Tlsr.usm.my
Tropical Life Sciences Research, 27(1), 135–144, 2016
Effectiveness of Ivermectin and Albendazole against Haemonchus
contortus in Sheep in West Java, Indonesia
1Silvia Puspitasari, 1Achmad Farajallah, 2Erni Sulistiawati and 3,4Muladno
1Animal Bioscience, Department of Biology, Faculty of Mathematics and Science,
2Veterinary Technician Diploma Program,
3Department of Animal Production and Technology, Faculty of Animal Science, Bogor Agricultural University, Bogor 16680, West Java, Indonesia
4Directorate General of Livestock and Animal Health, Ministry of Agriculture of Republic of
Indonesia, South Jakarta, Indonesia
Abstrak: Memberikan dos separuh antelmintik merupakan suatu kaedah untuk mengesan
daya tahan parasit yang menjangkiti haiwan ruminan kecil. Apabila satu antelmintik tidak
berkesan dalam kambing biri-biri asal daripada Indonesia, satu kombinasi antelmintik
daripada kelas kimia berbeza dengan cara tindakan berbeza diberikan sebagai satu
strategi pengawalan parasit alternatif. Kajian ini membezakan kemujaraban antelmintik
ivermectin (IVM) dan albendazole (ABZ) yang diberikan secara berasingan ataupun
separuh dos ataupun diberikan bersama kepada kambing biri-biri yang dijangkiti secara
semula jadi dengan
Haemonchus contortus. Dua belas kambing biri-biri daripada Bogor,
Jawa Barat, Indonesia telah dibahagikan kepada enam kumpulan rawatan: dos separuh
IVM, dos penuh IVM, dos separuh ABZ, dos penuh ABZ, kombinasi IVM + ABZ, dan
kawalan. Kemujaraban rawatan telah ditentukan menggunakan ujian pengurangan
pengiraan telur tinja (FECRT) pada hari 0 (pra-rawatan) dan pasca-rawatan pada hari-hari
7, 14, 21, 28, 35, dan 42. Kemujaraban separuh dos IVM, dos penuh IVM, dos separuh
ABZ, dos penuh ABZ, dan rawatan kombinasi bernilai daripada –1900% hingga 100%,
99% hingga 100%, –167% hingga 100%, –467% hingga 89%, dan –200% hingga 100%,
masing-masing. FECRT untuk separuh dos IVM, dos separuh ABZ, dos penuh ABZ
menunjukkan bahawa
H. contortus mempunyai daya tahan terhadap separuh dos IVM
dan ABZ. Dos penuh IVM berkesan terhadap
H. contortus. Rawatan kombinasi lebih
berkesan terhadap
H. contortus berbanding menggunakan ABZ sahaja.
Kata kunci: Ivermectin, Albendazole,
Haemonchus contortus, Jawa Barat Indonesia
Abstract: Administering a half dose of an anthelmintic is a simple method for detecting
resistance in parasites infesting small ruminants. When a single anthelmintic fails in native
sheep from Indonesia, a combination of anthelmintics from different chemical classes with
different modes of action are administered as an alternative parasite-control strategy. This
study compared the anthelmintic efficacy of ivermectin (IVM) and albendazole (ABZ) given
either separately as a single dose or half dose or co-administered to sheep naturally
infected with
Haemonchus contortus. Twelve sheep from Bogor, West Java, Indonesia
were divided into the following six treatment groups: half-dose IVM, full-dose IVM, half-
dose ABZ, full-dose ABZ, combined IVM + ABZ, and control. The treatment efficacy was
determined using the faecal egg count reduction test (FECRT) at day 0 (pre-treatment)
and post-treatment at days 7, 14, 21, 28, 35, and 42. The efficacies of half-dose IVM, full-
dose IVM, half-dose ABZ, full-dose ABZ, and the combination treatment ranged from
–1900% to 100%, 99% to 100%, –167% to 100%, –467% to 89%, and –200% to 100%,
respectively. The FECRT for the half-dose IVM, half-dose ABZ, full-dose ABZ showed that
*Corresponding author:
[email protected]
Penerbit Universiti Sains Malaysia, 2016
Silvia Puspitasari et al.
H. contortus is resistant to half-dose IVM and ABZ. Full-dose IVM was effective against
H. contortus. The combined treatment was more effective against
H. contortus than ABZ
alone.
Keywords: Ivermectin, Albendazole,
Haemonchus contortus, West Java Indonesia
INTRODUCTION
In the tropics, gastrointestinal nematodes are a major problem in small
ruminants, and they cause disease, death, and decreased production of milk,
meat, and wool (Bekele
et al. 1992; Zainalabidin
et al. 2014). The barber pole
worm,
Haemonchus contortus, causes haemonchosis, which results in anaemia
and digestive disorders. The adult worms live free in the abomasum of the host
and attack the mucosa by piercing with their buccal lancets to suck the blood.
Female worms produce eggs that are excreted in the faeces into the
environment, and the larvae orally infect the host as the ruminants feed on grass
(Monnig 1950).
In small ruminants, gastrointestinal nematodes can generally be
controlled using broad-spectrum anthelmintics. Widely available commercial anthelmintics commonly used by farmers include macrocyclic lactones (avermectin [AVM] and milbemycins [MLs]), benzimidazole (BZD), and imidazothiazoles (levamisole [LEV] and hydropyrimidines [pyrantel/morantel]). Ivermectin (IVM) is a macrocyclic lactone with broad activity against gastrointestinal nematodes (Egerton
et al. 1979) and ectoparasites (Campbell
et al. 1983). IVM acts by binding the α-subunit of glutamate-gated chloride channel receptors (GluCl) in nerve synapses (Wolstenholme 2011), which inhibits nematode feeding, fecundity, and motility (Yates
et al. 2003). Albendazole (ABZ) is a BZD with a methyl carbamate group that is effective against lung worms (McKellar & Scott 1990), gastrointestinal nematodes, tapeworms, and liver flukes (Campbell 1990). BZD acts by binding to β-tubulin, which inhibits dimerisation with α-tubulin during microtubule formation in nematode cells (Lacey 1990; Wolstenholme 2011).
The intensive use of anthelmintics has led to the development of
anthelmintic resistance in small ruminants (Kaplan 2004; Wolstenholme
et al. 2004). Resistance is considered present when a portion of a population is able to tolerate doses of a compound that are effective against other populations of the same species (Prichard
et al. 1980). IVM-resistant
H. contortus has been reported in Australia (Le Jambre 1993), Kenya (Mwamachi
et al. 1995; Waruiru
et al. 1998), South Africa (van Wyk
et al. 1989), and Brazil (Echevarria
et al. 1991; Vieira
et al. 1992). BZD-resistant
H. contortus has been reported in Australia (Green
et al. 1981), Malaysia (Rahman 1994), India (Uppal
et al. 1992), France (Kerboeuf
et al. 1988; Hubert
et al. 1991), Kenya (Mwamachi
et al. 1995), South Africa (Berger 1975), and Brazil (Echevarria
et al. 1991). The resistance problem is compounded by the fact that many parasite populations are resistant to more than one class of anthelmintic. It has been proposed that the combined use of anthelmintics might be more effective against gastrointestinal nematodes when a single anthelmintic has failed. In addition, anthelmintic combinations slow
Ivermectin and Albendazole against Haemonchus contortus
the development of resistance (Wolstenholme
et al. 2004; Coles 2005; Leathwick
et al. 2009).
This study compared the anthelmintic efficacy of IVM and ABZ given
separately in a single dose and half dose or co-administered to sheep naturally infected with
H. contortus.
MATERIALS AND METHODS
Animal
Twelve sheep (age 3–4 months, liveweight 13–20 kg) from Bogor, West Java,
were studied. The management system was intensive, the sheep were housed
colonially, fed hay, and water was provided ad libitum.
Experimental Design The sheep were divided randomly into six experimental groups (Table 1). Faecal
samples were collected directly from the rectums of the sheep at days 0 (pre-
treatment) and at days 7, 14, 21, 28, 35, and 42 post-treatment.
Table 1: The anthelmintic treatment.
Route of administration
½ mL/50 kg liveweight
1 mL/50 kg liveweight
½ mL/5 kg liveweight
1 mL/5 kg liveweight
Combined (IVM + ABZ)
Subcutaneous+oral
(½ mL/50 kg + ½ mL/5 kg) liveweight
Faecal Egg Counts (FEC)
The FEC were determined by a modified McMaster technique that is accurate to
50 eggs per gram. The total number of
H. contortus eggs counted in two
chambers of McMaster slide was multiplied by 50 to obtain the number of egg
per gram (epg) of faeces. The FEC were plotted in curves to calculate the
p-value of the regression. The identification of
H. contortus eggs was based on
the size, shape, and development stage of the eggs (Foreyt 2001).
Anthelmintic
The anthelmintics that were used for treatment were IVM (Ivomec Super, Merial,
Brazil) and ABZ (Kalbazen®-SG-Albendazole 19 mg/mL, PT. Kalbe Farma,
Bekasi, Indonesia).
Data Analysis
Susceptibility to the various anthelmintic drugs was quantified using the faecal
egg counts reduction (FECR) method. The FECR was calculated according to
the recommendations of the World Association for the Advancement of
Veterinary Parasitology (Coles
et al. 1992) to evaluate the effectiveness of each
Silvia Puspitasari et al.
anthelmintic treatment, using the following formula described by Kochapakdee
et al. (1995) and McKenna (2006):
FECRi (%) = 100 × [1 – (Ti/T0)]
where FECRi is FECR on days i, Ti is the arithmetic mean faecal egg counts after
treatment (day 7, 14, 21, 28, 35, and 42), and T0 is the arithmetic mean of the
faecal egg counts before treatment (day 0) in the treated group. Anthelmintic
effectiveness was based on the FECR (%) and the lower 95% confidence limits.
If the FECR percentage of an anthelmintic treatment was less than 95% and
the
lower 95% confidence limit for the reduction was less than 90%, resistance was
considered to be present. If only one of the two criteria was met, resistance was
suspected.
RESULTS
Full-dose IVM completely decreased the number of
H. contortus eggs on day 7.
However, the number of eggs was significantly different and actually increased in
the control group and the group that received the full-dose ABZ. The anthelmintic
combination decreased the number of eggs until day 35 (Fig. 1).
Figure 1: The
H. contortus faecal egg count (epg) for each treatment, before (day 0) and
after (day 7 to 42) treatment.
Note: Control and full-dose ABZ;
p<0.05.
Table 2 summarises the results of the FECR. The efficacy of half-dose
IVM, full-dose IVM, half-dose ABZ, full-dose ABZ, and the combined treatment ranged from –1900% to 100%, 99% to 100%, –167% to 100%, –467% to 89%, and –200% to 100%, respectively. Full-dose IVM had the greatest efficacy at
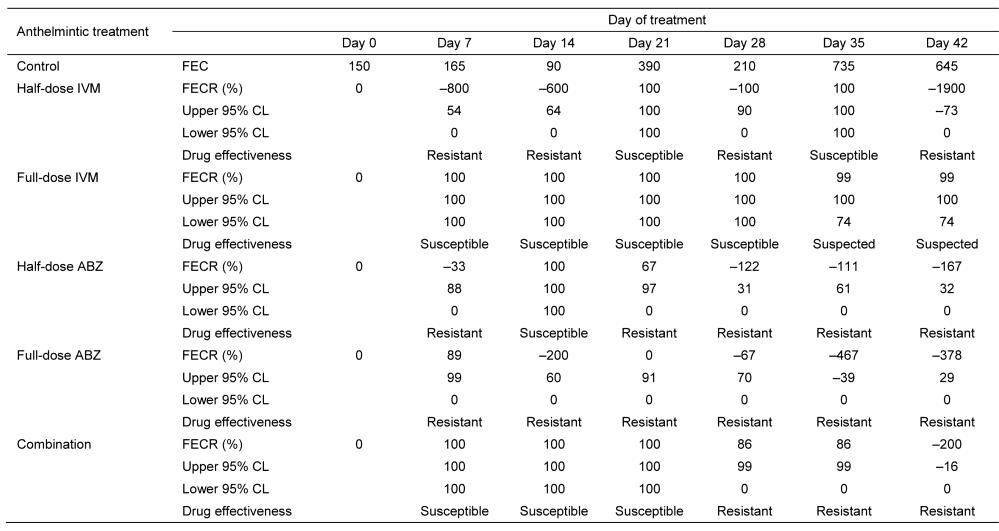
Ivermectin and Albendazole against Haemonchus contortus
99%–100%. The low overall percentage reduction showed that H. contortus is resistant to IVM and ABZ.
Table 2: FECR (%) and anthelmintic efficacy against H. contortus in sheep.
Notes: FEC = faecal egg counts; FECR = faecal egg count reduction; IVM = ivermectin; ABZ =
albendazole; Combination = half-dose ivermectin + half-dose albendazole; CL = confidence limit.
DISCUSSION
Faecal Egg Counts (FEC)
The FEC from each group prior to treatment (day 0) and after treatment (day 7 to
42) are shown in Figure 1. Based on the p-value, the significant treatments were
the control and full-dose ABZ groups. The control group demonstrated an
increase in the number of H. contortus eggs that was significant (p<0.05). The
egg counts increased on days 21 and 35 in the controls because it takes
approximately 18–21 days for H. contortus larvae to mature and produce eggs
(Monnig 1950). Adult female worms can lay an average of 6.582 eggs per day
(Coyne et al. 1991). By day 14, the control treatment had decreased the number
of H. contortus eggs. According to Dobson et al. (2012), counts of untreated
control animals may decrease because of density-dependent constraints on
fecundity that are exacerbated by any incoming infection in the period between
the pre- and post-treatment counts.
Full-dose ABZ treatment increased the number of H. contortus eggs to a
level that was significant (p<0.05). Cezar et al. (2010) showed that a full-dose ABZ treatment increased the number of H. contortus eggs by day 12.
Although the response to the full-dose IVM treatment was non-
significant, Figure 1 shows that the egg excretion dropped to 0 on day 7 after the administration of full-dose IVM. This result is in agreement with research from Yacob et al. (2009) that showed that IVM decreased the egg count to 0 on day 7 until day 21. Other studies have shown that IVM treatment decreased the
Silvia Puspitasari et al.
number of H. contortus eggs on days 5 (McKenna & Watson 1987), 10
(Borgsteede 1993), and 14 (Gogolewski et al. 1995).
Faecal Egg Count Reduction Test (FECRT)
Giving half the dose of an anthelmintic is recommended as a simple method for
the early detection of resistance (Hughes et al. 2005). According to Smith et al.
(1999), when given at below the recommended dose, the anthelmintic has a
shorter half-life. The parasite death rate due to the drug is seen as an
instantaneous decrease with the administration of an anthelmintic. On days 7
and 14, the IVM status was resistant, but by day 21, the anthelmintic status
became susceptible. Some important factors to be considered in the diagnosis of
anthelmintic resistance and the interpretation of results are the route of
administration and pharmacokinetic behaviour (Canga et al. 2009), host nutrition
(Coop & Kyriazakis 2001), and the host immune system and age (Lanusse &
Prichard 1993). Subcutaneous administration delays the absorption of IVM from
the injection site, resulting in the prolonged presence of the drug in the
bloodstream (Canga et al. 2009). Depending on the host nutrition, protein content
affects the development rate of immunity against parasitic infections, according
to Gibson (1983). Protein deficiency causes damage to T-lymphocytes (Beisel
1982). As demonstrated by Gill et al. (1993), T-lymphocytes play an important
role in combatting haemonchosis in sheep. Sheep under the age of 6 months
have very low protection from gastrointestinal nematodes (Lloyd & Soulsby
1987). The half-dose of ABZ was not effective against H. contortus. The activity
of ABZ depends on the ability of the drug to reach and sustain sufficient
concentrations at the site of infection (Lanusse & Prichard 1993).
The full-dose IVM treatment is very effective against gastrointestinal
nematodes that are resistant to ABZ, including H. contortus (George et al. 2011). The efficacy of a drug is also associated with the route of administration. The subcutaneous injection of IVM results in higher plasma IVM concentrations for a longer duration (Alvinerie et al. 1998). IVM paralyses the neuromuscular system in the target tissue by increasing the permeability of the plasma membrane to
chloride ions (Cl ). This causes hyperpolarisation and inhibits pharyngeal pumping, feeding, parasite spawning, and muscle motility. The pharynx is a muscular digestive organ that acts in nutrient ingestion and excretion and regulates turgor pressure in the parasite. Paralysis of the pharynx reduces the energy reserves of the parasites (Sangster & Gill 1999) and inhibits feeding by blocking pharyngeal pumping (Geary et al. 1993). Paralysis of the uterine muscles in female worms can suppress egg production and the release of eggs that are already present in the uterus. In addition, IVM can sterilise female nematodes in several hosts without killing them (Coles 2005). Le Jambre (1993) reported that IVM suppressed the fecundity of female H. contortus. IVM paralyses the somatic muscles in the parasite mid-body, reducing parasite motility and facilitating its expulsion from host intestinal tract (Sangster & Gill 1999).
In contrast, full-dose ABZ was less effective against H. contortus. George
et al. (2011) also reported the low efficacy of ABZ. The mechanism of resistance involves a reduction in the binding affinity of ABZ to tubulin (Sangster et al. 2002) due to a mutation in β-tubulin (Kohler 2001). In H. contortus, selection for
Ivermectin and Albendazole against Haemonchus contortus
resistance is enhanced by the high biotic potential. Their fecundity can enable small populations of resistant worms to become large populations in a short amount of time, especially if the climate is favourable for the free-living stages (Craig 1993). In this study, the farmer used ABZ as worm therapeutics for several years. Using the same class of drugs for several years (Coles 2005) ensures that the worms that survive treatment will have a greater chance than susceptible worms to contribute to the next generation.
In this study, the combined treatment with IVM and ABZ was effective
against H. contortus from days 7 to 21; then, resistance was seen on days 28 to 42, suggesting that H. contortus is cross-resistant to the combination treatment. According to Prichard et al. (1980), cross-resistance occurs when a population can fight anthelmintics in different chemical groups that have different modes of action. Miller and Craig (1996) reported that the combination of IVM and ABZ was more effective against H. contortus than either given alone. Multiple-drug resistance in H. contortus has also been reported (Traversa et al. 2007; Cezar et al. 2010).
CONCLUSION
The percentage reductions in the faecal egg count in the half-dose IVM, half-
dose ABZ, and full-dose ABZ groups showed that H. contortus is resistant to half-
dose IVM and ABZ. The full-dose IVM treatment was 99%–100%, effective
against H. contortus. The combined treatment was more effective against
H. contortus than ABZ alone.
ACKNOWLEDGEMENT
Thanks are due to the Directorate of General Higher Education (DIKTI) for the
scholarship Beasiswa Unggulan (BU) DIKTI 2012. The cooperation and
assistance of the farmers who agreed to take part in this study are gratefully
acknowledged.
REFERENCES
Alvinerie M, Escudero E, Sutra, J F, Eeckhoutte C and Galtier P. (1998). The
pharmacokinetics of moxidectin after oral and subcutaneous administration to sheep. Veterinary Research 29(2): 113–118.
Beisel W R. (1982). Synergism and antagonism of parasitic diseases and malnutrition.
Reviews of Infectious Disease 40(4): 746–750.
Bekele T, Woldeab T, Lahlou-Kassi A and Sherington J. (1992). Factors affecting
morbidity and motility on-farm and on-station in the Ethiopian highland sheep. Acta Tropica 52(2–3): 99–109.
Berger J. (1975). The resistance of a field strain of Haemonchus contortus to five
benzimidazole anthelmintic in current usetion 46(4): 369–372.
Silvia Puspitasari et al.
Borgsteede F H M. (1993). The efficacy and persistent anthelmintic effect of ivermectin in
sheep. Veterinari Parasitology 50(1–2): 117–124.
Campbell W C. (1990). Benzimidazoles: Veterinary uses. Parasitology Today 6(4): 130–
Campbell W C, Fisher M H, Stapley E O, Albers-Schonberg G and Jacob T A. (1983).
Ivermectin: A potent new antiparasitic agent. Science 221(4513): 823–828.
Canga A G, Prieto A M S, Liebana M J D, Martinez N F, Vega M S and Vieitez J J G.
(2009). The pharmacokinetics and metabolism of ivermectin in domestic animal species. The Veterinary Journal 179(1): 25–37.
Cezar A S, Toscan G, Camillo G, Sangioni L A, Ribas H O and Vogel F S F. (2010).
Multiple resistance of gastrointestinal nematodes to nine different drugs in a sheep flock in southern Brazil. Veterinary Parasitology 173(1–2): 157–160.
Coles G C. (2005). Anthelminthic resistance – Looking to the future: A UK perspective.
Research in Veterinary Science 78(2): 99–108.
Coles G C, Bauer C, Borgsteede F H, Geerts S, Klei T R, Tylor M A and Waller P J.
(1992). World Association for the Advancement of Veterinary Parasitology (WAAVP) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Veterinary Parasitology 44(1–2): 35–44.
Coop R L and Kyriazakis I. (2001). Influence of host nutrition on the development and
consequences of nematode parasitism in ruminants. Trends in Parasitology 17(7): 325–330.
Coyne M J, Smith G and Johnstone C. (1991). Fecundity of gastrointestinal
trichostrongylid nematodes of sheep in the field. American Journal of Veterinary Research 52(7): 1182–1188.
Craig T M. (1993). Anthelmintic resistance. Veterinary Parasitology 46(1–4): 121–131. Dobson R J, Hosking B C, Jacobson C L, Cotter J L, Besier R B, Stein P A and Reid S A.
(2012). Preserving new anthelmintics: A simple method for estimating faecal egg count reduction test (FECRT) confidence limits when efficacy and/or nematode aggregation is high. Veterinary Parasitology 186(1–2): 79–92.
Echevarria F A M, Armour J and Duncan J L. (1991). Efficacy of some anthelmintics on an
ivermectin-resistant strain of Haemonchus contortus in sheep. Veterinary Parasitology 39(3–4): 279–284.
Egerton J R, Ostlind D A, Blair L S, Eary C H, Suhayda D, Cifelli S, Riek R F and
Campbell W C. (1979). Avermectins, new family of potent anthelmintic agents: Efficacy of the B1a component. Antimicrobial Agents and Chemotherapy 15(3): 372–378.
Foreyt W J. (2001). Veterinary parasitology reference manual. Iowa, USA: Blackwell
Geary T G, Sims S M, Thomas E M, Vanover L, Davis J P, Winterrowd C A, Klein R D, Ho
N F H and Thompson D P. (1993). Haemonchus contortus: Ivermectin-induced paralysis of the pharynx. Experimental Parasitology 77(1): 88–96.
George N, Persad K, Sagam R, Offiah V N, Adesiyun A A, Harewood W, Lambie N and
Basu A K. (2011). Efficacy of commonly used anthelmintics: First report of multiple drug resistance in gastrointestinal nematodes of sheep in Trinidad. Veterinary Parasitology 183(1–2): 194–197.
Gibson T E. (1983). The influence of nutrition on the relationship between gastrointestinal
parasites and their hosts. Proceedings of the Nutrition Society 22(1): 15–20.
Gill H S, Watson D L and Brandon M R. (1993). Monoclonal antibody to CD4+ T cells
abrogates genetic resistaance to Haemonchus contortus in sheep. Immunology 78(1): 43–49.
Gogolewski R P, Allerton G R, Langholff W K, Cramer L G and Eagleson J S. (1995). An
ivermectin tablet for sheep: Efficacy against gastro-intestinal nematodes and a bioavailability comparison with a liquid ivermectin formulation. Veterinary Parasitology 60(3–4): 297–302.
Ivermectin and Albendazole against Haemonchus contortus
Green P E, Forsyth B A, Rowan K J and Payne G. (1981). The isolation of a field strain of
Haemonchus contortus in Queensland showing multiple anthelmintic resistance. Australian Veterinary Journal 57(2): 79–84.
Hubert J, Kerboeuf D, Nicolas J A, Dubost G and Gayaud C. (1991). Resistance of small
ruminant nematodes to benzimidazole derivative compounds in limousin france. Recueil De Medecine Veterinaire De L'Ecole D'Alfort 167(2): 135–140.
Hughes P L, McKenna P B and Dowling A F. (2005). A survey of the prevalence of
emerging macrocyclic lactone resistance and of benzimidaazole resistance in sheep nematodes in the lower North Island of New Zealand. New Zealand Veterinary Journal 53(1): 87–90.
Kaplan R M. (2004). Drug resistance in nematodes of veterinary importance: A status
report. Trends in Parasitology 20(10): 477–481.
Kerboeuf D, Beaumont-Schwartz C, Hubert J and Maillon M. (1988). Resistance to
anthelmintics of gastrointestinal nematodes. Results of a survey in Val de Loire (France). Recuil de Medicine Veterinaire 164(12): 1001–1006.
Kochapakdee S, Pandey V S, Pralomkarm W, Choldumrongkul S, Ngampongsai W and
Lawpetchara A. (1995). Anthelmintic resistance in goats in southern Thailand. Veterinary Record 137(5): 124–125.
Kohler P. (2001). The biochemical basis of anthelmintic action and resistance.
International Journal for Parasitology 31(4): 336–345.
Lacey E. (1990). Mode of action of benzimidazoles. Parasitology Today 6(4): 112–115. Lanusse C E and Prichard R K. (1993). Clinical pharmacokinetics and metabolism of
benzimidazole enthelmintics in ruminants. Drug Metabolism Review 25(3): 235–279.
Le Jambre L F. (1993). Ivermectin-resistant Haemonchus contortus in Australia. Australian
Veterinary Journal 70(9): 357.
Leathwick D M, Hosking B C, Bisset SA and McKay C H. (2009). Managing anthelmintic
resistance: Is it feasible in New Zealand to delay the emergence of resistance to a new anthelmintic class. New Zealand Veterinary Journal 57(4): 181–192.
Lloyd S and Soulsby E J L. (1987). Immunobiology of gastrointestinal nematodes of
ruminants. In E J L Soulsby (ed.). Immune response in parasitic infections: Immunology, immunopathology, and immunoprophylaxis, vol 1. Florida, USA: CRC Press Inc., 1–41.
McKellar Q and Scott E. (1990). The benzimidazole anthelmintic agents – A review.
Journal of Veterinary Pharmacology and Therapeutics 13(3): 223–247.
McKenna P B. (2006). Further comparison of faecal egg count reduction test procedures:
Sensitivity and specificity. New Zealand Veterinary Journal 54(6): 365–366.
McKenna P B and Watson T G. (1987). The comparative efficacy of four broad spectrum
anthelmintics against some experimentally induced trichostrongylid infections in sheep and goats. New Zealand Veterinary Journal 35(11): 192–195.
Miller D K and Craig T M. (1996). Use of anthelmintic combinations against multiple
resistant Haemonchus contortus in Angora goats. Small Ruminant Research 19(3): 281–283.
Monnig H O. (1950). Veterinary helminthology and entomology: The diseases of
domesticated animals caused by helminth and arthropod parasites. London: Baillière, Tindall & Cox.
Prichard R K, Hall C A, Kelly J D, Martin I C A and Donald D A. (1980). The problem of
anthelmintic resistance in nematodes. Australian Veterinary Journal 56(5): 239–250.
Mwamachi D M, Audho J O, Thorpe W and Baker R L. (1995). Evidence for multiple
anthelmintic resistance in sheep and goats reared under the same management in coastal Kenya. Veterinary Parasitology 60(3–4): 303–313.
Silvia Puspitasari et al.
Rahman W A. (1994). Resistance to benzimidazole anthelmintics by Haemonchus
contortus in goats in Peninsular Malaysia. Veterinary Parasitology 55(1–2): 155–157.
Sangster N C and Gill J. (1999). Pharmacology of anthelminthic resistance. Parasitology
Today 15(4): 141–146.
Sangster N, Betterham P, David Chapman H, Duraisingh M, Le Jambre L, Shirley M and
Upcroft P. (2002). Resistance to antiparasitic drugs: The role of molecular diagnosis. International Journal for Parasitology 32(5): 637–653.
Smith G, Grenfell B T, Isham V and Cornell S. (1999). Anthelmintic resistance revisited:
Under-dosing, chemoprophylactic strategies, and mating probabilities. International Journal for Parasitology 29(1): 77–91.
Traversa D, Paoletti B, Otranto D and Miller J. (2007). First report of multiple drug
resistance in trichostrongyles affecting sheep under field conditions in Italy. Parasitology Research 101(6): 1713–1716.
Uppal R P, Yadav C L, Godara P and Rana Z S. (1992). Multiple anthelmintic resistance
in a field strain of Haemonchus contortus in goats. Veterinary Research Communications 16(3): 195–198.
van Wyk J A, Malan F S, Gerber H M and Alves R M. (1989). The problem of escalating
resistance of Haemonchus contortus to the modern anthelmintics in South Africa. Thearch 56(1): 41–49.
Vieira L S, Berne M E A, Cavalcante A C R and Costa C A F. (1992). Haemonchus
contortus resistance to ivermectin and netobimin in Brazilian sheep. Veterinary Parasitology 45(1–2): 111–116.
Waruiru R M, Ngotho J W and Mukiri J G. (1998). Multiple and multigeneric anthelmintic
resistance on a sheep farm in Kenya. Tropical Animal Health and Production 30(3): 159–166.
Wolstenholme A J. (2011). Ion channels and receptor as targets for the control of parasitic
nematodes. International Journal for Parasitology: Drug and Drugs Resistance 1(1): 2–13.
Wolstenholme A J, Fairweather I, Prichard R, von Samson-Himmelstjerna G and Sangster
N C. (2004). Drug resistance in veterinary helminths. Trends in Parasitology 20(10): 469–476.
Yacob H T, Mistre C H, Adem A H and Basu A K. (2009). Parasitological and clinical
responses of lambs experimentally infected with Haemonchus contortus (L3) with and without ivermectin treatment. Veterinary Parasitology 166(1–2): 119–123.
Yates D M, Portillo V and Wolstenholme A J. (2003). The avermectin receptors of
Haemonchus contortus and Caenorhabditis elegans. International Journal for Parasitology 33(11): 1183–1193.
Zainalabidin F A, Raimy N, Yaacob M H, Musbah A, Bathmanaban P, Ismail E A, Mamat
Z C, Zahari Z, Ismail M I and Panchadcharam C. (2014). The prevalence of parasitic infestation of small ruminant farms in Perak, Malaysia. Tropical Life Sciences Research 25(2):1–8.
Source: http://www.tlsr.usm.my/tlsr27012016/27012016_08.pdf
Dynamic Energy-Aware Capacity Provisioning for Cloud Mohamed Faten Zhani University of Waterloo University of Waterloo National University of Defense Waterloo, ON, Canada Waterloo, ON, Canada Changsha, Hunan, China Joseph L. Hellerstein University of Illinois at University of Waterloo Google, Inc. Urbana-Champaign, USA Waterloo, ON, Canada Seattle, Washington, USA
Biophysical Journal Imaging Cells and Tissues with Refractive Index Radiology Y. Hwu,* W. L. Tsai,* H. M. Chang,y H. I. Yeh,z P. C. Hsu,* Y. C. Yang,* Y. T. Su,* H. L. Tsai,yG. M. Chow,§ P. C. Ho,{ S. C. Li,{ H. O. Moser,k P. Yang,k S. K. Seol,** C. C. Kim,**J. H. Je,** E. Stefanekova,yy A. Groso,yy and G. Margaritondoyy*Institute of Physics, Academia Sinica, Nankang, Taipei, Taiwan; yPandis Biomedical Research Association, Chupei, Taiwan;
