Ahsoman.com

A Quarterly Publication
Influenza today:
Still a hot topic on many fronts
Case Study Award:
Cepheid Xpert® Flu Assay Results:
Positive in More than One Way
Volume 6, Issue 1


CONTENTS WINTER 2013
COVER STORY 3
David Persing, M.D., Ph.D.
Influenza today:
Case Study Award:
Still a hot topic on
Cepheid Xpert® Flu Assay
Ellen Jo Baron, Ph.D.
Results: Positive in More
Contributing Authors
Fred Tenover, Ph.D.
Cepheid's ON DEMAND Report is distributed four times a year. We welcome communication from users of Cepheid systems and tests and invite suggestions for articles in future issues. Send correspondence to:
David Persing
M.D., Ph.D.
From the Editor
Cepheid ON DEMAND Report1327 Chesapeake Terrace
Chief Medical and
Sunnyvale, CA 94089
Technology Officer, Cepheid
[email protected] sign up for e-mail notification
In this issue of On Demand, Dr. Ellen Jo Baron provides a wealth of information on
of new issues of Cepheid's ON
our perennial foe, the influenza virus. If you want to get up to speed on this subject
DEMAND Report, visitwww.cepheidondemand.com
quickly, read this issue; it encapsulates much of the recent research on the flu viruses, their virulence factors, and approaches to their detection. As many of you know, rapid detection of this virus during the flu season is of significant medical value, but until
Contents are 2013 by Cepheid unless
recently, most of the rapid tests have not been sensitive enough to rule out influenza
otherwise indicated. Rights reserved on all guest columns. The contents of this
infection with a high degree of confidence. Next-generation molecular diagnostic
publication may not be reproduced in any form without written permission of
testing for influenza virus and specifically the Xpert® Flu cartridge represent game-
the editor. The mention of trade names,
changing technologies. I hope you enjoy reading this issue as much as I have.
commercial products, or organizations does not imply endorsement by Cepheid.

Influenza today: Still a hot topic on many fronts
Influenza today:
Still a hot topic on many fronts
It seems like just yesterday that influenza was on everyone's minds, but it has been almost a year since we featured influenza in an edition of On Demand. The novel influenza A H1N1 strain that caught everyone by surprise in 2009 seems to have become a very minor component of the circulating strains now, except in the Middle
Ellen Jo Baron
East and India, where it still predominates. This season, influenza B is prominent in
the Americas and Africa, and influenza A H3N2 comprises half the reported cases
Prof. Emerita, Stanford University
in Europe and Australia, and more than 75% of strains in ChinaA. The World Health
Director of Medical Affairs,Cepheid Organization reported that the most common strain worldwide, A(H3N2), is again
targeting the traditional at-risk age groups of >60 years and <2 years. In temperate South America, numbers of influenza cases began to increase in May, peaking around July with the largest numbers seen for A(H3N2), but showing a second peak in August with more influenza B strains and untyped influenza A strains (Figure 1). There was a sharp drop-off in September, heralded by the disappearance of almost all influenza A reports. Africa peaked in July, but numbers remained high into September, with influenza B assuming the majority of cases. The United States is just beginning its influenza season, so the epidemiology is not known yet (Figure 2). Overall, influenza activity is lower than historical levels. This may be a result of increasing numbers of Americans receiving their annual flu vaccine. However, a new virus, variant H3N2 (H3N2v), associated with pigs and originally discovered in 2011, has cropped up this year in some human outbreaks periodically since July, 2012.1,B Although the strains of influenza circulating globally now are genetically slightly different from those in the current vaccine, the CDC feels that there will be significant cross-reactive protection, so they recommend that the vaccines not be changed. The common viruses causing disease are generally susceptible to both neuraminidase inhibitor antiviral agents oseltamivir (Tamiflu®) and zanamivir (Relenza®). This correlates with the seeming disappearance of the previously circulating H1N1 strain (called "seasonal") that was resistant to oseltamivir. Of course, occasional resistance can arise and patients who fail to improve after a week of therapy should be evaluated for drug resistant strains, as well as for other complications. Zanamivir is not recommended for patients <7 years old or for those with underlying respiratory disease. The CDC treatment guidelines state that antiviral therapy should not be delayed while waiting for diagnostic test results when clinical indications suggest influenza and antiviral treatment is indicated.C
WINTER 2013 CEPHEID 3
Influenza today: Still a hot topic on many fronts
The current influenza activity is interesting to public health
In 2005, a huge die-off of wild birds in a large lake populated
authorities and of paramount importance to individual by numerous migratory species (Qinghai Lake) was patients and their caregivers and those close to them, but
determined to be due to a new, more lethal variant of H5N1.
the topic of most discourse in the literature this year is the
Japan and Philippines also reported disease in poultry and
ethics of performing certain types of research involving illness seemed to have spread to migratory birds as far influenza. The debate over this activity rose to the highest
away as Russia, Mongolia, and to poultry, pigs, and then
levels of academia and the national science community.
humans in Indonesia. Throughout the next few years, cases
Some readers may remember back in 1996 when reports
in humans or animals were reported throughout the world,
started trickling out of China about a new strain of avian
moving to Europe, India, and other areas. It appeared that
influenza that had a surprisingly high mortality rate in children and young adult patients were more susceptible to chickens, H5N1. In 1997 in Hong Kong there were 18 cases
infection than the elderly and very young patients, in whom
of the same virus infecting people who were in contact with
influenza is typically more common. Research was initiated to
sick poultry; and most unusual for influenza, one-third of the
explore the pathogenesis of the virus, now known as Highly
patients died. No one was particularly alarmed because all
Pathogenic Avian Influenza (HPAI) and one study showed
patients had contact with poultry and it was not thought
that human disease was diverted at least partly because the
that such viruses could transmit an infection from human to
virus preferentially adhered to epithelial cells deep in the lungs
human, and there were no more cases reported for several
rather than those cell types that it is most likely to encounter
years. But in 2003, the virus resurfaced in Hong Kong.
in the upper respiratory tract. By June, 2006, there had been 205 laboratory-confirmed human cases reported to the World Health Organization. By spring of this year (2012), several hundred human cases had been reported worldwide. WHO publishes a timeline of events that is updated often.D A major human outbreak has not occurred, but many people are concerned, and some scientists are studying this virus with
.if the current widely circulating
new technological and molecular tools.
strains of HPAI could acquire the Two specific groups of researchers, one collaboration between
the University of Wisconsin and several Japanese institutions
genetic determinants that would
and the other a collaboration between the National Institutes
allow the virus to infect humans
of Health, the Erasmus Medical Center in Netherlands and the University of Cambridge in UK, endeavored to determine
more easily…Such ability would
if the current widely circulating strains of HPAI could acquire the genetic determinants that would allow the virus to infect
surely contribute to the possibility humans more easily, including factors that would facilitate
binding to upper respiratory tract epithelial cells. Such
of a great pandemic of lethal
ability would surely contribute to the possibility of a great pandemic of lethal influenza, resembling that of 1918. The
influenza, resembling that of 1918. virus of the 1918 pandemic was similar to this virus, in that
it preferentially affected relatively healthy children and young adults. In fact, a number of genetic characteristics of the HPAI H5N1 resembled those of the 1918 strain.2 Those scientists who were studying the characteristics that would allow inter-species transmission by the airborne route had chosen ferrets
After that, cases began to spring up elsewhere and in other
as the animal model for their research. Ferrets are often used
species of animals. A zoo in Thailand fed fresh chicken
for this type of respiratory virus research because they can
carcasses to two leopards and two tigers, all four of which
develop a respiratory infection similar to human influenza;
died of fulminant disease in a short period of time. Poultry in
in fact, ferrets sneeze just like people do. The experiments
both Korea and Vietnam were dying and influenza A H5N1
were carried out with utmost care under rigorous scientific
was identified as the culprit. Human cases resulting in many
protocols. Mutations that developed in the viral strains grown
deaths in Vietnam began and continued, caused by the same
in the laboratory were carefully controlled and characterized.
virus. By 2004, the virus was widely disseminated throughout
Finally, the two independent groups of scientists were able
Southeast Asia. In early 2004, 9 million poultry were culled in
to modify A/H5N1 enough to allow ferret-to-ferret respiratory
China to stem the epidemic. A study published in January,
transmission. Does this mean that those genetic changes
2005 reported the first well-documented case of human to
could occur naturally in nature? Does it mean that even if such
human transmission, in which a young Thai girl passed the
changes were to occur, that they would behave the same way
infection to her mother. Sadly, the mother died.
in humans? Nobody knows the answers, but the prospect
4 VOLUME 6, ISSUE 1 CEPHEID
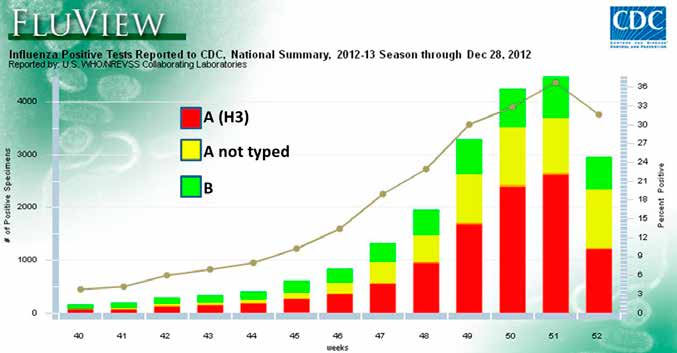
Influenza today: Still a hot topic on many fronts
FIGURE 1. Chart of influenza subtypes in temperate South American zone.
Source: Available in public domain
FIGURE 2. United States Influenza isolates for flu season beginning end of 2012. (http://www.cdc.gov/flu/weekly)
Source: Available in public domain
WINTER 2013 CEPHEID 5
Influenza today: Still a hot topic on many fronts
is disconcerting. Both manuscripts were submitted for The inevitable emergence of new
publication at the same time to Nature and Science, among the most prestigious and widely read scientific journals in the
strain variants fueled by genetic
world. These manuscripts were to generate a huge amount of public and private dialogue and controversy.3
reassortments among existing
After 9/11 and the anthrax distribution in the U.S., many
strains has important implications
scientists and government officials felt that the information necessary to weaponize anthrax spores could have for diagnostic tests.
been found in legitimate scientific literature (much like the information on how to build a bomb is available on the internet), and they wanted to censor any future publications that could be seen as helping potential bioterrorists achieve
Can the rapid antigen tests developed for circulating strains
their goals. This type of research is called Dual-Use Research
at the time of test creation detect the new variants? Clearly
of Concern (DURC), meaning that both peaceful and military
they have challenges, as illustrated by the very public failure
applications are possible based on the results. The academic
of existing rapid antigen tests to detect the influenza A 2009
and government communities agreed that bioterrorism was
novel strain8 and the H3N2v strain.9
a threat sufficient to warrant serious concern and put an official oversight function in place. The government created
Both the rapid antigen tests and the antibodies used in the
an advisory board, the National Science Advisory Board for
direct fluorescent antibody stains used by those laboratories
Biosecurity (NSABB), peopled by eminent and respected
performing DFA assays were developed before avian
scientists, whose job was to review publications of research
influenza was considered to be a threat. Genetic tests have
findings that could lead to potentially dangerous information
the advantage, as they can be tailored with the addition and
becoming available and being exploited for the wrong subtraction of specific primers and probes to match the purposes. The Board's focus was to decide how to protect
the public from harm while not inhibiting the advancement of science.
How do the current diagnostic tests fare with the influenza strains currently in circulation? The general laboratory
Clearly, the two submitted manuscripts describing the genetic
population was surprised to learn that the novel A H1N1
manipulations of the HPAI virus that allowed it to infect ferrets
2009 was not detected very well by the commonly used rapid
by the airborne route merited review by this board. After
antigen tests. Current recommendations from the CDC about
much deliberation, the NSABB decided that the full protocols
which diagnostic tests to use are based on that experience.
should be published and available to the general public. This
First of all, patients should be tested as early in their disease as
decision provoked an outcry from some scientists who felt
possible. Test results are less reliable at 4 days after onset of
that potential security risks outweighed the free dissemination
illness. The CDC recommendations state that rapid influenza
of research findings. The supporters of total publication diagnostic tests (RIDT) are not very sensitive, averaging felt that partial publication (holding back key experimental
sensitivities from 40-70% with a range of 10-80%.F A negative
details) would have a detrimental effect on other scientists
test should be confirmed with a more sensitive test, such as
contemplating similar research. They felt that the information
reverse transcriptase PCR or viral culture. These tests are
presented could potentially lead to better control, and more sensitive, but slower. Although a truly rapid test (15-perhaps a better vaccine. The final upshot of this highly vocal
20 minutes) that could unequivocally rule out influenza would
and visible rift within the influenza research community and
be ideal, the state of our technology still falls short of that
the broader scientific community was the publication, in full,
goal. Whether the patient presents at the height of influenza
of both manuscripts.2,4 Amidst many calls for better decision-
season or in the middle of the summer, major decisions
making in the future, the safeguards and oversight activities
will be made based on the test results. Should the patient
have been modified.5,6,E Still, at this time, the HPAI has not
be admitted into a respiratory isolation room? Should the
moved into the general population. Could our currently patient be sent home? Should the patient be given antiviral available rapid influenza tests detect H5N1 if it suddenly
medication or antibiotics? An accurate test result in a timely
swept into the human population? At least one group studied
fashion is important to that individual patient, his or her family,
two lateral flow antigen assays with samples from infected
and the institution. Fortunately, there are options available for
poultry.7 Rectal/genital (i.e., cloacal) swabs were less sensitive
the laboratory, although all have pros and cons.
for detection of HPAI H5N1 than feathers and neither sample was very sensitive compared with a molecular assay. Cloacal samples are appropriate, since infected birds shed the virus via the gastrointestinal tract. However, this result does not bode well for detection of the virus in human samples.
6 VOLUME 6, ISSUE 1 CEPHEID


Influenza today: Still a hot topic on many fronts
Some laboratories continue to use rapid antigen tests, usually immunochromatographic lateral flow format. A group from the Stanford University Medical School laboratory of Dr. Benjamin Pinsky, led by first author Dr. Mike Dimaio (Figure 3), has recently published an evaluation of two of the most popular such assays, BinaxNOW and BD Directigen, along with Cepheid Xpert Flu for their sensitivity and specificity with today's circulating virus strains.10 The Stanford laboratory, with its highly skilled and experienced virology staff, routinely used direct fluorescent antibody (DFA) testing and its own laboratory-validated real-time reverse-transcriptase PCR assays for influenza A (based on the CDC published protocol) and the primary mutation that confers oseltamivir resistance. In 2008, the laboratory had performed a comparison of the rapid direct antigen tests that it had been using in previous years and found the sensitivity and specificity to be so low (unpublished data) that it had discontinued use of those tests even before the novel H1N1 virus outbreak began in the spring of 2009, instead ramping up the number of times per day that DFA testing was performed. But physicians wanted a more rapid turnaround time. The group used 200 previously submitted samples (frozen) including 84 from children <18 years old. Samples had been previously identified by DFA
FIGURE 3. Dr. Mike Dimaio, the first author of the
FIGURE 4. Alejandro DeTorres, CLS, testing a
Stanford influenza study, hard at work at the Stanford
sample in the Kaiser-Permanente North Hollywood
Hospital GeneXpert® System.
WINTER 2013 CEPHEID 7
Influenza today: Still a hot topic on many fronts
as positive for influenza A, influenza B, respiratory syncytial virus, human metapneumovirus, adenovirus, or parainfluenza viruses or were negative for the above agents (57 samples). Results for the 74 influenza A positive specimens yielded sensitivity of 97.3% for Xpert® Flu, 95.9% for direct fluorescent antibody testing, 62.2% for BinaxNOW, and 71.6% for BD Directigen. All specimens positive for influenza A by rapid antigen testing were
The ease of use, the rapid
detected by the Xpert Flu assay. Specificity for influenza A was 100% (126/126) for Xpert Flu, BinaxNOW, and BD Directigen, and
turnaround time, and the
99.2% for direct fluorescent antibody testing. In this study, there were five samples with seasonal H1N1. All five were detected by
random access nature of the
Xpert Flu, BD Directigen detected one, but BinaxNOW failed to detect any. For the 33 influenza B positive samples, sensitivity
assay, which helps diminish
was 100% for Xpert Flu and direct fluorescent antibody testing, 54.5% for BinaxNOW, and 48.5% for BD Directigen. Specificity for
the chance of cross-
all three tests was 100%. The authors concluded that the Xpert
contamination, all serve
Flu was more accurate but as easy to use as the rapid antigen tests, and could eliminate the need for reflex testing. In addition,
to make the Xpert® Flu an
with a turnaround time of <90 minutes, results could be delivered in sufficient time to influence clinical decision-making.10 For
attractive test.
laboratories using molecular multi-analyte respiratory virus panels for certain at-risk patients, especially during influenza season, the Xpert Flu might be used as the assay of choice to triage influenza-positive patients and eliminate the need for the additional more expensive panel assay.
Novak-Weekley and colleagues (Figure 4) from five institutions in the U.S. and Australia provided another evaluation of Cepheid's new Xpert Flu test.11 Both nasopharyngeal swabs and nasal aspirates/washes, prospective and previously frozen, were included. More than 1500 samples were included, divided almost equally between fresh and frozen. The ProFlu+ molecular assay, viral cultures, and sequencing of viruses were all used as reference tests. Compared to the ProFlu+ molecular assay, frozen nasal wash samples tested by Xpert Flu had sensitivities of 98-100% for all influenza A and B viruses. For nasopharyngeal swabs, there were somewhat lower sensitivities (93.8%) for influenza B containing samples, although the results for influenza A were comparable to those seen with washings. Specificities were
99-100% for both aspirates and swabs. The prospective samples had 100% sensitivities for all samples except for nasal washings, for which slightly lower sensitivities were seen with seasonal influenza A, not including the 2009 H1N1 strains. The PCR assay performed better than culture, after discrepant analysis by sequencing. Novak-Weekley and colleagues noted that the ease of use, the rapid turnaround time, and the random access nature of the assay, which helps diminish the chance of cross-contamination, all serve to make the Xpert® Flu an attractive test for laboratories that lack molecular expertise or that require rapid turnaround time and do not wish to wait to accumulate a batch of samples for testing.11
8 VOLUME 6, ISSUE 1 CEPHEID
Influenza today: Still a hot topic on many fronts
The recently newsworthy influenza A H3N2v, another pig to human influenza virus, was probably not included in either of the Xpert Flu publications described above.
Call for Case Studies
This virus has become a risk especially for patients who tend pigs for prolonged
Sharing your interesting patient cases where
periods, and who frequent county fairs.
GeneXpert made a difference can be a rewarding
H3N2v is likely not well detected by any
experience. David Persing, Fred Tenover, and
current FDA-cleared tests. In fact, a recent study shows that a number of variant
Ellen Jo Baron invite you to send us your case
viruses were not detected by current rapid
for publication in On Demand.
antigen tests at all.12 So far, these viruses have all been detected by the current GeneXpert assay, including the H5N1
• The best case of the quarter
highly pathogenic strain, which is reported
will win a copy of the new
as "influenza A." Cepheid scientists are
definitive reference on
working now on the next generation
Molecular Microbiology from
influenza assay, which embodies our
ASM Press, signed by Dr.
commitment to continuously improve our
David Persing and Dr. Fred
assays, and in the case of influenza, to try
to keep up with this wily virus as it evolves.
• Your case and a photo of
The flu season has started, and
your lab will be published
laboratories have several choices of tests
in On Demand, Cepheid's
to employ. Knowing the pros and cons
quarterly newsletter:
of the options can help microbiologists
and physicians choose the best tests
• Write up the case including history, symptoms,
and testing algorithms for their needs.
initial findings, sample type received, assay(s)
The case report in the next section of this edition of On Demand describes one
performed (including routine laboratory tests), time
laboratory's approach.
to results, and outcomes.
• Describe how the GeneXpert® System made
• Photos or other images are especially welcome.
• Send it to [email protected].
A Quarterly Publication
esting for
History of Laboratory T
Sexually Transmitted Bacteria:
From Old School to Next Generation
Next-Generation T
A Quarterly Publication by Cepheid
Assay Should Y
Prize-Winning Case Report
From Dr
Clinique de L'Union, Toulouse, France
Summaries of Selected Abstracts
Presented in Europe Recently on the
Use of GeneXpert®
Volume 4, Issue 2
WINTER 2013 CEPHEID 9
Cepheid Xpert® Flu Assay Results: Positive in More than One Way
Case Study Award:
Cepheid Xpert® Flu Assay
Results: Positive in More
Jack L. Brothers, MT(ASCP), Microbiology Technical Supervisor, works at a surprisingly beautiful hospital (Figure 1) in an unlikely place: Anchorage, Alaska. The Joint Base Elmendorf-Richardson is the largest U.S. military installation in Alaska and was created when former Elmendorf Air Force and Fort Richardson Army bases were merged in 2010.
The base medical facility has 60 of a spinal tap). This is Jack's case inpatient beds but its larger mission is
to provide medical care to over 35,000 joint service members, dependents,
The parents of a two month
Veterans Administration (VA) patients,
old male infant brought their
Ellen Jo Baron
and retirees throughout Alaska. One
child to the 673rd Medical Group DOD/
usually does not think about VA VA Joint Venture Hospital Emergency
Prof. Emerita, Stanford University
hospitals as receiving many pediatric
Department late one night in March
Director of Medical Affairs,Cepheid patients, but in this case, families of of 2012. The chief complaint was a
service members are a large component
high fever with intermittent shortness
of the population served. For allowing
of breath and "gasping for air with
us to benefit from his experience with
a dry cough." Vital signs revealed a
a GeneXpert assay, Jack will receive a
rectal temperature of 101.1°F, pulse
copy of the recently published second
of 194, respirations of 40 per minute,
edition of the popular book published
and O Saturation of 98% on room
by ASM Press, Molecular Microbiology:
air. In addition to the tachycardia and
Diagnostic Principles and Practice, dyspnea, the physical exam noted hand signed by the two co-editors pharyngeal erythema, and a blanchable David Persing and Fred Tenover. Jack
rash on the cheeks and chest. Blood
Brothers elegantly described the was collected for a complete blood situation and how the Cepheid Xpert Flu
count (CBC) and culture, and an
assay, with final results delivered less
intravenous line was inserted. A nasal
than 2 hours from sample collection,
washing (using normal saline) was also
saved one tiny patient and his family a
obtained and sent to the laboratory
lot of grief and potential further trauma
for viral testing, and a chest X-ray
(notably enabling the cancellation was ordered.
10 VOLUME 6, ISSUE 1 CEPHEID
Cepheid Xpert® Flu Assay Results: Positive in More than One Way
FIGURE 1. The 673rd medical group Joint Venture
FIGURE 2. Senior Airman Jessica Green performing the
Department of Defense/Veterans Administration hospital
Xpert® Flu test, adding the sample to the cartridge in a biosafety cabinet
The CBC was essentially normal, as was the chest X-ray.
collected from inpatients. We define high risk for influenza
A rapid antigen detection assay for respiratory syncytial testing to be patients less than one year of age, patients virus (RSV) was performed on the nasal washings and was
over 65 years of age, and those with known conditions that
negative. Rapid antigen detection assays for influenza A and
would make them more vulnerable to viral infections, such
influenza B performed on the same sample were also negative
as chronic obstructive pulmonary disease (COPD) or immune
and were reported to the physician handling the case in the
suppression. We perform reflex testing using the real-time
Emergency Department.
Reverse Transcription Polymerase Chain Reaction (RT-PCR) Xpert Flu assay on a Cepheid 4-well GeneXpert instrument.
EJB comment: Luckily for the baby, Jack's laboratory policy requires reflexing samples from some patients that are Most PCR testing requires an extraction procedure that
negative by the rapid influenza antigen assay to testing with the
necessitates batching of samples. In this case the Cepheid
Cepheid Xpert® Flu assay, designed to detect both influenza
GeneXpert® enabled a busy Technician to perform an accurate,
A, B, and A[2009 H1N1], as he explains further below.
sensitive RT-PCR flu assay at 2:30 in the morning, while the patient was still in the Emergency Department. Less than
Per laboratory policy, Senior Airman Jessica Green pipetted
2 hours elapsed from the time the sample was
600µl of the original nasal wash into 3ml of Universal Transport
collected until the final PCR result was certified
Medium (UTM), and then used 300µl of the diluted sample to
and sent to the provider. As a result, additional
set up a Cepheid Xpert® Flu assay (Figure 2). The Cepheid®
expensive testing to determine the cause
RT-PCR assay was "influenza A positive, no influenza B
of a fever of unknown origin was avoided,
detected, no influenza A 2009 H1N1 (A/California/7/2009-
and the relieved parents were able to
like) detected", and was certified. A few minutes later, she
return home with an accurate diagnosis
received a call from the doctor. He wanted to make absolutely
for their child in minimum time. The
sure that he was interpreting the results correctly, and that the
Alaska State Virology Laboratory in
PCR test was indeed positive for Influenza A. Jessica explained
Fairbanks confirmed the Cepheid
that the rapid antigen tests can sometimes give false negative
Xpert Flu result (A/H3), but those results
results, and that the PCR is much more sensitive and also
were not available until the
more accurate. He thanked her, and said that there was no
following week.
longer a need to do a spinal tap on the baby since they now knew the origin of his fever. The child was given Tamiflu and sent home.
The policy at the 673rd Medical Group Laboratory is to confirm the results of rapid influenza antigen tests by performing reflex PCR testing on all samples obtained from patients considered at high risk for respiratory distress, and on all samples
WINTER 2013 CEPHEID 11
Influenza today: Still a hot topic on many fronts
1. Lindstrom, S., et al., Human infections with novel reassortant influenza
A(H3N2)v viruses, United States, 2011. Emerg Infect Dis. 18(5): p. 834-7.
2. Imai, M., et al., Experimental adaptation of an influenza H5 HA confers
respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets.
Nature. 486(7403): p. 420-8.
3. Osterholm, M.T. and D.A. Relman, Creating a mammalian-transmissible
A/H5N1 influenza virus: social contracts, prudence, and alternative
perspectives. J Infect Dis. 205(11): p. 1636-8.
4. Herfst, S., et al., Airborne transmission of influenza A/H5N1 virus between
ferrets. Science. 336(6088): p. 1534-41.
5. Fauci, A.S. and F.S. Collins, Benefits and risks of influenza research: lessons
learned. Science. 336(6088): p. 1522-3.
6. Lipsitch, M., et al., Evolution, safety, and highly pathogenic influenza viruses.
Science. 336(6088): p. 1529-31.
7. Slomka, M.J., et al., Evaluation of lateral flow devices for identification of
infected poultry by testing swab and feather specimens during H5N1 highly pathogenic avian influenza outbreaks in Vietnam. Influenza Other Respi Viruses. 6(5): p. 318-27.
8. Control, C.f.D., Performance of rapid influenza diagnostic tests during two
school outbreaks of 2009 pandemic influenza A (H1N1) virus infection - Connecticut, 2009. MMWR Morb Mortal Wkly Rep, 2009. 58(37): p. 1029-32.
9. Centers for Disease Control and Prevention. Evaluation of Rapid Influenza
Diagnostic Tests for Influenza A (H3N2)v Virus and Updated Case Count - United States, 2012. Morbidity and Mortality Weekly Report, 2012. 61: p. 1-3.
10. Dimaio, M.A., et al., Comparison of Xpert Flu rapid nucleic acid testing with
rapid antigen testing for the diagnosis of influenza A and B. J Virol Methods. 186(1-2): p. 137-140.
11. Novak-Weekley, S.M., et al., Evaluation of the Cepheid Xpert Flu Assay for
rapid identification and differentiation of influenza A, influenza A 2009 H1N1, and influenza B viruses. J Clin Microbiol. 50(5): p. 1704-10.
12. Balish, A., et al., Analytical detection of influenza A(H3N2)v and other A
variant viruses from the USA by rapid influenza diagnostic tests. Influenza Other Respi Viruses.
A better way.
1327 Chesapeake Terrace Sunnyvale, CA 94089 toll-free: 1.888.336.2743
Source: http://www.ahsoman.com/documents/catelogue/13.pdf
Pourquoi la maladie d'Alzheimer est-elle si grave ? Le dépistage de la maladie A. Les différentes méthodesB. Le test RL/RI-16C. Statistiques et résultats L'évolution de la maladie A. La manifestation de la maladie, les premiers instantsB. L'aggravation de la maladieC. Le terme de la maladie III- Comment lutter contre la maladie d'Alzheimer ?
Prescribing for Clinical Need Policy Version Control Policy Category: Medicines Optimisation CCG GP Member Practices Version history: Version No. Date Changes Made: Initial version based on policy from Eastbourne, Hailsham and Seaford /Hastings and Rother Clinical Commissioning Groups drafted by Medicines Management Team







