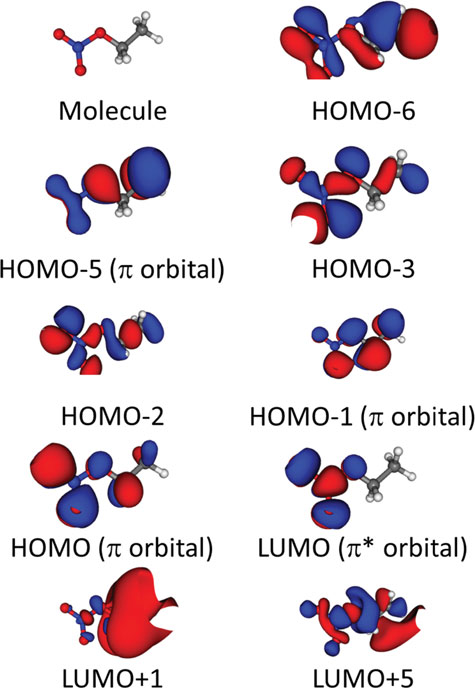
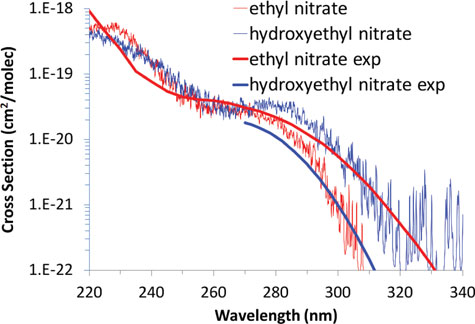
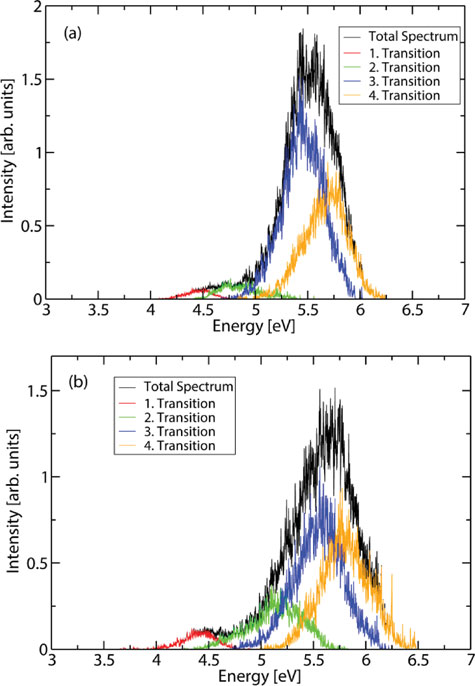
Absorption spectra and aqueous photochemistry of -hydroxyalkyl nitrates of atmospheric interest
Molecular Physics, 2015Vol. 113, Nos. 15–16, 2179–2190, Absorption spectra and aqueous photochemistry of β-hydroxyalkyl nitrates of atmospheric Dian E. RomonoskLucas Q. NguyDorit Tran B. NguyScott A. EDavid B.C. Mar Christopher D. VanderwaR. Benny Gand Sergey A. Nizkorodov aDepartment of Chemistry, University of California, Irvine, CA, USA; bFritz Haber Center for Molecular Dynamics, The Hebrew