Art. 1.136
European
Review for
Medical and
Pharmacological
Sciences
2008; 12(Suppl 1): 103-110
Novel treatments of GERD: focus on
the lower esophageal sphincter
Bioscience, AstraZeneca R&D, Mölndal (Sweden)
Abstract. – Up to 50% of patients with
gastroesophageal reflux disease (GERD) still
suffer from GERD symptoms despite proton
Gastroesophageal reflux disease (GERD) is a
pump inhibitor (PPI) therapy, indicating a need
common disorder that affects up to 20% of the
for new treatments. The lower esophageal
population worldwide1,2. The classic symptoms of
sphincter (LES) plays a crucial role in main-
taining the mechanical barrier necessary for
GERD, which include heartburn and acid regurgi-
prevention of gastric reflux. Transient LES re-
tation, are troublesome and have a substantial neg-
laxation (TLESR) is an important factor behind
ative impact on patients' health-related quality of
the occurrence of reflux, and preclinical stud-
life3,4. Consequently, effective treatment to provide
ies have identified a number of targets for
enduring control of GERD symptoms is necessary.
pharmacologic modification of TLESR. Howev-
Acid-suppressive therapy currently forms the
er, only γ
-aminobutyric acid (GABA) type B re-
ceptor (GABA ) agonists and metabotropic
mainstay of treatment for GERD and proton
glutamate receptor 5 (mGluR5) modulators
pump inhibitors (PPIs) are the treatment-of-
have shown positive proof of concept in the
choice in this regard5. However, GERD symp-
clinical setting. The mGluR5 negative allosteric
toms often persist despite PPI therapy in a con-
modulator ADX10059 improved symptoms in
siderable number of patients2,6. Recent survey da-
GERD patients, but was associated with cen-
ta, for example, indicate that approximately 50%
tral side effects such as dizziness. Baclofen, a
of patients diagnosed with GERD continued to
receptor agonist, reduces the inci-
dence of TLESR and improves GERD symp-
experience symptoms despite PPI treatment7, and
toms in both adult and pediatric GERD pa-
around one-quarter (22%) of PPI users report
tients. However, the utility of baclofen is simi-
taking additional over-the-counter (OTC) medi-
larly limited by poor tolerability and recent re-
cines to control their symptoms8.
search has focused on the development of
Possible reasons for a lack of effect with PPI
GABA receptor agonists with improved tolera-
therapy include inadequate dosing and/or poor
bility. XP19986, a prodrug of R-baclofen, re-
duced the number of reflux episodes in a dose-
compliance (possibly resulting from lack of effi-
ranging study and was similarly tolerated to
cacy), pharmacokinetic characteristics (such as
placebo. AZD3355 and AZD9343 are GABA re-
poor oral bioavailability or rapid metabolism due
ceptor agonists with limited central nervous
to genetic polymorphisms or cytochrome P450
system activity that have been shown in pre-
induction), and incorrect diagnosis6,9,10.
clinical studies to reduce the incidence of
Esophageal hypersensitivity and weakly acidic or
TLESR and decrease esophageal acid expo-
weakly alkaline reflux may also contribute to
sure; data from clinical studies of these agents
in GERD patients are awaited with interest.
symptoms, outlining a need for new treatments
Agents that target TLESR activity may there-
for GERD in addition to PPIs. The aim of this ar-
fore offer a promising new add-on treatment
ticle is to discuss emerging novel treatments for
for patients who suffer from GERD symptoms
GERD, with a focus on the lower esophageal
despite PPI therapy.
sphincter (LES) as the therapeutic target.
Key Words:
Reflux Mechanisms
Gastroesophageal reflux disease, Lower esophageal
The LES, along with the crural diaphragm
sphincter, Novel treatments.
(CD), forms a mechanical barrier that prevents
Corresponding Author: Anders Lehmann, Ph.D; e-mail:
[email protected]
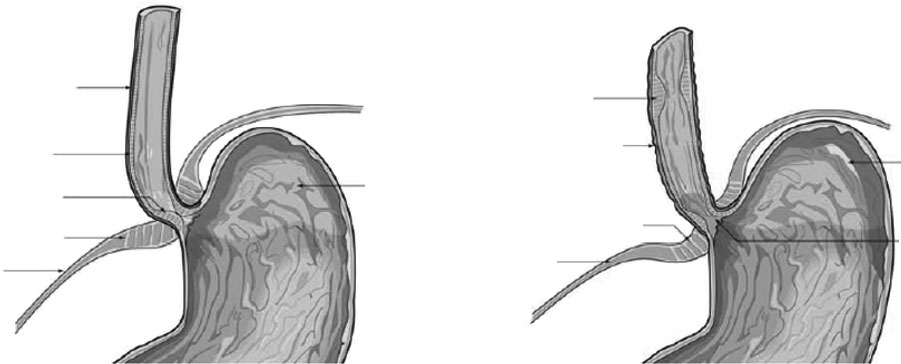
reflux at the gastroesophageal junction11. The
More recently, transient LES relaxation
LES is a 3-4 cm region of circular smooth mus-
(TLESR) has been identified as an important fac-
cle that is maintained at high resting basal pres-
tor in the pathophysiology of GERD (Figure 1)17.
sure (>10 mm Hg) via neural, myogenic and hor-
TLESR is characterized by rapid LES relaxation
monal input12. Transport of esophageal contents
in the absence of swallowing or esophageal peri-
into the stomach occurs with neurally mediated
stalsis18-20. Neural control of TLESR is via a va-
relaxation of the LES, and concurrent partial in-
go-vagal reflex initiated in response to gastric
hibition of CD activity; the CD usually contracts
distension by activation of mechanosensitive va-
with respiration. The retrograde flow of gastric
gal afferent neurons that terminate in the dorsal
contents is prevented by LES contraction11. Basal
vagal complex of the brainstem12,19. Efferent va-
LES pressure of at least 3 mm Hg is required to
gal nerve signals stimulate the release of nitric
prevent gastric reflux13.
oxide, pituitary adenylate cyclase activating pep-
Low tonic basal LES pressure was previously
tide, and vasoactive intestinal peptide from en-
a favored mechanism to explain the pathogenesis
teric neurons, resulting in smooth muscle relax-
of GERD14. However, this theory was largely
ation12,17. The efferent neural pathway in TLESR
based on flawed measurements of LES function,
is thought to be the same as that for swallow-in-
and it is now established that reflux episodes can
duced LES relaxation, only more intense and
occur in patients with normal LES pressure.
prolonged; TLESR are of greater magnitude and
Moreover, the poor clinical performance of
duration than the LES relaxation occurring dur-
agents supposedly acting on LES pressure, such
ing swallowing. Additionally, extragastric factors
as the 5-HT partial receptor agonists cisapride
may influence the rate and occurrence of
and tegaserod, and motilides such as ery-
TLESR. Increased TLESR occurs with mechani-
thromycin, provides further evidence that low
cal stimulation of the pharynx, hyperglycemia,
tonic LES pressure is not a major mechanism in
duodenal nutrient infusions, intracolonic lactose,
the pathogenesis of GERD10. For example, a ran-
and bronchoconstriction (in asthmatics). Con-
domized, double-blind study in healthy volun-
versely, TLESR is inhibited during sleep or anes-
teers found no significant differences between
thesia, indicating higher central nervous system
tegaserod and placebo in terms of the number of
(CNS) activity, and in response to supine posture,
acid and weakly acidic or weakly alkaline reflux
and cold stress12,17.
episodes, bolus transit time or distal esophageal
TLESR is a normal physiologic response to
contraction amplitude15. Similarly, Champion et
postprandial gastric distension and the rate of
al. reported that "standard" doses of ery-
TLESR increases after meals in healthy volun-
thromycin had no relevant effects on esophageal
teers21,22. Some studies have shown a similar rate
function or acid reflux parameters in patients
and incidence of TLESR among healthy individu-
with GERD16.
als and GERD patients14,18,23; however, other stud-
Focal contraction of
Longitudinal muscle
Sustained contraction
of longitudinal muscle
esophageal sphincter
Figure 1. Transient relaxation of the lower esophageal sphincter (TLESR) and associated motility events. Events in boldface
denote defining features of TLESR; the other changes are common but not obligatory components of a TLESR. Reproduced
with permission17.
Novel treatments of GERD: focus on the lower esophageal sphincter
ies indicate an increased prevalence of TLESR
hibitors, cholecystokinin receptor 1 (CCK ) an-
among those with GERD24-26. In particular,
tagonists, metabotropic glutamate receptor 5
GERD patients may experience more instances of
(mGluR5) antagonists, and γ-aminobutyric acid
TLESR in the supine position that are associated
(GABA) type B receptor (GABA ) agonists
with acid reflux25. Since acid secretion is un-
(Table I)17. Among these, only mGluR5 receptor
changed in GERD patients, possible reasons for
antagonists and GABA receptor agonists have
the higher likelihood of reflux during a TLESR in
shown positive results in proof-of-concept stud-
those with GERD include altered distribution of
ies in the clinical setting, as described in the fol-
gastric juices27 or a larger opening of the gastroe-
lowing section.
sophageal junction during relaxation28.
The role of TLESR versus other mechanical
Clinical Findings on Reflux Inhibitors
factors such as low or absent tonic basal LES
pressure, straining and swallowing varies between
ADX10059 is an orally available small mole-
endoscopy-positive and endoscopy-negative
cule that acts as a negative allosteric modulator
GERD patients17. Non-TLESR mechanisms may
of mGluR5 receptors. In a single-blind, placebo-
be equally or more important among patients with
controlled, proof-of-concept study in 24 GERD
endoscopy-positive GERD in the presence of hi-
patients, single doses of ADX10059 reduced acid
atal hernia, in which the position of the gastric re-
reflux (as measured by pHmetry) and improved
ceptors with the lowest threshold for initiation of
GERD symptoms31. Compared with placebo,
TLESR has shifted proximally29,30. However, data
ADX10059 250 mg three times daily was not on-
are controversial and further clarification of the
ly associated with significantly lower percent-
role of non-TLESR mechanisms is required.
ages of time with esophageal pH <4 over 24hours (and at night) but also fewer symptomatic
Pharmacologic Targets for
acid reflux episodes (Figure 2). The duration of
Reflux Inhibition
such episodes was also shorter with ADX10059;
Theoretically, pharmacologic modification of
this was a somewhat surprising finding as there is
both the frequency and quality (ie, degree and
no evidence or theoretical basis that TLESRs that
duration) of TLESR can be achieved anywhere
occur during partial pharmacological inhibition
along the pathway from gastric mechanorecep-
are not identical to those seen in control condi-
tors to the smooth muscle cells of the LES. How-
tions. The administration of ADX10059 was as-
ever, observations that drugs with TLESR-modi-
sociated with a very high rate of side effects, in-
fying activity do not affect swallow-induced LES
cluding dizziness and nausea, which indicates the
relaxation suggest that these agents act on the af-
need to discover new modulators of mGluR5 re-
ferent, rather than efferent, pathway. A site of ac-
ceptors in order to improve tolerability.
tion in the brain also seems possible in some cas-es. Targets for pharmacologic TLESR modifica-
tion therapy have emerged based on preclinical
Baclofen is a selective GABA receptor ago-
evidence and include nitric oxide synthase in-
nist with well established efficacy in the treat-
Table I. Pharmacologic targets of reflux inhibition.
Drugs used to verify target
Proposed site of action
WIN55, 212-2, ∆-9-tetrahydrocannibinol (agonists)
Central and/or peripheral
Devazepide, loxiglumide (antagonists)
Baclofen (agonist)
Central and/or peripheral
MPEP, MTEP (antagonists)
Central and/or peripheral
mGluR group III receptors
L-AP4, (S)-3,4-DCPG (agonists)
Central and/or peripheral
Muscarinic receptor(s)
Atropine (antagonist)
µ opioid receptor
Morphine (agonist)
L-NAME, L-NMMA (inhibitors)
CB = cannabinoid type 1; CCK = cholecystokinin type 1; GABA = γ-aminobutyric acid type B; mGluR5 = metabotropic
glutamate receptor type 5; NO = nitric oxide.
p = 0.0028
No. of symptomatic
acid reflux episodes
reflux episodes (min)
0 5 10 15 20
Figure 2. Effect of the mGluR5 negative allosteric modulator, ADX10059 (250 mg three times daily), on esophageal pH and
clinical symptoms (heartburn, acid regurgitation or burping) in patients with gastroesophageal reflux disease31.
ment of spasticity. Numerous studies, in adult
However, the utility of baclofen in the man-
healthy volunteers and patients with GERD (in-
agement of GERD is limited by the requirement
cluding children), indicate that this agent reduces
for frequent dosing, due to its short half-life (3-4
the number of TLESR and improves GERD
hours), and poor tolerability with a high rate of
symptoms (Table II)32-40. In adult healthy volun-
CNS side effects such as sedation and dizziness.
teers, for example, a single dose of oral baclofen
This is explained by the ability of baclofen to en-
significantly decreased the incidence of TLESR
ter the CNS and act on the high concentrations of
(by up to 64%) and increased basal LES pressure
GABA receptors in the brain17.
(by up to 40%)32,37. This effect was confirmed inadult GERD patients, in whom a single 40 mg
XP19986, AZD9343 and AZD3355
dose inhibited up to 40% of instances of
Efforts to overcome tolerability issues associ-
TLESR34,35. Baclofen also reduced the occur-
ated with baclofen have resulted in the develop-
rence of acid reflux episodes and decreased acid
ment of agents such as XP19986, an investiga-
exposure in adult GERD patients after adminis-
tional prodrug of R-baclofen. In a dose-ranging,
tration of a single 40 mg dose33-35,38 or multiple
placebo-controlled study in GERD patients,
doses of 10-20 mg three or four times daily36. In
XP19986 significantly reduced the number of re-
pediatric GERD patients, a single dose of ba-
flux episodes (P ≤ 0.05 vs. placebo) and showed
clofen 0.5 mg/kg provided 51% inhibition of
a similar rate of side effects to placebo42. Howev-
TLESR40 and administration of baclofen 0.7
er, it remains to be shown that XP19986 has an
mg/kg once daily for 1 week reduced acid reflux
acceptable tolerability profile during long-term
episodes by 39%39.
use. Indeed, while the main advantage of
Add-on treatment with baclofen appears to be
XP19986 is related to improved pharmacokinet-
effective in patients with GERD symptoms de-
ics, there is little reason to expect it to substan-
spite PPI therapy. Indeed, the addition of ba-
tially differ from baclofen with respect to CNS
clofen 20 mg three times daily reduced the cu-
side effects.
mulative severity score for 14 GERD symptoms
Research has also focused on the development
from 10.3 to 5.8 (P < 0.01) in patients with per-
of GABA receptor agonists with primarily pe-
sistent GERD symptoms while treated with
ripheral, rather than central, activity. Promising
omeprazole (Table II)41. This evidence suggests a
preclinical results have been obtained with two
potential role for add-on treatment with a
such agents, AZD9343 and AZD3355.
GABA receptor agonist in patients who experi-
AZD9343 showed low permeation into the CNS
ence GERD symptoms despite PPI therapy.
in rats and, in dogs, it dose-dependently inhibit-
Novel treatments of GERD: focus on the lower esophageal sphincter
urn (day and night) and
icant* reductions in nausea
gastric distension
related symptoms and 75%*
reduction in non-acid-related
All symptoms reduced, including
44%* reduction in o
78%* reduction in acid reflux-
Reduced emesis in 6/8 patients
% Inhibition
% Inhibition
Bile reflux: 48*;
icant; SD = single dose; tid = three times daily; od = once daily; qid = f
Increase in
LES outcomes
Inhibition
olunteers or GERD patients.
= not assessed; ns = not signifA
5 mg tid increasing
0.0001 vs placebo. N≤P
aarden et al.
Clinical studies of baclofen in adult healthy v
Lidums et al.
Ciccaglione et al.
GERD patients (including childr
Cange et al.
Zhang et al.
Ciccaglione et al.
Omari et al.
ed TLESR43. Similarly, AZD3355 has also been
shown to have low CNS activity in rats and
sophageal reflux symptoms and well-being in a
mice44. In dogs, the latter agent demonstrated
random sample of the general population of aSwedish community. Am J Gastroenterol 2006;
dose-dependent inhibition of TLESR44 and re-
duced esophageal acid exposure time45. The po-
tentially better tolerability of these agents versus
DEVAULT KR, CASTELL DO. Updated guidelines forthe diagnosis and treatment of gastroesophageal
baclofen makes them promising new therapies
reflux disease. Am J Gastroenterol 2005; 100:
for reflux inhibition in patients who experience
GERD symptoms despite PPI therapy. Results
6) FASS R, SHAPIRO M, DEKEL R, SEWELL J. Systematic
from clinical studies of these agents are there-
review: proton-pump inhibitor failure in gastro-oe-
fore awaited with interest.
sophageal reflux disease–where next? AlimentPharmacol Ther 2005; 22: 79-94.
7) JONES R, LIKER HR, DUCROTTÉ P. Relationship be-
tween symptoms, subjective well-being and med-
ication use in gastro-oesophageal reflux disease.
Int J Clin Pract 2007; 61: 1301-1307.
It is apparent that, despite the success of
8) JONES R, ARMSTRONG D, MALFERTHEINER P, DUCROTTÉ P.
PPIs, there is a significant unmet medical need
Does the treatment of gastroesophageal refluxdisease (GERD) meet patients' needs? A survey-
in GERD. The role of TLESR as an important
based study. Curr Med Res Opin 2006; 22: 657-
factor behind reflux has prompted the discov-
ery of several targets for TLESR inhibition, but
9) MAINIE I, TUTUIAN R, SHAY S, VELA M, ZHANG X, SIFRIM
most have a low utility for pharmacologic ther-
D, CASTELL DO. Acid and non-acid reflux in pa-
apy of GERD. To date, only GABA agonists
tients with persistent symptoms despite acid sup-
and mGluR5 antagonists have shown positive
pressive therapy: a multicentre study using com-
proof of concept in the clinical setting, and fur-
bined ambulatory impedance-pH monitoring. Gut
ther studies are clearly required to investigate
2006; 55: 1398-1402.
the promising utility of such agents for patients
10) CORON E, HATLEBAKK JG, GALMICHE J-P. Medical ther-
who suffer from GERD symptoms despite PPI
apy of gastroesophageal reflux disease. CurrOpin Gastroenterol 2007; 23: 434-439.
11) TYTGAT GNJ, EDITOR. Gastroesophageal reflux and
gastric stasis. Chester: Adis International Ltd, 1991.
IPAN MJ, REIDENBERG JS, LAITMAN JT. Anatomy of re-
flux: a growing health problem affecting structuresof the head and neck. Anat Rec B New Anat
The author thanks Vicki Oldfield, from Wolters Kluw-
2006; 289: 261-270.
er Health, who provided medical writing support fund-ed by AstraZeneca.
13) HUNT RH, EDITOR. Proton pump inhibitors and acid-
related disorders. Osaka: Adis International Ltd,1994.
14) DENT J, HOLLOWAY RH, TOOULI J, DODDS WJ. Mecha-
nisms of lower oesophageal sphincter incompe-tence in patients with symptomatic gastrooe-
sophageal reflux. Gut 1988; 29: 1020-1028.
15) TUTUIAN R, MAINIE I, ALLAN R, HARGREAVES K, AGRAWAL
1) VAKIL N, VAN ZANTEN SV, KAHRILAS P, DENT J, JONES R,
A, FREEMAN J, GALE J, CASTELL DO. Effects of a 5-HT4
ON BEHALF OF THE GLOBAL CONSENSUS GROUP. The
receptor agonist on oesophageal function and
Montreal definition and classification of gastroe-
gastro-oesophageal reflux: studies using com-
sophageal reflux disease: a global evidence-
bined impedance-manometry and combined im-
based consensus. Am J Gastroenterol 2006; 101:
pedance-pH. Aliment Pharmacol Ther 2006; 24:
2) DENT J, EL-SERAG HB, WALLANDER MA, JOHANSSON S.
16) CHAMPION G, RICHTER JE, SINGH S, SCHAN C, NELLANS
Epidemiology of gastro-oesophageal reflux dis-
H. Effects of oral erythromycin on esophageal pH
ease: a systematic review. Gut 2005; 54: 710-
and pressure profiles in patients with gastroe-
sophageal reflux disease. Dig Dis Sci 1994; 39:129-137.
3) WIKLUND I. Review of the quality of life and burden
of illness in gastroesophageal reflux disease. Dig
17) LEHMANN A. Inhibitors of transient lower
Dis 2004; 22: 108-114.
esophageal sphincter relaxations ("reflux in-
Novel treatments of GERD: focus on the lower esophageal sphincter
hibitors") in the future treatment of GERD. Gas-
30) VAN HERWAARDEN MA, KATZKA DA, SMOUT AJ, SAMSOM
troenterol Hepatol Ann Rev 2006; 1: 109-117.
M, GIDEON M, CASTELL DO. Effect of different re-cumbent positions on postprandial gastroe-
18) DENT J, DODDS WJ, FRIEDMAN RH, SEKIGUCHI T,
sophageal reflux in normal subjects. Am J Gas-
HOGAN WJ, ARNDORFER RC, PETRIE DJ. Mechanism
troenterol 2000; 95: 2731-2736.
of gastroesophageal reflux in recumbent asymp-tomatic human subjects. J Clin Invest 1980; 65:
31) KEYWOOD CGA, WAKEFIELD M. A single-blind, proof-
of-concept study of the effect of the mGluR nega-tive allosteric modulator, ADX10059, on 24-hour
19) HOLLOWAY RH, DENT J. Pathophysiology of gas-
esophageal pH and clinical symptoms, in patients
with gastroesophageal reflux disease (GERD)
sphincter dysfunction in gastroesophageal re-
[abstract]. Gut 2007; 56 Suppl. III: A6.
flux disease. Gastroenterol Clin North Am1990; 19: 517-535.
32) LIDUMS I, LEHMANN A, CHECKLIN H, DENT J, HOLLOWAY
RH. Control of transient lower esophageal sphinc-
20) MITTAL RK, FISHER MJ. Electrical and mechanical in-
ter relaxations and reflux by the GABA agonist
hibition of the crural diaphragm during transient
baclofen in normal subjects. Gastroenterology
relaxation of the lower esophageal sphincter.
2000; 118: 7-13.
Gastroenterology 1990; 99: 1265-1268.
33) CANGE L, JOHNSSON E, RYDHOLM H, LEHMANN A,
21) FREIDEN N, REN J, SLUSS J. The effect of a large
FINIZIA C, LUNDELL L, RUTH M. Baclofen-mediated
meal on the frequency and quality of transient
gastro-oesophageal acid reflux control in patients
LES relaxations (TLESR) [abstract]. Gastrointest
with established reflux disease. Aliment Pharma-
Mot 1989; 1: 62.
col Ther 2002; 16: 869-873.
22) HOLLOWAY RH, KOYCAN P, DENT J. Meals provoke
34) VAN HERWAARDEN MA, SAMSOM M, RYDHOLM H,
gastroesophageal reflux by increasing the rate
SMOUT AJ. The effect of baclofen on gastro-oe-
of transient lower esophageal sphincter relax-
sophageal reflux, lower oesophageal sphincter
ations [abstract]. Gastroenterology 1989; 96:
function and reflux symptoms in patients with re-
flux disease. Aliment Pharmacol Ther 2002; 16:
23) IWAKIRI K, HAYASHI Y, KOTOYORI M, TANAKA Y, KAWAKAMI
A, SAKAMOTO C, HOLLOWAY RH. Transient lower
35) ZHANG Q, LEHMANN A, RIGDA R, DENT J, HOL-
esophageal sphincter relaxations (TLESRs) are
the major mechanism of gastroesophageal reflux
sophageal sphincter relaxations and reflux by
but are not the cause of reflux disease. Dig Dis
the GABA agonist baclofen in patients with
Sci 2005; 50: 1072-1077.
gastro-oesophageal reflux disease. Gut 2002;
24) GROSSI L, CICCAGLIONE AF, TRAVAGLINI N, MARZIO L.
Transient lower esophageal sphincter relaxations
36) CICCAGLIONE AF, MARZIO L. Effect of acute and
and gastroesophageal reflux episodes in healthy
chronic administration of the GABA agonist ba-
subjects and GERD patients during 24 hours. Dig
clofen on 24 hour pH metry and symptoms in
Dis Sci 2001; 46: 815-821.
control subjects and in patients with gastro-oe-
25) SIFRIM D, HOLLOWAY R. Transient lower esophageal
sophageal reflux disease. Gut 2003; 52: 464-570.
sphincter relaxations: how many or how harmful?
37) LEE K-J, VOS R, JANSSENS J, TACK J. Differential ef-
Am J Gastroenterol 2001; 96: 2529-2532.
fects of baclofen on lower oesophageal sphincter
26) TRUDGILL NJ, RILEY SA. Transient lower esophageal
pressure and proximal gastric motility in humans.
sphincter relaxations are no more frequent in pa-
Aliment Pharmacol Ther 2003; 18: 199-207.
tients with gastroesophageal reflux disease than
38) VELA MF, TUTUIAN R, KATZ PO, CASTELL DO. Baclofen
in asymptomatic volunteers. Am J Gastroenterol
decreases acid and non-acid post-prandial gas-
2001; 96: 2569-2574.
tro-oesophageal reflux measured by combined
27) FLETCHER J, WIRZ A, YOUNG J, VALLANCE R, MCCOLL
multichannel intraluminal impedance and pH. Ali-
KE. Unbuffered highly acidic gastric juice exists at
ment Pharmacol Ther 2003; 17: 243-251.
the gastroesophageal junction after a meal. Gas-
39) KAWAI M, KAWAHARA H, HIRAYAMA S, YOSHIMURA N,
troenterology 2001; 121: 775-783.
Ida S. Effect of baclofen on emesis and 24-
28) PANDOLFINO JE, SHI G, TRUEWORTHY B, KAHRILAS PJ.
hour esophageal pH in neurologically impaired
Esophagogastric junction opening during relax-
children with gastroesophageal reflux disease.
ation distinguishes nonhernia reflux patients, her-
J Pediatr Gastroenterol Nutr 2004; 38: 317-
nia patients, and normal subjects. Gastroenterol-
ogy 2003; 125: 1018-1024.
40) OMARI TI, BENNINGA MA, SANSOM L, BUTLER RN, DENT
29) KAHRILAS PJ, SHI G, MANKA M, JOEHL RJ. Increased
J, DAVIDSON GP. Effect of baclofen on esopha-
frequency of transient lower esophageal sphinc-
gogastric motility and gastroesophageal reflux in
ter relaxation induced by gastric distention in re-
children with gastroesophageal reflux disease: a
flux patients with hiatal hernia. Gastroenterology
randomized controlled trial. J Pediatr 2006; 149:
2000; 118: 688-695.
41) KOEK GH, SIFRIM D, LERUT T, JANSSENS J, TACK J. Ef-
ceptor agonist and reflux inhibitor [abstract].
fect of the GABA agonist baclofen in patients
Gastroenterology 2008; 134(Suppl 1): A-131.
with symptoms and duodeno-gastro-oesophageal
reflux refractory to proton pump inhibitors. Gut
EHMANN A, BLACKSHAW LA, ELEBRING T, JENSEN J,
2003; 52: 1397-1402.
ATTSSON JP, NILSSON KA, SARANSAARI P, VON UNGE
S. The new reflux inhibitor AZD3355 has a low
42) CASTELL DO, GERSON LB, HIROTA WK, REILLEY SF, HI-
propensity for inducing central side effects due
LA A, AGRAWAL A, LAL R, HUFF FJ. XP19986 de-
to its affinity for the GABA carrier [abstract].
creases reflux and is well tolerated in GERD
Gastroenterology 2008; 134(Suppl 1): A-715.
patients [abstract]. Annual Scientific Meeting of
the American College of Gastroenterology
RÄNDÉN L, CARLSSON A, JENSEN J, LEHMANN A. The
novel GABA receptor agonist AZD3355 inhibits
2006; Las Vegas, NV.
acid reflux and reduces esophageal acid expo-
43) LEHMANN A, ELEBRING T, JENSEN J, MATTSSON JP, NILSSON
sure as measured by 24h pHmetry in dogs [ab-
KA, SARANSAARI P, VON UNGE S. In vivo and in vitro
stract]. Gastroenterology 2008; 134(Suppl 1): A-
characteristics of AZD9343, a novel GABA re-
Source: http://www.europeanreview.org/wp/wp-content/uploads/521.pdf
NOMBRE DEL MEDICAMENTO: Arava 10 mg, Arava 20 mg, Arava 100 mg comprimidos recubiertos con película COMPOSICIÓN CUALITATIVA Y CUANTITATIVA: Arava 10 mg. Cada comprimido contiene 10 mg de leflunomida. Excipiente(s) con efecto conocido: cada comprimido contiene 78 mg de lactosa monohidrato Arava 20 mg. Cada comprimido contiene 20 mg de leflunomida. Excipiente(s) con efecto conocido: cada comprimido contiene 72 mg de lactosa monohidrato. Arava 100 mg. Cada comprimido contiene 100 mg de leflunomida. Excipiente(s) con efecto conocido: cada comprimido contiene 138,42 mg de lactosa monohidrato. FORMA FARMACÉUTICA: Comprimido recubierto con película. Arava 10 mg: comprimido recubierto con película blanco o blanquecino, redondo, con la inscripción ZBN en una cara. Arava 20 mg: comprimido recubierto con película amarillento a ocre, triangulares, con la inscripción ZBO en una cara. Arava 100 mg: comprimido recubierto con película blanco o blanquecino, redondo, con la inscripción ZBP en una cara. DATOS CLÍNICOS. Indicaciones terapéuticas: La leflunomida está indicada para el tratamiento de pacientes adultos con: artritis reumatoide activa como un "fármaco antirreumático modificador de la enfermedad" (FARME), artritis psoriásica activa. El tratamiento reciente o concomitante con FARMEs hepatotóxicos o hematotóxicos (por ejemplo, metotrexato) puede producir un aumento del riesgo de aparición de reacciones adversas graves; por tanto, en estos casos, el inicio del tratamiento con leflunomida debe considerarse en función del balance beneficio/riesgo. Más aún, el sustituir la leflunomida por otro FARME sin realizar el procedimiento de lavado, puede incrementar el riesgo de aparición de reacciones adversas graves incluso durante un largo período de tiempo después del cambio. Posología y forma de administración: El tratamiento se debe iniciar y supervisar por especialistas con experiencia en el tratamiento de artritis reumatoide y artritis psoriásica. Los niveles de alanina transaminasa (ALT) o transaminasa piruvato glutamato sérico (SGPT) y un recuento hemático completo, incluyendo un recuento diferencial de leucocitos y un recuento de plaquetas, deben determinarse simultáneamente, y con la misma frecuencia en las siguientes situaciones: antes de iniciar el tratamiento con leflunomida, cada dos semanas durante los primeros seis meses de tratamiento, y posteriormente, cada ocho semanas. Posología: En artritis reumatoide: el tratamiento con leflunomida se inicia normalmente con una dosis de carga de 100 mg una vez al día durante 3 días. La omisión de la dosis de carga puede disminuir el riesgo de reacciones adversas. La dosis de mantenimiento recomendada es de 10 mg a 20 mg de leflunomida una vez al día dependiendo de la gravedad (actividad) de la enfermedad. En artritis psoriásica: el tratamiento con leflunomida se inicia con una dosis de carga de 100 mg una vez al día durante 3 días. La dosis de mantenimiento recomendada es de 20 mg de leflunomida una vez al día. El efecto terapéutico normalmente empieza después de 4 ó 6 semanas y puede mejorar posteriormente hasta los 4 ó 6 meses. No hay un ajuste de dosis recomendable en pacientes con insuficiencia renal leve. No se requiere realizar un ajuste de la dosis en los pacientes con edad superior a 65 años. Población pediátrica: No se recomienda la utilización de Arava en pacientes menores de 18 años, ya que no se ha establecido la eficacia y la seguridad en la artritis reumatoide juvenil (ARJ). Forma de administración: Los comprimidos de Arava deben ingerirse enteros con suficiente líquido. La ingesta de alimentos no modifica la absorción de la leflunomida. Contraindicaciones: Hipersensibilidad al principio activo (especialmente con historial previo de síndrome de Stevens-Johnson, necrólisis epidérmica tóxica, eritema multiforme) o a alguno de los excipientes. Pacientes con insuficiencia hepática. Pacientes con estados de inmunodeficiencia grave, por ejemplo, SIDA. Pacientes con afectación significativa de la función de la médula ósea o con anemia, leucopenia, neutropenia o trombocitopenia importante debida a causas distintas de la artritis reumatoide o psoriásica. Pacientes con infecciones graves. Pacientes con insuficiencia renal de moderada a grave, debido a que la experiencia clínica de la que se dispone en este grupo de pacientes es insuficiente. Pacientes con hipoproteinemia grave, por ejemplo en el síndrome nefrótico. Mujeres embarazadas o mujeres en edad fértil que no utilicen un método anticonceptivo eficaz durante el tratamiento con leflunomida y después de finalizar el mismo mientras los niveles plasmáticos del metabolito activo estén por encima de 0,02 mg/l. Antes de iniciar el tratamiento con leflunomida, debe descartarse el embarazo. Mujeres que se encuentren en periodo de lactancia. Advertencias y precauciones especiales de empleo: No se aconseja la administración conjunta con FARMEs hepatotóxicos o hematotóxicos (por ejemplo metotrexato). El metabolito activo de leflunomida, A771726, tiene una vida media larga, generalmente de 1 a 4 semanas. Pueden producirse efectos adversos graves (por ejemplo: hepatotoxicidad, hematotoxicidad o reacciones alérgicas, ver más abajo), aunque se haya interrumpido el tratamiento con leflunomida. Por tanto, cuando aparezcan estos efectos adversos o si por cualquier otro motivo se
Cell Motility and the Cytoskeleton 60:24 –34 (2005) Effects of Substrate Stiffness on Cell Morphology, Cytoskeletal Structure, Tony Yeung,1 Penelope C. Georges,1 Lisa A. Flanagan,2 Beatrice Marg,2 Miguelina Ortiz,1 Makoto Funaki,1 Nastaran Zahir,1 Wenyu Ming,1 Valerie Weaver,1 and Paul A. Janmey1,2* 1Institute for Medicine and Engineering, University of Pennsylvania, Philadelphia
