Wnt5a can both activate and repress wnt/β-catenin signaling during mouse embryonic development


Contents lists available at
Developmental Biology
journal homepage:
Wnt5a can both activate and repress Wnt/b-catenin signaling duringmouse embryonic development
Rene´e van Amerongen n,1, Christophe Fuerer 2, Makiko Mizutani, Roel Nusse n
Department of Developmental Biology and Howard Hughes Medical Institute, Lorry I. Lokey Stem Cell Research Building, 265 Campus Drive, Stanford University,Stanford, CA 94305, USA
Embryonic development is controlled by a small set of signal transduction pathways, with vastly
Received 6 April 2012
different phenotypic outcomes depending on the time and place of their recruitment. How the same
Received in revised form
molecular machinery can elicit such specific and distinct responses, remains one of the outstanding
questions in developmental biology. Part of the answer may lie in the high inherent genetic complexity
Accepted 27 June 2012
of these signaling cascades, as observed for the Wnt-pathway. The mammalian genome encodes
Available online 4 July 2012
multiple Wnt proteins and receptors, each of which show dynamic and tightly controlled expression
patterns in the embryo. Yet how these components interact in the context of the whole organism
remains unknown. Here we report the generation of a novel, inducible transgenic mouse model that
allows spatiotemporal control over the expression of Wnt5a, a protein implicated in many develop-
mental processes and multiple Wnt-signaling responses. We show that ectopic Wnt5a expression from
Hair folliclesCalvarial mesenchyme
E10.5 onwards results in a variety of developmental defects, including loss of hair follicles and reduced
bone formation in the skull. Moreover, we find that Wnt5a can have dual signaling activities duringmouse embryonic development. Specifically, Wnt5a is capable of both inducing and repressingb-catenin/TCF signaling in vivo, depending on the time and site of expression and the receptorsexpressed by receiving cells. These experiments show for the first time that a single mammalian Wntprotein can have multiple signaling activities in vivo, thereby furthering our understanding of howsignaling specificity is achieved in a complex developmental context.
& 2012 Elsevier Inc. All rights reserved.
obvious intricacy of the organism under study: At any one time agiven cell will find itself capable of receiving simultaneous inputs
In all multicellular animals, tissue morphogenesis is regulated
from multiple sources, including the extracellular environment
by the concerted activities of a limited number of developmental
and neighboring cells, and in a developing organism these
signal transduction pathways, including Wnt, Hedgehog and
surroundings are rapidly and continually changing. Equally
Notch ). Over the past thirty years our knowledge
important however, is the high complexity of the developmental
regarding the molecular nature of the intracellular signaling
signaling pathways themselves. An illustrative example is that of
events triggered by pathway activation has steadily increased.
the Wnt-pathway, a major player in development, physiology and
However, we have only begun to scratch the surface when it
comes to understanding how these same molecular pathways can
which uses a large number of extra-
be used over and over again, at multiple developmental sites
cellular ligands (Wnts) and transmembrane receptors (Fzd) to
and time points, with vastly different phenotypic outcomes.
elicit a diverse array of intracellular signaling responses.
One reason for our apparent lack of understanding signal trans-
Both ligands and receptors belong to large, multi-gene
duction in the context of an intact, multicellular animal, is the
families: The mammalian genome encodes 19 Wnt and 10 Fzdhomologues, totaling 190 different potential ligand/receptor pair-ings. Of note, this complexity is conserved in lower organisms,
n Corresponding authors.
with the sea anemone Nematostella showing conservation of 11 of
E-mail addresses:
the 12 Wnt subfamilies (). Further complica-
tion arises from the presence of additional (co-)receptors,
1 Present Address: Netherlands Cancer Institute, Plesmanlaan 121, 1066 CX
and secreted inhibitors or co-activators, which help shape the
Amsterdam, The Netherlands.
signaling response (see for instance
Present Address: Ecole Polytechnique Fe´de´rale de Lausanne (EPFL) SV ISREC,
CH-1015 Lausanne, Switzerland.
Furthermore, Wnt proteins can elicit multiple intracellular
0012-1606/$ - see front matter & 2012 Elsevier Inc. All rights reserved.
R. van Amerongen et al. / Developmental Biology 369 (2012) 101–114
responses The best-
b-catenin signaling in vivo. However, it is unknown whether this
characterized response downstream of Wnt/Fzd binding results in
is the sole or dominant activity of Wnt5a. In particular, the
the activation of b-catenin/TCF transcriptional complexes (here-
question of whether mammalian Wnt5a also has the capacity to
after ‘Wnt/b-catenin signaling') and requires the recruitment of
activate Wnt/b-catenin signaling in vivo remains unexplored.
LRP5/6 co-receptors (; ;
The severe developmental phenotype observed in conventional
For many years, Wnt proteins themselves
Wnt5a-knockout mice precludes a straightforward analysis of Wnt5a-
were classified as either ‘canonical' or ‘non-canonical' based
signaling. To uncover the potential signaling activities of Wnt5a
during mammalian development, we therefore generated a novel,
inducible transgenic mouse model that allows tight spatiotemporal
subdivision was challenged, however, by the finding that a given
control over Wnt5a expression. Our experiments reveal multiple
Wnt can be turned into an activator of Wnt/b-catenin signaling
phenotypes caused by ectopic Wnt5a expression, some of which
when provided with the appropriate receptor
can be linked to the inhibition of Wnt/b-catenin signaling and others
In light of this complexity, a key question then is how signaling
to its activation. First, overexpression of Wnt5a in the developing skin
specificity is achieved in a developmental context. One step towards
results in a loss of hair follicle formation, coinciding with a decrease in
answering this question will come from determining whether differ-
b-catenin/TCF reporter activity in the dermis and revealing a pre-
ent, and if so which, combinations of ligands and receptors trigger
viously unrecognized role for Wnt5a/Ror2 in patterning of the skin.
specific signaling outcomes. To date however, it has proven a
Second, Wnt5a overexpression causes delayed bone formation in the
challenge to determine binding affinities for specific Wnt/Fzd com-
developing skull. Unexpectedly, this is preceded by an increase in
plexes. Most efforts have stalled due to the hydrophobic nature of the
b-catenin/TCF reporter activity in the calvarial mesenchyme, suggest-
Wnt proteins and the concomitant difficulty of purifying them.
ing that Wnt5a can induce Wnt/b-catenin signaling in vivo. Taken
Although this hurdle has been overcome for some Wnt proteins
together, our studies uncover novel roles for Wnt5a and its receptors
; ), it has prevented
during development and reveal a dual signaling capacity for Wnt5a in
comprehensive quantitative biochemical analyses in vitro. Both Wnts
an intact, complex organism.
and Fzds display tightly regulated and highly dynamic spatiotemporalexpression patterns within the developing organism ; ; suggestingequally dynamic ligand/receptor interactions. Although some Wnt/
Materials and methods
receptor pairings appear to be more likely than others ; ;
; , at present it remains largely unknownwhich ligands engage which receptors under which circumstances.
The tetO-Flag-Wnt5A transgenic construct was generated by clon-
One particularly intriguing Wnt protein is Wnt5a, which has
ing a Flag-tagged version of mouse Wnt5a downstream of an artificial
been implicated in many developmental processes and which can
signal sequence into the pTRE-tight vector (Clontech). Fourteen
activate multiple intracellular signaling responses. Our lab pre-
independent mouse lines (F5A-1 through F5A-14) were generated
viously showed that mouse Wnt5a, which is typically not found
initially by injecting the transgenic construct into FVB oocytes. Four
to activate a b-catenin/TCF responsive reporter gene, was able to
additional founder lines (F5A-15 through F5A-18) were generated in a
induce Wnt/b-catenin signaling in 293 cells overexpressing Fzd4
second round of injection. The vast majority of experiments in this
and LRP5 In their absence, however, the
paper were performed with line F5A-5, which we have since replaced
cells responded to Wnt5a by inhibiting Wnt3A-mediated signal-
with line F5A-17. TetO-Flag-Wnt5A mice were kept on an FVB back-
ing through b-catenin/TCF. The capacity of Wnt5a to inhibit Wnt/
ground, although all complex crosses were performed on a mixed
b-catenin signaling has been reported in multiple systems and
background. Rosa26-rtTA-M2 mice ) were
obtained from Jackson Laboratories (stock #006965). Axin2-lacZ
) and is mediated through the receptor
and TOPGAL reporter
tyrosine kinase Ror2, which has been reported to function as
mice were obtained from Dr. W. Birchmeier (Max Delbruck Center,
either a co-receptor or a bona-fide Wnt-receptor
Berlin-Buch, Germany) and Dr. E. Fuchs (Rockefeller University, New
York, USA), respectively. The tetO-Dkk mice ) were
In addition to the above, Wnt5a can also induce alternative
obtained from Dr. S. Millar (University of Pennsylvania, Philadelphia,
signaling responses that appear to proceed independently from
USA), Ror2-knockout mice from Dr. Y. Minami
b-catenin. Although many of these remain poorly characterized at
(Kobe University, Kobe, Japan) and Wnt5a-knockout mice (
the biochemical level, they too have been reported to involve Fzd
were a gift from Dr. E. Vladar and Dr. J. Axelrod (Stanford
as well as Ror2 receptors
University, USA). Mice were housed at the Stanford University
Medical Center. All experiments were approved by the Stanford
Wnt5a-knockout mice have a variety of phenotypes, including
University Animal Care and Use Committee and performed according
skeletal defects and multiple defects
to NIH guidelines.
associated with internal organs (; ; ;; ; ;
). Interestingly, the phenotype of Ror-knockout mice resembles that of Wnt5a-knockout mice. Both
Tail clippings were lysed overnight at 55 1C in Direct PCR lysis
display dwarfism, craniofacial defects, limb abnormalities and
reagent (Viagen Biotech, Inc., Los Angeles, CA, USA) supplemented
intestinal elongation defects ; ;
with proteinase K (100 ug/ml, Roche). Following heat inactivation
In addition, both Ror2
at 85 1C, the lysate was used for PCR with the following primers:
and Wnt5a loss-of-function alleles have been associated withincreased Wnt/b-catenin signaling
tetO-Wnt5A (transgene specific product, annealing at 55 1C):
). Taken together, these studies
Fwd: ACAAAGACGATGACGACAAGC Rev: CGCACCTTCTCCAAT-
suggest the existence of a Wnt5a/Ror2 pathway that inhibits Wnt/
R. van Amerongen et al. / Developmental Biology 369 (2012) 101–114
Rosa26-rtTA-M2 (transgene specific product, annealing at
PAGE gel. Following Western blot transfer, membranes were
60 1C): Fwd: CTGGGAGTTGAGCAGCCTAC Rev: AGAGCAC-
blocked in TBST with 3% milk and 2% BSA. Wnt5a protein was
detected using a primary antibody directed against the Flag tag
Axin2-lacZ and TOPGAL (transgene specific product, annealing
(M1, Sigma) or Wnt5a (RnD), a secondary HRP-conjugated anti-
at 55 1C): Fwd: ATCCTCTGCATGGTCAGGTC Rev: CGTGGC-
body (Santa Cruz Biotechnology) and ECL (Perkin Elmer). Ror2
protein expression was detected using hybridoma supernatant
Ror2-knockout (WT band 500 bp; KO band 200 bp, annealing
(1:100) containing the anti-Ror2 mouse monoclonal previously
at 61 1C): WT Fwd: CTTAACTGTTCTAGGTCAAGTATG WT Rev:
generated by our lab ((), available through the
CCTACTATAGACTCTGATCCTTCTGCC. Mutant Rev: ATCGCCTTC-
Developmental Studies Hybridoma Bank). An antibody detecting
tubulin (Sigma) was used as a loading control.
Wnt5a-knockout (WT band 484 bp; KO band 400 bp, annealingat 55 1C): WT Fwd: GAGGAGAAGCGCAGTCAATC WT Rev:
Wnt5a protein purification and luciferase assays
CATCTCAACAAGGGCCTCAT Mutant Fwd: GCCAGAGGCCACTT-GTGTAG.
Flag-tagged Wnt5a protein was purified from stably transfected
S2 cells by consecutive affinity chromatography and gel filtrationsteps as described previously
Induction of tetO-Wnt5a transgene expression in vivo
. Peak fractions were identified by Western blot analysisand used in luciferase assays. To test the efficacy of Flag-Wnt5a in
Timed matings were set up and the morning on which a
inhibiting the activity of Wnt3a, 293T cells transfected with the
vaginal plug was discovered was designated E0.5. Induction of
SuperTOPFLASH luciferase reporter and CMV-b-galactosidase for
transgene expression during embryogenesis from the indicated
normalization were treated with different combinations of purified
timepoints onwards was achieved by dissolving doxycycline
Wnt3a and Wnt5a. To test the ability of Flag-Wnt5a to induce b-
(Sigma) in the drinking water of pregnant dams. Unless otherwise
catenin/TCF signaling, 293T cells were transfected with Super-
indicated, mice received a final concentration of 1–2 mg/ml
TOPFLASH, CMV-b-galactosidase, Fzd4 and LRP5, and treated with
doxycycline. The doxycycline containing drinking water was
purified Wnt3a or Wnt5a. Following overnight stimulation with
refreshed three times per week.
purified Wnt proteins, cells were lysed and luciferase and b-galactosidase activities were measured using the Tropix Dual Light
Induction of tetO-Wnt5a transgene expression in vitro
Reporter Gene Assay System (Applied Biosystems) on a Bertholdluminometer (Berthold Technologies).
Primary mouse embryo fibroblasts (MEFs) were isolated and
cultured in a 3T3 protocol according to . Early
Histology and immunohistochemistry
passage MEFs from tetO-Wnt5A transgenic embryos or wildtypelittermates were infected with a mix of pBabe-TBX2 (blasticidin
Tissue samples were fixed in 4% paraformaldehyde, washed in
resistance) and pBabe-rtTA3 (puromycin resistance) retroviruses to
PBS, dehydrated through a graded ethanol series, cleared in
allow for immortalization and inducible expression of the tetO-
orange terpene and embedded in paraffin. For wholemount
Wnt5a transgene, respectively. Alternatively, transgene expression
analysis of dorsal skins, samples were flat mounted and imaged
was induced in double-heterozygous tetO-Wnt5a;R26-rtTA MEFs.
under a dissecting scope (Leica) when they were in 70% ethanol.
Cells were grown in Dulbecco's modified Eagle's medium supple-
Contrast and color in these images were enhanced in Photoshop
mented with 10% fetal bovine serum, glutamine, penicillin/strepto-
in order to better visualize pigmentation. Tissue blocks were
mycin and 50 mM b-mercaptoethanol under 5% CO2 at 37 1C in
sectioned on a microtome at 3–6 mm thickness, mounted on
humidifying conditions. To induce Wnt5a transgene expression, cells
Superfrost glass microscope slides (Fisher Scientific) and left to
were cultured in the presence of 1 ug/ml doxycycline overnight
dry at 37 1C overnight. For further processing, sections were
(TBX2-infected MEFs), or in the presence of a concentration series of
deparaffinized in orange terpene and rehydrated in a graded
doxycycline for 3–5 days (tetO-Wnt5a;R26-rtTA MEFs), after which
ethanol series. For H&E staining, slides were stained in hematox-
cells were lysed to extract RNA or protein.
ilin (Sigma) and eosin Y (Sigma). For immunohistochemistry,antigen retrieval was performed in Tris/EDTA (pH 9.0). Endogen-
Quantitative RT-PCR
ous peroxidase activity was blocked by incubating the sectionswith 0.3% H2O2. Slides were blocked using the Vector M.O.M. kit
RNA was isolated using an RNeasy Mini Kit (Qiagen) with on
(Vectorlabs). Primary antibody incubation was performed with
column DNAse digestion. cDNA was synthesized using random
the anti-Ror2 mouse monoclonal (1:1000) described previously
hexamer primers and the Thermoscript II RT-PCR system for First-
() or a rabbit polyclonal antibody raised against
Strand Synthesis (Invitrogen). Quantitative PCRs were performed
Wnt5a (previously generated in our lab) at room temperature for
on a Roche LightCycler (Roche) using the LightCycler Fast Start DNA
4 h or overnight. After this, tissue sections were further processed
Master Plus SYBR green I mix (Roche) with the following primers:
using the Vectastain ABC system (Vectorlabs). Antibody bindingwas detected with VIP or DAB substrate (Vectorlabs). Following
tetO-Wnt5a: Fwd: GCGTGGCTATGACCAGTTTA Rev:
dehydration in a graded ethanol series and orange terpene,
coverslips were sealed with Cytoseal-60 (Thermo Scientific).
mGAPDH: Fwd: CTGGTGCTGCCAAGGCT Rev:
Images were acquired on a Zeiss upright microscope equipped
with an Axiocam CCD camera.
Detection of endogenous AP and lacZ reporter gene activity
Protein isolation and Western blotting
To visualize endogenous alkaline phosphatase (AP) activity in
Cells were lysed in RIPA buffer supplemented with Complete
primary hair follicles, E14.5 embryos were processed according to
Protease Inhibitor Cocktail (Roche). Equal amounts of lysate
Nagy et al. with minor modifications (Embryos
(or purified Wnt5a protein; see below) were run on a 10% SDS/
were fixed in 4% PFA for 30 min, rinsed in PBS and AP buffer, after
R. van Amerongen et al. / Developmental Biology 369 (2012) 101–114
which AP activity was detected with BM Purple substrate (Roche)in the dark at room temperature. Wholemount X-gal staining wasperformed according to with minor modifications.
Organs from E14.5 or E16.5 embryos were microdissected follow-ing fixation (in PBS with 0.2% glutaraldehyde, 5 mM EGTA (pH8.0) and 2 mM MgCl2). Tissues were washed in detergent rinse(PBS with 2 mM MgCl2, 0.01% sodium deoxycholate and 0.02% NP-40)and stained in staining solution (PBS with 2 mM MgCl2, 0.01% sodiumdeoxycholate, 0.02% NP-40, 5 mM potassium ferricyanide, 5 mMpotassium ferrocyanide and 1 mg/ml X-gal) in the dark at roomtemperature overnight. Following staining, tissues were washed indetergent rinse, post-fixed in 4% paraformaldehyde and processed forparaffin embedding. Wholemount tissues were photographed undera dissecting scope (Leica) when samples were in 70% ethanol. Paraffinsections of X-gal stained tissues were counterstained with nuclearfast red. In all cases, tetO-Wnt5a;R26rtTA double-transgenics werecompared to control mice from the same litter, the samples of whichwere processed at the same time and for the same duration andanalyzed simultaneously.
Skeletal stainings
Skulls and skeletons from newborn mice were processed for
Alizarin Red and Alcian Blue staining to detect bone and cartilage(In short, tissues were fixed in 100% ethanol,washed in 100% acetone, stained in Alizarin Red and Alcian Bluefor 3 days at 37 1C, cleared in 1% KOH and processed through agraded glycerol series. Cleared specimens were stored in 100%glycerol and imaged under a dissecting scope (Leica).
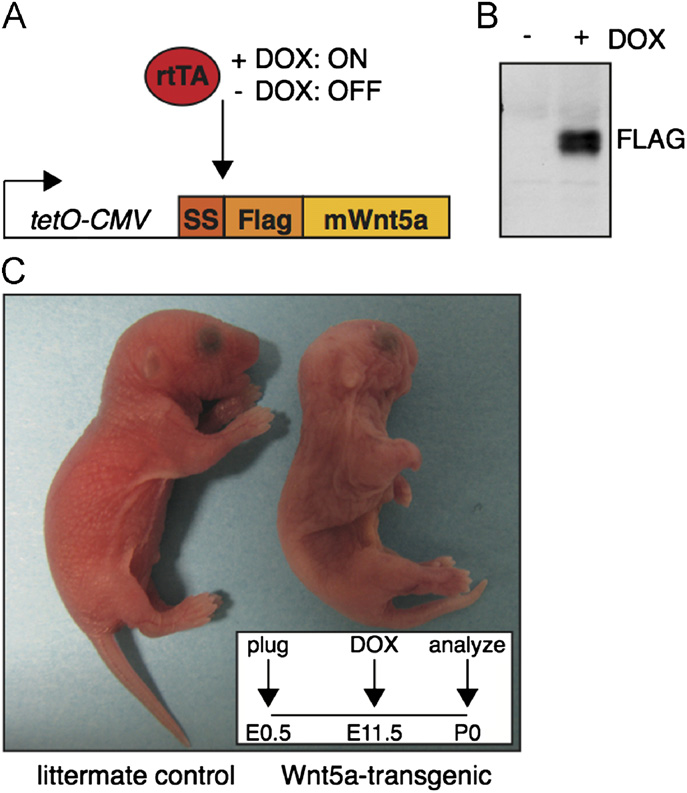
Fig. 1. A novel transgenic mouse model allowing inducible Wnt5a overexpression.
(A) Schematic representation of the tetO-Wnt5A transgenic construct and theexperimental strategy. Mouse Wnt5a was cloned downstream of an artificial
Quantification of hair follicle number, animal size and bone length
signal sequence (SS) in frame with an N-terminal FLAG tag under the control of adoxycycline inducible promoter. Transgene expression is only switched on in the
To quantify the number of hair follicles in newborn skin,
presence of both an rtTA driver and doxycycline (DOX). (B) Western blot analysis
images of H&E stained sections were imported in Image J.
illustrating that Wnt5a protein can be detected with an anti-Flag antibody in tetO-Wnt5A transgenic mouse embryo fibroblasts infected with pBabe-rtTA3 in the
A freehand line was drawn to trace and measure the length (in
presence (right) but not in the absence (left) of doxycycline. (C) External
pixels) of a continuous stretch of longitudinally oriented skin (as
appearance of a newborn tetO-Wnt5a;R26rtTA double-transgenic animal (right)
determined by the orientation of the sub-dermal muscle fibers).
and a littermate control (left) following treatment with doxycycline from E11.5-
The number of hair follicles in this stretch of skin was counted by
P0. Wnt5a overexpressing mice are smaller, have shortened limbs and an easily
hand. Hair follicle number was divided by the length of the skin in
identifiable craniofacial phenotype. Insert depicts treatment schedule.
pixels. The resulting ratio, designated the ‘number of hair folliclesper unit length' was plotted.
To determine the relative size of newborn mice, images of
supplementary data), allowing us to routinely purify large quan-
double-heterozygotes photographed together with littermate
tities of bioactive Flag-Wnt5a according to previously published
controls were imported in Image J. A freehand line was drawn
protocols (Transgenic mice were gen-
to trace and measure (in pixels) the distance from the nose, along
erated by oocyte injection of the tetO-Flag-Wnt5a (hereafter tetO-
the back of the animal to the tip of the tail. For each control
Wnt5a) construct. Mouse embryo fibroblasts from individual
littermate, this measurement was set at 100% and the size
transgenic founder lines were tested in vitro for doxycycline-
reduction in double-heterozygotes was calculated accordingly.
inducible expression of the transgene by quantitative RT-PCR
To quantify the reduction in bone length, images of Alazarin
in the supplementary data) and Western blot analysis
Red and Alcian Blue stained limbs were imported in Image
B) to identify lines that showed robust induction of Flag-
J. A straight line was drawn from the distal to the proximal end
Wnt5a expression. Furthermore, transgene induction was shown
of the bone. The measured distance (in pixels) was then used as a
to be dose-dependent by testing a concentration series of dox-
relative measure for bone length.
ycycline (in the supplementary data). All phenotypesdiscussed below were confirmed to be present in at least twoindependent tetO-Wnt5a lines.
Generalized overexpression of Wnt5A throughout embryonic
Generation and characterization of tetO-Wnt5a transgenic mice
development is lethal
To uncover the potential signaling activities of Wnt5a in vivo,
Transgene expression can be induced in vivo by crossing tetO-
we generated an inducible transgenic mouse model allowing tight
Wnt5a mice to a strain expressing the appropriate rtTA driver, after
spatiotemporal control over Wnt5a expression. For this purpose,
which the expression of Wnt5A can be switched on during embryo-
we cloned a Flag-tagged version of the mouse Wnt5a gene
nic development by the administration of doxycycline in the
downstream of a tetracycline inducible promoter and
drinking water of pregnant dams (. To determine the effect
in the supplementary data). Activity of Flag-tagged Wnt5a
of broad Wnt5a overexpression during embryonic development,
was comparable to that of the untagged protein (in the
we set up timed matings between tetO-Wnt5a mice and mice
R. van Amerongen et al. / Developmental Biology 369 (2012) 101–114
overexpressing Wnt5a to that of mice overexpressing the known
Mendelian ratios of tetO-Wnt5a;R26rtTA mice. Offspring from a cross between
secreted Wnt/b-catenin inhibitor Dickkopf1 (Dkk1) (
parents heterozygous for either tetO-Wnt5a or R26rtTA was born at the expected
). Since the effects of global Dkk1 overexpression have not
Mendelian ratios when doxycycline treatment of pregnant mothers was started
been reported, we crossed both the tetO-Wnt5a mice and pre-
from E13.5 onwards. In contrast, no double-transgenic offspring was recovered atbirth when Wnt5a overexpression was induced from, or prior to, E7.5.
viously generated tetO-Dkk mice (to R26rtTAdriver mice and compared newborn tetO-Wnt5a;R26rtTA and tetO-
Wnt5a inducedrE7.5n
Wnt5a induced Z E13.5nn
Dkk;R26rtTA pups following doxycycline treatment from E10.5onwards. To our surprise, the gross phenotype of generalized
Dkk overexpression was much less severe than that of Wnt5a
overexpression. TetO-Dkk;R26rtTA mice were born alive at the
expected Mendelian ratios and could not be distinguished from
control littermates based on their overall appearance (data
not shown). Moreover, tetO-Dkk;R26rtTA double-transgenic mice
n n¼34, P¼0.0015 (chi square test).
were readily recovered when doxycycline treatment was initiated
nn n¼36, P ¼0.34 (chi square test).
at E7.5, in contrast to what we observed for tetO-Wnt5a;R26rtTAmice (data not shown).
expressing the rtTA-M2 driver from the Rosa26 locus
Overexpression of Dkk1 in the basal layers of the developing
hereafter R26rtTA). Expectant mothers were treated with
epidermis was previously reported to cause a complete loss of
doxycycline for various amounts of time during pregnancy, after
hair follicles in the dorsal skin and to result in aberrant and
which newborn mice were analyzed at birth. In the absence of
reduced vibrissiae formation This phenotype
doxycycline, the different genotypes were observed at the expected
was recapitulated in tetO-Dkk;R26rtTA double-transgenic mice
Mendelian ratios (data not shown). However, when doxycycline
that were treated with doxycycline from E10.5 onwards
treatment was started on or prior to E7.5, no tetO-Wnt5A;R26rtTA
and B and data not shown). Interestingly, we observed a similar
double-heterozygotes were recovered among the offspring
phenotype in the skin of double-transgenic tetO-Wnt5a;R26rtTA
P¼0.0015). In contrast, treatment from E13.5 onwards again yielded
mice (and in the supplementary data). Loss of hair
the expected ratios of tetO-Wnt5A;R26rtTA double-heterozygotes at
follicle formation is not only observed in mice overexpressing
birth ). Since we were able to recover tetO-Wnt5A;R26rtTA
Dkk1, but also in mice conditionally deficient for b-catenin
double-transgenic mice at birth when doxycycline treatment was
(and in mice displaying the combined loss
started on or after E10.5 (see below), this suggests a critical time
of TCF3/TCF4 (). Therefore, this phenotype
window prior to E10.5 when generalized overexpression of Wnt5a is
bears the hallmarks of a typical Wnt/b-catenin loss-of-function
Wnt5a overexpression during the second half of pregnancy results in
Wnt5a overexpression inhibits the second wave of hair follicle
a variety of defects
Double heterozygous tetO-Wnt5A;R26rtTA mice that were born
Whereas Dkk overexpression resulted in the complete loss of
following transgene induction from E13.5 onwards were slightly
hair follicles in the dorsal skin (B), some hair follicles were
smaller than their control littermates, had trouble breathing and
found to be remaining in the skin of Wnt5a-overexpressing
did not survive (data not shown). We next determined that the
mice (D). In most cases however, these follicles failed
earliest timepoint at which doxycycline treatment could be
to progress beyond the embryonic hair germ or hair peg stage
initiated while still allowing double-heterozygotes to be recov-
(white arrowheads in D). Quantification of the number of
ered at birth, was E10.5. These mice were stillborn and were
remaining follicles in the dorsal skin of tetO-Wnt5A;R26rtTA
easily identified by their external appearance. When doxycycline
mice showed a clear decrease at the time of birth (Of
treatment was started at E11.5 or E12.5, double heterozygous
note, the absolute reduction in hair follicle number did not
animals showed a similar aberrant gross morphology and an
change with earlier onset of doxycyline treatment. Regardless of
approximate 10% reduction in size compared to their control
when Wnt5a overexpression was initiated between E10.5 and
littermates (C and in the supplementary data). Closer
E13.5, there was a 58% (7 8%) decrease in the number of hair
inspection of these newborn tetO-Wnt5A;R26rtTA mice revealed
follicles that could be counted at birth. In addition, the extent to
phenotypes in multiple tissues. Most strikingly, virtually all
which remaining hair follicles developed was also comparable
double-transgenic mice lacked most of their intestinal tract.
(Fig. 2 E–H). While some hairs appeared to mature normally
Whereas these mice had a stomach and hindgut, the small
(arrowhead in F), on average the growth of remaining hair
intestine was largely absent (in the supplementary data).
follicles was delayed compared to control skin (compare for
In addition, double heterozygotes had lung defects (RvA and RN,
unpublished data) as well as abnormalities in the skin and
Hair follicle formation in the mouse skin occurs in three waves
multiple skeletal malformations (see below).
(reviewed in ), the first of whichtakes place around E14.5. When we compared sections from the
Global Wnt5a overexpression causes a Wnt/b-catenin loss-of-
developing skin of E14.5 embryos treated with doxycycline from
function phenotype in the skin
E10.5 onwards, hair placodes were readily detectable in bothcontrol and Wnt5a-overexpressing skin suggesting
Given the reported role for Wnt5a in repressing Wnt/b-catenin
that this primary wave of hair follicle formation was initiated
signaling in vivo, both in normal development and disease
normally. This was confirmed by wholemount analysis of E14.5
embryos, in which primary hair follicle formation can be visua-
hypothesized that at least some of the phenotypes observed in
lized by alkaline phosphatase staining C). At E16.5 however,
tetO-Wnt5a;R26rtTA mice would reflect this particular signaling
when the second wave of hair follicle formation commences, the
activity of Wnt5a. To distinguish between this and potential other
total number of hair follicles in the skin from Wnt5a overexpres-
activities of Wnt5a, we decided to compare the phenotype of mice
sing mice appeared to be reduced (D–E). Taken together
R. van Amerongen et al. / Developmental Biology 369 (2012) 101–114
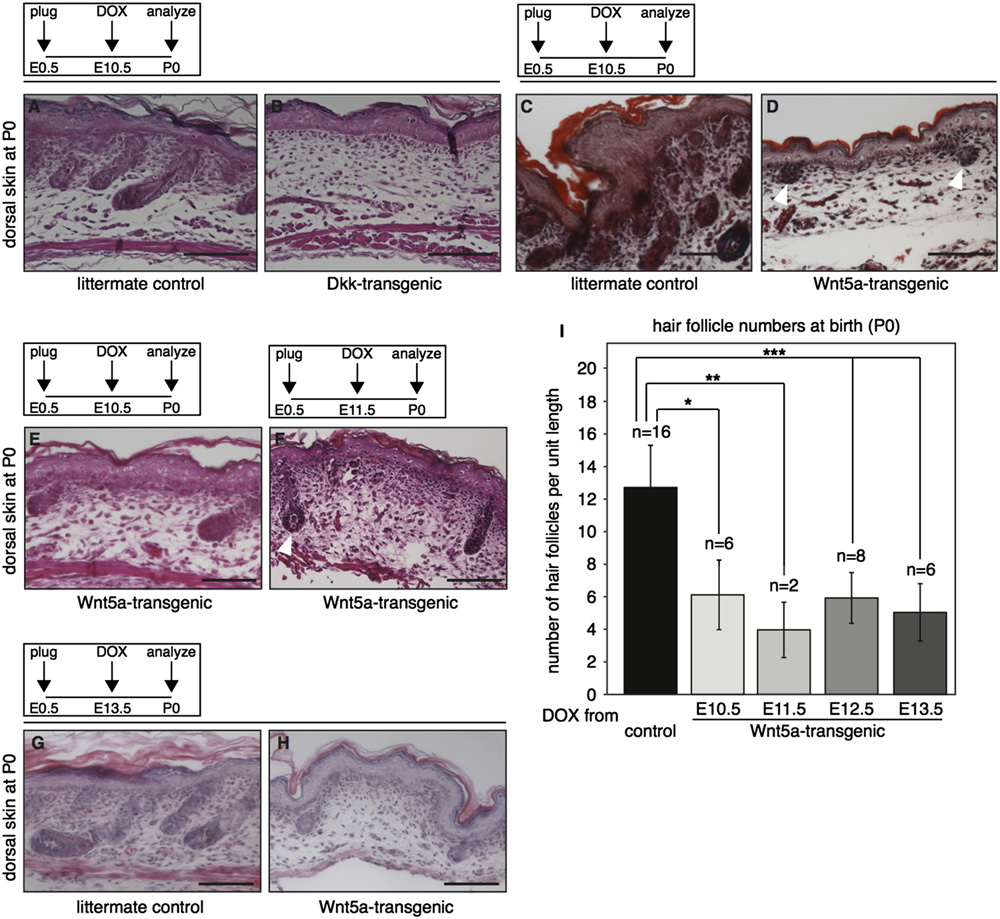
Fig. 2. Global overexpression of Wnt5a or Dkk1 results in loss of hair follicle formation in the dorsal skin. (A–D) H&E stained histological tissue sections from the dorsalskin of tetO-Dkk;R26rtTA (B) and tetO-Wnt5a;R26rtTA (D) double-transgenic mice and their respective littermate controls (A and C), showing that both Wnt5a and Dkk1overexpression results in loss of hair follicle formation. Transgene induction was achieved by administering doxycycline from E10.5 to P0. (E–H) H&E stained histologicaltissue sections showing representative images of the dorsal skin from newborn tetO-Wnt5a;R26rtTA mice, illustrating that a reduction in hair follicle numbers occursregardless of whether doxycycline treatment was initiated at E10.5 (E), E11.5 (F) or E13.5 (G–H). In addition to their overall number being lower, remaining hair follicleswere less mature, although some did show signs of keratinization (white arrowhead in F). (I) Graph depicting the quantification of hair follicle numbers in the skin ofnewborn mice overexpressing Wnt5a from E10.5-P0, E11.5-P0, E12.5-P0 or E13.5-P0 relative to littermate controls. Sagittal sections from a total of 38 skins were counted.
Control samples (n ¼16) derived from the different doxycycline treated litters were pooled to calculate the average number of hair follicles per unit length in control skin.
A comparable and statistically significant reduction in hair follicle numbers was observed in all Wnt5a overexpressing newborn skins (np o1 � 10�4 for onset at E10.5,nnpo5 � 10�4 for onset at E11.5 and nnnpo1 � 10�5 for onset at E12.5 and E13.5, T-test). Error bars indicate standard deviation. Scale bar is 100 mm in A–H.
these results demonstrate that Wnt5a overexpression does not
Wnt5a-transgenic mice. At E16.5, Axin2-lacZ expression in control
affect the initial formation of hair placodes at E14.5, but results in
mice is widespread and observed in all layers of the epidermis, as
a reduction in the number of hair follicles at birth. Thus, we
well as in the dermal papilla of growing hair follicles and in the
conclude that Wnt5a overexpression affects the second and third,
first five cell layers of the dermis (In mice overexpres-
but not the first, wave of hair follicle formation in the skin.
sing Wnt5a, growing hair follicles showed strong, and apparentlyunchanged, Axin2-lacZ staining in the dermal papilla (D,
Wnt5a overexpression affects hair follicle spacing through inhibition
white arrowheads). Similarly, expression in most of the epidermis
of Wnt/b-catenin signaling in the dermis
appears to be unaffected. In contrast, Axin2-lacZ expression isspecifically reduced in the upper dermis of tetO-Wnt5a;R26rtTA
To test if Wnt5a overexpression indeed resulted in inhibition
double-transgenic
of Wnt/b-catenin signaling in the skin, we crossed a known Wnt/
The tyrosine kinase receptor Ror2 can transduce a Wnt5a
b-catenin reporter strain, Axin2-lacZ (into our
signal resulting in inhibition of Wnt/b-catenin signaling in vitro.
Wnt5a-transgenic mouse model and analyzed double-heterozy-
We therefore hypothesized that Ror2 might play a similar role in
gous tetO-Wnt5a;R26rtTA embryos carrying an Axin2-lacZ allele
the developing skin. Ror2 has been reported to be expressed in
for changes in reporter gene expression. At E14.5, when hair
the skin, but the pattern has not been studied in detail
follicle formation has been initiated, Axin2-lacZ is broadly
expressed in wildtype skin (with the strongest expres-
antibody directed against mouse Ror2, we were able to detect
sion seen in dermal condensates adjacent to hair placodes and in
endogenous Ror2 protein expression in the developing skin at
the outer layers of the epidermis. In addition, prominent Axin2-
both E14.5 (data not shown) and E16.5 in control, but
lacZ expression can be observed in a thin layer of dermal cells
not Ror2-deficient animals. The highest levels of Ror2 were
immediately underlying the basal epidermis. The overall expression
expressed in the upper dermis, precisely where Wnt5a-over-
of Axin2-lacZ is markedly reduced in the skin of tetO-Wnt5a;R26rtTA
expression results in inhibition of the Wnt/b-catenin dependent
double-transgenic littermates (B). In particular, most of the
luciferase reporter Axin2-lacZ. Of note, we found that Axin2-lacZ
expression observed in the dermis of control embryos is absent in
reporter gene expression in this stripe of cells was unchanged in
R. van Amerongen et al. / Developmental Biology 369 (2012) 101–114
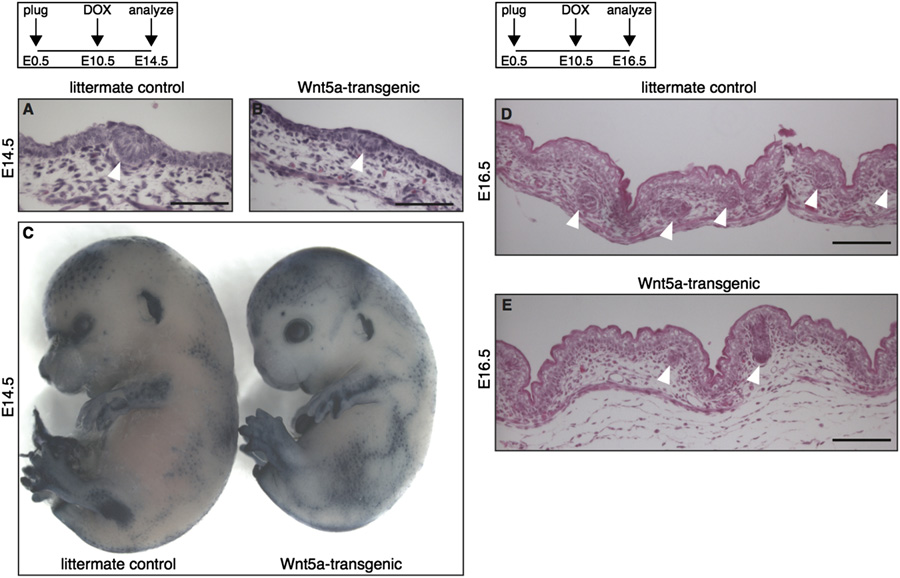
Fig. 3. Wnt5a overexpression inhibits the second, but not the first wave of hair follicle formation. (A–B) H&E stained histological tissue sections of E14.5 dorsal skin,showing that hair placodes (white arrowheads) are formed in both tetO-Wnt5a;R26rtTA double-heterozygous embryos and littermate controls when doxycycline isadministered from E10.5-E14.5. (C) Wholemount alkaline phosphatase staining of E14.5 embryos, revealing a normal pattern of primary hair follicle induction in Wnt5a-transgenic mice (right) following doxycycline administration from E10.5-E14.5. (D–E) H&E stained histological tissue sections of E16.5 dorsal skin, showing a reduction inthe number of hair follicles (white arrowheads) in Wnt5a-transgenic mice following doxycycline administration from E10.5-E16.5. Scale bars are 100 mm in A–B and D–E.
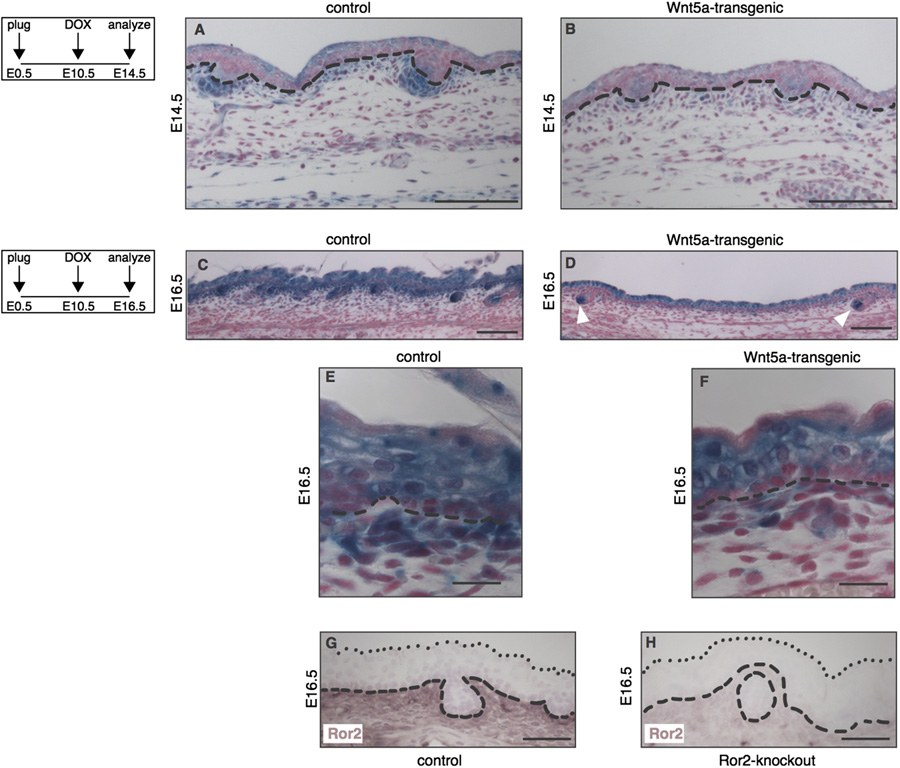
Fig. 4. Loss of Wnt/b-catenin signaling at sites of Ror2 expression in the dermis of Wnt5a-overexpressing mice. (A–F) Histological tissue sections of wholemount X-galstained dorsal skin, demonstrating that expression of the Wnt/b-catenin reporter Axin2-lacZ is markedly reduced in Wnt5a-transgenic embryos at both E14.5 (A–B) andE16.5 (C–F). Mice depicted in A and B are littermates, as are the mice depicted in C and D. The activity of an independent Wnt/b-catenin reporter strain, TOPGAL, was alsoreduced at these timepoints (data not shown). (A–B) At E14.5 the overall activity of Axin2-lacZ is lower throughout the epidermis and dermis of Wnt5a overexpressingmice, but expression of the reporter is particularly reduced in the dermal condensates. (C–D) At E16.5 the Axin2-lacZ signal in the dermal papilla of existing hair folliclesremains largely unaffected (white arrowheads in D). (E–F) Close-ups of the control and Wnt5a overexpressing skins shown in C and D, showing that the most dramaticreduction in Wnt/b-catenin reporter gene expression is seen in a thin layer of cells just underlying the basal layer of the epidermis in tetO-Wnt5a;R26rtTA double-heterozygous mice (F) but not in littermate controls (E). (G–H) Immunohistochemical detection of endogenous Ror2 protein expression in the dermis of control (G) but notRor2-knockout (H) skin at E16.5. Dashed lines indicate the boundary between epidermis and dermis. Dotted lines in G and H indicate the outermost cell layer of theepidermis. Scale bars are 100 mm in A–B, 20 mm in C and D, and 50 mm in E–H.
tetO-Dkk;R26rtTA mice, which is in agreement with the fact that
support a model in Wnt5a/Ror2 inhibits dermal Wnt/b-catenin
Dkk inhibits b-catenin/TCF signaling in a Ror2-independent fash-
signaling to control the progression of hair follicle formation
ion (in the supplementary data). Taken together, our data
during embryonic development.
R. van Amerongen et al. / Developmental Biology 369 (2012) 101–114
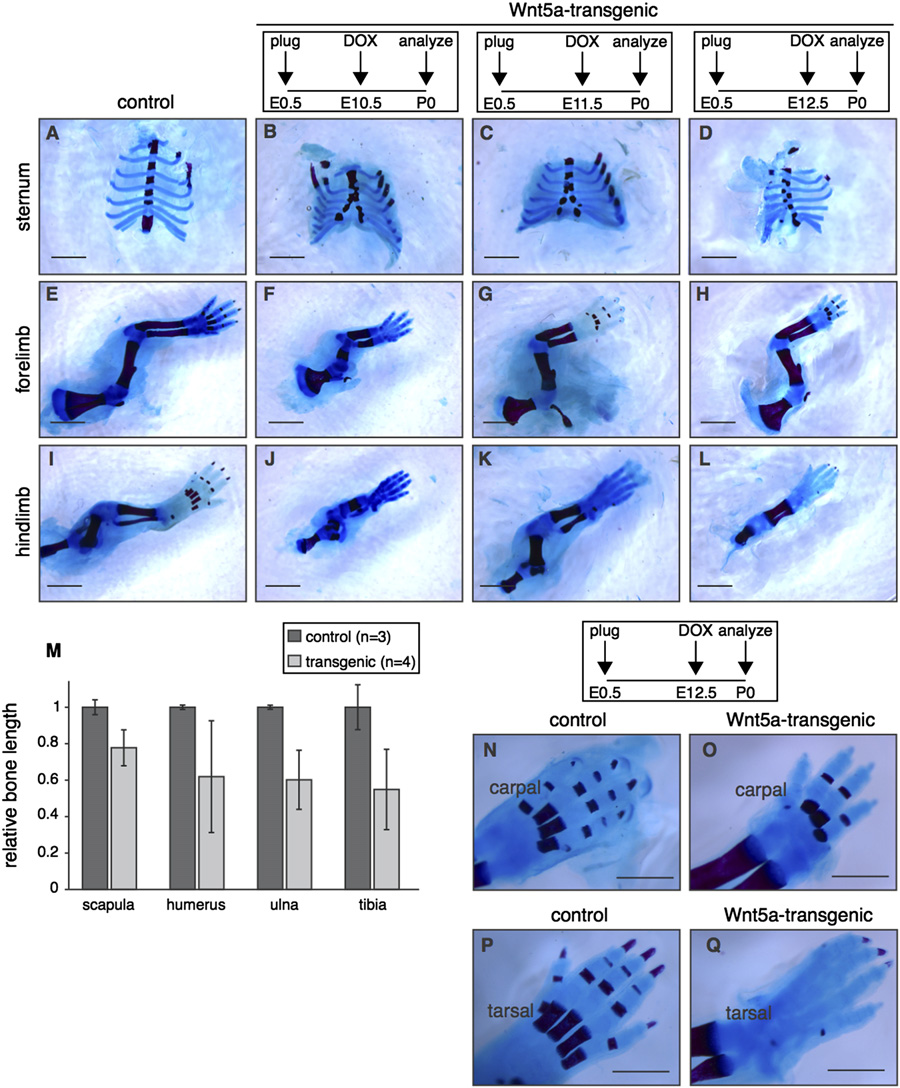
Fig. 5. Global overexpression of Wnt5a causes multiple skeletal defects. (A–L) Wholemount preparations of newborn Wnt5a-overexpressing and control skeletons, stainedwith Alizarin Red and Alcian Blue to visualize bone (red) and cartilage (blue) following transgene expression from E10.5-P0 (B,F,J), E11.5-P0 (C,G,K) or E12.5-P0 (D,H,L).
TetO-Wnt5a;R26rtTA double-heterozygotes develop a split sternum (A–D) and have shortened bones in the fore (E–H) and hindlimbs (I–L) at the time of birth. (M) Graphdepicting the relative length of flat (scapula) and long bones (humerus, ulna and tibia) in control (n¼ 3) and Wnt5a-overexpressing mice (n¼ 4), revealing that limboutgrowth is more severely impaired than overall body size (in the supplementary data). Data were pooled for animals treated with doxycycline from E10.5-P0,E11.5-P0 and E12.5-P0. Error bars indicate standard deviation. (N–Q) Close-up images, demonstrating that bone formation in the extremities is delayed in Wnt5a-overexpressing mice compared to littermate controls. This effect is more pronounced for tarsal (compare Q to P) than for carpal bones (compare O to N). Scale bar is 2 mmin (A–L) and 1 mm in (N–Q).
To test the requirement for Ror2 in the Wnt5a-mediated loss of
doxycycline) or low (0.2 mg/ml doxycycline) levels of Wnt5a-trans-
hair follicle formation, we sought to rescue the effects of Wnt5a
gene expression (in the supplementary data). In contrast, some
overexpression in the skin by introducing a Ror2 loss-of-function
of these animals showed quite aberrant patterning, in which areas
allele Unfortunately, the interpretation of
with hair follicles at low density were bordered by regions in which
these rescue experiments was not straightforward. In agreement with
hair follicles appeared to be totally absent O in the supple-
published data (), we initially did not detect any
mentary data). This observation suggests a precarious balance in
obvious defects in the initiation or progression of hair follicle
Wnt5a/Ror2 signaling in the developing skin, the disruption of which
formation in sections from the dorsal skin of Ror2-deficient mice at
affects hair follicle patterning and spacing.
either E16.5 or P0 in the supplementary data). However,upon wholemount analyses of the dorsal skin from newborn animals,
Wnt5a overexpression inhibits bone formation
we noticed a large variation in hair follicle density in Ror2-null micein the supplementary data). Since hair follicles in (early)
The external appearance of tetO-Wnt5A;R26rtTA transgenic
anagen are easily identifiable as dark spots in wholemount prepara-
mice also suggested prominent skeletal defects. Compared to
tions of the skin from newborn, pigmented animals, it thus appears
control littermates, mice overexpressing Wnt5a had shortened
that Ror2-deficient mice show aberrant hair patterning or pigmenta-
limbs and prominent craniofacial abnormalities (C and
tion, albeit with incomplete penetrance. Consequently, we did not
in the supplementary data), reminiscent of the phenotype pre-
observe restoration to wildtype numbers of hair follicles in Ror2-
viously reported for Col2a1-Wnt5a transgenic mice (
deficient tetO-Wnt5a;R26rtTA animals compared to tetO-Wnt5a;
). Closer inspection of skeletons from newborn mice revealed
R26rtTA littermates that were wildtype or heterozygous for Ror2
that Wnt5a overexpression in the embryo (from either E10.5,
at either high (1 mg/ml doxycycline), intermediate (0.4 mg/ml
E11.5 or E12.5 until birth) caused a split sternum and
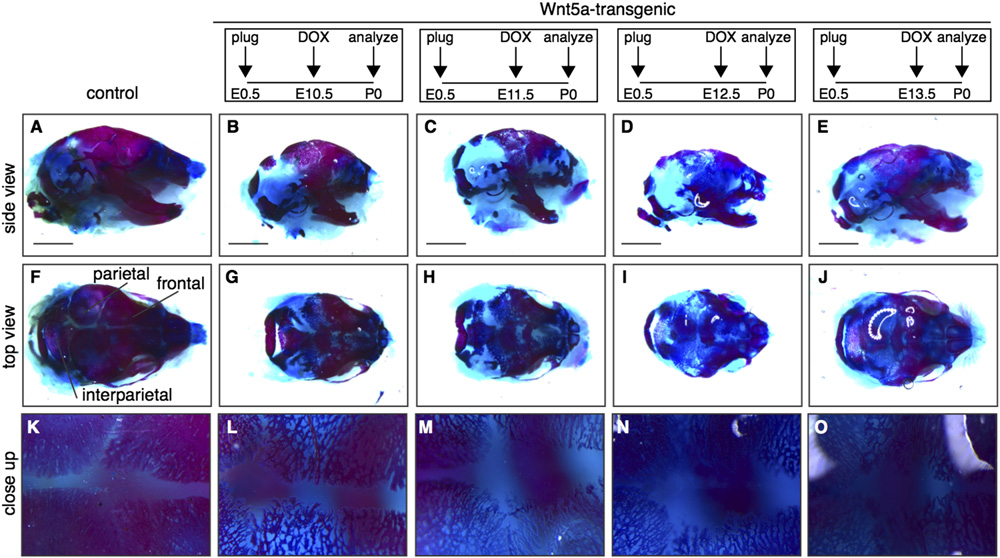
R. van Amerongen et al. / Developmental Biology 369 (2012) 101–114
Fig. 6. Delayed calvarial ossification in Wnt5a-transgenic mice. Wholemount preparations of newborn Wnt5a-overexpressing and control skulls, stained with Alizarin Redand Alcian Blue to visualize bone (red) and cartilage (blue) following transgene expression from E10.5-P0 (B,G,L) to E11.5 (C,H,M) or E12.5 (D,I,N) or E13.5 (E,J,O),demonstrating that calvarial bone formation is reduced in the skull of Wnt5a-overexpressing mice compared to control littermates (A,F,K). (F–J) are top views of the skullsdepicted in (A–E) and (K–O) are close-ups of the same samples. Scale bar is 2 mm.
resulted in reduced outgrowth of the long bones in both fore- and
the skull of newborn tetO-Wnt5a;R26rtTA double-heterozygous
hindlimbs M). Although shortened, long bones were fully
mice might be due to a separate signaling activity of Wnt5a,
ossified at the time of birth. In contrast, ossification in the digits was
distinct from its ability to inhibit b-catenin/TCF signaling.
visibly delayed in Wnt5a-transgenic neonates, with reduced bone
To test this, we again crossed the Axin2-lacZ reporter mice into
formation in the metacarpal and phalangeal bones. This delay was
the tetO-Wnt5a;R26rtTA background to score changes in Wnt/b-
more prominent in the hindlimb than in the forelimb: When mice
catenin activity. Independently, we also crossed in TOPGAL, a second
were treated with doxycycline from E12.5 onwards, bone formation
Wnt/b-catenin reporter strain . To our
was detectable in carpal N–O), but absent in tarsal bones
surprise, we observed a consistent increase in b-catenin/TCF signal-
Q). We observed the most prominent reduction in bone
ing in the heads of mice overexpressing Wnt5a at E14.5 and E16.5
formation in the calvaria. Here, both the neural crest-derived frontal
(and in the supplementary data). Both Axin2-lacZ
bones and the mesoderm-derived parietal and interparietal bones
(F and M–N) and TOPGAL (reporter activity
were affected A–J). Compared to control littermates, the
showed an increase in tetO-Wnt5a;R26rtTA double-transgenics com-
meshwork of bone in the calvaria of newborn tetO-Wnt5a;R26rtTA
pared to littermate controls. Thus, in contrast to the skin, where we
mice was less condensed (, suggesting either a delay or a
found Wnt5a overexpression to inhibit b-catenin/TCF signaling,
block in ossification. This phenotype was most apparent when
Wnt5a causes activation of Wnt/b-catenin signaling in the develop-
doxycline treatment was commenced between E10.5 and E12.5,
ing skull (see also in the supplementary data).
but even Wnt5a transgene expression from E13.5 onwards resulted
Upon isolating the tissue with increased b-catenin/TCF signal-
in reduced density of the calvarial bones O). Since bone
ing by microdissection, we found it to be located between the
formation in the limbs occurs through endochondral ossification of
surface ectoderm and the brain, consistent with the location of
cartilage intermediates, whereas bone formation in the skull occurs
the developing calvarial mesenchyme. However, when we ana-
directly through intramembranous ossification of a mesenchymal
lyzed Axin2-lacZ reporter gene expression in paraffin embedded
progenitor, these results suggest that Wnt5a overexpression affects
tissue sections of E14.5 and E16.5 heads, we found that the
both processes.
increase in Wnt/b-catenin signaling was restricted to the duramater, which is the outermost layer of the developing meninges
Reduced calvarial ossification is preceded by gain of Wnt/b-catenin
(and O–P). The meninges is a neural crest derived tissue
signaling in the meninges
that develops in close association with the underlying brain andthe overlying frontal and parietal bones ().
Wnt/b-catenin signaling is known to control multiple steps of
Of note, meningeal defects have previously been shown to
bone development (for reviews see ;
adversely affect bone formation during embryonic development
). It is generally considered to promote bone
(; Conversely, an intact
formation, as illustrated by the association of LRP5 gain and loss
dura mater has been shown to aid in osteogenic repair following
of function mutations with familial high and low bone mass
calvarial defects ). These studies, as well as our
phenotypes, respectively (;
own, suggest important crosstalk between the meninges and the
In fact, administration of Wnt
developing or healing calvaria. Our data suggest a previously
ligands is seen as a powerful tool to potentially increase bone
unrecognized role for Wnt/b-catenin signaling in this process.
mass upon tissue damage or aging AlthoughDkk1 has previously been shown to negatively affect boneformation ), we did not observe any defects
in calvarial ossification in tetO-Dkk;R26rtTA-transgenic mice fol-lowing doxycycline administration from E10.5-P0 (
It has long been debated whether individual Wnt ligands have
We therefore hypothesized that the reduced bone formation in
multiple signaling activities in vivo and, if so, how signaling
R. van Amerongen et al. / Developmental Biology 369 (2012) 101–114
Fig. 7. Global Wnt5a overexpression causes an increase in Wnt/b-catenin signaling in the developing meninges. (A–D) Wholemount preparations of the skulls fromnewborn mice, stained with Alizarin Red and Alcian Blue to visualize bone (red) and cartilage (blue), showing that overexpression of Wnt5a (compare B to A), but not Dkk1(compare D to C) from E10.5-P0 results in reduced calvarial ossification at birth. (E–H) Wholemount preparations of X-gal stained E14.5 embryos, demonstrating thatexpression of the Wnt/b-catenin reporters Axin2-lacZ (compare F to E) and TOPGAL (compare H to G) is increased in the developing skull of Wnt5a-overexpressing micecompared to control littermates. The skin was removed to better visualize the calvarial mesenchyme. (I–L) Histological tissue sections of the samples depicted in (E–F)following paraffin embedding, showing that the increase in Axin2-lacZ activity is restricted to a thin layer of cells immediately underlying the calvarial mesenchyme andoverlying the brain, consistent with the location of the developing meninges. The sagittal (I–J) and coronal (K–L) planes of sectioning are indicated with the black lines inpanel (E). (M–N) Wholemount preparations of X-gal stained E16.5 embryos, demonstrating that expression of the Wnt/b-catenin reporter Axin2-lacZ (compare N to M) isincreased in the developing skull of Wnt5a-overexpressing mice compared to control littermates. The skin was removed to better visualize the developing skull. (O–P)Independent coronal tissue sections of wholemount X-gal stained E16.5 embryos, showing an increase in Axin2-lacZ activity in the meninges. Scale bars are 2 mm in A–D,1 mm in E–H and M–N, 100 mm in I–J and 50 mm in K–L and O–P. (br) brain, (cm) calvarial mesenchyme, (dm) dura mater, (sk) skin.
specificity is achieved in a complex, developing multicellular
Wnt5a-knockout and -transgenic mouse lines (
organism. In recent years it has become evident that a single
Wnt protein can indeed initiate multiple downstream signaling
), including tight spatial and temporal control over the onset
events. For instance, both Wnt5a and Wnt11 are required for axis
and duration of Wnt5a overexpression. For our first analysis,
specification in the early Xenopus embryo (;
we chose to study the effects of broad Wnt5a overexpression
), a process that is driven by Wnt/b-catenin
during embryonic development, using a Rosa26-rtTA-M2 driver
signaling. Yet each protein is also well known to have a
). We found that elevated levels of
b-catenin-independent role in controlling convergent extension
Wnt5a expression caused a variety of developmental defects that
movements at later developmental stages ;
diminished in severity with lower levels (achieved by lowering
). The finding that purified mouse Wnt5a
the concentration of doxycycline) as well as later onset of
was able to both activate and inhibit Wnt/b-catenin signaling
transgene induction. By focusing on two of these phenotypes in
in vitro suggested that a similar paradigm
more detail, we were able to uncover previously unrecognized
might apply to mammalian Wnt proteins. Existing mouse models
roles for the dermis in determining hair follicle formation and
however, did not allow perturbation of Wnt5a expression in a
patterning of the skin, as well as for Wnt/b-catenin signaling in
controlled manner, precluding the testing of this hypothesis in vivo.
the meninges in controlling calvarial bone formation. Interest-
Here we describe the generation of a novel, inducible trans-
ingly, we were able to link these phenotypes to different signaling
genic mouse model, which offers many advantages over existing
activities of Wnt5a: On the one hand, we found that Wnt5a/Ror2
R. van Amerongen et al. / Developmental Biology 369 (2012) 101–114
signaling inhibits Wnt/b-catenin signaling in the dermis ),
calvarial osteoblasts ). Whereas Wnt/b-catenin
thereby affecting the second and third wave of hair follicle
signaling therefore appears to be required for bone formation, earlier
formation. On the other hand, Wnt5a caused an increase in
activation of the pathway might result in the prolonged mainte-
Wnt/b-catenin signaling in the meninges preceding
nance of a mesenchymal progenitor state. Although at present we
diminished ossification of the calvarial mesenchyme. To our
have no proof that in tetO-Wnt5a;R26rtTA double-transgenic animals
knowledge, this is the first report of potential dual signaling
the increase in b-catenin/TCF signaling in the dura mater at E14.5
activities for a mammalian Wnt ligand during embryonic
and E16.5 is directly responsible for the reduced bone formation
observed at birth, it is generally well accepted that the dura mater
Wnt-signaling controls multiple aspects of hair follicle mor-
affects osteogenesis in the overlying calvarial mesenchyme through
phogenesis, both during embryonic development and in postnatal
the secretion of paracrine growth factors
hair turnover. In addition to directing hair follicle formation, Wnt/
. We therefore hypothesize that
b-catenin signaling has also been shown to control hair follicle
the enhanced Wnt/b-catenin signaling in the dura mater alters the
spacing. Establishment of the hair follicle pattern in the skin
production of one or more of these factors. Although the endogen-
occurs according to a reaction-diffusion model, with Wnt proteins
ous role of Wnt-signaling in dura mater biology has not been
serving as activators and Dkk proteins as diffusible inhibitors
studied, both ligands and receptors are expressed in the developing
meninges as suggested by publicly available expression databases
find that the Wnt5a-mediated inhibition of hair follicle formation
(e.g. Gene Expression Database at
coincides with a reduction in dermal Wnt/b-
These include Fzd4, which can induce Wnt/b-catenin signaling in
catenin signaling (Ror2 is normally expressed throughout
response to Wnt5a ) and which might
the dermis during this time () and endogenous Wnt5a
therefore be responsible for the observed increase in Axin2-lacZ and
is similarly expressed from E14.5 onwards, although its expres-
TOPGAL reporter activity in our Wnt5a overexpressing mice. At
sion later becomes concentrated in dermal condensates (
present however, we cannot exclude that other signaling activities
We therefore propose that endogenous Wnt5a/Ror2
of Wnt5a (such as a Ror2-mediated induction of osteoclastogenesis)
signaling might help control hair follicle spacing by globally
underlie the bone loss observed in the skull and limbs of tetO-
dampening Wnt/b-catenin signaling, thereby preventing the pre-
Wnt5a;R26rtTA double-transgenic mice ).
cocious or extraneous hair follicle formation that can result from
Of note, the block in calvarial ossification was more sensitive
ectopic b-catenin/TCF signaling ().
to dose than the loss of hair follicles in the skin or the loss of
The fact that we were unable to rescue the loss of hair follicle
intestinal tissue. The latter two were still observed when mice
formation in tetO-Wnt5a;R26rtTA animals by introducing a Ror2-
were treated with lower levels of doxycycline (Figs. S4 and S8 in
null allele is puzzling in light of our earlier findings: Ror2 directly
the supplementary data). In contrast, loss of bone formation in
binds Wnt5a through its CRD domain and expression of Ror2 is
the skull was only detected when mice received 1–2 mg/ml
sufficient to confer Wnt5a-mediated inhibition of b-catenin/TCF
doxycycline. This suggests that the Wnt/b-catenin loss of function
signaling, suggesting that it can act as a genuine Wnt receptor
phenotype is preserved at lower levels, whereas the induction of
However, other studies have proposed
Wnt/b-catenin signaling requires higher levels of Wnt5a trans-
that Ror2 functions as a co-receptor for Fzd (
gene expression. It is tempting to speculate that this might reveal
something about the affinity of Wnt5a for its receptors: Much
As such, Ror2 might be involved in, but not be critically required
higher levels of Wnt5a might be required to induce activation of
for, the Wnt5a-mediated inhibition of b-catenin/TCF signaling,
Wnt/b-catenin signaling through a particular Fzd receptor than to
especially when the levels of Wnt5a are not limiting. One
inhibit it via Ror2. By incorporating loss-of-function alleles for
complicating factor in interpreting these experiments is our
different receptors, as we have done here for Ror2, the tetO-Wnt5a
finding that loss of Ror2 alone causes a reduction in the number
mouse model might help elucidate the factors that control the
of hair follicles in the skin of some, but not all, animals in
specificity of ligand-receptor interactions. Furthermore, as the
the supplementary data). Incomplete penetrance is something we
arsenal of rtTA drivers expands, it will be possible to induce
have also observed for other phenotypes in the Ror2-knockout
tissue-specific overexpression at multiple sites, both in the
mice (RvA and RN, unpublished data) and might reflect functional
embryo and in the adult. We have already observed vast differ-
redundancy with Ror1, which shows overlapping expression with
ences in phenotypic outcome depending on the promoter used to
Ror2 in some tissues during embryonic development (
drive Wnt5a overexpression in the developing lung (RvA and RN,
; ). At present, we have no
unpublished data).
explanation for the defects in hair follicle spacing in Ror2-
Although our system relies on the ectopic expression of
deficient mice, although it leaves open the possibility of a non-
Wnt5a, which may exert its activities at different sites than the
cell autonomous role for Ror2, in which it could function to
endogenous protein, tight control over the onset and levels of
sequester multiple Wnt ligands (both activators and inhibitors of
ligand expression offers an important advantage in dissecting the
Wnt/b-catenin signaling) similar to its Caenorhabditis elegans
signaling responses in a complex, developing organism. Follow-up
studies using conditional knockout mice will be required to test
We uncovered a second signaling activity for Wnt5a in the
the requirement for endogenous Wnt5a, or another Wnt protein,
developing skull. Here, Wnt5a overexpression caused the induction
in the biological processes discussed here. In conclusion, although
of Wnt/b-catenin signaling in the meninges This was
Wnt5a is generally not associated with signaling through
associated with reduced calvarial ossification at birth
b-catenin/TCF, our current study suggests that it is able to do so
. Compared to endochondral bone formation, which occurs
in vivo. While it remains to be determined whether Wnt5a indeed
through a cartilage intermediate, the process of intramembranous
activates Wnt/b-catenin signaling under physiological conditions
ossification remains relatively poorly understood. As first glance our
in mammals, this finding is of special interest given the dual, and
findings appear counterintuitive to a paradigm in which Wnt/b-
confusing, roles reported for Wnt5a in oncogenesis. Wnt5a has
catenin signaling is generally considered to promote bone forma-
been ascribed both oncogenic and tumor suppressor properties in
tion. However, it was recently shown that high levels of Wnt/b-
human malignancies
catenin signaling inhibit the osteogenic differentiation of embryonic
calvarial mesenchymal cells, while inducing osteogenesis in mature
R. van Amerongen et al. / Developmental Biology 369 (2012) 101–114
The current data suggest that especially when overexpressed at
Duverger, O., Morasso, M.I., 2009. Epidermal patterning and induction of different
ectopic sites, its signaling activities should be carefully analyzed
hair types during mouse embryonic development. Birth Defects Res. C EmbryoToday 87, 263–272.
and the possibility of Wnt5a driving b-catenin/TCF signaling
Fischer, T., Guimera, J., Wurst, W., Prakash, N., 2007. Distinct but redundant
should be considered.
expression of the Frizzled Wnt receptor genes at signaling centers of thedeveloping mouse brain. Neuroscience 147, 693–711.
Gao, B., Song, H., Bishop, K., Elliot, G., Garrett, L., English, M.A., Andre, P., Robinson,
J., Sood, R., Minami, Y., Economides, A.N., Yang, Y., 2011. Wnt signaling
gradients establish planar cell polarity by inducing Vangl2 phosphorylationthrough Ror2. Dev. Cell 20, 163–176.
Gerhart, J., 1999. 1998 Warkany lecture: signaling pathways in development.
We thank Orly Liel Wapinski for help in the initial character-
Teratology 60, 226–239.
ization of the Wnt5a-transgenic founder lines, Tim Blauwkamp for
Glinka, A., Wu, W., Delius, H., Monaghan, A.P., Blumenstock, C., Niehrs, C., 1998.
valuable discussions, and Catriona Logan and Xinhong Lim for
Dickkopf-1 is a member of a new family of secreted proteins and functions in
comments on the manuscript. RvA was supported by an EMBO
head induction. Nature 391, 357–362.
Gong, Y., Slee, R.B., Fukai, N., Rawadi, G., Roman-Roman, S., Reginato, A.M., Wang, H.,
Long Term Fellowship (ALTF 122-2007) and a KWF fellowship
Cundy, T., Glorieux, F.H., Lev, D., Zacharin, M., Oexle, K., Marcelino, J., Suwairi, W.,
from the Dutch Cancer Society. CF was supported by the Swiss
Heeger, S., Sabatakos, G., Apte, S., Adkins, W.N., Allgrove, J., Arslan-Kirchner, M.,
National Science Foundation. RN is an Investigator of the Howard
Batch, J.A., Beighton, P., Black, G.C., Boles, R.G., Boon, L.M., Borrone, C., Brunner, H.G.,Carle, G.F., Dallapiccola, B., De Paepe, A., Floege, B., Halfhide, M.L., Hall, B.,
Hughes Medical Institute.
Hennekam, R.C., Hirose, T., Jans, A., Juppner, H., Kim, C.A., Keppler-Noreuil, K.,Kohlschuetter, A., LaCombe, D., Lambert, M., Lemyre, E., Letteboer, T., Peltonen, L.,Ramesar, R.S., Romanengo, M., Somer, H., Steichen-Gersdorf, E., Steinmann, B.,Sullivan, B., Superti-Furga, A., Swoboda, W., van den Boogaard, M.J., Van Hul, W.,
Appendix A. Supporting information
Vikkula, M., Votruba, M., Zabel, B., Garcia, T., Baron, R., Olsen, B.R., Warman, M.L.,2001. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye develop-
Supplementary data associated with this article can be found in
ment. Cell 107, 513–523.
Green, J.L., Inoue, T., Sternberg, P.W., 2007. The C. elegans ROR receptor tyrosine
the online version at
kinase, CAM-1, non-autonomously inhibits the Wnt pathway. Development134, 4053–4062.
Grumolato, L., Liu, G., Mong, P., Mudbhary, R., Biswas, R., Arroyave, R., Vijayaku-
mar, S., Economides, A.N., Aaronson, S.A., 2010. Canonical and noncanonical
Wnts use a common mechanism to activate completely unrelated coreceptors.
Genes Dev. 24, 2517–2530.
He, F., Xiong, W., Yu, X., Espinoza-Lewis, R., Liu, C., Gu, S., Nishita, M., Suzuki, K.,
Adamska, M., Billi, A.C., Cheek, S., Meisler, M.H., 2005. Genetic interaction between
Yamada, G., Minami, Y., Chen, Y., 2008. Wnt5a regulates directional cell
Wnt7a and Lrp6 during patterning of dorsal and posterior structures of the
migration and cell proliferation via Ror2-mediated noncanonical pathway in
mouse limb. Dev. Dyn. 233, 368–372.
mammalian palate development. Development 135, 3871–3879.
Al-Shawi, R., Ashton, S.V., Underwood, C., Simons, J.P., 2001. Expression of the Ror1
He, X., Saint-Jeannet, J.P., Wang, Y., Nathans, J., Dawid, I., Varmus, H., 1997. A
and Ror2 receptor tyrosine kinase genes during mouse development. Dev.
member of the Frizzled protein family mediating axis induction by Wnt-5A.
Genes Evol. 211, 161–171.
Science 275, 1652–1654.
Allgeier, S.H., Lin, T.M., Vezina, C.M., Moore, R.W., Fritz, W.A., Chiu, S.Y., Zhang, C.,
Ho, H.Y., Susman, M.W., Bikoff, J.B., Ryu, Y.K., Jonas, A.M., Hu, L., Kuruvilla, R.,
Peterson, R.E., 2008. WNT5A selectively inhibits mouse ventral prostate
Greenberg, M.E., 2012. Wnt5a-Ror-Dishevelled signaling constitutes a core
development. Dev. Biol. 324, 10–17.
developmental pathway that controls tissue morphogenesis. Proc. Natl. Acad.
Andersson, E.R., Prakash, N., Cajanek, L., Minina, E., Bryja, V., Bryjova, L., Yama-
Sci. USA 109, 4044–4051.
guchi, T.P., Hall, A.C., Wurst, W., Arenas, E., 2008. Wnt5a regulates ventral
Hochedlinger, K., Yamada, Y., Beard, C., Jaenisch, R., 2005. Ectopic expression of
midbrain morphogenesis and the development of A9-A10 dopaminergic cells
Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial
in vivo. PLoS One 3, e3517.
tissues. Cell 121, 465–477.
Andl, T., Reddy, S.T., Gaddapara, T., Millar, S.E., 2002. WNT signals are required for
Hogan, B., 1994. Manipulating the Mouse Embryo a Laboratory Manual, 2nd ed.
the initiation of hair follicle development. Dev. Cell 2, 643–653.
Cold Spring Harbor Press, Plaqinview, NY.
Baxley, S.E., Jiang, W., Serra, R., 2011. Misexpression of wingless-related MMTV
Hsieh, J.C., Rattner, A., Smallwood, P.M., Nathans, J., 1999. Biochemical character-
integration site 5A in mouse mammary gland inhibits the milk ejection
ization of Wnt-frizzled interactions using a soluble, biologically active verte-
response and regulates connexin43 phosphorylation. Biol Reprod.
brate Wnt protein. Proc. Natl. Acad. Sci. USA 96, 3546–3551.
Carmon, K.S., Loose, D.S., 2010. Development of a bioassay for detection of Wnt-
Hu, B., Lefort, K., Qiu, W., Nguyen, B.C., Rajaram, R.D., Castillo, E., He, F., Chen, Y.,
binding affinities for individual frizzled receptors. Anal. Biochem. 401,
Angel, P., Brisken, C., Dotto, G.P., 2010. Control of hair follicle cell fate by
underlying mesenchyme through a CSL-Wnt5a-FoxN1 regulatory axis. Genes
Cervantes, S., Yamaguchi, T.P., Hebrok, M., 2009. Wnt5a is essential for intestinal
Dev. 24, 1519–1532.
elongation in mice. Dev. Biol. 326, 285–294.
Huelsken, J., Vogel, R., Erdmann, B., Cotsarelis, G., Birchmeier, W., 2001. beta-
Cha, K.B., Douglas, K.R., Potok, M.A., Liang, H., Jones, S.N., Camper, S.A., 2004.
Catenin controls hair follicle morphogenesis and stem cell differentiation in
WNT5A signaling affects pituitary gland shape. Mech. Dev. 121, 183–194.
the skin. Cell 105, 533–545.
Cha, S.W., Tadjuidje, E., Tao, Q., Wylie, C., Heasman, J., 2008. Wnt5a and Wnt11
Ito, Y., Yeo, J.Y., Chytil, A., Han, J., Bringas Jr., P., Nakajima, A., Shuler, C.F., Moses, H.L.,
interact in a maternal Dkk1-regulated fashion to activate both canonical and
Chai, Y., 2003. Conditional inactivation of Tgfbr2 in cranial neural crest causes
non-canonical signaling in Xenopus axis formation. Development 135,
cleft palate and calvaria defects. Development 130, 5269–5280.
Jacobs, J.J., Kieboom, K., Marino, S., DePinho, R.A., van Lohuizen, M., 1999. The
Chu, E.Y., Hens, J., Andl, T., Kairo, A., Yamaguchi, T.P., Brisken, C., Glick, A.,
oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and
Wysolmerski, J.J., Millar, S.E., 2004. Canonical WNT signaling promotes
senescence through the ink4a locus. Nature 397, 164–168.
mammary placode development and is essential for initiation of mammary
James, R.G., Conrad, W.H., Moon, R.T., 2008. Beta-catenin-independent Wnt path-
gland morphogenesis. Development 131, 4819–4829.
ways: signals, core proteins, and effectors. Methods Mol. Biol. 468, 131–144.
Croce, J.C., McClay, D.R., 2008. Evolution of the Wnt pathways. Methods Mol. Biol.
Jiang, X., Iseki, S., Maxson, R.E., Sucov, H.M., Morriss-Kay, G.M., 2002. Tissue origins
469, 3–18.
and interactions in the mammalian skull vault. Dev Biol 241, 106–116.
Cui, Y., Niziolek, P.J., Macdonald, B.T., Zylstra, C.R., Alenina, N., Robinson, D.R.,
Kim, G.H., Her, J.H., Han, J.K., 2008. Ryk cooperates with Frizzled 7 to promote
Zhong, Z., Matthes, S., Jacobsen, C.M., Conlon, R.A., Brommage, R., Liu, Q.,
Wnt11-mediated endocytosis and is essential for Xenopus laevis convergent
Mseeh, F., Powell, D.R., Yang, Q.M., Zambrowicz, B., Gerrits, H., Gossen, J.A., He,
extension movements. J. Cell. Biol. 182, 1073–1082.
X., Bader, M., Williams, B.O., Warman, M.L., Robling, A.G., 2011. Lrp5 functions
Kim, H.J., Schleiffarth, J.R., Jessurun, J., Sumanas, S., Petryk, A., Lin, S., Ekker, S.C.,
in bone to regulate bone mass. Nat. Med. 17, 684–691.
2005. Wnt5 signaling in vertebrate pancreas development. BMC Biol. 3, 23.
DasGupta, R., Fuchs, E., 1999. Multiple roles for activated LEF/TCF transcription
Klaus, A., Birchmeier, W., 2008. Wnt signalling and its impact on development and
complexes during hair follicle development and differentiation. Development
cancer. Nat. Rev. Cancer 8, 387–398.
126, 4557–4568.
Komiya, Y., Habas, R., 2008. Wnt signal transduction pathways. Organogenesis 4,
DeChiara, T.M., Kimble, R.B., Poueymirou, W.T., Rojas, J., Masiakowski, P., Valen-
zuela, D.M., Yancopoulos, G.D., 2000. Ror2, encoding a receptor-like tyrosine
Kurayoshi, M., Oue, N., Yamamoto, H., Kishida, M., Inoue, A., Asahara, T., Yasui, W.,
kinase, is required for cartilage and growth plate development. Nat. Genet. 24,
Kikuchi, A., 2006. Expression of Wnt-5a is correlated with aggressiveness of
gastric cancer by stimulating cell migration and invasion. Cancer Res. 66,
Du, S.J., Purcell, S.M., Christian, J.L., McGrew, L.L., Moon, R.T., 1995. Identification of
distinct classes and functional domains of Wnts through expression of wild-
Kusserow, A., Pang, K., Sturm, C., Hrouda, M., Lentfer, J., Schmidt, H.A., Technau, U., von
type and chimeric proteins in Xenopus embryos. Mol. Cell. Biol. 15,
Haeseler, A., Hobmayer, B., Martindale, M.Q., Holstein, T.W., 2005. Unexpected
complexity of the Wnt gene family in a sea anemone. Nature 433, 156–160.
R. van Amerongen et al. / Developmental Biology 369 (2012) 101–114
Kwee, M.L., Balemans, W., Cleiren, E., Gille, J.J., Van Der Blij, F., Sepers, J.M., Van
The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK
Hul, W., 2005. An autosomal dominant high bone mass phenotype in
signalling pathway. Genes Cells 8, 645–654.
association with craniosynostosis in an extended family is caused by an
Olson, D.J., Gibo, D.M., 1998. Antisense wnt-5a mimics wnt-1-mediated C57MG
LRP5 missense mutation. J. Bone Miner. Res. 20, 1254–1260.
mammary epithelial cell transformation. Exp. Cell Res. 241, 134–141.
Leucht, P., Minear, S., Ten Berge, D., Nusse, R., Helms, J.A., 2008. Translating
Peng, C., Zhang, X., Yu, H., Wu, D., Zheng, J., 2011. Wnt5a as a predictor in poor
insights from development into regenerative medicine: the function of Wnts
clinical outcome of patients and a mediator in chemoresistance of ovarian
in bone biology. Semin. Cell Dev. Biol. 19, 434–443.
cancer. Int. J. Gynecol. Cancer 21, 280–288.
Levi, B., Nelson, E.R., Li, S., James, A.W., Hyun, J.S., Montoro, D.T., Lee, M., Glotzbach,
Pinson, K.I., Brennan, J., Monkley, S., Avery, B.J., Skarnes, W.C., 2000. An LDL-
J.P., Commons, G.W., Longaker, M.T., 2011. Dura mater stimulates human
receptor-related protein mediates Wnt signalling in mice. Nature 407,
adipose-derived stromal to undergo bone formation in mouse calvarial
defects. Stem Cells.
Plikus, M.V., Baker, R.E., Chen, C.C., Fare, C., de la Cruz, D., Andl, T., Maini, P.K.,
Li, C., Xiao, J., Hormi, K., Borok, Z., Minoo, P., 2002. Wnt5a participates in distal lung
Millar, S.E., Widelitz, R., Chuong, C.M., 2011. Self-organizing and stochastic
morphogenesis. Dev. Biol. 248, 68–81.
behaviors during the regeneration of hair stem cells. Science 332, 586–589.
Li, J., Ying, J., Fan, Y., Wu, L., Ying, Y., Chan, A.T., Srivastava, G., Tao, Q., 2010.
Qian, D., Jones, C., Rzadzinska, A., Mark, S., Zhang, X., Steel, K.P., Dai, X., Chen, P.,
WNT5A antagonizes WNT/beta-catenin signaling and is frequently silenced by
2007. Wnt5a functions in planar cell polarity regulation in mice. Dev. Biol. 306,
promoter CpG methylation in esophageal squamous cell carcinoma. Cancer
Biol. Ther. 10, 617–624.
Quarto, N., Behr, B., Longaker, M.T., 2010. Opposite spectrum of activity of
Li, S., Quarto, N., Longaker, M.T., 2007. Dura mater-derived FGF-2 mediates
canonical Wnt signaling in the osteogenic context of undifferentiated and
mitogenic signaling in calvarial osteoblasts. Am. J. Physiol. Cell Physiol. 293,
differentiated mesenchymal cells: implications for tissue engineering. Tissue
Eng. Part A 16, 3185–3197.
Little, R.D., Carulli, J.P., Del Mastro, R.G., Dupuis, J., Osborne, M., Folz, C., Manning,
Reddy, S., Andl, T., Bagasra, A., Lu, M.M., Epstein, D.J., Morrisey, E.E., Millar, S.E.,
S.P., Swain, P.M., Zhao, S.C., Eustace, B., Lappe, M.M., Spitzer, L., Zweier, S.,
2001. Characterization of Wnt gene expression in developing and postnatal
Braunschweiger, K., Benchekroun, Y., Hu, X., Adair, R., Chee, L., FitzGerald, M.G,
hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair
Tulig, C., Caruso, A., Tzellas, N., Bawa, A., Franklin, B., McGuire, S., Nogues, X.,
follicle morphogenesis. Mech. Dev. 107, 69–82.
Gong, G., Allen, K.M., Anisowicz, A., Morales, A.J., Lomedico, P.T., Recker, S.M.,
Roarty, K., Baxley, S.E., Crowley, M.R., Frost, A.R., Serra, R., 2009. Loss of TGF-beta or
Van Eerdewegh, P., Recker, R.R., Johnson, M.L., 2002. A mutation in the LDL
Wnt5a results in an increase in Wnt/beta-catenin activity and redirects
receptor-related protein 5 gene results in the autosomal dominant high-bone-
mammary tumour phenotype. Breast Cancer Res. 11, R19.
mass trait. Am. J. Hum. Genet. 70, 11–19.
Roman-Gomez, J., Jimenez-Velasco, A., Cordeu, L., Vilas-Zornoza, A., San Jose-
Lustig, B., Jerchow, B., Sachs, M., Weiler, S., Pietsch, T., Karsten, U., van de Wetering,
Eneriz, E., Garate, L., Castillejo, J.A., Martin, V., Prosper, F., Heiniger, A., Torres, A.,
M., Clevers, H., Schlag, P.M., Birchmeier, W., Behrens, J., 2002. Negative
Agirre, X., 2007. WNT5A, a putative tumour suppressor of lymphoid malignan-
feedback loop of Wnt signaling through upregulation of conductin/axin2 in
cies, is inactivated by aberrant methylation in acute lymphoblastic leukaemia.
colorectal and liver tumors. Mol. Cell. Biol. 22, 1184–1193.
Eur. J. Cancer 43, 2736–2746.
MacDonald, B.T., Tamai, K., He, X., 2009. Wnt/beta-catenin signaling: components,
Sato, A., Yamamoto, H., Sakane, H., Koyama, H., Kikuchi, A., 2010. Wnt5a regulates
mechanisms, and diseases. Dev. Cell 17, 9–26.
distinct signalling pathways by binding to Frizzled2. Embo. J. 29, 41–54.
Maeda, K., Kobayashi, Y., Udagawa, N., Uehara, S., Ishihara, A., Mizoguchi, T.,
Schlake, T., Sick, S., 2007. Canonical WNT signalling controls hair follicle spacing.
Kikuchi, Y., Takada, I., Kato, S., Kani, S., Nishita, M., Marumo, K., Martin, T.J.,
Cell Adh. Migr. 1, 149–151.
Minami, Y., Takahashi, N., 2012. Wnt5a-Ror2 signaling between osteoblast-
Shimizu, H., Julius, M.A., Giarre, M., Zheng, Z., Brown, A.M., Kitajewski, J., 1997.
lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat. Med.
Transformation by Wnt family proteins correlates with regulation of beta-
18, 405–412.
catenin. Cell Growth Differ. 8, 1349–1358.
Matsuda, T., Nomi, M., Ikeya, M., Kani, S., Oishi, I., Terashima, T., Takada, S.,
Sick, S., Reinker, S., Timmer, J., Schlake, T., 2006. WNT and DKK determine hair follicle
Minami, Y., 2001. Expression of the receptor tyrosine kinase genes, Ror1 and
spacing through a reaction-diffusion mechanism. Science 314, 1447–1450.
Ror2, during mouse development. Mech. Dev. 105, 153–156.
Spector, J.A., Greenwald, J.A., Warren, S.M., Bouletreau, P.J., Crisera, F.E., Mehrara,
McDonald, S.L., Silver, A., 2009. The opposing roles of Wnt-5a in cancer. Br. J.
B.J., Longaker, M.T., 2002. Co-culture of osteoblasts with immature dural cells
Cancer 101, 209–214.
causes an increased rate and degree of osteoblast differentiation. Plast.
McLeod, M.J., 1980. Differential staining of cartilage and bone in whole mouse
Reconstr. Surg. 109, 631–642; discussion 643–634.
fetuses by alcian blue and alizarin red S. Teratology 22, 299–301.
Summerhurst, K., Stark, M., Sharpe, J., Davidson, D., Murphy, P., 2008. 3D
Mii, Y., Taira, M., 2009. Secreted Frizzled-related proteins enhance the diffusion
representation of Wnt and Frizzled gene expression patterns in the mouse
of Wnt ligands and expand their signalling range. Development 136,
embryo at embryonic day 11.5 (Ts19). Gene Expr. Patterns 8, 331–348.
Tai, C.C., Sala, F.G., Ford, H.R., Wang, K.S., Li, C., Minoo, P., Grikscheit, T.C., Bellusci, S.,
Mikels, A., Minami, Y., Nusse, R., 2009. Ror2 receptor requires tyrosine kinase
2009. Wnt5a knock-out mouse as a new model of anorectal malformation. J. Surg.
activity to mediate Wnt5A signaling. J. Biol. Chem. 284, 30167–30176.
Res. 156, 278–282.
Mikels, A.J., Nusse, R., 2006. Purified Wnt5a protein activates or inhibits beta-
Takada, R., Hijikata, H., Kondoh, H., Takada, S., 2005. Analysis of combinatorial
catenin-TCF signaling depending on receptor context. PLoS Biol. 4, e115.
effects of Wnts and Frizzleds on beta-catenin/armadillo stabilization and
Minear, S., Leucht, P., Jiang, J., Liu, B., Zeng, A., Fuerer, C., Nusse, R., Helms, J.A.,
Dishevelled phosphorylation. Genes Cells 10, 919–928.
2010. Wnt proteins promote bone regeneration. Sci. Transl. Med. 2, 29ra30.
Takeuchi, S., Takeda, K., Oishi, I., Nomi, M., Ikeya, M., Itoh, K., Tamura, S., Ueda, T.,
Morvan, F., Boulukos, K., Clement-Lacroix, P., Roman Roman, S., Suc-Royer, I.,
Hatta, T., Otani, H., Terashima, T., Takada, S., Yamamura, H., Akira, S., Minami,
Vayssiere, B., Ammann, P., Martin, P., Pinho, S., Pognonec, P., Mollat, P., Niehrs,
Y., 2000. Mouse Ror2 receptor tyrosine kinase is required for the heart
C., Baron, R., Rawadi, G., 2006. Deletion of a single allele of the Dkk1 gene leads
development and limb formation. Genes Cells 5, 71–78.
to an increase in bone formation and bone mass. J. Bone Miner. Res. 21,
Tamai, K., Semenov, M., Kato, Y., Spokony, R., Liu, C., Katsuyama, Y., Hess, F., Saint-
Jeannet, J.P., He, X., 2000. LDL-receptor-related proteins in Wnt signal
Nagy, A., Gertsenstein, M., Vintersten, K., Behringer, R., 2007. Staining mouse
transduction. Nature 407, 530–535.
embryos for alkaline phosphatase activity. CSH Protoc 2007, pdb prot4776.
Tao, Q., Yokota, C., Puck, H., Kofron, M., Birsoy, B., Yan, D., Asashima, M., Wylie, C.C.,
Narhi, K., Jarvinen, E., Birchmeier, W., Taketo, M.M., Mikkola, M.L., Thesleff, I.,
Lin, X., Heasman, J., 2005. Maternal wnt11 activates the canonical wnt
2008. Sustained epithelial beta-catenin activity induces precocious hair
signaling pathway required for axis formation in Xenopus embryos. Cell 120,
development but disrupts hair follicle down-growth and hair shaft formation.
Development 135, 1019–1028.
Topol, L., Jiang, X., Choi, H., Garrett-Beal, L., Carolan, P.J., Yang, Y., 2003. Wnt-5a
Nemeth, M.J., Topol, L., Anderson, S.M., Yang, Y., Bodine, D.M., 2007. Wnt5a
inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-
inhibits canonical Wnt signaling in hematopoietic stem cells and enhances
catenin degradation. J. Cell Biol. 162, 899–908.
repopulation. Proc. Natl. Acad. Sci. USA 104, 15436–15441.
Torres, M.A., Yang-Snyder, J.A., Purcell, S.M., DeMarais, A.A., McGrew, L.L., Moon, R.T.,
Nguyen, H., Merrill, B.J., Polak, L., Nikolova, M., Rendl, M., Shaver, T.M., Pasolli, H.A.,
1996. Activities of the Wnt-1 class of secreted signaling factors are antagonized
Fuchs, E., 2009. Tcf3 and Tcf4 are essential for long-term homeostasis of skin
by the Wnt-5A class and by a dominant negative cadherin in early Xenopus
epithelia. Nat. Genet. 41, 1068–1075.
development. J. Cell Biol. 133, 1123–1137.
Nishita, M., Itsukushima, S., Nomachi, A., Endo, M., Wang, Z., Inaba, D., Qiao, S.,
Vivatbutsiri, P., Ichinose, S., Hytonen, M., Sainio, K., Eto, K., Iseki, S., 2008. Impaired
Takada, S., Kikuchi, A., Minami, Y., 2010. Ror2/Frizzled complex mediates
meningeal development in association with apical expansion of calvarial bone
Wnt5a-induced AP-1 activation by regulating Dishevelled polymerization.
osteogenesis in the Foxc1 mutant. J. Anat. 212, 603–611.
Mol. Cell. Biol. 30, 3610–3619.
Wallingford, J.B., Vogeli, K.M., Harland, R.M., 2001. Regulation of convergent
Nomachi, A., Nishita, M., Inaba, D., Enomoto, M., Hamasaki, M., Minami, Y., 2008.
extension in Xenopus by Wnt5a and Frizzled-8 is independent of the canonical
Receptor tyrosine kinase Ror2 mediates Wnt5a-induced polarized cell migra-
Wnt pathway. Int. J. Dev. Biol. 45, 225–227.
tion by activating c-Jun N-terminal kinase via actin-binding protein filamin A.
Wehrli, M., Dougan, S.T., Caldwell, K., O'Keefe, L., Schwartz, S., Vaizel-Ohayon, D.,
J. Biol. Chem. 283, 27973–27981.
Schejter, E., Tomlinson, A., DiNardo, S., 2000. arrow encodes an LDL-receptor-
O'Connell, M.P., Fiori, J.L., Xu, M., Carter, A.D., Frank, B.P., Camilli, T.C., French, A.D.,
related protein essential for Wingless signalling. Nature 407, 527–530.
Dissanayake, S.K., Indig, F.E., Bernier, M., Taub, D.D., Hewitt, S.M., Weeraratna,
Willert, K., Brown, J.D., Danenberg, E., Duncan, A.W., Weissman, I.L., Reya, T., Yates
A.T., 2010. The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A
3rd, J.R., Nusse, R., 2003. Wnt proteins are lipid-modified and can act as stem
signaling in metastatic melanoma. Oncogene 29, 34–44.
cell growth factors. Nature 423, 448–452.
Oishi, I., Suzuki, H., Onishi, N., Takada, R., Kani, S., Ohkawara, B., Koshida, I., Suzuki, K.,
Williams, B.O., Insogna, K.L., 2009. Where Wnts went: the exploding field of Lrp5
Yamada, G., Schwabe, G.C., Mundlos, S., Shibuya, H., Takada, S., Minami, Y., 2003.
and Lrp6 signaling in bone. J. Bone Miner. Res. 24, 171–178.
R. van Amerongen et al. / Developmental Biology 369 (2012) 101–114
Witte, F., Dokas, J., Neuendorf, F., Mundlos, S., Stricker, S., 2009. Comprehensive
Yamamoto, H., Oue, N., Sato, A., Hasegawa, Y., Matsubara, A., Yasui, W., Kikuchi, A.,
expression analysis of all Wnt genes and their major secreted antagonists
2010. Wnt5a signaling is involved in the aggressiveness of prostate cancer and
during mouse limb development and cartilage differentiation. Gene Expr.
expression of metalloproteinase. Oncogene 29, 2036–2046.
Patterns 9, 215–223.
Yamamoto, S., Nishimura, O., Misaki, K., Nishita, M., Minami, Y., Yonemura, S.,
Wong, G.T., Gavin, B.J., McMahon, A.P., 1994. Differential transformation of
Tarui, H., Sasaki, H., 2008. Cthrc1 selectively activates the planar cell polarity
mammary epithelial cells by Wnt genes. Mol. Cell. Biol. 14, 6278–6286.
pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev. Cell
Wu, C.H., Nusse, R., 2002. Ligand receptor interactions in the Wnt signaling
15, 23–36.
pathway in Drosophila. J. Biol. Chem. 277, 41762–41769.
Yang, Y., Topol, L., Lee, H., Wu, J., 2003. Wnt5a and Wnt5b exhibit distinct activities
Yamada, M., Udagawa, J., Matsumoto, A., Hashimoto, R., Hatta, T., Nishita, M.,
in coordinating chondrocyte proliferation and differentiation. Development
Minami, Y., Otani, H., 2010. Ror2 is required for midgut elongation during
130, 1003–1015.
mouse development. Dev. Dyn. 239, 941–953.
Ying, J., Li, H., Chen, Y.W., Srivastava, G., Gao, Z., Tao, Q., 2007. WNT5A is
Yamaguchi, T.P., Bradley, A., McMahon, A.P., Jones, S., 1999. A Wnt5a pathway
epigenetically silenced in hematologic malignancies and inhibits leukemia
underlies outgrowth of multiple structures in the vertebrate embryo. Devel-opment 126, 1211–1223.
cell growth as a tumor suppressor. Blood 110, 4130–4132.
Source: http://documents.epfl.ch/users/c/cf/cfuerer/www/papers/2012_van_Amerongen_et%20al.pdf
for All ELIQUIS Indications Please see Important Safety Information throughout and Full Prescribing Information, including Boxed WARNINGS, following page 15. ELIQUIS® (apixaban) INDICATIONS to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial for the treatment of deep vein thrombosis (DVT) for the treatment of pulmonary embolism (PE)
Prevalence of chronic obstructivepulmonary disease in rural women ofTamilnadu: implications for refiningdisease burden assessmentsattributable to household biomasscombustion Priscilla Johnson1Kalpana Balakrishnan2,Padmavathi Ramaswamy1, Santu Ghosh2,Muthukumar Sadhasivam3, Omprakash Abirami1,Bernard W. C. Sathiasekaran4, Kirk R. Smith5,Vijayalakshmi Thanasekaraan6 and Arcot S. Subhashini1







