA plot and all other points in the plot at the same time point are compared between time points using the Kruskal–Wallis testwith Dunn's post hoc analysis: high values of the sum of Euclidean distances represent a lower degree of cluster tightness at a given time point, and vice versa.
(A) Circulating immunological parameters: baseline samples were less tightly clustered than follow-up samples (median sum of Euclidean distances at 0 vs. 4 vs.
8 wk, 253.5 vs. 197.1 vs. 203.4, respectively; P < 0.0001). (B) rESAT-6–stimulated responses: samples converged from a loosely clustered pattern at baselinetoward a more tightly clustered pattern at 4 wk, and then back to a more loosely clustered pattern at 8 wk (median sum of Euclidean distances at 0 vs. 4 vs.
8 wk, 85.4 vs. 66.7 vs. 83.3, respectively; P < 0.0001). (C) rCFP-10–stimulated responses: pattern as for rESAT-stimulated responses (median sum of Euclideandistances at 0 vs. 4 vs. 8 wk, 91.9 vs. 67.0 vs. 89.3, respectively; P < 0.0001). Lines at median. ***P < 0.0001. NS, P > 0.05.
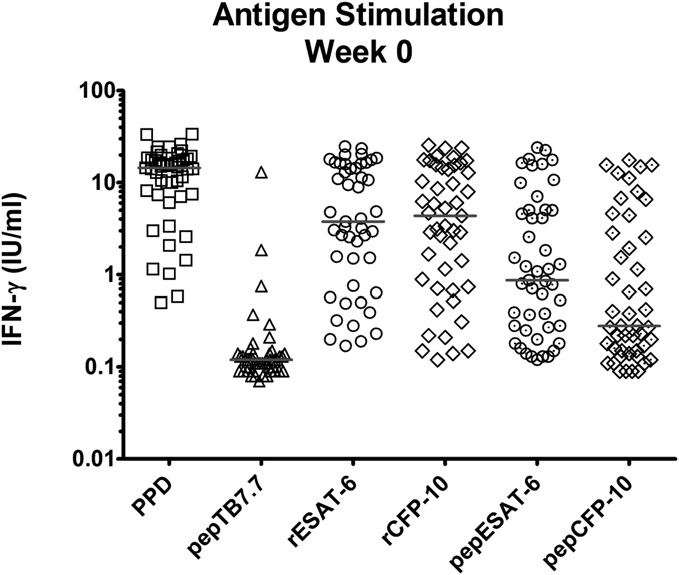
Concentrations of IFN-γ in supernatants of antigen-stimulated whole blood. Blood was stimulated for 72 h with the following antigens: tuberculin-
purified protein derivative (PPD), peptide (pep) TB7.7, recombinant (r) and peptide ESAT-6 and CFP-10. Assays were performed on blood samples of 47 patientssampled within 1 wk of initiating antituberculous therapy before administration of adjunctive vitamin D or placebo. Horizontal bar at median. 1 IU/mL IFN-γapproximates to 40 pg/mL IFN-γ.
Antigen-stimulated IFN-γ secretion is not influenced by administration of antimicrobials or adjunctive vitamin D during intensive phase antituber-
culous therapy. Data from 47 patients (28 allocated to adjunctive placebo, dotted line, and 19 allocated to adjunctive vitamin D, solid line) sampled at 0, 2, 4, 6,and 8 wk into therapy are presented. (A) Total rESAT-6–stimulated IFN-γ secretion by allocation. (B) Total rCFP-10–stimulated IFN-γ secretion by allocation. (C)rESAT-6–stimulated IFN-γ secretion per 106 lymphocytes by allocation. (D) rCFP-10–stimulated IFN-γ secretion per 106 lymphocytes by allocation. Medians andinterquartile (IRQ) ranges are presented.
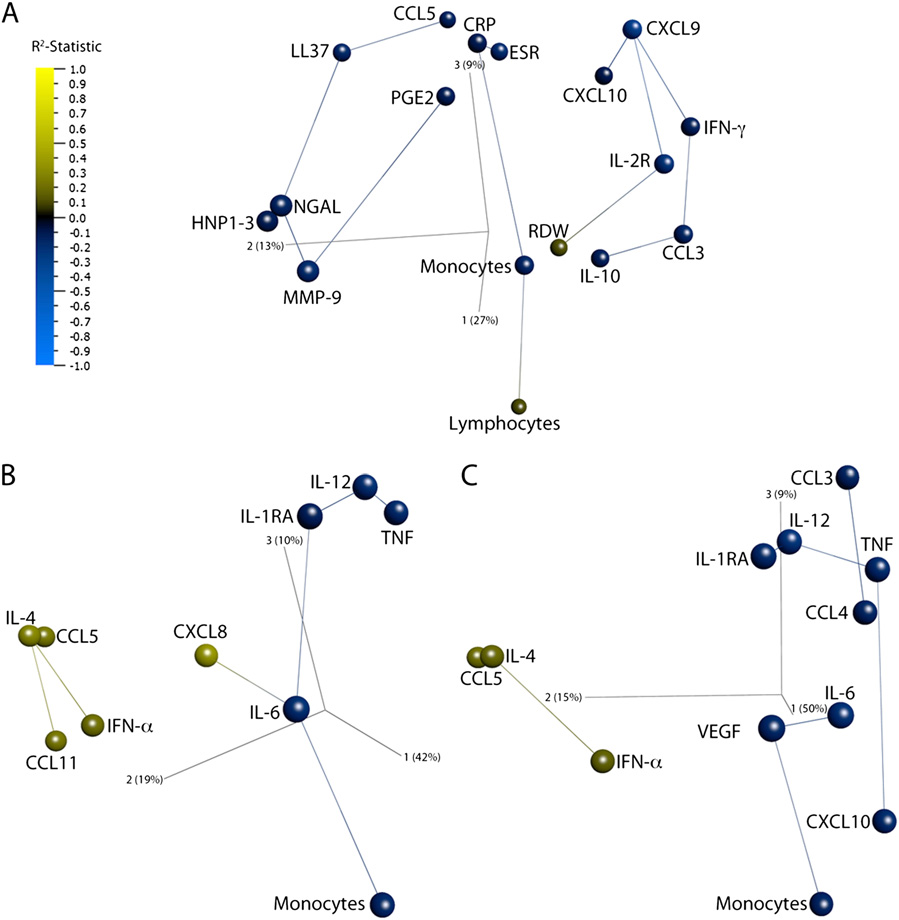
Three-dimensional network PCA plots showing the effect of adjunctive vitamin D supplementation on the immune response to intensive-phase
antituberculous therapy. Individual points represent a single analyte. The distance between analytes is a measure of their Pearson correlation coefficients. Lineslink analytes to their nearest neighbor, the analyte with the most similar pattern of change during the course of treatment. Analytes are colored by R2-statistic:blue represents suppression and yellow augmentation by vitamin D. Component vectors are displayed, along with a percent figure signifying the proportion ofthe variability in the data that each component accounts for. (A) Circulating immune parameters. (B) rESAT-6–stimulated whole-blood responses. (C) rCFP-10–stimulated whole-blood responses.
Baseline characteristics of participants entering analyses of circulating and antigen-stimulated responses by allocation
Circulating responses (n = 95)
Antigen-stimulated responses (n = 47)
Vitamin D (n = 44)
Vitamin D (n = 19)
Median age, years (IQR)
31.8 (24.4–41.8)
30.1 (24.7–36.6)
29.4 (23.7–37.5)
31.0 (23.9–36.6)
Asian/Asian British
Black/Black British
White/Latin American
Median symptom duration prediagnosis,
Median duration of treatment pre-enrolment,
Baseline sputum smear
≤3 AFB per high-power field
>3 AFB per high-power field
Serum 25(OH)D, nmol/L
Serum 25(OH)D <20 nmol/L
Serum 25(OH)D <75 nmol/L
Body mass index, kg/m2
Baseline chest radiograph
TaqI vitamin D receptor genotype
Data are number (%) or mean (SD) except where stated. AFB, acid-fast bacilli; IQR, interquartile range; 25(OH)D, 25-hydroxyvitamin D.
Influence of intensive-phase antituberculous therapy on circulating and antigen-stimulated immune responses in adults
with smear-positive pulmonary tuberculosis
Circulating responses (n = 51)
responses (n = 28)
responses (n = 28)
Factors and parameters
Angiogenic factors
Antimicrobial peptides
Acute phase proteins
Red blood cell parameters
White blood cell parameters
Neutrophil count{
Lymphocyte count{
Eosinophil count{
MCV, mean cell volume; PCV, packed cell volume; RDW, red cell distribution width; —, no data.
*t-Statistic (regression coefficient/SD) represents magnitude of change over time of treatment; sign indicates direction of this change. t-statistic value notpresented when P > 0.05.
†P values derived using principal component analysis and rank regression on treatment duration; all q values were < 0.1.
‡Median baseline concentration below limit of detection in serum samples.
§Median baseline concentration below limit of detection in whole blood assay supernatants.
{Not determined in whole blood assay supernatants.
Influence of adjunctive vitamin D supplementation on circulating and antigen-stimulated immune responses
to intensive-phase antituberculous therapy
Circulating responses
responses (n = 47)
responses (n = 47)
Factors and parameters
Angiogenic factor
Antimicrobial peptides
Matrix Metalloproteinase
Acute phase protein
Red blood cell parameter
White blood cell parameters
Lymphocyte count§
RDW, red cell distribution width; —, no data.
*t-statistic (regression coefficient/SD) represents magnitude of difference between patients receiving adjunctive vitamin D vs. placebo;sign indicates direction of this difference for vitamin D vs. placebo arms. t-statistic value not presented when P > 0.05.
†P values derived using principal component analysis and rank regression on the interaction term "treatment duration*allocation"; all qvalues were < 0.1.
‡Median baseline concentration below limit of detection in serum samples.
§Not determined in whole blood assay supernatants.
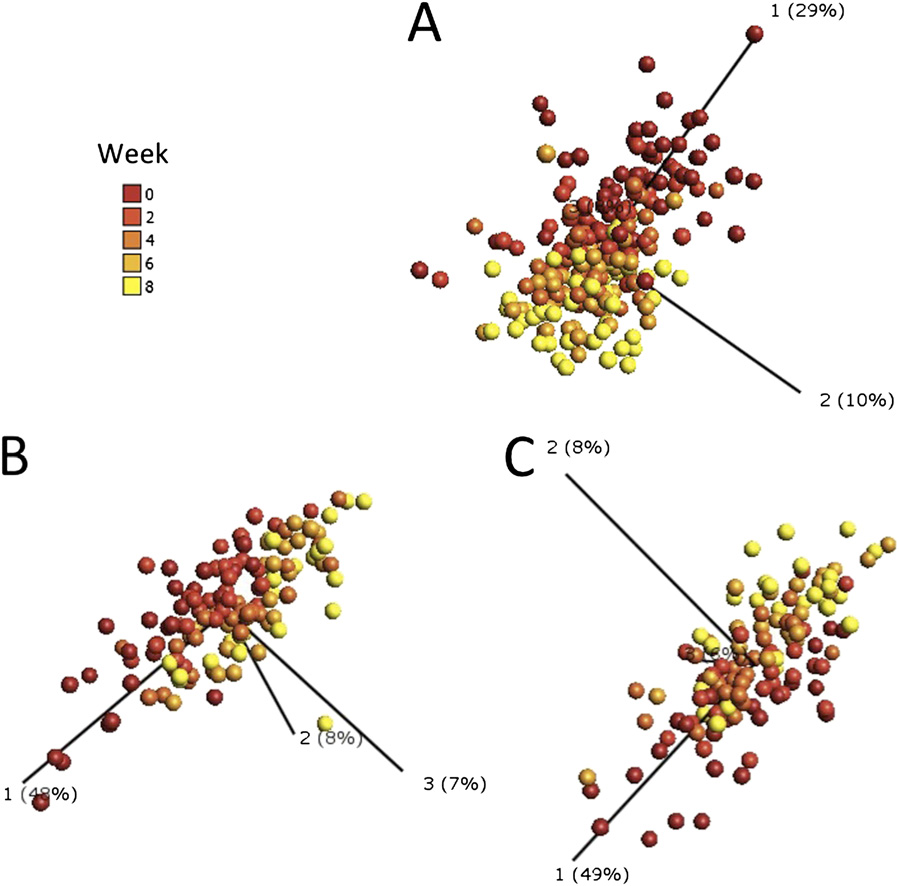
Three dimensional principal component analysis plots generated using parameters that significantly contribute to the effect of intensive-phase
antituberculous therapy on the immune response in patients with smear-positive pulmonary tuberculosis. Individual points represent one patient at one timepoint and their position in the plot is determined by the combined effects of all parameters measured for that patient sample that contribute significantly tothe effect of antituberculous therapy. The distance between sample points presents Euclidean distance and is calculated using all parameters which contributesignificantly to the effect of antituberculous therapy. Component vectors are displayed, along with a percent figure signifying the proportion of the variabilityin the data that each component accounts for. Color gradient denotes timing of sample, with baseline samples in red and 8-wk samples in yellow. (A) Cir-culating immunological parameters; (B) rESAT-6–stimulated responses; (C) rCFP-10–stimulated responses.
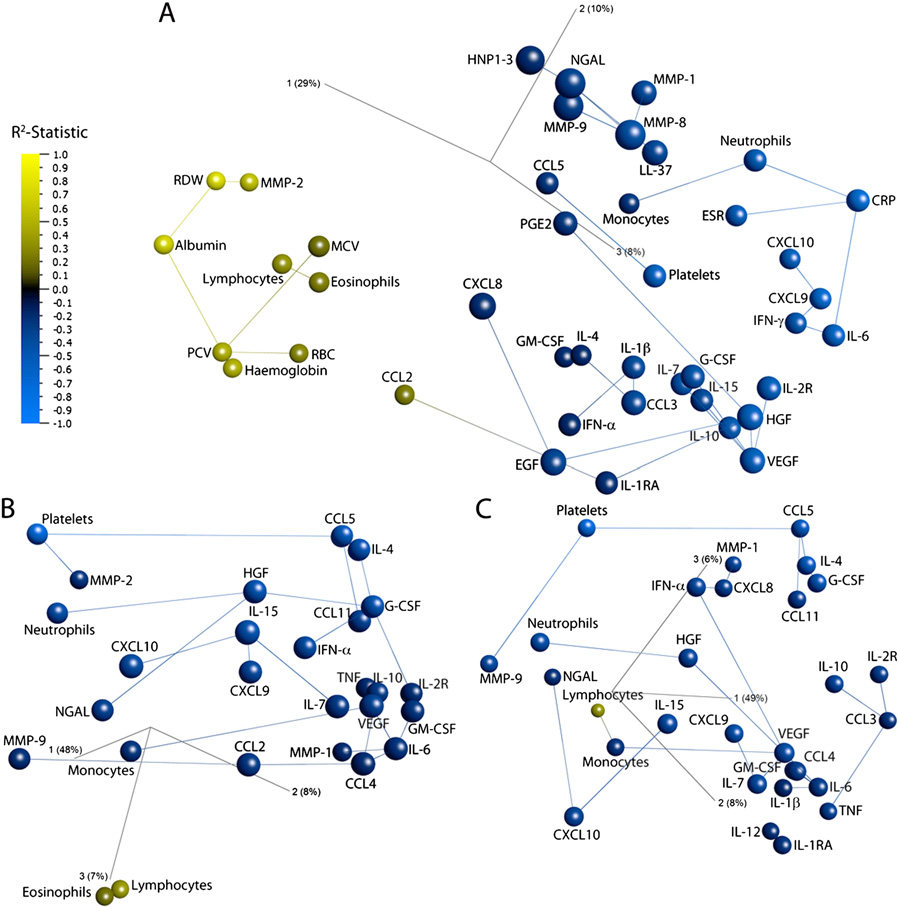
Three dimensional network principal component analysis plots showing the effect of intensive-phase antituberculous therapy on the immune
response in patients with smear-positive pulmonary tuberculosis. Individual points represent a single analyte. The distance between analytes is a measure oftheir Pearson correlation coefficients. Lines link analytes to their nearest neighbor, the analyte which has the most similar pattern of change during the courseof treatment. Analytes are colored by R2-statistic: blue represents a decrease, and yellow an increase, with time of treatment. Component vectors are displayed,along with a percent figure signifying the proportion of the variability in the data for which each component accounts. (A) Circulating immune parameters; (B)rESAT-6–stimulated responses; (C) rCFP-10–stimulated responses.
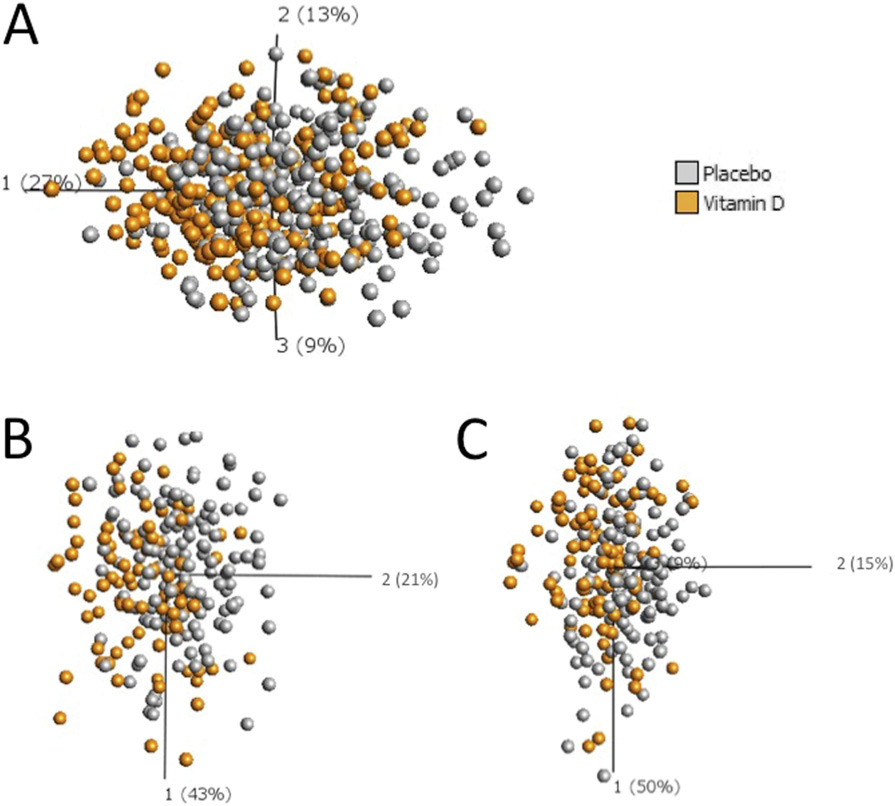
Three dimensional principal component analysis plots generated using parameters that significantly contribute to the effect of adjunctive vitamin
D on the immune response to intensive-phase antituberculous therapy. Individual points represent one patient at one time point and their position in the plotis determined by the combined effects of all parameters measured for that patient sample that contribute significantly to the effects of adjunctive vitamin D;data from all five time points are displayed. The distance between sample points presents Euclidean distance and is calculated using all parameters whichcontribute significantly to the effects of adjunctive vitamin D. Component vectors are displayed, along with a percent figure signifying the proportion of thevariability in the data for which each component accounts. (A) Circulating immunological parameters in patients randomized to vitamin D (orange, n = 44) vs.
placebo (gray, n = 51). (B) rESAT-6–stimulated responses in patients randomized to vitamin D (orange, n = 19) vs. placebo (gray, n = 28). (C) rCFP-10–stimulatedresponses in patients randomized to vitamin D (orange, n = 19) vs. placebo (gray, n = 28).
Source: http://www.theoldexchangesurgery.co.uk/website/D81623/files/Vitamin_D_info.pdf
Comparison of the Efficiency and Accuracy of Three Estrous Detection Methods to Indicate Ovulation in Beef Cattle 1 George A. Perry2Department of Animal and Range Sciences BEEF 2005 - 24 of estrous detection aids can correctly identify the majority of animals that will ovulate. The ability to successfully artificially inseminate
Catamaran Home Delivery 1 Member information: Please verify or provide member information below. Please send me e-mail notices about the status of the enclosed Member ID: prescription(s) and online ordering at: New Shipping Address: Catamaran Home Delivery will keep this address on file for all orders from City, State, Zip:




