Cannabinoid cb1 receptor in the modulation of stress coping behavior in mice: the role of serotonin and different forebrain neuronal subpopulations


Contents lists available at
Cannabinoid CB1 receptor in the modulation of stress coping behavior in mice:The role of serotonin and different forebrain neuronal subpopulations
M. Häring , M. Grieb , K. Monory , B. Lutz F.A. Moreira
a Institute of Physiological Chemistry, University Medical Center of the Johannes Gutenberg University Mainz, Duesbergweg 6, 55128 Mainz, Germanyb Department of Pharmacology, Institute of Biological Sciences, Universidade Federal de Minas Gerais, Av. Antônio Carlos 6627, Belo Horizonte, MG 31270-901, Brazil
The endocannabinoid system (ECS) may either enhance or inhibit responses to aversive stimuli, possibly
Received 7 July 2011
caused by its modulatory activity on diverse neurotransmitters. The aim of this work was to investigate
Received in revised form
the involvement of serotonin (5-HT) and catecholamines, as well as the role of glutamatergic and
GABAergic cannabinoid type 1 (CB
Accepted 2 September 2012
1) receptor, in responses to the antidepressant-like doses of the CB1
receptor agonist D9-tetrahydrocannabinol (THC) and the antagonist rimonabant in the forced swim test(FST). Mice received acute injections of low doses of THC (0.1 or 0.5 mg/kg) or high dose of rimonabant (3
or 10 mg/kg) after treatment with the 5-HT synthesis inhibitor pCPA (100 mg/kg, 4 days), the 5-HT
receptor antagonist WAY100635 (1 mg/kg, acute) or the non-selective blocker of catecholamine
synthesis, AMPT (20 mg/kg, acute). THC and rimonabant were also tested in mutant mice lacking CB1
receptor in specific forebrain neuronal subpopulations.
Both THC and rimonabant induced antidepressant-like effects, quantified as immobility in the FST.
However, only THC effects were reversed by pCPA or WAY100635. In contrast, only AMPT could attenuatethe rimonabant effect. We also found decreased immobility in mice lacking the CB1 receptor in gluta-matergic cortical neurons, but not in forebrain GABAergic neurons, as compared with wild-type controls.
The effect of THC persisted in mutant mice with CB1 receptor inactivation in GABAergic neurons, whereasrimonabant effects were alleviated in these mutants. Thus, employing both pharmacological and genetictools, we could show that the ECS regulates stress responses by influencing GABAergic, glutamatergicand monoaminergic transmission. The antidepressant-like action of THC depends on serotonergicneurotransmission, whereas rimonabant effects are mediated by CB1 receptor on GABAergic neurons andby catecholamine signaling.
Ó 2012 Elsevier Ltd. All rights reserved.
terminals. As the CB1 receptor is present on both GABAergic andglutamatergic terminals (the endocannabinoid
The herb Cannabis sativa induces a diversity of emotional
system (ECS) is able to control the activation of both inhibitory and
responses ranging from anxiolytic and relaxing effects to the
excitatory neurotransmission. Therefore, depending on its specific
induction of acute panic attacks (). Similarly,
spatio-temporal activation within neuronal circuits, this system can
divergent emotional responses have been observed in both humans
act as a major "bi-directional" neuromodulator (for a review, see
and rodents after the administration of D9-tetrahydrocannabinol
(THC), the main psychoactive compound from this plant
This "dual" role of endocannabinoid signaling has likely been
the reason for a number of contradictory results in rodent models of
). Postsynaptically produced endocannabinoids, the
anxiety and depression (
endogenous counterparts of THC, including anandamide and 2-
). This is supported by recent studies, using conditional
arachidonoyl glycerol, function as retrograde modulators of
mutant mice lacking the CB1 receptor either on GABAergic or glu-
synaptic activity, which, through activation of presynaptic CB1
tamatergic neurons, supporting the notion that the two pop-
receptor, restrain neurotransmitter release from presynaptic
ulations might be important for the biphasic effect ; ). Another explanationmight be a variation in the initial baseline stress level of an animal,which depends on a multitude of genetic, environmental and
* Corresponding author. Tel.: þ49 (0)6131 39 25912; fax: þ49 (0)6131 39 23536.
E-mail address: (B. Lutz).
experimental factors. This baseline might alter the activity of the
0028-3908/$ e see front matter Ó 2012 Elsevier Ltd. All rights reserved.
M. Häring et al. / Neuropharmacology 65 (2013) 83e89
Altogether, accumulating evidence supports
the involvement of CB1 receptor signaling in the regulation ofmonoaminergic neurotransmission, which could, in turn, mediate
a-methyl para-tyrosine
endocannabinoid effects in the FST.
receptor, cannabinoid type 1 receptor
approaches, we aimed at investigating the contradictory findings
endocannabinoid system
of ECS modulation in emotion and how serotonergic and cate-
cholamine transmission might be involved in. To address this
g-amino buryric acid
issue, we studied the antidepressant-like effects of the CB1
receptor agonist THC, and the antagonist/inverse agonist rimo-
nabant in combination with drugs disrupting serotonergic (with
parachlorophenylalanine, pCPA; ) and
catecholamine (with a-methyl-para-tyrosine, AMPT;
prefrontal cortex
) transmission. Both drugs were also shown to block
the antidepressant-like effects of 5-HT or dopamine reuptake
inhibitors Furthermore, we included mice
3-carboxamide (also called SR141716)
lacking the CB1 receptor in specific neuronal subpopulations,
namely in GABAergic forebrain neurons (GABA-CB1 mouse line)
and glutamatergic cortical neurons (Glu-CB1 mouse line) to
investigate the role of these cells in THC and rimonabant effects.
ECS, thus, resulting in the same behavioral effect induced by
This study was performed on adult (3e5 months old) male C57BL/6N mice, as
signaling blockade or enhancement) (;
well as mutants and littermate controls in a predominant C57BL/6N background.
). Such a dose dependent cannabinoid-induced biphasic
Animals were housed in a temperature- and humidity-controlled room with a 12 h
effect on the behavioral performance can also be seen in the forced
lightedark cycle (lights on at 1 am) and had access to food and water ad libitum. The
swim test (FST). One of the most widely used behavioral paradigms
experimental protocols were carried out in accordance with the EuropeanCommunities Council Directive of 24 November 1986 (86/609/EEC) and approved by
to detect antidepressant-like activities of drugs (
the Ethical Committee on animal care and use of Rhineland-Palatinate, Germany.
). It is based on the observation that
Generation, breeding and genotyping of the mutant lines were performed according
rodents, when exposed to an inescapable situation (immersion in
to previous publications: CBflox/flox;Nex-cre
mice (referred to as Glu-CB1
a beaker filled with water), will cease over several minutes to
mice (referred to as GABA-CB1
engage in escape-oriented movements and adopt an immobile
Animals were in a predominant C57BL/6Nbackground (at least 7 backcrosses) and were group housed (3e5 animals per cage)
passive "floating" posture. Acquired immobility is often interpreted
until one week before behavioral testing, when they were single housed to avoid
as "behavioral despair", mimicking psychomotor impairments
behavioral differences between dominant and subordinate animals. All experiments
experienced by depressed patients ().
were performed during the second half of the light phase.
A reduction of immobility time in the FST is especially observedafter treatment with a broad range of antidepressants, which
2.2. Drug treatments
increase serotonergic and/or noradrenergic neurotransmission(). In this model, CB
Injections were given intraperitoneally (i.p.) in a volume of 10 ml/kg body
1 receptor activa-
weight. Stock solution of rimonabant (SR141716; NIMH Chemical Synthesis and
tion can lead to a decrease or increase of immobility
Drug Supply Program) was prepared by solving the lyophilized drug in DMSO (Sigma
; ). Blocking the
Aldrich). Working solution contained the respective rimonabant concentration
CB1 receptor with SR141716 (rimonabant) can also induce either an
dissolved in a 0.9 w/v % NaCl solution containing 2 vol % DMSO and 2.5 vol % pol-
antidepressant-like effect ()
yoxyethylenesorbitan monooleate (Tween-80; Sigma Aldrich). THC (THC Pharm,
or increase immobility behavior, depending on the dose
Frankfurt, Germany) was warmed and dissolved in 100% ethanol. Working solutioncontained the respective THC concentration dissolved in a 0.9 w/v % NaCl solution
containing 0.5 vol % ethanol and 2.5 vol % Tween-80. WAY100635 (SigmaeAldrich)
Thus, before we consider the ECS as a valid strategy for devel-
was diluted in 0.9 w/v % NaCl solution. pCPA (SigmaeAldrich) was suspended in
oping new drugs for the treatment of mood disorders
a 0.9 w/v % NaCl solution containing 2.5 vol % Tween-80. AMPT (SigmaeAldrich) was
suspended in a 0.9 w/v % NaCl solution containing 10 vol % Tween-80. All vehiclecontrols contained the respective concentration of Tween-80, DMSO and/or ethanol
understand the reasons for these complex responses
dissolved in a 0.9 w/v % NaCl solution.
Surprisingly, even though marihuana has been used for
Single drug injections were given 30 min prior to the experiment. If the mice
recreational purposes since centuries, studies on antidepressive
were exposed to two different drugs, first drug was applied 45 min and the second
potentials of its major component, THC, are still sparse.
drug 30 min before the experiment. pCPA was injected every 24 h for 4 days with the
One possible mechanism through which cannabinoids interfere
last injection on the day of the FST. For each experiment, vehicle treatment wasgiven as control in the same injection schedule as the respective drug treated mice.
with stress-related responses might be through monoaminergic
The doses were selected based on previous works, WAY100635 (
mechanisms. Several studies connected the ECS with serotonergic
AMPT pCPA ); SR141716
transmission. Indeed, the CB1 receptor antagonist rimonabant was
shown to increase the efflux of 5-HT and noradrenaline in the ratprefrontal cortex (). CB1 receptor is expressed in
mouse serotonergic raphe neurons (and innoradrenergic nerve terminals in the rat frontal cortex (
To evaluate potential effects by the drugs on locomotor activity we performed an
open field test. The open field was an H 40 cm � W 40 cm � L 40 cm box illuminated
). In addition, CB1 receptor signaling influences the
at 200 lux, in which the animal was placed for 5 min to allowed free exploration. The
firing rate of serotonergic and noradrenergic neurons in the rat
animal movement was recorded, and the distance moved was scored by the SMART
raphe nuclei and locus coeruleus, respectively (
program (PanLab, Spain).
M. Häring et al. / Neuropharmacology 65 (2013) 83e89
2.4. Forced swim test (FST)
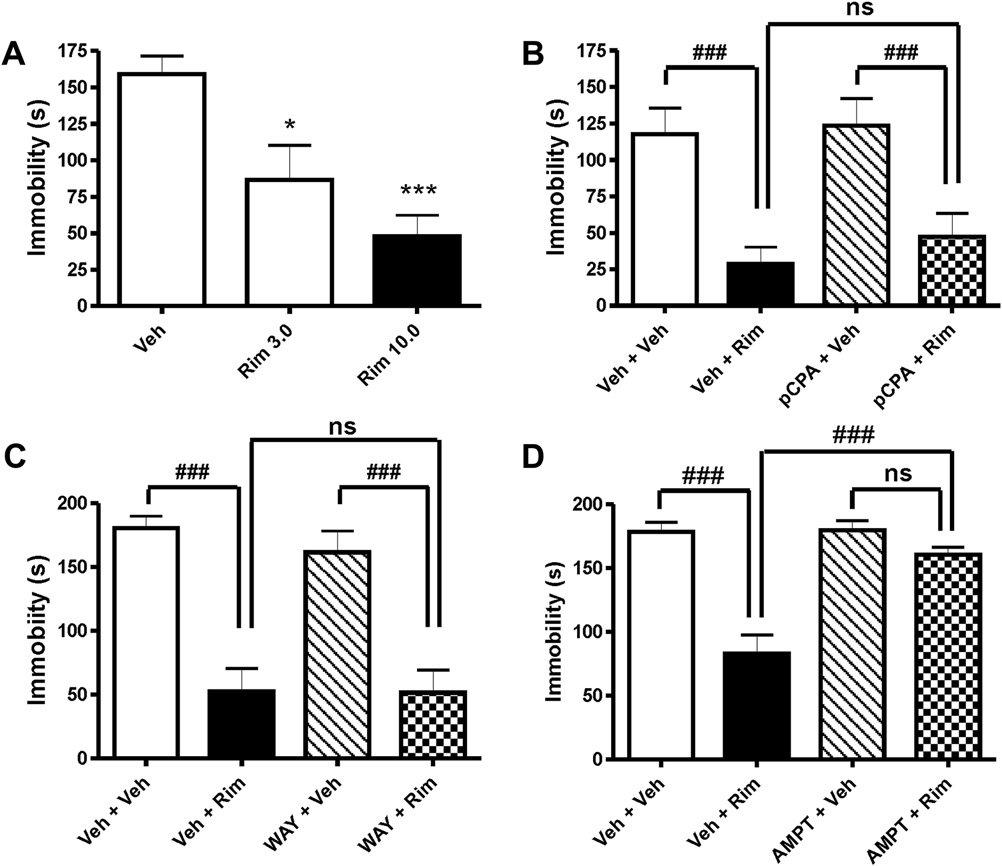
1 mg/kg (Pretreatment factor: F1,35 ¼ 3.41; p > 0.05; THC factor:F1,35 ¼ 3.07; p > 0.05; Interaction[Pretreatment � THC]: F1,35 ¼ 3.79;
The paradigm was performed in a round glass beaker (18 cm in diameter and
p ¼ 0.0596; Column comparison
30 cm in height) filled with tap water at 25 � 0.5 �C. The water level was
approximately 20 cm to prevent the animal from touching the bottom of the glass.
NewmaneKeuls Multiple Comparison post-test q ¼ 3.846; p < 0.05;
The mouse was also unable to climb out off the beaker. The animal was carefully
C). In addition, the effect of THC (0.1 mg/kg) was
lowered into the water and recorded on DVD for 6 min. The first 2 min were not
prevented by a pre-treatment with a low dose (0.5 mg/kg) of
evaluated; however, floating behavior was scored for the following 4 min by an
rimonabant (Pretreatment factor: F
experimenter blind to genotype and treatment. Floating was defined by immo-
1,90 ¼ 5.619; p < 0.05; THC
bility of the animal and minimal movements to keep the body's balance. The
factor; F1,90 ¼ 1.638; p > 0.05; Interaction[Pretreatment � THC]:
functionality of the paradigm was successfully tested by acute i.p. injection of the
F1,90 ¼ 4.669; p < 0.05; see Applying rimonabant alone in
antidepressant drug imipramine (30 mg/kg), which resulted in a significant
higher dose (3 and 10 mg/kg) also resulted in a decreased immo-
decrease in floating behavior as compared to saline treated animals (t16 ¼ 10.45;
bility (F2,19 ¼ 10.74; p < 0.001; A). Co-administration of pCPA
p < 0.001; n ¼ 9).
100 mg/kg (Pretreatment factor: F1,36 ¼ 0.57; p > 0.05; rimonabant
2.5. Statistical analysis
factor: F1,36 ¼ 25.97; p < 0.0001; Interaction: F1,36 ¼ 0.15; p > 0.05;B) and WAY100635 (Pretreatment factor: F1,33 ¼ 0.38;
Data are presented as mean � standard error of the mean (SEM). All behavioral
p > 0.05; rimonabant factor: F1,33 ¼ 52.32; p < 0.0001;
endpoints of the open field and FST were analyzed using Student t-test, one-way
Interaction[Pretreatment � rimonabant]: F1,33 ¼ 0.29; p > 0.05; C),
ANOVA or two-way ANOVA followed by the NewmaneKeuls Multiple Comparison
respectively, failed to block the effect of 10 mg/kg rimonabant. In
post-test depending on the combination of genotype and treatment factors. Graphsand statistics were generated by GraphPad Prism 4.03 Software. Results were
contrast, a per se ineffective dose of 20 mg/kg AMPT attenuated
considered to be significant at p < 0.05.
the effect of rimonabant (Pretreatment factor: F1,44 ¼ 14.18;p > 0.05; Rimonabant factor: F1,44 ¼ 30.49; p < 0.01;
Interaction[Pretreatment � rimonabant]: F1,44 ¼ 13.43; p < 0.001;
In order to test whether THC and rimonabant effects depend on
3.1. Locomotor activity
CB1 receptor activation on specific glutamatergic or GABAergicneuronal population, we tested these drugs in conditional mutant
To avoid potential disturbing factors related to locomotor
mice lacking CB1 receptor specifically in these neuronal subpopu-
activity, all drugs were tested in the open field test. In fact none of
lations. However, we first characterized the phenotype of these
the drugs and doses applied altered the distance moved as
animals in the FST, without any treatment. Analyzing the floating
compared to respective control groups ().
behavior in the conditional CB1 receptor knock-out mice revealeda significant decrease in floating time for Glu-CB�/�
3.2. Antidepressant-like effects
(mean � SEM: WT ¼ 82.9 � 16 s and Glu-CB�/�
t10 ¼ 3.02; p < 0.01; n ¼ 6; Pre-test not shown but is similar
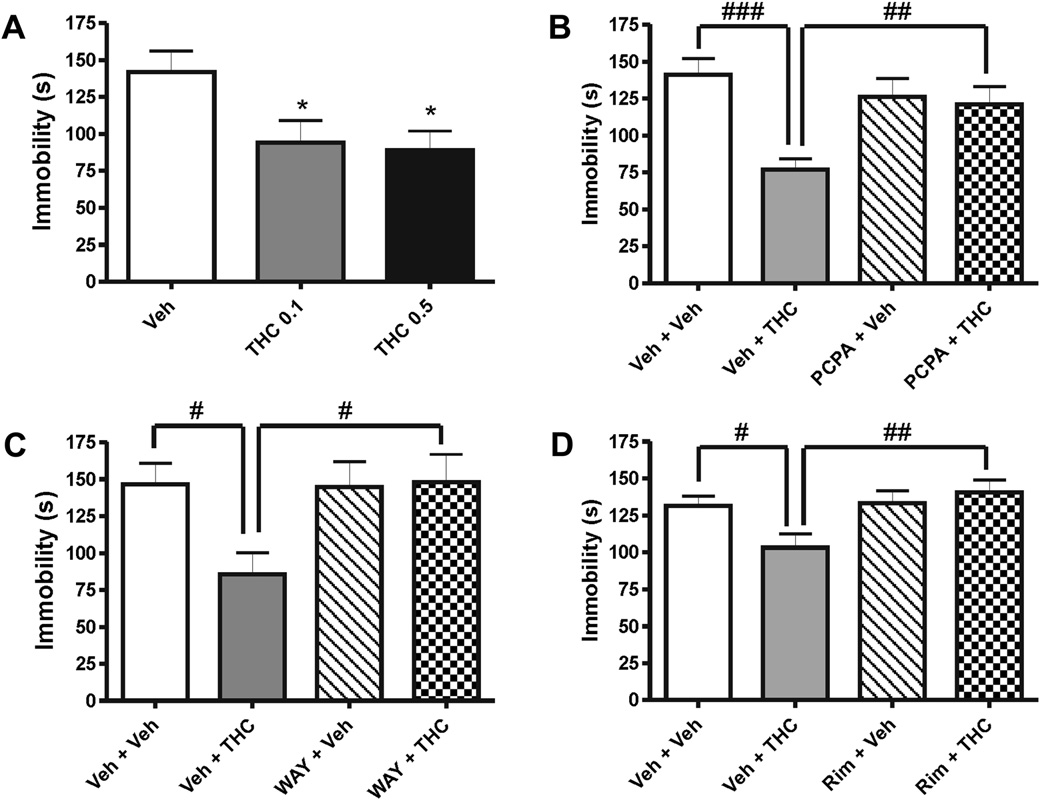
Animals treated with a low dose of THC (0.1 and 0.5 mg/kg)
as depicted in without changes in open field activity
showed a significant reduction in floating behavior (F2,23 ¼ 4.17;
� SEM: WT ¼ 1331 � 202 cm and Glu-
p < 0.05; A). The THC effect of 0.1 mg/kg was prevented by
¼ 1544 � 498 cm; t16 ¼ 0.39, ns; n ¼ 9). The difference in
a pretreatment with the 5-HT synthesis inhibitor pCPA 100 mg/kg
phenotype in these animals was annulled by the pretreatment with
(Pretreatment factor: F1,68 ¼ 1.77; p > 0.05; THC factor:
pCPA (Genotype factor: F1,46 ¼ 1.47; ns; Treatment factor:
F1,68 ¼ 10.12; p < 0.01; Interaction[Pretreatment � THC]: F1,68 ¼ 7.33;
F1,46 ¼ 1.72; ns; Interaction[Genotype � Treatment]: F1,46 ¼ 4.33;
p < 0.01; B) and the 5-HT1A receptor antagonist WAY100635
p < 0.05; On the other hand, CB1 receptor deletion from
Table 1Locomotor activity (cm moved in 5 min) in the open field after different pharmacological treatments.
Experimental groups
Effects of THC and rimonabant
F4,38 ¼ 0.59; ns
Interaction of THC with serotonin release
Vehicle þ Vehicle
Interaction (Rim/pCPA): F1,36 ¼ 0.001; ns
Vehicle þ THC [0.1 mg/kg]
Vehicle � Rim: F1,36 ¼ 0.56; ns
pCPA [100 mg/kg] þ Vehicle
Vehicle � pCPA: F1,36 ¼ 0.06; ns
pCPA [100 mg/kg] þ THC [0.1 mg/kg]
Interaction (Rim/WAY): F1,36 ¼ 0.01; ns
WAY [1 mg/kg] þ Vehicle
Vehicle � Rim: F1,36 ¼ 0.46; ns
WAY [1 mg/kg] þ THC [0.1 mg/kg]
Vehicle � WAY: F1,36 ¼ 1.77; ns
Interaction of rimonabant with serotonin release
Vehicle þ Vehicle
Interaction (Rim/pCPA): F1,36 ¼ 0.93; ns
Vehicle þ Rim [10 mg/kg]
Vehicle � Rim: F1,36 ¼ 0.03; ns
pCPA [100 mg/kg] þ Vehicle
Vehicle � pCPA: F1,36 ¼ 0.26; ns
pCPA [100 mg/kg] þ Rim [10 mg/kg]
Interaction (Rim/WAY): F1,36 ¼ 3.63; ns
WAY [1 mg/kg] þ Vehicle
Vehicle � Rim: F1,36 ¼ 0.003; ns
WAY [1 mg/kg] þ Rim [10 mg/kg]
Vehicle � WAY: F1,36 ¼ 2.09; ns
Interaction of rimonabant with catecholamine release
Vehicle þ Vehicle
Interaction: F1,41 ¼ 0.103; ns
Vehicle þ Rim [10 mg/kg]
Vehicle � Rim: F1,41 ¼ 0.097; ns
AMPT [20 mg/kg] þ Vehicle
Vehicle � AMPT: F1,41 ¼ 1.202; ns
AMPT [20 mg/kg] þ Rim [10 mg/kg]
M. Häring et al. / Neuropharmacology 65 (2013) 83e89
Fig. 1. Antidepressant-like effects of THC and the role of serotonin. Treatment with (A) THC (0.1 mg/kg and 0.5 mg/kg) decreased immobility in the forced swim test. The effect ofTHC (0.1 mg/kg) was attenuated when combined (B) with the serotonin synthesis inhibitor pCPA (100 mg/kg), (C) with the 5-HT1A receptor antagonist WAY100635 (WAY; 1 mg/kg),and (D) with a per se non-effective dose of rimonabant (Rim; 0.5 mg/kg). Data are expressed as mean � SEM. n ¼ 9e24; *p < 0.05 (students t-test); #p < 0.05, ##p < 0.01, ###p <0.001 (NewmaneKeuls Multiple Comparison post-test following two-way ANOVA).
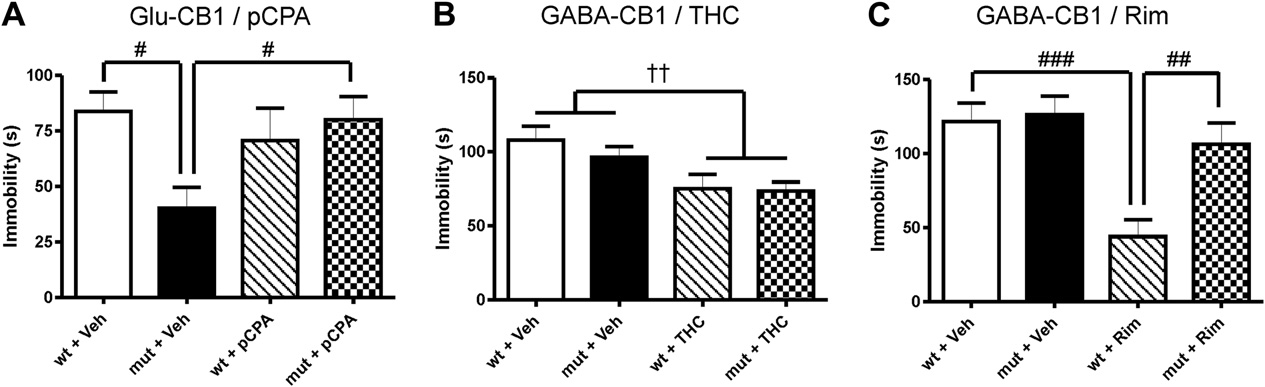
forebrain GABAergic neurons had no effect on the performance in
situation that Glu-CB�/�
mice showed already an antidepressant-
the FST (mean � SEM: WT ¼ 90.8 � 26.3 and GABA-CB�/�
like behavior which cannot be further enhanced by THC.
65.3 � 17.2; t11 ¼ 0.83, ns; n ¼ 6e7; Pre-test not shown but is
These findings suggest that, even though both drugs have
similar as depicted in and C). By testing drugs in these
antidepressant-like properties, they seem to interfere with
animals, we were able to show that the THC effect was still present
different circuits, serotonergic transmission being important for the
mutants (Genotype factor: F1,67 ¼ 0.622; ns; Treat-
behavioral response to low doses of THC, and catecholamines for
ment factor: F1,67 ¼ 10.89; p < 0.01; Interaction[Genotype � Treatment]:
the rimonabant effects. The antidepressant-like effect of THC is in
F1,68 ¼ 0.371; ns; On the contrary, the decrease in floating
line with previous data obtained with other cannabinoids
induced by a dose of 10 mg/kg rimonabant was not detect-
able in GABA-CB�/�
animals (Genotype factor: F1,33 ¼ 6.97;
addition, the role of 5-HT was also proposed previously for this
p < 0.05; Treatment factor: F1,33 ¼ 14.64; p < 0.001;
class of substances. For instance, demon-
Interaction[Genotype � Treatment]: F1,33 ¼ 5.17; p < 0.05;
strated that the antidepressant-like effects of WIN-55,212-2,a synthetic cannabinoid agonist, was blocked by pCPA in rats.
Likewise, WAY100635 blocked the effect of cannabidiol, a non-psychotomimetic phytocannabinoid, in mice in the FST (
Our results confirm previous findings on the contradictory roles
Finally, the same 5-HT1A receptor antagonist also
of the ECS activation and inhibition regarding stress coping. We
blocked the anxiolytic-like effects of THC in rats
could show that low dose of THC (0.1 and 0.5 mg/kg) or high dose of
). The latter result is highly congruent with our findings, as
the CB1 receptor antagonist rimonabant (3 and 10 mg/kg) led to
anxiolytic drugs can also have antidepressent-like effects and vice
a decrease of immobility, indicating an antidepressant-like
behavior. THC effects were prevented by a per se ineffective dose
A relevant neuronal circuit in respect to our finding might be the
of rimonabant (0.5 mg/kg; proving the CB1 receptor
projection between prefrontal cortex (PFC) and serotonergic
dependence of the THC effect. Remarkably, inhibition of 5-HT
neurons in the raphe nuclei, which is modulated by cannabinoids, as
synthesis, and 5-HT1A receptor blockade, respectively, was also
proposed by . The PFC, a region highly involved
able to prevent the effects of THC, but not of rimonabant. On the
in the processing and evaluation of a stressful situation, has strong
other hand, using a genetic approach, we could show that the
glutamatergic connections with the raphe nuclei
antidepressant-like effects of rimonabant, but not of THC for the
Interestingly, the connection seems to be indirect,
doses used in this study, seem to depend exclusively on CB1 receptor
as decrease in excitatory drive leads to an increased 5-HT trans-
in GABAergic neurons. Low doses of THC might act mainly via other
mission. Thus, the local CB1 receptor activation on glutamatergic
CB1 receptor populations, potentially on glutamatergic neurons,
terminals in the PFC by the synthetic cannabinoid receptor agonist
even though there is no final proof for this notion, due to the
WIN55,212 resulted in an increased firing of serotonergic neurons
M. Häring et al. / Neuropharmacology 65 (2013) 83e89
Fig. 2. Antidepressant-like effects of rimonabant and the role of serotonin and catecholamines. Treatment with (A) rimonabant (Rim; 3 and 10 mg/kg) both decreased immobility inthe forced swim test. The decrease in immobility induced by rimonabant (10 mg/kg) was not altered by (B) the serotonin synthesis inhibitor pCPA (100 mg/kg) or (C) the 5-HT1Areceptor antagonist WAY100635 (WAY; 1 mg/kg), however, by (D) the catecholamine synthesis inhibitor AMPT (20 mg/kg). Data are expressed as mean � SEM. n ¼ 9e12; *p < 0.05,***p < 0.001 (students t-test); ###p < 0.001 (NewmaneKeuls Multiple Comparison post-test following two-way ANOVA); ns, non significant.
Earlier studies already suggested that an
However, the acute/sub-
reduced excitatory input from the PFC is followed by a decreased
chronic antidepressant-like effect of this CB1 receptor antagonist
activation of inhibitory neurons in the raphe nuclei, leading to an
was shown previously in rodents exposed to the FST (
increased 5-HT transmission and subsequently to decreased anxiety
; One explanation could be the chronic
and depressive-like behavior
use in clinical applications, resulting in the negative side effects.
Regarding the effects of high doses of rimonabant, this seems to
Also one should keep in mind the clinical intent to reduce obesity
be in contrast with the clinical effects of this drug, which may
using rimonabant. Obesity might sensitize the body to an increased
induce anxiety and depression in patients (for reviews, see
susceptibility toward depressive behavior. Nevertheless, our data
Fig. 3. Behavioral phenotype after cell type-specific CB1 receptor deletion, and pharmacological effects with THC and rimonabant. (A) Inactivation of CB1 receptor in corticalglutamatergic neurons led to a decrease in immobility (black bar), which was blocked by the treatment with pCPA (100 mg/kg), comparable as in wild-type controls. (B) Inactivationof CB1 receptor in forebrain GABAergic neurons did not alter immobility (black bar), and the effect of THC (0.1 mg/kg) on immobility is still detectable in mutant mice. (C) The effectof rimonabant (Rim; 10 mg/kg), however, was not present in GABA-CB�/�
mice. Data are expressed as mean � SEM; n ¼ 10e15; #p < 0.05; ##p < 0.01; ###p < 0.001 (Newmane
Keuls Multiple Comparison post-test following two-way ANOVA); yyp<0.01 (two-way ANOVA treatment factor). ns, non significant. Abbreviations: mut, mutant, i.e. Glu-CB�/�
; wt, wild-type littermate control, i.e. Glu-CB1
or GABA-CB1 , respectively.
M. Häring et al. / Neuropharmacology 65 (2013) 83e89
strongly suggest that this antagonist/inverse agonist acts via the
of antagonist/inverse agonist, on the other hand, dominantly acted
inhibition of CB1 receptor on GABAergic terminals, since the
via CB1 receptor on GABAergic neurons and depended at least not
decrease in floating induced by rimonabant was abolished when
mainly on serotonergic transmission, but on catecholamine trans-
injected into GABA-CB�/�
mutant mice. Why inhibiting 5-HT
mission. Our data further suggest a two-neuronal subpopulation
transmission had no effect on the action of rimonabant is not
model in which glutamatergic and GABAergic neurons, under the
clear. This seems to be in contrast with neurochemical data,
control of the CB1 receptor, seem to be differently sensitive to
showing that similar doses of rimonabant increased 5-HT in the
prefrontal cortex (). One possibility could be thesystemic increase in GABAergic transmission as the result of the
Statement of conflicts of interest
blockade of CB1 receptor, which could attenuate the effect of anincreased serotonergic transmission downstream in the stress
The authors declare to have no conflicts of interest.
circuit. Also possible would be an assisting role of 5-HT trans-mission after rimonabant treatment. In this respect, Tzavara andcolleagues also showed an increased release of catecholamines as
response to rimonabant treatment, thus potentially covering thebehavioral effect of blocking 5-HT transmission
We would like to thank Andrea Conrad, Danka Dormann, Anisa
). In consistence with this finding, we were able to attenuate
Kosan, and Anne Rohrbacher for genotyping of mutant mice. This
the antidepressant-like effect of rimonabant by applying a per se
research was supported in part by the German Research Foundation
ineffective dose of AMPT, a blocker of catecholamine synthesis. The
DFG (to B.L. and K.M.; FOR926, subproject SP3), and by a stipend
additional absence of the behavioral effects of rimonabant in the
from the Humboldt Foundation (to F.M.).
mice suggests a mechanism for the action of this drug,
mediated by catecholamine signaling and controlled by GABA
release. Recent finding also suggest an important role for the opioidsystem regarding the antidepressant-like effects of rimonabant
Bambico, F.R., Katz, N., Debonnel, G., Gobbi, G., 2007. Cannabinoids elicit
(Thus, they were able to block the rimonabant-
antidepressant-like behaviour and activate serotonergic neurons through themedial prefrontal cortex. J. Neurosci. 27, 11700e11711.
induced decrease in immobility by interfering with opioid
Bambico, F.R., Cassano, T., Dominguez-Lopez, S., Katz, N., Walker, C.D., Piomelli, D.,
signaling, in particular by blocking the k-opioid receptor
Gobbi, G., 2010. Genetic deletion of fatty acid amide hydrolase alters emotional
). Interestingly, the activation of this receptor was also
behaviour and serotonergic transmission in the dorsal raphe, prefrontal cortex,and hippocampus. Neuropsychopharmacology 35, 2083e2100.
suggested to increase the noradrenergic drive in the hypothalamic
Bambico, F.R., Hattan, P.R., Garant, J.P., Gobbi, G., 2012. Effect of delta-9-
paraventricular nucleus ).
tetrahydrocannabinol on behavioral despair and on presynaptic and post-
Contrasting with the rimonabant treatment, which seems to
Psychiatry 38, 88e96.
depend on CB1 receptors on GABAergic neurons, specific deletion of
Berrendero, F., Maldonado, R., 2002. Involvement of the opioid system in the
the receptor in GABAergic neurons (GABA-CB�/�
anxiolytic-like effects induced by D9-tetrahydrocannabinol. Psychopharma-
induce an antidepressant-like response. However, GABA-CB�/�
cology (Berl) 163, 111e117.
Beyer, C.E., Dwyer, J.M., Piesla, M.J., Platt, B.J., Shen, R., Rahman, Z., Chan, K.,
mice are still responsive to THC, suggesting that GABAergic CB1
Manners, M.T., Samad, T.A., Kennedy, J.D., Bingham, B., Whiteside, G.T., 2010.
receptor is unlikely to be the target of low doses of THC. Therefore,
Depression-like phenotype following chronic CB1 receptor antagonism. Neu-
we suggest a possible connection to CB
robiol. Dis. 39, 148e155.
1 receptor on other neuronal
Braida, D., Limonta, V., Malabarba, L., Zani, A., Sala, M., 2007. 5-HT
populations. Recent findings in our group have highlighted the
involved in the anxiolytic effect of D9-tetrahydrocannabinol and AM404, the
importance of glutamatergic CB1 receptors, showing that compa-
anandamide transport inhibitor, in Sprague-Dawley rats. Eur. J. Pharmacol. 555,
rable doses of CB1 receptor agonist failed to induce an anxiolytic
effect in Glu-CB�/�
Celada, P., Puig, M.V., Casanovas, J.M., Guillazo, G., Artigas, F., 2001. Control of dorsal
mutants tested in the elevated plus maze
raphe serotonergic neurons by the medial prefrontal cortex: involvement of
Due to the fact that Glu-CB�/�
serotonin-1A, GABAA, and glutamate receptors. J. Neurosci. 21, 9917e9929.
a decrease in floating behavior without treatment, it was not
Corrodi, H., Hanson, L.C., 1966. Central effects of an inhibitor of tyrosine hydrox-
possible to test the equivalent hypothesis in the FST. A similar
ylation. Psychopharmacologia 10, 116e125.
Cryan, J.F., Mombereau, C., 2004. In search of a depressed mouse: utility of models
decrease in immobility was previously observed in Glu-CB�/�
for studying depression-related behaviour in genetically modified mice. Mol.
(). This increased stress-coping behavior in Glu-
Psychiatry 9, 326e357.
Egashira, N., Matsuda, T., Koushi, E., Higashihara, F., Mishima, K., Chidori, S.,
mice is in contrast with other studies in which these mutants
Hasebe, N., Iwasaki, K., Nishimura, R., Oishi, R., Fujiwara, M., 2008. D9-tetra-
actually showed increased anxiety-like responses (
hydrocannabinol prolongs the immobility time in the mouse forced swim test:
; ; ). Thus, the decrease in
involvement of cannabinoid CB1 receptor and serotonergic system. Eur. J.
floating behavior may rather be an inadequate fear response than
Pharmacol. 589, 117e121.
El-Alfy, A.T., Ivey, K., Robinsonm, K., Ahmed, S., Radwan, M., Slade, D., Khan, I.,
a positive stress coping behavior. This situation would prevent
ElSohly, M., Ross, S., 2010. Antidepressant-like effect of D9-tetrahydrocannab-
a stringent interpretation of a possible antidepressant-like of THC
inol and other cannabinoids isolated from Cannabis sativa L. Pharmacol. Bio-
effect in Glu-CB�/�
chem. Behav. 95, 434e442.
Gobbi, G., Bambico, F.R., Mangieri, R., Bortolato, M., Campolongo, P., Solinas, M.,
The present data also suggest that the behavioral changes in the
Cassano, T., Morgese, M.G., Debonnel, G., Duranti, A., Tontini, A., Tarzia, G.,
may depend on serotonergic transmission, as it
Mor, M., Trezza, V., Goldberg, S.R., Cuomo, V., Piomelli, D., 2005. Antide-
was blocked by pCPA. Why the inhibition of 5-HT transmission
pressant-like activity and modulation of brain monoaminergic transmission
blocks this effect is not clear. One possibility might be a continuous
by blockade of anandamide hydrolysis. Proc. Natl. Acad. Sci. U. S. A. 102,18620e18625.
over-excitation of the serotonergic neurons via a different pathway
Gorzalka, B.B., Hill, M.N., 2011. Putative role of endocannabinoid signaling in the
(independent of inhibitory interneurons in the raphe) as suggested
above, caused by a general elevated excitatory drive.
psychopharmacol. Biol. Psychiatry 35, 1575e1585.
Griebel, G., Stemmelin, J., Scatton, B., 2005. Effects of the cannabinoid CB1 receptor
In summary, by using both pharmacological and genetic
antagonist rimonabant in models of emotional reactivity in rodents. Biol.
approaches, we could provide new insights into how to reconcile
Psychiatry 57, 261e267.
the contradictory findings on antidepressant-like effect of CB
Hall, W., Solowij, N., 1998. Adverse effects of cannabis. Lancet 352, 1611e1616.
Häring, M., Marsicano, G., Lutz, B., Monory, K., 2007. Identification of the cannabi-
receptor agonist and antagonist/inverse agonist. Low doses of
noid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience
agonist clearly depended on serotonergic transmission. High doses
146, 1212e1219.
M. Häring et al. / Neuropharmacology 65 (2013) 83e89
Häring, M., Kaiser, N., Monory, K., Lutz, B., 2011. Circuit specific functions of
endocannabinoid system controls key epileptogenic circuits in the hippo-
cannabinoid CB1 receptor in the balance of investigatory drive and exploration.
campus. Neuron 51, 455e466.
PLoS One 6, e26617.
Moreira, F.A., Crippa, J.A., 2009. The psychiatric side effects of rimonabant. Rev. Bras.
Häring, M., Guggenhuber, S., Lutz, B., 2012. Neuronal populations mediating the
Psiquiatr 31, 145e153.
effects of endocannabinoids on stress and emotionality. Neuroscience 204,
Moreira, F.A., Lutz, B., 2008. The endocannabinoid system: emotion, learning and
addiction. Addict. Biol. 13, 196e212.
Hill, M.N., Gorzalka, B.B., 2005. Pharmacological enhancement of cannabinoid CB1
Moreira, F.A., Grieb, M., Lutz, B., 2009. Central side effects of therapies based on CB1
receptor activity elicits an antidepressant-like response in the rat forced swim
cannabinoid receptors agonist and antagonists: focus on anxiety and depres-
test. Eur. Neuropsychopharmacol. 15, 593e599.
sion. Best Pract. Res. Clin. Endocrinol. Metab. 23, 133e144.
Höschl, C., Svestka, J., 2008. Escitalopram for the treatment of major depression and
Muntoni, A.L., Pillolla, G., Melis, M., Perra, S., Gessa, G.L., Pistis, M., 2006. Cannabi-
anxiety disorders. Expert Rev. Neurother. 8, 537e552.
noids modulate spontaneous neuronal activity and evoked inhibition of locus
Jacob, W., Yassouridis, A., Marsicano, G., Monory, K., Lutz, B., Wotjak, C.T., 2009.
coeruleus noradrenergic neurons. Eur. J. Neurosci. 23, 2385e2394.
Endocannabinoids render exploratory behaviour largely independent of the
O'Leary, O.F., Bechtholt, A.J., Crowley, J.J., Hill, T.E., Page, M.E., Lucki, I., 2007.
test aversiveness: role of glutamatergic transmission. Genes Brain Behav. 8,
Depletion of serotonin and catecholamines block the acute behavioral response
to different classes of antidepressant drugs in the mouse tail suspension test.
Jankowski, M.P., Sesack, S.R., 2004. Prefrontal cortical projections to the rat dorsal
Psychopharmacology (Berl) 192, 357e371.
raphe nucleus: ultrastructural features and associations with serotonin and
Oropeza, V.C., Mackie, K., Van Bockstaele, E.J., 2007. Cannabinoid receptors are
gamma-aminobutyric acid neurons. J. Comp. Neurol. 468, 518e529.
localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res.
Jesse, C.R., Wilhelm, E.A., Bortolatto, C.F., Nogueira, C.W., 2010. Evidence for the
1127, 36e44.
involvement of the noradrenergic system, dopaminergic and imidazoline
Patel, S., Hillard, C.J., 2006. Pharmacological evaluation of cannabinoid receptor
receptors in the antidepressant-like effect of tramadol in mice. Pharmacol.
ligands in a mouse model of anxiety: further evidence for an anxiolytic role for
Biochem. Behav. 95, 344e350.
endogenous cannabinoid signaling. J. Pharmacol. Exp. Ther. 318, 304e311.
Jiang, W., Zhang, Y., Xiao, L., Van Cleemput, J., Ji, S.P., Bai, G., Zhang, X., 2005.
Rey, A.A., Purrio, M., Viveros, M.P., Lutz, B., 2012. Biphasic effects of cannabinoids in
Cannabinoids promote embryonic and adult hippocampus neurogenesis
anxiety responses: CB1 and GABAB receptors in the balance of GABAergic and
and produce anxiolytic and antidepressant-like effects. J. Clin. Invest. 115,
Kano, M., Ohno-Shosaku, T., Hashimotodani, Y., Uchigashima, M., Watanabe, M.,
Steiner, M.A., Marsicano, G., Nestler, E.J., Holsboer, F., Lutz, B., Wotjak, C.T., 2008a.
2009. Endocannabinoid-mediated control of synaptic transmission. Physiol.
Antidepressant-like behavioral effects of impaired cannabinoid receptor type 1
Rev. 89, 309e380.
signaling coincide with exaggerated corticosterone secretion in mice. Psycho-
Kaster, M.P., Santos, A.R., Rodrigues, A.L., 2005. Involvement of 5-HT1A receptors in
neuroendocrinology 33, 54e67.
the antidepressant-like effects of adenosine in the forced swim test. Brain Res.
Steiner, M.A., Wanisch, K., Monory, K., Marsicano, G., Borroni, E., Bächli, H.,
Bull. 67, 53e61.
Holsboer, F., Lutz, B., Wotjak, C.T., 2008b. Impaired cannabinoid receptor type 1
Lafenêtre, P., Chaouloff, F., Marsicano, G., 2009. Bidirectional regulation of novelty-
signaling interferes with stress-coping behaviour in mice. Pharmacogenomics J.
induced behavioural inhibition by the endocannabinoid system. Neurophar-
macology 57, 715e721.
Steiner, M.A., Marsicano, G., Wotjak, C.T., Lutz, B., 2008c. Conditional cannabinoid
Laorden, M.L., Castells, M.T., Martínez, M.D., Martínez, P.J., Milanés, M.V., 2000.
receptor type 1 mutants reveal neuron subpopulation-specific effects on behavioral
Activation of c-fos expression in hypothalamic nuclei by mu- and kappa-
and neuroendocrine stress responses. Psychoneuroendocrinology 33, 1165e1170.
receptor agonists: correlation with catecholaminergic activity in the hypotha-
Tzavara, E.T., Davis, R.J., Perry, K.W., Li, X., Salhoff, C., Bymaster, F.P., Witkin, J.M.,
lamic paraventricular nucleus. Endocrinologist 141, 1366e1376.
Nomikos, G.G., 2003. The CB1 receptor antagonist SR141716A selectively
Lockie, S.H., Czyzyk, T.A., Chaudhary, N., Perez-Tilve, D., Woods, S.C., Oldfield, B.J.,
increases monoaminergic neurotransmission in the medial prefrontal cortex:
Statnick, M.A., Tschöp, M.H., 2011. CNS opioid signaling separates cannabinoid
implications for therapeutic actions. Br. J. Pharmacol. 138, 544e553.
receptor 1-mediated effects on body weight and mood-related behavior in
Viveros, M.P., Marco, E.M., File, S.E., 2005. Endocannabinoid system and stress and
mice. Endocrinology 152, 3661e3667.
anxiety responses. Pharmacol. Biochem. Behav. 81, 331e342.
Lucki, I., Dalvi, A., Mayorga, A.J., 2001. Sensitivity to the effects of pharmacologically
Weissman, A., Koe, B.K., 1965. Behavioral effects of L-alpha-methyltyrosine, an
selective antidepressants in different strains of mice. Psychopharmacology
inhibitor of tyrosine hydroxylase. Life Sci. 4, 1037e1048.
(Berl) 155, 315e322.
Wotjak, C.T., 2005. Role of endogenous cannabinoids in cognition and emotionality.
Massa, F., Mancini, G., Schmidt, H., Steindel, F., Mackie, K., Angioni, C., Oliet, S.H.,
Mini. Rev. Med. Chem. 5, 659e670.
Geisslinger, G., Lutz, B., 2010. Alterations in the hippocampal endocannabinoid
Zanelati, T., Biojone, C., Moreira, F.A., Guimaraes, F.S., Joca, S.R., 2010. Antidepres-
system in diet-induced obese mice. J. Neurosci. 30, 6273e6281.
sant-like effects of cannabidiol in mice: possible role of 5-HT1A receptors. Br. J.
Monory, K., Massa, F., Egertova, M., Eder, M., Blaudzun, H., Westenbroek, R.,
Pharmacol. 159, 122e128.
Kelsch, W., Jacob, W., Marsch, R., Ekker, M., Long, J., Rubenstein, J.L., Goebbels, S.,
Zuardi, A.W., Shirakawa, I., Finkelfarb, E., Karniol, I.G., 1982. Action of cannabidiol on
Nave, K.A., During, M., Klugmann, M., Wolfel, B., Dodt, H.U., Zieglgansberger, W.,
the anxiety and other effects produced by D9-THC in normal subjects.
Wotjak, C.T., Mackie, K., Elphick, M.R., Marsicano, G., Lutz, B., 2006. The
Psychopharmacology (Berl) 76, 245e250.
Source: http://www.for926.uni-bonn.de/kunden/for926/content/e229/e1305/e1342/2012_Haering_Neuropharmacology.pdf
Réponses concernant le questionnaire sur l'hyperprolactinémie, MC Wimmer, interne de gynécologie-obstétrique CHU Rennes 1 « Evaluation des connaissances concernant l'hyperprolactinémie, dans la population des internes de gynécologie-obstétrique » 2 Quelques résultats et discussion Cette étude épidémiologique s'est intéressée aux connaissances des internes de gynécologie-obstétrique en France sur le thème de l'hyperprolactinémie. Le taux de réponse au questionnaire est de 17.7 % des internes. Ce taux de participation est proche d'études publiées dans ce domaine : 19 % concernant l'enseignement du siège en 2006 [15], 33.6 % concernant le formation sur la dystocie des épaules [16]. Une nouvelle relance devrait permettre d'améliorer le taux de réponse à notre questionnaire. Il est intéressant de noter une disparité au sein des régions de France, parmi les réponses. Le taux des internes de la région de Rennes est le plus élevé, traduisant qu'une motivation locale au remplissage du questionnaire peut être utile. Il serait intéressant de connaître les motifs de non remplissage du questionnaire. Le principal résultat de cette étude, est la mise en évidence de lacunes chez les internes de gynécologie obstétrique, concernant le sujet de l'hyperprolactinémie. En effet, la moyenne globale de 5,7 sur 20 (+/- 2.4). Les lacunes dominent dans le contexte de la grossesse que ce soit en physiologie ou lors d'un adénome à prolactine. Les connaissances pratiques semblent elles mieux maitrisées notamment sur la réalisation d'IRM hypophysaire en urgence lors d'une suspicion d'adénome à prolactine, sur la conduite thérapeutique en première intention, et sur les traitements hyperprolactinémiants. Près de 20 % des réponses enregistrées étaient des « ne sais pas », mettant en évidence leur clairvoyance sur leur manque de connaissances sur le sujet. Il est intéressant de noter que le cursus ne semble pas permettre une meilleure courbe d'apprentissage. En effet, les internes de fin de cursus n'ont pas obtenus de meilleurs résultats que les plus jeunes. En revanche, les internes à destinée médicale semblent avoir des connaissances plus importantes à l'inverse des internes mentionnant un cursus plus chirurgical ou orienté en obstétrique. Les résultats n'ont qu'une tendance significative due aux plus faible nombre de personne dans les groupes gynécologie médicale +/- PMA et échographie, DAN. Quelles peuvent être les principales hypothèses de ces mauvais résultats ? Les internes de gynécologie-obstétriques ne rencontrent probablement pas ou peu ces pathologies lors de leurs stages pratiques, car elles sont principalement gérées en consultation. Lors des cours théoriques, les thèmes de gynécologie endocrinienne sont peut- être abordés moins fréquemment que ceux de chirurgie ou d'obstétrique [11]. De plus, ces connaissances s'acquièrent plutôt dans des stages spécialisés (gynécologie-endocrinologie) qui ne peuvent s'intégrer dans la maquette de tous les internes. Une deuxième hypothèse est que les connaissances restent potentiellement mal intégrées et/ou mal utilisées. En 2015, Mesdag et collaborateurs ont réalisé un état des lieux sur l'enseignement en gynécologie-obstétrique en France [11]. L'offre d'enseignement est très variable sur le plan de la méthodologie employée, des formateurs et de la périodicité. L'accès est difficile de par l'éloignement géographique des lieux de stages, et des impératifs de service. Dans ce travail les étudiants auraient souhaités des cours plus axés sur des conduites à tenir en pratique, et des ateliers de formation aux gestes techniques. Cette étude concluait à la nécessité d'une réforme de l'enseignement avec comme pistes de travail l'uniformisation de l'enseignement en France, la possibilité d'un
alz_nach_der_diagnose_D:Layout 1 10.5.2010 15:46 Uhr Seite 24 Rue des Pêcheurs 8E1400 Yverdon-les-BainsTel. 024 426 20 00Fax 024 426 21 [email protected]: 024 426 06 06 Ich möchte mehr InformationenBitte senden Sie mir: Infoblatt zur Vorsorgevollmacht und Patientenverfügung (gratis) die Broschüre «Leben mit Demenz. Tipps für Angehörige und Betreuende» (gratis)




