Mxn008 15.34
Epidemiologic Reviews
ª The Author 2008. Published by the Johns Hopkins Bloomberg School of Public Health.
All rights reserved. For permissions, please e-mail:
[email protected].
Advance Access publication July 16, 2008
Dementia of the Alzheimer Type
Jessica J. Jalbert1, Lori A. Daiello1,2, and Kate L. Lapane1
1 Department of Community Health – Epidemiology, Warren Alpert School of Medicine at Brown University, Providence, RI.
2 Alzheimer's Disease and Memory Disorders Center, Rhode Island Hospital, Providence, RI.
Accepted for publication May 12, 2008.
Dementia of the Alzheimer type is a progressive, fatal neurodegenerative condition characterized by
deterioration in cognition and memory, progressive impairment in the ability to carry out activities of daily living,and a number of neuropsychiatric symptoms. This narrative review summarizes the literature regardingdescriptive epidemiology, clinical course, and characteristic neuropathological changes of dementia of theAlzheimer type. Although there are no definitive imaging or laboratory tests, except for brain biopsy, for diagnosis,brief screening instruments and neuropsychiatric test batteries used to assess the disease are discussed.
Insufficient evidence exists for the use of biomarkers in clinical practice for diagnosis or disease management, butpromising discoveries are summarized. Optimal treatment requires both nonpharmacological and pharmacolog-ical interventions, yet none have been shown to modify the disease's clinical course. This review describes thecurrent available options and summarizes promising new avenues for treatment. Issues related to the care ofpersons with dementia of the Alzheimer type, including caregiver burden, long-term care, and the proliferation ofdementia special care units, are discussed. Although advances have been made, more research is needed toaddress the gaps in our understanding of the disease.
Alzheimer disease; dementia; drug therapy; review
Abbreviations: APOE, apolipoprotein E; DAT, dementia of the Alzheimer type; DSM-IV-TR, Diagnostic and Statistical Manualof Mental Disorders, fourth edition text revision; MMSE, Mini-Mental State Examination; NINCDS/ADRDA, National Institute ofNeurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association.
dition if current trends persist and no preventive treatmentsbecome available (4). The cognitive, behavioral, and func-
Dementia of the Alzheimer type (DAT) is a progressive,
tional decline in patients with DAT places a considerable
fatal neurodegenerative condition characterized by deterio-
burden on the health care system and caregivers (5). DAT is
ration in cognition and memory, progressive impairment in
therefore a growing medical, social, and economic problem.
the ability to carry out activities of daily living, and a number
Despite the urgency of the situation, many questions re-
of neuropsychiatric and behavioral symptoms (1). DAT is
main unanswered in DAT research. For instance, although
the most common form of dementia among elderly persons
advanced age, female gender, carrying the apolipoprotein E
and accounts for approximately two thirds of cases of de-
(APOE) e4 allele, current smoking, family history of DAT or
mentia and between 60 percent and 70 percent of cases of
other dementia, fewer years of formal education, lower in-
progressive cognitive impairment in older adults (2, 3). The
come, and lower occupational status have been associated
prevalence of DAT is expected to increase as the population
with an increased risk of developing the condition, the path-
ogenesis of Alzheimer's disease is still largely unknown (6–8).
In 2000, approximately 4.5 million people in the United
Although progress is being made in developing new therapies
States were living with DAT; by 2050, more than 13 million
for DAT, no therapeutic interventions to cure or substantially
older Americans are projected to be afflicted with the con-
modify disease progression currently exist.
Correspondence to Jessica J. Jalbert, Department of Community Health – Epidemiology, Warren Alpert School of Medicine at Brown University,121 South Main, Box G, Providence, RI 02912 (e-mail:
[email protected]).
Epidemiol Rev 2008;30:15–34
Jalbert et al.
This review provides an update on the current state of
In the United States, 12 percent of the population is at
knowledge on DAT. With a focus on findings generated from
least 65 years of age (13). By 2020, 16 percent of the pop-
studies conducted in the United States, it describes the ep-
ulation will be 65 years of age or older, and adults over 80
idemiology of DAT, its characteristic clinical course, neuro-
years of age are expected to account for 3.7 percent of the
psychiatric symptoms, factors associated with accelerated
population (14, 15). Growth will occur in all racial and ethnic
cognitive decline, characteristic neuropathological changes
groups (4). By 2050, the number of persons with DAT is
of Alzheimer's disease, diagnostic tools to assess DAT, and
expected to increase to 13.2 million, and it is estimated that
biomarkers and neuroimaging, and it provides an overview
more than 8.0 million cases will be older than age 85 years (4).
of pharmacological and nonpharmacological treatments. Wealso summarize the ramifications of DAT for caregivers anddiscuss long-term care.
Patients with DAT are likely to exhibit neuropsychiatric
symptoms, also commonly referred to in the literature as
The following definitions were adapted from the position
behavioral and psychological symptoms of dementia, such
statement of the American Association for Geriatric Psychi-
as aggression/agitation, depression, apathy, anxiety, delu-
sions, and hallucinations at some point during the course
Dementia is a clinical syndrome characterized by global
of the illness. Neuropsychiatric symptoms are common in
cognitive decline with memory and one other area of cog-
all stages of dementia with prevalence estimates between 60
nition affected that interfere significantly with the person's
percent and 80 percent, depending on whether patients are
ability to perform the tasks of daily life and meet the
community dwelling or institutionalized, and a lifetime risk
Diagnostic and Statistical Manual of Mental Disorders,
of nearly 100 percent (16–20). The prevalence of neuropsy-
fourth edition text revision (DSM-IV-TR) criteria.
chiatric symptoms in persons with DAT or dementia is
Dementia resulting from Alzheimer's disease or DAT is
greater than the background prevalence in the general pop-
characterized by decline primarily in cortical aspects of
ulation (16, 17, 21–24). Those symptoms most commonly
cognition (i.e., memory, language, praxis) and follows a char-
seen in patients with DAT or dementia are apathy, depres-
acteristic time course of gradual onset and progression.
sion, anxiety, aggression/agitation, and psychosis (delusions
Alzheimer's disease is a specific degenerative brain disease
and hallucinations). The prevalence of apathy ranges from
characterized by senile plaques, neuritic tangles, and progres-
20 percent to 51 percent and the 5-year prevalence is esti-
sive loss of neurons, the presumptive cause of Alzheimer's
mated as 71 percent (16, 18, 21, 23); the respective preva-
lences are 15–54 percent and 77 percent for depression(16, 18, 21, 22, 24–27) and 10–59 percent and 62 percent
GLOBAL INCIDENCE AND PREVALENCE OF DAT
for anxiety (16, 18, 21, 23, 28). The prevalence estimates foraggression/agitation and psychosis range from 13 percent to
Data documenting the incidence of DAT indicate that it is
30 percent and from 12 percent to 74 percent, respectively
a global problem that will become more severe as the pop-
(16, 18, 21, 29, 30). The considerable variation in the prev-
ulation ages. Table 1 summarizes population-based studies
alence estimates results from the different operational def-
estimating the incidence and prevalence of DAT. Studies
initions of dementia and neuropsychiatric symptoms, the
from different parts of the world (North America, Europe,
different types of dementia studied, and the heterogeneity
Asia, and Africa) were selected if they were population
of the study populations. Other less-common and less-
based and large. The estimates from the Delphi Consensus
studied neuropsychiatric symptoms include irritability, ela-
Study are for dementia rather than DAT but were included
tion, disinhibition, wandering, and aberrant motor behavior.
because prevalence/incidence estimates were generated
Neuropsychiatric symptoms in DAT may be better cap-
from a systematic review of population-based studies.
tured by grouping individual symptoms into various clusters
Regardless of country of origin, age-specific incidence rates
(20, 31–34). The motivation behind identifying symptom
of DAT increase exponentially with advancing age. In the
clusters is that they may form syndromes, with each DAT–
United States, the incidence rate of DAT is 1 per 1,000
neuropsychiatric symptom subtype having a different prev-
person-years among individuals aged 60–64 years and 25
alence and time course as well as distinct biologic correlates
per 1,000 person-years among those older than age 85 years
and psychosocial determinants (32). If neuropsychiatric
(10). Although DAT is not a normal part of the aging pro-
symptom clusters reflect differences in brain regions af-
cess, the prevalence of DAT also increases with advancing
fected by the disease, pharmacological and nonpharmaco-
age. While less than 1 percent of individuals aged 60–64
logical treatment opportunities could be optimized (20, 32).
years are deemed to be affected, it is estimated that up to 40
Neuropsychiatric symptoms in DAT may be classified into
percent of those over the age of 85 years have the condition
three groups: an affective syndrome, a psychotic syndrome,
(11). Similar trends were observed in a population-based
and other neuropsychiatric disturbances (20). Diagnostic
European study of persons aged 65 years or older. The
criteria for DAT-associated affective disorder and DAT-
age-standardized prevalence of DAT was 4.4 percent, and
associated psychotic disorder have been proposed (34). Other
the prevalence increased with age (0.6 percent for those
neuropsychiatric symptom classification systems, all of
aged 65–69 years; 22.2 percent for those aged 90 years or
which have identified clusters of mood or psychotic neuro-
older) (12).
psychiatric symptoms, have also been advanced (17, 35–39).
Epidemiol Rev 2008;30:15–34
Dementia of the Alzheimer Type
NEUROPATHOLOGICAL FEATURES OF ALZHEIMER'S
retrospectively determined symptom onset and 5.7 years
(standard deviation, 0.1) from initial clinic presentation(79). Baseline level of cognition may not predict mortality,
Alzheimer's disease can be definitively diagnosed only at
but mortality is strongly related to rate of cognitive decline
brain autopsy or biopsy, when neuritic plaques reach a cer-
(76). Indeed, the lack of effective predictors of the rate of
tain number in the most severely affected regions of the
deterioration extends to the earliest stages of dementia (80).
neocortex (40, 41). More stringent research criteria require
In the early clinical stage, deficits occur in episodic mem-
the presence of neuritic plaques and neurofibrillary tangles
ory, verbal abilities, visuospatial functions, attention, and
in the neocortex (42–44).
executive functions (81). Sensory-motor performance and
Neuritic plaques consist of a central core of beta-amyloid
procedural memory seem to be intact, and only slight im-
peptides clumped together with fibrils of beta-amyloid, dys-
pairment may be seen in primary memory (81). Cognitive
trophic neurites, reactive astrocytes, phagocytic cells, and
decline stems from unifunctional to global deficits (81).
other proteins and protein fragments derived from degener-
Performance falls off rapidly in all areas of cognitive func-
ating cells or liberated from neurons (45, 46). The accumu-
tioning, but abilities thought to be subserved by the medial
lation of beta-amyloid seen in Alzheimer's disease brains may
and lateral temporal lobes (episodic and semantic memory,
be the result of faulty beta-amyloid clearance (47), cleaving
respectively) appear to be substantially more impaired than
of the amyloid precursor protein by enzymes to yield free
those abilities thought to be subserved by the frontal lobes
beta-amyloid peptides (48), or overproduction of beta-
(82). Yearly cognitive decline varies from a loss of 2.7–4.5
amyloid peptides caused by mutations in the amyloid pre-
points on the Mini-Mental State Examination (MMSE), 1.8–
cursor protein or the presenilins (49–54) or in the presence
4.2 points on the Blessed Dementia Scale, and 12–13 points
of the APOE e4 genotype (55, 56). Beta-amyloid fibrils
on the Cambridge Cognitive Examination to a gain of 2.6–
aggregate and neuritic plaques form, triggering a locally
4.5 points on the Blessed Test of Information, Memory, and
induced, non-immune-mediated, chronic inflammatory re-
Concentration (83).
sponse involving microglial cell activation and stimulation
The presence of one or more APOE e4 alleles is a signif-
of a cerebral acute-phase reaction (57) (figure 1). Activated
icant predictor of the incidence of delusions during the
microglial cells release potentially neurotoxic proinflamma-
course of DAT (84). The frequency and intensity of neuro-
tory cytokines (e.g., interleukin-6), reactive oxygen and ni-
psychiatric symptoms may increase with declining cognitive
trogen species, and proteolytic enzymes that may exacerbate
function in patients with DAT (76, 85–87) or may simply be
neuronal damage (58, 59). Beta-amyloid fibrils also appear
correlated with duration of disease (88). Curvilinear associ-
to exert direct neurotoxic effects (60–62).
ations between dementia severity and neuropsychiatric
Oxidative stress resulting from free radical damage may
symptoms such as forgetfulness and emotional and impul-
also be caused when soluble, aggregated amyloid fibrils are
sive behaviors have been reported (89, 90). Consensus has
inserted into neuronal membranes, inducing lipid perioxida-
yet to be reached on whether the prevalence of individual
tion, protein oxidation, and formation of reactive oxygen
neuropsychiatric symptoms remains constant at all stages of
and nitrogen species (63). APOE may, in e4 allele carriers,
dementia or whether it varies systematically depending on
exacerbate oxidative stress through its association with the
the stage of the disease (21, 30, 91–94).
catabolism of polyunsaturated fatty acids (63). Oxidative
Some DAT patients appear to have neuropsychological
stress results in loss of cell potential, accumulation of ex-
deficits more prominent in one domain than in other do-
citotoxic molecules, and decreased neuronal viability (61).
mains (95). Language impairment in DAT may be associ-
Healthy neurons have microtubules stabilized by the tau
ated with two distinct neuropsychological abnormalities:
protein; in Alzheimer's disease, this protein is hyperphos-
1) a lexical/semantic impairment unrelated to onset or
phorylated and aggregates as paired helical filaments, caus-
2) progression of symptoms and a syntactic impairment that
ing the dissociation of microtubules and the formation of
may be associated with earlier onset and more rapid pro-
neurofibrillary tangles that result in neurotransmitter deficits
gression of dementia (96, 97). The annual decline in lan-
and neuronal cell death (45, 64–66). Beta-amyloid deposits
guage composite score was approximately 0.71 standard
may accelerate the formation of neurofibrillary tangles in
units, which did not differ by gender (98).
brain areas associated with Alzheimer's disease (67, 68).
Declining cholinergic function (69–71), reductions in syn-aptic density (71, 72), and the loss of neurons (71, 73–75)
CORRELATES OF MORE RAPID COGNITIVE DECLINE
are also consistent features of Alzheimer's disease.
CHARACTERISTICS AND CLINICAL COURSE OF DAT
Progressive cognitive decline is the principal clinical
manifestation of DAT, and a faster rate of decline is strongly
DAT is associated with increased mortality, but survival
associated with mortality (76). The rates at which people
among those with the disease varies widely (76, 77). Esti-
decline, however, differ substantially between affected per-
mates of mean survival time are hampered by lack of de-
sons, are difficult to predict, and are still not well understood
finitive onset-of-disease dates. In a study that followed
persons with DAT for an average of 4 years, 54 percent were
The APOE e4 allele, a strong genetic risk factor for DAT, is
institutionalized and 49 percent died (78). Median survival
associated with a greater risk of developing DAT (odds ratio 5
is estimated at 11.8 years (standard deviation, 0.6) since
14.9, 95 percent confidence interval: 10.8, 20.6 for persons
Epidemiol Rev 2008;30:15–34
Jalbert et al.
Summary of studies estimating incidence and prevalence of DAT* and dementia
Population/study design
Measure of disease frequency
Incidence studies
Canadian Study of Health and
Population-based Canadian cohort study
Women 65–69 years of age: 1.4 per 1,000 person-years
Aging, Canadian Study of
of 5,432 community-dwelling and 210
(95% CI*: 0.1, 3.3); men 65–69 years of age: 0; women
Health and Aging Working
institutionalized persons 65 years of
85 years of age or older: 49.0 per 1,000 person-years
(95% CI: 40.7, 57.2); men 85 years of age or older:44.2 per 1,000 person-years (95% CI: 31.0, 57.5);women all ages: 7.4 per 1,000 person-years (95% CI:4.4, 10.4); men all ages: 5.9 per 1,000 person-years(95% CI: 2.0, 9.8)
Cache County Study, Miech
US population-based cohort study of
68 years of age or less: 2.2 per 1,000 person-years;
3,308 persons aged 65 years or older
84–86 years of age: 57.9 per 1,000 person-years;all ages: 16.8 per 1,000 person-years
Monongahela Valley
US population-based cohort study of
65–69 years of age: 2.1 per 1,000 person-years
Independent Elders Survey
1,298 rural persons aged 65 years
(95% CI: 0.6, 7.8); 90 years of age or older: 50.9 per
(MoVIES), Ganguli et al.
1,000 person-years (95% CI: 23.3, 111.0); all ages:
11.6 per 1,000 person-years (95% CI: 9.5, 14.2)
North America/Africa
Hendrie et al. (294)
2,459 Yoruba residents of Ibadan,
Annual age-standardized incidence rate of DAT:
Nigeria, aged 65 years or older; 2,147
Nigeria: 1.15% (95% CI: 0.96, 1.35); Indiana:
African Americans residing in
2.52% (95% CI: 1.4, 3.64)
Indianapolis, Indiana, aged 65 yearsor older
Fratiglioni et al. (295)
Estimates of DAT incidence in persons
65–69 years of age: 1.2 per 1,000 person-years
65 years of age or older obtained by
(95% CI: 0.6, 2.3); over 90 years of age:
pooling population-based data from
53.5 per 1,000 person-years (95% CI: 36.5, 55.8)
European population-based studies
Neurologic Disorders in
Population-based Spanish survey of
65–69 years of age: 1.5 per 1,000 person-years
Central Spain Survey,
3,891 persons aged 65–90 years
(95% CI: 0.3, 4.4); 90 years of age or older: 52.6 per
Bermejo-Pareja et al. (296)
1,000 person-years (95% CI: 31.7, 82.2); age-adjustedincidence rate: 7.4 per 1,000 person-years (95% CI:6.0, 8.8)
Conselice Study of Brain
Italian prospective population-based
65–74 years of age: 11.3 per 1,000 person-years
Imaging, Ravaglia et al.
study of 927 persons aged 65 years
(95% CI: 7.1, 17.9); 85–94 years of age: 75.8 per
1,000 person-years (95% CI: 49.4, 116.2); all ages:23.8 per 1,000 person-years (95% CI: 17.3, 31.7)
Rotterdam Study, Ruitenberg
Population-based Dutch study of 7,046
65–69 years of age: 1.3 per 1,000 person-years
persons aged 55 years or older
(95% CI: 0.7, 2.3); 85–89 years of age: 34.8 per1,000 person-years (95% CI: 27.7, 43.9); all ages:7.2 per 1,000 person-years (95% CI: 6.4, 8.1)
Chinese cohort of 1,593 persons aged
All ages: 5.4 per 1,000 person-years
60 years or older residing in Beijing
Indo-US Cross-National
Population-based Indian study of 5,126
65–74 years of age: 1.2 per 1,000 person years
Dementia Epidemiology
persons aged 55 years or older
(95% CI: 0.25, 3.57); 85 years of age or older:
Study, Chandra et al. (300)
24.8 per 1,000 person-years (95% CI: 5.1, 72.5);65 years of age or older: 3.24 per 1,000person-years (95% CI: 1.48, 6.14)
homozygous for the e4 genotype; persons with only one
process regarding development of DAT and that cognitive
copy of e4 are also at increased risk—odds ratio 5 2.6, 95
decline should progress more rapidly in these patients (102),
percent confidence interval: 1.6, 4.0 for those with an e2/e4
but studies have provided conflicting evidence on whether
genotype; odds ratio 5 3.2, 95 percent confidence interval:
the APOE e4 allele is associated with an accelerated rate of
2.8, 3.8 for those with an e3/e4 genotype, relative to persons
cognitive decline (102–107).
with an e3/e3 genotype) (100). Earlier age at onset is ob-
High educational attainment is also associated with an
served in a dose-dependent fashion (the average age at onset
accelerated rate of cognitive deterioration in DAT patients
for persons with genotype e4/e4, only one e4 allele, and no
(108, 109), and the cognitive reserve hypothesis has been
e4 allele is 68 years, 76 years, and 84 years, respectively)
proposed to explain this association. For instance, someone
(101). These findings have led to the hypothesis that APOE e4
with a higher number of neuronal synapses or neurons could
allele carriers may experience a more rapid degenerative
withstand a higher degree of neuropathological change
Epidemiol Rev 2008;30:15–34
Dementia of the Alzheimer Type
Population/study design
Measure of disease frequency
Delphi Consensus Study,
Estimates of annual incidence of
North America: 10.5; Latin America: 9.2; western
Ferri et al. (301)
dementia (per 1,000) in persons
Europe: 8.8; eastern Europe: 7.7–8.1; North Africa
60 years of age or older derived by
and Middle Eastern Crescent: 7.6; Africa: 3.5; India
using the Delphi consensus approach
and south Asia: 4.3; Indonesia, Thailand, and
and guided by a systematic review of
Sri Lanka: 5.9; China and developing western
Pacific: 8.0; developed western Pacific: 7.0; worldannual incidence: 7.5
Prevalence studies
Health and Retirement Study,
Nationally representative sample of the
71–79 years of age: 5.0%; 90 years of age or
Plassman et al. (302)
US population (N 5 856) aged 71
older: 37.4%; all ages: 9.7%
Framingham Study, Bachman
US population-based cohort study
Men: 1.17%; women: 3.01%
North America/Africa
Hendrie et al. (304)
2,494 Yoruba residents of Ibadan,
Age-adjusted prevalence in Nigeria: 1.41%;
Nigeria, aged 65 years or older;
age-adjusted prevalence in Indiana for community-
2,212 African Americans residing in
dwelling persons: 3.69%; age-adjusted prevalence in
Indianapolis, Indiana, aged 65 years
Indiana for persons living in the community and in
nursing homes: 6.24%
Farrag et al. (305)
Population-based Egyptian study of
4.5% (95% CI: 3.6, 5.4)
persons older than age 60 years
Prevalence estimate for DAT in persons
Age-standardized prevalence: 4.4%; 65–69 years of
65 years of age or older obtained by
age: 0.6%; 90 years of age or older: 22.2%
pooling population-based data fromEuropean population-based studies
Rotterdam Study, Ott
Population-based study of Dutch
55–64 years of age: 0.20%; 85 years of age or older:
persons aged 55 years or older
26.8%; all ages: 4.5%
Gurvit et al. (307)
Population-based Turkish study of
11.0% (95% CI: 7.0, 15.0)
persons older than age 70 years
Dong et al. (308)
Estimate of prevalence of DAT in
1.6% (95% CI: 1.0, 2.7)
China among persons aged 60 yearsor older derived from systematicanalysis of work published between1980 and 2004
Zhang et al. (309)
Prevalence of DAT among persons
65 years of age or older across fourregions in China: Beijing, Xian,Shanghai, Chengdu
Delphi Consensus Study,
Estimates of prevalence of dementia in
North America: 6.4%; Latin America: 4.6%; western
Ferri et al. (301)
persons 60 years of age or older
Europe: 5.4%; eastern Europe: 3.8%23.9%;
derived by using the Delphi
North Africa and Middle Eastern Crescent: 3.6%;
consensus approach and guided by
Africa: 1.6%; India and south Asia: 1.9%; Indonesia,
a systematic review of published work
Thailand, and Sri Lanka: 2.7%; China and developingwestern Pacific: 4.0%; developed western Pacific:4.3%; world prevalence 2001: 3.9%
* DAT, dementia of the Alzheimer type; CI, confidence interval.
before becoming symptomatic (45, 109, 110). If patients
toms such as aggression/agitation, depression, psychosis,
with higher educational levels also have a higher cognitive
delusions, and hallucinations (30, 78, 111–113), but these
reserve, then, when DAT symptoms become apparent, the
findings have been challenged (114–118). The neurobiology
pathological burden will already be more severe and wide-
underlying the emergence of neuropsychiatric symptoms is
spread, and, with the cognitive reserve depleted, the patient
far from being understood, as is the mechanism by which
would appear to experience cognitive decline at a more
these symptoms may accelerate cognitive decline (119–123).
rapid rate (45, 109). Rapid cognitive decline has also been
Antipsychotic medications, widely used to treat neuro-
observed among patients exhibiting neuropsychiatric symp-
psychiatric symptoms (124, 125), have also been identified
Epidemiol Rev 2008;30:15–34
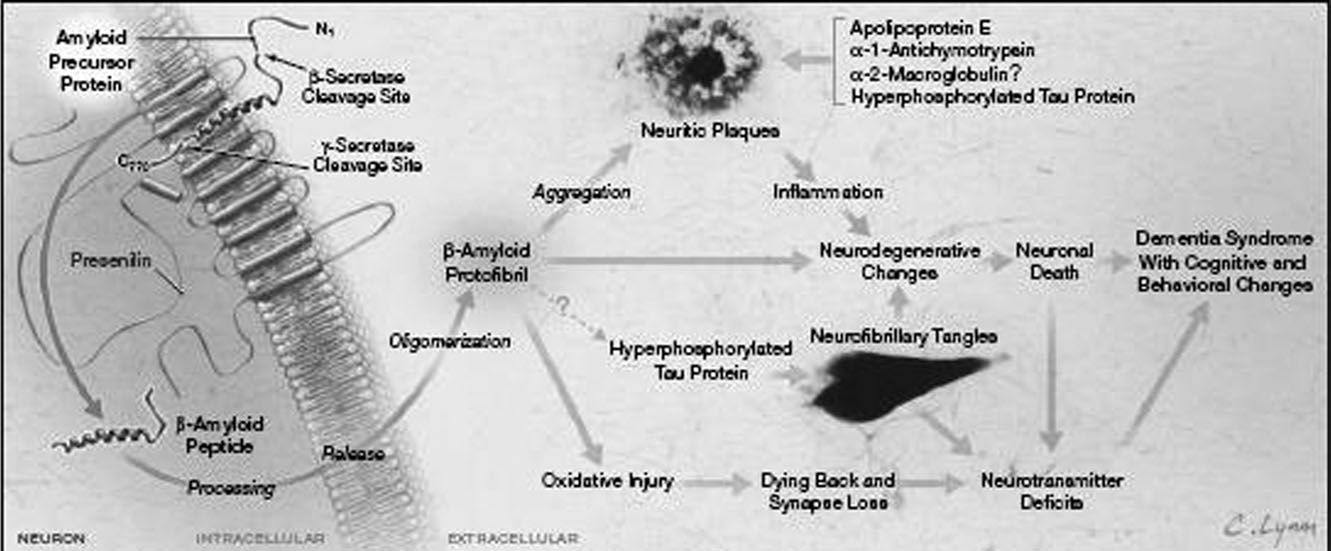
Jalbert et al.
Cascade of neuropathological events leading to the behavioral and cognitive features of dementia. Reproduced with permission from
primary author J. L. Cummings and from JAMA 2002;287:2335–2338. Copyright ª 2002, American Medical Association. All rights reserved.
as a factor accelerating cognitive decline (114, 126).
to 96 percent and that the specificity of these criteria for
Although it is possible that antipsychotics could exacerbate
DAT against other dementia ranges from 23 percent to 88
cognitive deficits through their anticholinergic effects (117),
some studies have failed to corroborate the findings that
Table 2 briefly summarizes the screening instruments
antipsychotic medications are associated with more rapid
used to determine the need for further evaluation. The
cognitive decline in DAT patients (117, 127–129). Although
MMSE (140), consisting of a brief assessment of language,
there is little information on the effects of other commonly
memory, praxis, and orientation, is the most widely used and
used psychotropic medications on cognition in patients with
has been the most extensively studied with respect to its
DAT (125, 129), a positive association between certain psy-
accuracy and validity (141–146). MMSE scores are affected
chotropic medications (sedatives and anxiolytics) and cogni-
by gender, educational attainment, age, and cultural back-
tive deterioration has been reported (129).
ground (143, 147, 148); the sensitivity of the MMSE is poorfor patients with mild dementia (2, 149); the instrument isconsidered too time-consuming to administer in routine
ASSESSMENT METHODS
clinical practice (150, 151); not all changes in MMSE scoresnecessarily reflect true clinical improvement or decline
In practice and in research, DAT is diagnosed by applying
(152, 153); and the MMSE exhibits floor effects in patients
the DSM-IV-TR criteria (130) and/or those of the National
with severe impairment and ceiling effects in those who are
Institute of Neurological and Communicative Diseases and
mildly impaired (154). Modifications, alternatives, and sup-
Stroke/Alzheimer's Disease and Related Disorders Associ-
plements to the MMSE such as the modified version of the
ation (NINCDS/ADRDA) (40). Whereas the DSM-IV-TR
MMSE (155); the Montreal Cognitive Assessment (156);
criteria require the presence of memory impairment and
the Memory Impairment Screen (157); the Blessed Test of
cognitive deterioration in one other domain such as lan-
Information, Memory, and Concentration (158) and its
guage, perception, or motor skills, or disturbances in exec-
abridged version, the Short Blessed Test (159); the One-
utive functioning (130), the NINCDS/ADRDA criteria
Minute Verbal Fluency Test for Animals; and the Clock
classify the likelihood of DAT into one of three categories:
Drawing Test (160) have been advanced.
definite (clinical diagnosis coupled with a histologic confir-
Although the lines between screening instruments and
mation of Alzheimer's disease), probable (clinical diagnosis
neuropsychological battery tests are sometimes blurred,
without a histologic confirmation), and possible (atypical
generally the former are much less time-consuming and de-
symptoms with no apparent alternative diagnosis in the ab-
tailed than the latter; battery tests often combine multiple
sence of a histologic confirmation) (40). The NINCDS/
screening tests so that more cognitive symptoms in DAT can
ADRDA clinical diagnostic criteria, similar to those of the
be covered in one assessment; and battery tests may allow
DSM-IV-TR, require a subtle onset and a gradual worsening
for discrimination between DAT and other illnesses affect-
of cognitive function and that other etiologies (e.g., thyroid
ing cognitive function. One of the most commonly used
diseases) be ruled out. Studies validating the NINCDS/
neuropsychological instruments in clinical trials of antide-
ADRDA and DSM-IV-TR against a variety of ‘‘gold stand-
mentia medications in the United States is the Alzheimer's
ards'' have found that the sensitivity ranges from 65 percent
Disease Assessment Scale-Cognitive Subscale (161, 162).
Epidemiol Rev 2008;30:15–34
Dementia of the Alzheimer Type
Summary of commonly used DAT* screening instruments
Cognitive domains assessed
Language, memory, praxis,
8–13 minutes in duration (310), covers
Sensitivity poor in those with mild
a wide variety of cognitive domains
dementia (2, 149); performance
in a brief test, reliable (149, 311)
affected by age, educationalattainment, gender, and culturalbackground (2, 143, 148); too long toadminister routinely in clinical practice(150, 151); ceiling effects in mildimpairment and floor effects in severeimpairment (154)
Language, memory, praxis,
10–15 minutes in duration (310), samples
Takes longer to administer than the
orientation, executive
a broader variety of cognitive domains
than the MMSE (155), enhanced
reliability and validity relative to theMMSE (155, 312)
Language, memory, praxis,
High specificity and sensitivity for mild
Useful primarily for mild cognitive
cognitive impairment and mild DAT
impairment and mild DAT (156),
abilities, attention,
(156), can detect mild cognitive
longer to administer than the MMSE
concentration, executive
impairment (156), reliable (156)
4 minutes in duration (310); performance
Covers few cognitive domains in DAT
not affected by age, education, or
patients, sensitivity influenced by
severity of dementia (157)
Performance not correlated with
Covers few cognitive domains in DAT
educational background (151), does
patients, demonstrates intermediate
not require a specific form to administer
sensitivity (151)
5 minutes in duration (310), reliable
Covers few cognitive domains in DAT
(314, 315), can differentiate between
patients, should be used in conjunction
mild cognitive impairment and normal
with other screens
subjects (159), highly sensitive andspecific for dementia (313), highcorrelation with the MMSE (163, 314)
Language, semantic
1 minute in duration, less time-consuming
Covers few cognitive domains in DAT
than most screens, correlates well with
patients, demonstrates intermediate
the MMSE (163), demonstrates good
sensitivity (151), should be used in
discrimination between persons with
conjunction with other screens
dementia and normal controls (316),does not require a specific form toadminister
Praxis, executive
2 minutes in duration (310), less
Covers few cognitive domains in DAT
functioning, attention,
time-consuming than most screens,
patients, subjective interpretation of
visuospatial abilities
high interrater reliability (317)
clock drawing, intermediate sensitivityand specificity (313, 317), should beused in conjunction with other screens
* DAT, dementia of the Alzheimer type; MMSE, Mini-Mental State Examination.
This instrument assesses memory, language, praxis, and ori-
calculation, and perception). Screening instruments, partic-
entation with a total score ranging from zero (no impair-
ularly the MMSE, and more in-depth neuropsychological
ment) to 70 (severely impaired). The Neuropsychological
tests are also often used to chart the rate of cognitive
Battery of the Consortium to Establish a Registry for
Alzheimer's Disease (163) consists of seven tests, includingthe MMSE and three others adapted from the Alzheimer'sDisease Assessment Scale-Cognitive Subscale (162) and
BIOMARKERS AND NEUROIMAGING
measures memory, language, praxis, and orientation. Otherneuropsychological tests used in dementia include, but are
Disease-modifying drugs are likely to be more efficacious
not limited to, the Syndrom-Kurztest (164) (assessing mem-
in the early or preclinical stage of the disease (168, 169).
ory, attention, naming, and object arrangement), the Seven-
Promising biomarkers and neuroimaging could have a sub-
Minute Neurocognitive Screening Battery (165) (assessing
stantial public health impact if new drug candidates, such as
memory, orientation, visual abilities, praxis, and language
beta-amyloid immunotherapy or beta-sheet breakers, were
skills), the Addenbrooke's Cognitive Examination (166)
found to have disease-arresting effects (170). Evidence is
(assessing orientation, attention, memory, language, and vi-
currently insufficient to support or direct the use of bio-
suospatial abilities), and the Cambridge Cognitive Exami-
markers (table 3) in usual clinical practice for dementia di-
nation (167) (assessing orientation, language, memory, praxis,
agnosis or disease management purposes.
Epidemiol Rev 2008;30:15–34
Jalbert et al.
Summary of the rationale and the disadvantages of selected CSF* and plasma biomarkers
Decreased levels of beta-amyloid in CSF may
Requires a lumbar puncture; invasive and uncomfortable
reflect increased deposition of beta-amyloid
procedure; despite availability of a commercial test
in the brain (171).
with high sensitivity and specificity, this biomarker isunderutilized (318).
tau is released from dying neurons, so total tau
Requires a lumbar puncture; invasive and uncomfortable
concentration in the CSF is thought to reflect
procedure; nonspecific for Alzheimer's disease because
the intensity of the neuronal damage and
elevated levels of tau are observed in other
degeneration (168).
degenerative CNS* conditions (168); despite theavailability of a commercial test with high sensitivityand specificity, this biomarker is underutilized (318).
Concentration of phosphorylated tau in the
Requires a lumbar puncture; invasive and uncomfortable
CSF may reflect the formation of tangles in
procedure; despite the availability of a commercial test
the brain because there is no increase in
with high sensitivity and specificity, this biomarker is
phosphorylated tau in other diseases with
intense neuronal degeneration (e.g.,Creutzfeldt-Jakob disease) (168).
Plasma beta-amyloid
Beta-amyloid is produced in the brain and
Plasma beta-amyloid levels do not correlate well with
cleared to the plasma via the CSF and the
biochemical or pathological measures of cerebral
blood brain barrier (319).
beta-amyloid deposition (181); there is broad overlapin the plasma levels of beta-amyloid peptides inpersons with DAT* and controls, making discriminationof persons with and without Alzheimer's diseasedifficult (171).
Plasma amyloid-beta
Antibodies against neuritic plaques may
Titer of beta-amyloid antibodies has been found to be
protect against Alzheimer's disease
significantly higher in healthy controls than in patients
with DAT (322); some studies have found nocorrelation between antibody titer and prevalence ofDAT (323); immune response to beta-amyloid 40 andtolerance of beta-amyloid 42 occurs naturally inhumans and is not related to the neuritic plaqueburden in the brain (171).
The APOE e4 allele is associated with
Studies of levels of APOE in DAT have been
increased neuritic plaque load and elevated
contradictory; some have reported elevated APOE
levels of beta-amyloid in the brain
levels in DAT (328), no difference (329–331), or
(324–326); the APOE e4 allele is associated
reduced (332, 333) levels compared with controls.
with less APOE protein in plasma (327).
Plasma isoprostanes
Increased levels of lipid oxidation in the
Isoprostanes appear to be elevated in DAT patients
Alzheimer's disease brain support a role for
relative to controls (334), but these findings have been
oxidative stress in DAT (171); free-radical-
challenged (335).
mediated peroxidation of polyunsaturatedfatty acids creates isoprostanes (171).
Inflammatory molecules
Amyloid deposition in the Alzheimer's disease
Many of the proteins involved in the inflammatory
such as interleukin-6
brain elicits a range of inflammatory
response do not cross the blood-brain barrier (188);
responses (57, 336); interleukin-6 is a
controversy exists regarding the levels of cytokine and
cytokine implicated in inflammation.
acute-phase reaction reactants in the blood followingan inflammatory response (187); findings from studiescomparing the levels of plasma interleukin-6 in peoplewith DAT and in healthy controls have been inconsistent(182, 185, 186, 337).
* CSF, cerebrospinal fluid; CNS, central nervous system; DAT, dementia of the Alzheimer type; APOE, apolipoprotein E.
Beta-amyloid 42, a more aggregate-prone peptide derived
Plasma levels of beta-amyloid peptides in persons with
from the amyloid precursor protein, a key molecule in
DAT overlap those found in controls (171, 178–180) and do
Alzheimer's disease pathology (171), total tau, and phosphor-
not reflect neuropathological or neurochemical measures of
ylated tau in the cerebrospinal fluid are biomarkers with
the levels of beta-amyloid deposition in the brain (181).
high diagnostic sensitivity and specificity for Alzheimer's
Some studies reported increased levels of interleukin-6, a cy-
disease (172, 173). The decrease in beta-amyloid 42 in the
tokine implicated in inflammation, in serum and plasma of
cerebrospinal fluid, presumably a result of its decreased
persons with DAT (182, 183), whereas others did not (184–
clearance from the brain into the cerebrospinal fluid (174),
186). Cytokine and acute-phase-reaction reactant levels in
has recently been added as one of the supportive features of
the plasma or serum remain controversial (187), and many
the proposed revisions of NINCDS/ADRDA criteria for
of these proteins do not cross the blood-brain barrier (188).
Alzheimer's disease (131). Cerebrospinal fluid biomarkers
Cerebrospinal fluid measures of beta-amyloid, total tau, and
may be able to identify preclinical Alzheimer's disease even
hyperphosphorylated tau are currently the best biomarkers
before the onset of mild cognitive impairment (175–177).
Epidemiol Rev 2008;30:15–34
Dementia of the Alzheimer Type
Within the medial temporal lobe, the disease consistently
quent use in clinical practice, few have been studied in
manifests itself through atrophy of the hippocampus and
controlled trials. Much of the published evidence is charac-
parahippocampal gyrus (189), which can be visualized by
terized by a number of limitations such as inadequate sample
using structural magnetic resonance imaging (190). Mag-
sizes, short study duration, use of nonstandardized evaluation
netic resonance imaging measurements of the medial tem-
methods, and lack of information on persistence of treatment
poral lobe include the qualitative appraisal of atrophy in the
effects (209). Although short-term adverse consequences of
hippocampal formation (191) as well as quantitative tech-
nonpharmacological interventions, such as agitation and cat-
niques analyzing tissue segmentation and computing cerebral
astrophic reactions, have been reported in some studies, these
volume (192). Sensitivity ranges from 80 percent to 100
outcomes have not been a focus of research (210).
percent (193–195) and specificity is over 90 percent (194)
The 2007 American Psychiatric Association guidelines
when magnetic resonance imaging–based estimates of the
for treatment of patients with DAT and other dementias
volume of various regions of the medial temporal lobe are
categorize nonpharmacological or psychosocial treatments
used to discriminate between patients with Alzheimer's dis-
into four broad areas: emotion oriented (reminiscence ther-
ease and normal controls. Functional magnetic resonance
apy, validation therapy, supportive psychotherapy, sensory
imaging may also allow for earlier detection of Alzheimer's
integration, and simulated-presence therapy), stimulation ori-
disease (189).
ented (recreational activities, art therapies, exercise), cogni-
Single-photon emission computed tomography has been
tion oriented (reality orientation, cognitive retraining, skills
used to measure regional cerebral blood flow, which corre-
training), and behavior oriented (211). The growing interest
lates well with severity of DAT (196, 197) and prognosis
in newer, nonpharmacological interventions such as cognitive
(198), although its diagnostic accuracy for distinguishing
rehabilitation and retraining techniques in the early stages of
between DAT and non-DAT in studies including healthy
DAT is focused on developing therapies that enhance present
controls is quite low (pooled weighted sensitivities ranged
capabilities and possibly augment the effects of cholinester-
from 65 percent to 71 percent with a specificity of 79 per-
ase inhibitors (212, 213). While some studies have reported
cent) (199). Computed tomography, the oldest technique for
treatment-related improvements in specific cognitive do-
scanning the brain, is generally used to exclude other causes
mains, well-designed, randomized trials of these interven-
of dementia (e.g., subdural hematomas) (189) but is worse
tions are lacking, and the effect on real-life skills, required
than cognitive screening in identifying dementia (200).
for independent living, is largely unknown (214).
Positron emission tomography can assess hypometabo-
Nonpharmacological interventions for dementia-related
lism and hypoperfusion and, when conducted with fluoro-
neuropsychiatric symptoms have been more widely studied
deoxyglucose, can measure the regional cerebral metabolic
and target predominantly neuropsychiatric symptoms in
rate of glucose (201). Approved in the United States as a di-
mild to moderate stages of dementia (e.g., communication
agnostic tool, fluorodeoxyglucose2positron emission to-
techniques, environmental alterations) (215). Studies of
mography is highly sensitive and specific in detecting
patient-centered behavioral interventions such as sensory
Alzheimer's disease in its early stages (202). Positron emis-
stimulation or music therapy have reported positive, but
sion tomography techniques in combination with use of an
short-lived effects on agitation and other symptoms (216).
amyloid-specific tracer may also provide in vivo visualiza-
Caregiver interventions may have long-term benefits be-
tion of neuritic plaques. Studies using the Pittsburgh Com-
cause several well-designed trials of psychoeducation pro-
pound B, a molecule that binds preferentially to beta-
grams for caregivers of persons with dementia reported
amyloid fibrils (203), demonstrated that brains of DAT pa-
a decrease in the frequency of neuropsychiatric symptoms
tients had a two- to threefold greater Pittsburgh Compound
and a delay in the time to institutionalization (217, 218).
B retention on positron emission tomography scans relative
The currently available DAT pharmacotherapeutic agents
to cognitively intact age-matched controls, and retention was
are symptomatic rather than disease-modifying treatments.
consistent with Alzheimer's disease pathology (204–206).
Symptomatic treatments such as cholinesterase inhibitors
Pittsburgh Compound B–positron emission tomography im-
and memantine, an N-methyl-D-aspartate receptor antago-
aging of amyloid deposits may have the potential to increase
nist, may stabilize or slow the progression of DAT, but these
diagnostic accuracy of DAT and could serve as a tool for
effects are lost after discontinuation (219, 220). Disease-
monitoring the changes in beta-amyloid pathology over the
modifying therapies are being designed to target various
course of DAT (206).
aspects of DAT neuropathology and confer benefits thatpersist beyond the course of treatment. The three broad in-vestigational classes of disease-modifying treatments are
TREATMENTS FOR DAT
antiamyloid agents, neuroprotective agents that reduce orprotect against neuronal injury associated with amyloid de-
Optimal treatment of DAT requires both nonpharmaco-
position, and neurorestorative strategies such as nerve
logical and pharmacological interventions (207). Given the
growth factors and cell transplantation (221). Experts in
progressive nature of the illness, interventions must be pe-
the field have theorized that the most effective DAT medi-
riodically reviewed and revised to meet the changing needs
cation regimens of the future will combine symptomatic and
of the patient.
disease-modifying agents (221, 222).
Nonpharmacological methods are appropriately used
Cholinesterase inhibitors have been the cornerstone of
throughout the severity spectrum of DAT and are used alone
contemporary DAT pharmacotherapy for over a decade
or in combination with pharmacotherapy (208). Despite fre-
and were developed based on the cholinergic hypothesis
Epidemiol Rev 2008;30:15–34
Jalbert et al.
of memory dysfunction (223). Degeneration of cholinergic
controlled trials of mild-moderate disease, however, have
neurons in the basal forebrain and declining levels of cho-
failed to show conclusive evidence of benefit (231, 232).
line acetyltransferase, the enzyme responsible for acetyl-
In a 6-month, placebo-controlled, monotherapy trial, mem-
choline synthesis, are associated with progressive decline
antine was associated with improvements in cognition and
of cholinergic transmission in the cerebral cortex and hip-
function (233). In addition, memantine or placebo added to
pocampus (223). Cholinesterase inhibitors block the degra-
a stable regimen of donepezil resulted in significant treat-
dation of acetylcholine and are associated with modest
ment effects favoring memantine in cognitive, functional,
benefits in the domains of cognition, function, and behavior
neuropsychiatric, and global outcomes over a 6-month period
in DAT clinical trials (224). Two drugs in this class, donep-
(234). To our knowledge, no head-to-head trials comparing
ezil and galantamine, inhibit acetylcholinesterase, whereas
memantine monotherapy with cholinesterase inhibitors
tacrine and rivastigmine block both acetylcholinesterase and
therapy in moderate-to-severe DAT have been conducted.
butyrylcholinesterase, an enzyme that plays a lesser role in
Adverse-effect rates from placebo-controlled dementia
the breakdown of acetylcholine (225). Galantamine is also
trials indicate that memantine is generally well tolerated
an allosteric nicotinic receptor modulator and enhances the
effect of acetylcholine on nicotinic receptors (226). Donepezil,
Considerable debate over the value of the pharmacolog-
rivastigmine, and galantamine have supplanted tacrine because
ical treatment of DAT continues and is fueled by difficulties
of more convenient dosing, greater tolerability, and the
in translating the modest effects observed in controlled trials
absence of significant hepatotoxicity (224).
into meaningful clinical and economic benefits (235). The
A recent meta-analysis of 13 double-blind, placebo-
United Kingdom's National Institute for Health and Clinical
controlled trials using donepezil, rivastigmine, or galant-
Excellence recently revised its previous position and ap-
amine treatments for 6 months to 1 year in patients with
proved the use of cholinesterase inhibitors for moderate-
mild, moderate, or severe DAT reported improvements over
stage DAT only (235). Memantine is not recommended as
placebo in cognition averaging 2.7 points (95 percent con-
a treatment for DAT under the guidelines, except for patients
fidence interval: 23.0, 22.3) on the 70-point Alzheimer's
participating in clinical trials. Without solid evidence to
Disease Assessment Scale-Cognitive Subscale and 1.37 points
elucidate the optimal duration of therapy, the impact of
(95 percent confidence interval: 1.13, 1.61) on the 30-point
treatment on outpatient and institutional caregiver burden,
MMSE scale (227). Modest, but statistically significant bene-
and the effects of therapy on patient and caregiver quality of
fits were also observed for global clinical ratings, activities of
life, payers will continue to question the utility of treating
daily living functioning, and neuropsychiatric symptoms.
DAT with the currently available agents.
More patients dropped out of cholinesterase inhibitor treat-
Another area of controversy in DAT pharmacotherapy is
ment groups because of adverse effects (29 percent) than
what constitutes appropriate treatment of neuropsychiatric
placebo-treated patients (18 percent), and fewer patients
symptoms. Pharmacological treatment of neuropsychiatric
experienced adverse events with donepezil compared with
symptoms may be warranted when nonpharmacological in-
terventions fail or when the nature or severity of neuropsy-
Despite the structural differences between various cholin-
chiatric symptoms endangers the safety of the patient or
esterase inhibitors, there is no evidence to suggest clinical
others (211). Before considering any pharmacological ther-
differentiation in efficacy trials (227, 228). Of the four cho-
apy to treat neuropsychiatric symptoms, it is essential that
linesterase inhibitor comparative clinical trials that have
physiologic (hunger, thirst, need to void) and medical causes
been conducted, there is only one double-blind study: a
of the behavior be investigated and treated because these
2-year comparison of donepezil with rivastigmine in patients
antecedents can trigger or exacerbate neuropsychiatric
with moderate DAT (range of MMSE scores: 10–20) (229).
symptoms (236, 237).
No significant treatment differences were observed be-
Pharmacotherapeutic management of neuropsychiatric
tween donepezil and rivastigmine regarding ratings of cog-
symptoms poses complex challenges for clinicians and care-
nitive function, activities of daily living performance, and
givers because an increasing body of evidence has revealed
neuropsychiatric symptoms (229). However, compared with
that the potential ‘‘cost'' in the form of adverse effects may
rivastigmine-treated patients, fewer donepezil patients dis-
offset marginal therapeutic benefits for many patients (238).
continued treatment (odds ratio 5 0.64, 95 percent confi-
A recent meta-analysis of antipsychotic, antidepressant, and
dence interval: 0.50, 0.83) (229).
anticonvulsant clinical trials for dementia-related neuropsy-
More recently, dysregulation of glutamatergic neurotrans-
chiatric symptoms concluded that these medications offer
mission in DAT was hypothesized to play a role in abnormal
modest benefits and a considerable risk of adverse effects
information processing, storage, and retrieval (230). Meman-
(239). No medication has been approved by the US Food
tine, a low-to-moderate-affinity, noncompetitive, N-methyl-
and Drug Administration to treat dementia-related neuro-
D-aspartate glutamate receptor antagonist, blocks excitotoxic
neuronal toxicity associated with excessive release of gluta-
The second generation of antipsychotics, atypical antipsy-
mate (230). Memantine has been used in the treatment of
chotics, is the best-studied and most commonly prescribed
a variety of neurologic disorders for more than 25 years in
class of psychoactive medications for neuropsychiatric symp-
Europe and, in 2003, was approved in the United States to
toms. A number of recent placebo-controlled clinical trials of
treat moderate-to-severe DAT.
atypical antipsychotics for neuropsychiatric symptoms have
Studies of memantine in patients with more advanced
reported small treatment effects coupled with adverse effects
DAT have reported favorable treatment effects; randomized,
at rates that exceed those observed among placebo-treated
Epidemiol Rev 2008;30:15–34
Dementia of the Alzheimer Type
patients (238, 240). Results from some randomized con-
stage of the illness (254). Increasing dependency, personality
trolled trials in dementia and subsequent meta-analyses have
changes, and neuropsychiatric symptoms such as aggression/
identified an increased risk of mortality and cerebrovascular-
agitation and depression are also highly distressing to the
adverse events associated with atypical antipsychotic treat-
caregiver (255, 256). Providing assistance to a loved one
ment (241, 242). Conventional antipsychotics may not be
afflicted with DAT comes at a considerable emotional, psy-
safer than atypical antipsychotics; subsequent analyses have
chological, and physical cost to the caregiver. Informal care-
reported an elevated risk of mortality associated with the use
givers report higher levels of depression and anxiety (255–
of older antipsychotics in patients with dementia and other
259), lower overall life satisfaction (257, 260), and engaging
psychiatric illnesses (243, 244). These developments have
in fewer preventive health behaviors (261), and they are at
fueled an ongoing debate over the appropriate prescribing
increased risk of illness (262–265) and mortality (266).
of antipsychotics (242, 245).
Informal caregivers also often experience social isolation
Better understanding of the safety issues associated with
(256), financial strain (251, 259, 267), employment compli-
antipsychotic therapy and the lack of safer and more effec-
cations (258, 268), and disruption of relationships (258).
tive alternatives have stimulated interest in the effects of
Research has predominantly focused on the negative and
dementia-specific medication on neuropsychiatric symp-
deleterious aspects of caregiving, but some studies have
toms. Modest reductions in neuropsychiatric symptoms
found that caregivers of persons with dementia perceive
have been reported from trials of cholinesterase inhibitors,
their caregiving as providing them with positive and satis-
fying experiences (269–272).
donepezil in DAT patients (227, 246). Studies of small num-
The decision to institutionalize a loved one afflicted with
bers of patients in open trials of cholinesterase inhibitors
DAT is difficult and complex but has been found to be as-
(donepezil, rivastigmine, galantamine) and in one double-
sociated with the patient's manifestation of neuropsychiatric
blind, placebo-controlled trial with rivastigmine have re-
symptoms, caregiver exhaustion, and the increased need for
ported varying degrees of improvement of neuropsychiatric
patient supervision (273–276). As many as 90 percent of
symptoms and psychosis in dementia with Lewy bodies
patients with dementia will be institutionalized before death
(247). Delusions, hallucinations, apathy, and agitation/
(277). Among new admissions to nursing homes, the prev-
aggression are the symptoms most likely to show significant
alence of dementia is nearly 70 percent (278); in 1999,
improvement in trials of DAT or dementia with Lewy bodies
approximately 214,200 nursing home residents were living
(246), but there is considerable intertrial heterogeneity in
with DAT (279).
neuropsychiatric symptoms domains showing the greatest
In the last few decades, there has been a rapid prolifera-
response to treatment. Current treatment guidelines suggest
tion of dementia special care units in nursing homes (280).
a trial of a cholinesterase inhibitor and/or memantine in
Approximately 10 percent of nursing homes had a special
the management of nonacute neuropsychiatric symptoms
unit for people with dementia in the 1990s; this figure has
risen to 20 percent (281, 282). There is no consensus defi-
A substantial number of patients with dementia experi-
nition of what constitutes ‘‘special care'' for dementia, but
ence severe and persistent neuropsychiatric symptoms that
a modified physical environment, special programs for res-
may require the use of medication for varying periods of
idents and families, and additional staff training and cover-
time throughout the course of DAT (248). The optimal treat-
age have become standard features (283). Studies evaluating
ment of neuropsychiatric symptoms is an essential research
the impact of living in a special care unit on improved
focus. More thoughtfully designed randomized controlled
resident outcomes (slower cognitive and functional decline
trials of pharmacological agents as monotherapy and in
and fewer neuropsychiatric symptoms) have been contradic-
combination with innovative nonpharmacological interven-
tory (284–287). In contrast, research has shown that the use
tions are urgently needed.
of psychotropic medications is higher among residents ofspecial care units (288–290).
CAREGIVING AND LONG-TERM CARE
As persons with DAT become more cognitively and func-
tionally impaired, many lose the ability to care for them-
Alzheimer's disease is a complex neurodegenerative ill-
selves and become dependent on others for their care (249).
ness, but much progress has been made in understanding it.
The majority of informal DAT caregivers are caring for
Research on the use of neuroimaging and biomarkers is
a relative, usually a parent, because the spouse of a DAT
promising and may allow for earlier and more accurate de-
patient may be deceased or unable to provide the level of
tection of Alzheimer's disease cases. Most studies across the
care needed without substantial help from his or her children
world indicate that the incidence and prevalence of DAT are
(250, 251). The burden of care is often borne by one in-
increasing. The majority of persons afflicted with DAT will
dividual (252).
exhibit neuropsychiatric symptoms, but symptom-specific
Caregiving generally requires a significant investment of
prevalence estimates vary widely and it is unclear how
time, energy, and money that often needs to be sustained
and if stage-specific prevalence of individual symptoms
over a period of years (252, 253). The number of hours per
changes. Current pharmacological treatments for DAT ap-
week spent providing care increases from 13.1 for patients
pear to slow progression of the disease but are not disease
with mild dementia to 46.1 for those in the more advanced
modifying. Further research on disease-modifying therapies
Epidemiol Rev 2008;30:15–34
Jalbert et al.
is needed if the prevalence and clinical course of the condition
Rockville, MD: Agency for Healthcare Research and Quality,
are to be altered. This review underscores that much more
2000. (Fact sheet; AHRQ publication no. 00-P012).
needs to be done before the mystery of DAT is unraveled.
15. Older Americans 2004: key indicators of well-being. Federal
Interagency Forum on Aging-Related Statistics, 2004.
16. Lyketsos CG, Lopez O, Jones B, et al. Prevalence of
neuropsychiatric symptoms in dementia and mild cognitiveimpairment: results from the cardiovascular health study.
The authors thank Dr. Andrea Gruneir for her help and
guidance regarding the ‘‘Caregiving and Long-term Care''
17. Mega MS, Cummings JL, Fiorello T, et al. The spectrum of
section of the manuscript.
behavioral changes in Alzheimer's disease. Neurology 1996;
Jessica J. Jalbert is a predoctoral fellow at Pfixer Inc.
(New York, New York). This program is supported by a grant
18. Steinberg M, Shao H, Zandi P, et al. Point and 5-year period
on which Kate L. Lapane is the principal investigator. Lori
prevalence of neuropsychiatric symptoms in dementia: theCache County Study. Int J Geriatr Psychiatry 2008;23:170–7.
A. Daeillo has served as a consultant for Eli Lilly and
19. Zuidema S, Koopmans R, Verhey F. Prevalence and pre-
Company (Indianapolis, Indiana), Forest Laboratories, Inc.
dictors of neuropsychiatric symptoms in cognitively impaired
(New York, New York), and Pfixer Inc.
nursing home patients. J Geriatr Psychiatry Neurol 2007;20:41–9.
20. Lyketsos CG, Sheppard JM, Steinberg M, et al. Neuropsy-
chiatric disturbance in Alzheimer's disease clusters into three
groups: the Cache County study. Int J Geriatr Psychiatry2001;16:1043–53.
1. Cummings JL. Alzheimer's disease. N Engl Med J 2004;351:
21. Lyketsos CG, Steinberg M, Tschanz JT, et al. Mental and
behavioral disturbances in dementia: findings from the Cache
2. Cummings JL, Cole G. Alzheimer disease. JAMA 2002;287:
County Study on Memory in Aging. Am J Psychiatry 2000;
3. Jorm AF, Jolley D. The incidence of dementia: a meta-
22. McCormick WC, Kukull WA, van Belle G, et al. Symptom
analysis. Neurology 1998;51:728–33.
patterns and comorbidity in the early stages of Alzheimer's
4. Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in
disease. J Am Geriatr Soc 1994;42:517–21.
the US population: prevalence estimates using the 2000
23. Porter VR, Buxton WG, Fairbanks LA, et al. Frequency and
census. Arch Neurol 2003;60:1119–22.
characteristics of anxiety among patients with Alzheimer's
5. Sadik K, Wilcock G. The increasing burden of Alzheimer
disease and related dementias. J Neuropsychiatry Clin
disease. Alzheimer Dis Assoc Disord 2003;17(suppl):S75–9.
6. Reitz C, den Heijer T, van Duijn C, et al. Relation between
24. Zubenko GS, Zubenko WN, McPherson S, et al. A collab-
smoking and risk of dementia and Alzheimer disease: the
orative study of the emergence and clinical features of the
Rotterdam Study. Neurology 2007;69:998–1005.
major depressive syndrome of Alzheimer's disease. Am J
7. Huang W, Qiu C, von Strauss E, et al. APOE genotype, family
history of dementia, and Alzheimer disease risk: a 6-year
25. Migliorelli R, Teson A, Sabe L, et al. Prevalence and
follow-up study. Arch Neurol 2004;61:1930–4.
correlates of dysthymia and major depression among patients
8. Launer LJ, Andersen K, Dewey ME, et al. Rates and risk
with Alzheimer's disease. Am J Psychiatry 1995;152:37–44.
factors for dementia and Alzheimer's disease: results from
26. Vida S, Des Rosiers P, Carrier L, et al. Prevalence of
EURODEM pooled analyses. EURODEM Incidence
depression in Alzheimer's disease and validity of Research
Research Group and Work Groups. European Studies of
Diagnostic Criteria. J Geriatr Psychiatry Neurol 1994;7:
Dementia. Neurology 1999;52:78–84.
9. Lyketsos CG, Colenda CC, Beck C, et al. Position statement
27. Burns A, Jacoby R, Levy R. Psychiatric phenomena in
of the American Association for Geriatric Psychiatry re-
Alzheimer's disease. III: disorders of mood. Br J Psychiatry
garding principles of care for patients with dementia resulting
from Alzheimer disease. Am J Geriatr Psychiatry 2006;14:
28. Landes AM, Sperry SD, Strauss ME. Prevalence of apathy,
dysphoria, and depression in relation to dementia severity in
10. van Duijn CM. Epidemiology of the dementias: recent
Alzheimer's disease. J Neuropsychiatry Clin Neurosci 2005;
developments and new approaches. J Neurol Neurosurg
29. Burns A, Jacoby R, Levy R. Psychiatric phenomena in
11. Breteler MM, Claus JJ, van Duijn CM, et al. Epidemiology of
Alzheimer's disease. IV: disorders of behaviour. Br J
Alzheimer's disease. Epidemiol Rev 1992;14:59–82.
12. Lobo A, Launer LJ, Fratiglioni L, et al. Prevalence of
30. Ropacki SA, Jeste DV. Epidemiology of and risk factors for
dementia and major subtypes in Europe: a collaborative study
psychosis of Alzheimer's disease: a review of 55 studies
of population-based cohorts. Neurologic Diseases in the
published from 1990 to 2003. Am J Psychiatry 2005;162:
Elderly Research Group. Neurology 2000;54(suppl):S4–9.
13. Summary file 1. 2000 Census of population and housing.
31. Jeste DV, Finkel SI. Psychosis of Alzheimer's disease and
Washington, DC, US Census Bureau, 2007.
related dementias. Diagnostic criteria for a distinct syndrome.
Am J Geriatr Psychiatry 2000;8:29–34.
14. Health services research on aging: building on biomedical
32. Robert PH, Verhey FR, Byrne EJ, et al. Grouping for
and clinical research. Translating research into practice.
behavioral and psychological symptoms in dementia: clinical
Epidemiol Rev 2008;30:15–34
Dementia of the Alzheimer Type
and biological aspects. Consensus paper of the European
5th ed. Philadelphia, PA: Lippincott Williams & Wilkins,
Alzheimer disease consortium. Eur Psychiatry 2005;20:
49. Mullan M, Crawford F, Axelman K, et al. A pathogenic
33. Olin JT, Katz IR, Meyers BS, et al. Provisional diagnostic
mutation for probable Alzheimer's disease in the APP gene at
criteria for depression of Alzheimer disease: rationale and
the N-terminus of beta-amyloid. Nat Genet 1992;1:345–7.
background. Am J Geriatr Psychiatry 2002;10:129–41.
50. Scheuner D, Eckman C, Jensen M, et al. Secreted amyloid
34. Lyketsos CG, Breitner JC, Rabins PV. An evidence-based
beta-protein similar to that in the senile plaques of
proposal for the classification of neuropsychiatric disturbance in
Alzheimer's disease is increased in vivo by the presenilin
Alzheimer's disease. Int J Geriatr Psychiatry 2001;16:1037–42.
1 and 2 and APP mutations linked to familial Alzheimer's
35. Aalten P, de Vugt ME, Lousberg R, et al. Behavioral problems
disease. Nat Med 1996;2:864–70.
in dementia: a factor analysis of the neuropsychiatric in-
51. Sherrington R, Rogaev EI, Liang Y, et al. Cloning of a gene
ventory. Dement Geriatr Cogn Disord 2003;15:99–105.
bearing missense mutations in early-onset familial Alz-
36. Cook SE, Miyahara S, Bacanu SA, et al. Psychotic symptoms
heimer's disease. Nature 1995;375:754–60.
in Alzheimer disease: evidence for subtypes. Am J Geriatr
52. De Strooper B, Saftig P, Craessaerts K, et al. Deficiency of
presenilin-1 inhibits the normal cleavage of amyloid pre-
37. Tariot PN, Mack JL, Patterson MB, et al. The Behavior
cursor protein. Nature 1998;391:387–90.
Rating Scale for Dementia of the Consortium to Establish
53. Hardy J. The Alzheimer family of diseases: many etiologies,
a Registry for Alzheimer's Disease. The Behavioral Pathol-
one pathogenesis? Proc Natl Acad Sci U S A 1997;94:2095–7.
ogy Committee of the Consortium to Establish a Registry for
54. Beyreuther K, Pollwein P, Multhaup G, et al. Regulation and
Alzheimer's Disease. Am J Psychiatry 1995;152:1349–57.
expression of the Alzheimer's beta/A4 amyloid protein
38. Frisoni GB, Rozzini L, Gozzetti A, et al. Behavioral
precursor in health, disease, and Down's syndrome. Ann N Y
syndromes in Alzheimer's disease: description and correlates.
Acad Sci 1993;695:91–102.
Dement Geriatr Cogn Disord 1999;10:130–8.
55. Bales KR, Verina T, Dodel RC, et al. Lack of apolipoprotein
39. Benoit M, Staccini P, Brocker P, et al. Behavioral and
E dramatically reduces amyloid beta-peptide deposition. Nat
psychologic symptoms in Alzheimer's disease: results of the
REAL.FR study. (In French). Rev Med Interne 2003;
56. Carter DB, Dunn E, McKinley DD, et al. Human apolipo-
protein E4 accelerates beta-amyloid deposition in APPsw
40. McKhann G, Drachman D, Folstein M, et al. Clinical
transgenic mouse brain. Ann Neurol 2001;50:468–75.
diagnosis of Alzheimer's disease: report of the NINCDS-
57. Eikelenboom P, Veerhuis R. The importance of inflammatory
ADRDA Work Group under the auspices of Department of
mechanisms for the development of Alzheimer's disease. Exp
Health and Human Services Task Force on Alzheimer's
Disease. Neurology 1984;34:939–44.
58. Kalaria RN. Microglia and Alzheimer's disease. Curr Opin
41. Mirra SS, Heyman A, McKeel D, et al. The Consortium to
Establish a Registry for Alzheimer's Disease (CERAD). Part
59. Griffin WS, Sheng JG, Royston MC, et al. Glial-neuronal
II. Standardization of the neuropathologic assessment of
interactions in Alzheimer's disease: the potential role of
Alzheimer's disease. Neurology 1991;41:479–86.
a ‘cytokine cycle' in disease progression. Brain Pathol 1998;
42. Arnold SE, Hyman BT, Flory J, et al. The topographical and
neuroanatomical distribution of neurofibrillary tangles and
60. Pike CJ, Cummings BJ, Cotman CW. beta-Amyloid induces
neuritic plaques in the cerebral cortex of patients with
neuritic dystrophy in vitro: similarities with Alzheimer
Alzheimer's disease. Cereb Cortex 1991;1:103–16.
43. Consensus recommendations for the postmortem diagnosis of
pathology. Neuroreport 1992;3:769–72.
Alzheimer's disease. The National Institute on Aging, and
61. Varadarajan S, Yatin S, Aksenova M, et al. Review:
Reagan Institute Working Group on Diagnostic Criteria for
Alzheimer's amyloid beta-peptide-associated free radical
the Neuropathological Assessment of Alzheimer's Disease.
oxidative stress and neurotoxicity. J Struct Biol 2000;130:
Neurobiol Aging 1997;18:S1–2.
44. Jellinger KA, Bancher C. Proposals for re-evaluation of
62. Kowall NW, McKee AC, Yankner BA, et al. In vivo
current autopsy criteria for the diagnosis of Alzheimer's
neurotoxicity of beta-amyloid [beta(1-40)] and the
disease. Neurobiol Aging 1997;18(suppl):S55–65.
beta(25-35) fragment. Neurobiol Aging 1992;13:537–42.
45. Cummings JL, Vinters HV, Cole GM, et al. Alzheimer's
63. Butterfield DA, Drake J, Pocernich C, et al. Evidence of
disease: etiologies, pathophysiology, cognitive reserve, and
oxidative damage in Alzheimer's disease brain: central role
treatment opportunities. Neurology 1998;51(suppl):S2–17,
for amyloid beta-peptide. Trends Mol Med 2001;7:548–54.
64. Alonso AC, Grundke-Iqbal I, Iqbal K. Alzheimer's disease
46. Mann DM, Brown SM, Owen F, et al. Amyloid beta protein
hyperphosphorylated tau sequesters normal tau into tangles
(A beta) deposition in dementia with Lewy bodies: pre-
of filaments and disassembles microtubules. Nat Med 1996;
dominance of A beta 42(43) and paucity of A beta 40
compared with sporadic Alzheimer's disease. Neuropathol
65. Geula C. Abnormalities of neural circuitry in Alzheimer's
Appl Neurobiol 1998;24:187–94.
disease: hippocampus and cortical cholinergic innervation.
47. Bell RD, Sagare AP, Friedman AE, et al. Transport pathways
Neurology 1998;51(suppl):S18–29, S65–7.
for clearance of human Alzheimer's amyloid beta-peptide
66. Lee VM, Balin BJ, Otvos L Jr, et al. A68: a major subunit of
and apolipoproteins E and J in the mouse central nervous
paired helical filaments and derivatized forms of normal Tau.
system. J Cereb Blood Flow Metab 2007;27:909–18.
48. Nixon RA. Cell and molecular neuropathology of Alzheimer
67. Gotz J, Chen F, van Dorpe J, et al. Formation of neurofi-
disease. In: Davis KL, Charney D, Coyle JT, et al, eds.
brillary tangles in P301l tau transgenic mice induced by
Neuropsychopharmacology: the fifth generation of progress.
Abeta 42 fibrils. Science 2001;293:1491–5.
Epidemiol Rev 2008;30:15–34
Jalbert et al.
68. Lewis J, Dickson DW, Lin WL, et al. Enhanced neurofibril-
89. McCarty HJ, Roth DL, Goode KT, et al. Longitudinal course
lary degeneration in transgenic mice expressing mutant tau
of behavioral problems during Alzheimer's disease: linear
and APP. Science 2001;293:1487–91.
versus curvilinear patterns of decline. J Gerontol A Biol Sci
69. Davies P. Neurotransmitter-related enzymes in senile de-
Med Sci 2000;55:M200–6.
mentia of the Alzheimer type. Brain Res 1979;171:319–27.
90. Mitnitski AB, Graham JE, Mogilner AJ, et al. The rate of
70. Bierer LM, Haroutunian V, Gabriel S, et al. Neurochemical
decline in function in Alzheimer's disease and other
correlates of dementia severity in Alzheimer's disease:
dementias. J Gerontol A Biol Sci Med Sci 1999;54:M65–9.
relative importance of the cholinergic deficits. J Neurochem
91. Paulsen JS, Salmon DP, Thal LJ, et al. Incidence of and risk
factors for hallucinations and delusions in patients with
71. Francis PT, Palmer AM, Snape M, et al. The cholinergic
probable AD. Neurology 2000;54:1965–71.
hypothesis of Alzheimer's disease: a review of progress.
92. Kuzis G, Sabe L, Tiberti C, et al. Neuropsychological
J Neurol Neurosurg Psychiatry 1999;66:137–47.
correlates of apathy and depression in patients with dementia.
72. DeKosky ST, Scheff SW. Synapse loss in frontal cortex
biopsies in Alzheimer's disease: correlation with cognitive
93. Bierman EJ, Comijs HC, Jonker C, et al. Symptoms of
severity. Ann Neurol 1990;27:457–64.
anxiety and depression in the course of cognitive decline.
73. Whitehouse PJ, Price DL, Struble RG, et al. Alzheimer's
Dement Geriatr Cogn Disord 2007;24:213–19.
disease and senile dementia: loss of neurons in the basal
94. Chen JC, Borson S, Scanlan JM. Stage-specific prevalence
forebrain. Science 1982;215:1237–9.
of behavioral symptoms in Alzheimer's disease in a multi-
74. Mann DM. Pyramidal nerve cell loss in Alzheimer's disease.
ethnic community sample. Am J Geriatr Psychiatry 2000;8:
75. Gomez-Isla T, Hollister R, West H, et al. Neuronal loss
95. Hutchinson AD, Mathias JL. Neuropsychological deficits in
correlates with but exceeds neurofibrillary tangles in
frontotemporal dementia and Alzheimer's disease: a meta-
Alzheimer's disease. Ann Neurol 1997;41:17–24.
analytic review. J Neurol Neurosurg Psychiatry 2007;78:
76. Hui JS, Wilson RS, Bennett DA, et al. Rate of cognitive
decline and mortality in Alzheimer's disease. Neurology
96. Rebok G, Brandt J, Folstein M. Longitudinal cognitive
decline in patients with Alzheimer's disease. J Geriatr
77. Eaker ED, Vierkant RA, Mickel SF. Predictors of nursing
Psychiatry Neurol 1990;3:91–7.
home admission and/or death in incident Alzheimer's disease
97. Becker JT, Huff FJ, Nebes RD, et al. Neuropsychological
and other dementia cases compared to controls: a population-
function in Alzheimer's disease. Pattern of impairment and
based study. J Clin Epidemiol 2002;55:462–8.
rates of progression. Arch Neurol 1988;45:263–8.
78. Scarmeas N, Brandt J, Albert M, et al. Delusions and
98. Hebert LE, Wilson RS, Gilley DW, et al. Decline of language
hallucinations are associated with worse outcome in
among women and men with Alzheimer's disease. J Gerontol
Alzheimer disease. Arch Neurol 2005;62:1601–8.
B Psychol Sci Soc Sci 2000;55:P354–60.
79. Roberson ED, Hesse JH, Rose KD, et al. Frontotemporal
99. Wilson RS, Gilley DW, Bennett DA, et al. Person-specific
dementia progresses to death faster than Alzheimer disease.
paths of cognitive decline in Alzheimer's disease and their
relation to age. Psychol Aging 2000;15:18–28.
80. Storandt M, Grant EA, Miller JP, et al. Rates of progression in
100. Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex,
mild cognitive impairment and early Alzheimer's disease.
and ethnicity on the association between apolipoprotein E
genotype and Alzheimer disease. A meta-analysis. APOE
81. Almkvist O. Neuropsychological features of early
Alzheimer's disease: preclinical and clinical stages. Acta
and Alzheimer Disease Meta Analysis Consortium. JAMA
Neurol Scand Suppl 1996;165:63–71.
82. Mickes L, Wixted JT, Fennema-Notestine C, et al. Pro-
101. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose
gressive impairment on neuropsychological tasks in a longi-
of apolipoprotein E type 4 allele and the risk of Alzheimer's
tudinal study of preclinical Alzheimer's disease.
disease in late onset families. Science 1993;261:921–3.
102. Hirono N, Hashimoto M, Yasuda M, et al. Accelerated
83. Aguero-Torres H, Fratiglioni L, Winblad B. Natural history
memory decline in Alzheimer's disease with apolipoprotein
of Alzheimer's disease and other dementias: review of the
epsilon4 allele. J Neuropsychiatry Clin Neurosci 2003;15:
literature in the light of the findings from the Kungsholmen
Project. Int J Geriatr Psychiatry 1998;13:755–66.
103. Craft S, Teri L, Edland SD, et al. Accelerated decline in
84. Scarmeas N, Brandt J, Albert M, et al. Association between
apolipoprotein E-epsilon4 homozygotes with Alzheimer's
the APOE genotype and psychopathologic symptoms in
disease. Neurology 1998;51:149–53.
Alzheimer's disease. Neurology 2002;58:1182–8.
104. Stern Y, Brandt J, Albert M, et al. The absence of an
85. Jost BC, Grossberg GT. The evolution of psychiatric
apolipoprotein epsilon4 allele is associated with a more
symptoms in Alzheimer's disease: a natural history study.
aggressive form of Alzheimer's disease. Ann Neurol 1997;
J Am Geriatr Soc 1996;44:1078–81.
86. Teri L, Larson EB, Reifler BV. Behavioral disturbance in
105. Kleiman T, Zdanys K, Black B, et al. Apolipoprotein E
dementia of the Alzheimer's type. JAm Geriatr Soc 1988;36:1–6.
epsilon4 allele is unrelated to cognitive or functional decline
87. Agniel A, Celsis P, Puel M, et al. Psychiatric disorders in
in Alzheimer's disease: retrospective and prospective analy-
dementia of Alzheimer type: cognitive and hemodynamic
sis. Dement Geriatr Cogn Disord 2006;22:73–82.
correlates. Dementia 1990;1:215–21.
106. Jonker C, Schmand B, Lindeboom J, et al. Association
88. Devanand DP, Brockington CD, Moody BJ, et al. Behavioral
between apolipoprotein E epsilon4 and the rate of cognitive
syndromes in Alzheimer's disease. Int Psychogeriatr 1992;
decline in community-dwelling elderly individuals with and
without dementia. Arch Neurol 1998;55:1065–9.
Epidemiol Rev 2008;30:15–34
Dementia of the Alzheimer Type
107. Slooter AJ, Houwing-Duistermaat JJ, van Harskamp F, et al.
127. Livingston G, Walker AE, Katona CL, et al. Antipsychotics
Apolipoprotein E genotype and progression of Alzheimer's
and cognitive decline in Alzheimer's disease: the LASER-
disease: the Rotterdam Study. J Neurol 1999;246:304–8.
Alzheimer's disease longitudinal study. J Neurol Neurosurg
108. Wilson RS, Li Y, Aggarwal NT, et al. Education and the
course of cognitive decline in Alzheimer disease. Neurology
128. Caballero J, Hitchcock M, Scharre D, et al. Cognitive effects
of atypical antipsychotics in patients with Alzheimer's
109. Scarmeas N, Albert SM, Manly JJ, et al. Education and rates
disease and comorbid psychiatric or behavioral problems:
of cognitive decline in incident Alzheimer's disease. J Neurol
a retrospective study. Clin Ther 2006;28:1695–700.
Neurosurg Psychiatry 2006;77:308–16.
129. Ellul J, Archer N, Foy CM, et al. The effects of commonly
110. Scarmeas N, Stern Y. Cognitive reserve: implications for
prescribed drugs in patients with Alzheimer's disease on the
diagnosis and prevention of Alzheimer's disease. Curr Neurol
rate of deterioration. J Neurol Neurosurg Psychiatry 2007;
Neurosci Rep 2004;4:374–80.
111. Teri L, McCurry SM, Edland SD, et al. Cognitive decline in
130. American Psychiatric Association. Diagnostic and statisti-
Alzheimer's disease: a longitudinal investigation of risk
cal manual of mental disorders: DSM-IV-TR. 4th ed, text
factors for accelerated decline. J Gerontol A Biol Sci Med Sci
rev. Washington, DC: American Psychiatric Association,
112. Wilson RS, Gilley DW, Bennett DA, et al. Hallucinations,
131. Dubois B, Feldman HH, Jacova C, et al. Research criteria for
delusions, and cognitive decline in Alzheimer's disease.
the diagnosis of Alzheimer's disease: revising the NINCDS-
J Neurol Neurosurg Psychiatry 2000;69:172–7.
ADRDA criteria. Lancet Neurol 2007;6:734–46.
113. Bassuk SS, Berkman LF, Wypij D. Depressive symptom-
132. Kukull WA, Larson EB, Reifler BV, et al. The validity of 3
atology and incident cognitive decline in an elderly com-
clinical diagnostic criteria for Alzheimer's disease. Neurol-
munity sample. Arch Gen Psychiatry 1998;55:1073–81.
114. McShane R, Keene J, Gedling K, et al. Do neuroleptic drugs
133. Blacker D, Albert MS, Bassett SS, et al. Reliability and
hasten cognitive decline in dementia? Prospective study with
validity of NINCDS-ADRDA criteria for Alzheimer's dis-
necropsy follow up. BMJ 1997;314:266–70.
ease. The National Institute of Mental Health Genetics
115. Burns A, Jacoby R, Levy R. Psychiatric phenomena in
Initiative. Arch Neurol 1994;51:1198–204.
Alzheimer's disease. I: disorders of thought content. Br J
134. Hogervorst E, Barnetson L, Jobst KA, et al. Diagnosing
Psychiatry 1990;157:72–6, 92–4.
dementia: interrater reliability assessment and accuracy of
116. Lopez OL, Boller F, Becker JT, et al. Alzheimer's disease and
the NINCDS/ADRDA criteria versus CERAD histopatho-
depression: neuropsychological impairment and progression
logical criteria for Alzheimer's disease. Dement Geriatr Cogn
of the illness. Am J Psychiatry 1990;147:855–60.
117. Lopez OL, Wisniewski SR, Becker JT, et al. Psychiatric
135. Hogervorst E, Bandelow S, Combrinck M, et al. The validity
medication and abnormal behavior as predictors of pro-
and reliability of 6 sets of clinical criteria to classify
gression in probable Alzheimer disease. Arch Neurol 1999;
Alzheimer's disease and vascular dementia in cases con-
firmed post-mortem: added value of a decision tree approach.
118. Stern Y, Tang MX, Albert MS, et al. Predicting time to
Dement Geriatr Cogn Disord 2003;16:170–80.
nursing home care and death in individuals with Alzheimer
136. Lim A, Tsuang D, Kukull W, et al. Clinico-neuropathological
disease. JAMA 1997;277:806–12.
correlation of Alzheimer's disease in a community-based
119. Mega MS, Lee L, Dinov ID, et al. Cerebral correlates of
case series. J Am Geriatr Soc 1999;47:564–9.
psychotic symptoms in Alzheimer's disease. J Neurol
137. Petrovitch H, White LR, Ross GW, et al. Accuracy of
Neurosurg Psychiatry 2000;69:167–71.
clinical criteria for AD in the Honolulu-Asia Aging
120. Zubenko GS, Moossy J, Martinez AJ, et al. Neuropathologic
Study, a population-based study. Neurology 2001;57:
and neurochemical correlates of psychosis in primary de-
mentia. Arch Neurol 1991;48:619–24.
138. Varma AR, Snowden JS, Lloyd JJ, et al. Evaluation of the
121. Farber NB, Rubin EH, Newcomer JW, et al. Increased
NINCDS-ADRDA criteria in the differentiation of
neocortical neurofibrillary tangle density in subjects with
Alzheimer's disease and frontotemporal dementia. J Neurol
Alzheimer disease and psychosis. Arch Gen Psychiatry
Neurosurg Psychiatry 1999;66:184–8.
139. Kazee AM, Eskin TA, Lapham LW, et al. Clinicopathologic
122. Lanctot KL, Herrmann N, Mazzotta P. Role of serotonin in
correlates in Alzheimer disease: assessment of clinical and
the behavioral and psychological symptoms of dementia.
pathologic diagnostic criteria. Alzheimer Dis Assoc Disord
J Neuropsychiatry Clin Neurosci 2001;13:5–21.
123. Herrmann N, Lanctot KL, Khan LR. The role of norepi-
140. Folstein MF, Folstein SE, McHugh PR. Mini-mental state.
nephrine in the behavioral and psychological symptoms of
J Psychiatr Res 1975;12:189–98.
dementia. J Neuropsychiatry Clin Neurosci 2004;16:261–76.
141. Aevarsson O, Skoog I. A longitudinal population study of the
124. Semla TP, Cohen D, Freels S, et al. Psychotropic drug use in
mini-mental state examination in the very old: relation to
relation to psychiatric symptoms in community-living per-
dementia and education. Dement Geriatr Cogn Disord
sons with Alzheimer's disease. Pharmacotherapy 1995;15:
142. Cossa FM, Sala SD, Musicco M, et al. The Milan overall
125. Alexopoulos GS, Streim J, Carpenter D, et al. Using
dementia assessment and the mini-mental state examination
antipsychotic agents in older patients. J Clin Psychiatry
compared: an epidemiological investigation of dementia. Eur
J Neurol 1999;6:289–94.
126. Ballard C, Margallo-Lana M, Juszczak E, et al. Quetiapine
143. Dufouil C, Clayton D, Brayne C, et al. Population norms for
and rivastigmine and cognitive decline in Alzheimer's
the MMSE in the very old: estimates based on longitudinal
disease: randomised double blind placebo controlled trial.
data. Mini-Mental State Examination. Neurology 2000;55:
BMJ 2005;330:874.
Epidemiol Rev 2008;30:15–34
Jalbert et al.
144. Flicker L, Logiudice D, Carlin JB, et al. The predictive value
166. Mathuranath PS, Nestor PJ, Berrios GE, et al. A brief
of dementia screening instruments in clinical populations. Int
cognitive test battery to differentiate Alzheimer's disease and
J Geriatr Psychiatry 1997;12:203–9.
frontotemporal dementia. Neurology 2000;55:1613–20.
145. Monsch AU, Foldi NS, Ermini-Funfschilling DE, et al.
167. Roth M, Tym E, Mountjoy CQ, et al. CAMDEX. A stand-
Improving the diagnostic accuracy of the Mini-Mental State
ardised instrument for the diagnosis of mental disorder in the
Examination. Acta Neurol Scand 1995;92:145–50.
elderly with special reference to the early detection of
146. Tangalos EG, Smith GE, Ivnik RJ, et al. The Mini-Mental
dementia. Br J Psychiatry 1986;149:698–709.
State Examination in general medical practice: clinical utility
168. Blennow K, Hampel H. CSF markers for incipient Alz-
and acceptance. Mayo Clin Proc 1996;71:829–37.
heimer's disease. Lancet Neurol 2003;2:605–13.
147. Crum RM, Anthony JC, Bassett SS, et al. Population-based
169. DeKosky ST, Marek K. Looking backward to move forward:
norms for the Mini-Mental State Examination by age and
early detection of neurodegenerative disorders. Science
educational level. JAMA 1993;269:2386–91.
148. Grigoletto F, Zappala G, Anderson DW, et al. Norms for the
170. Hansson O, Zetterberg H, Buchhave P, et al. Association
Mini-Mental State Examination in a healthy population.
between CSF biomarkers and incipient Alzheimer's disease
in patients with mild cognitive impairment: a follow-up
149. Tombaugh TN, McIntyre NJ. The mini-mental state exam-
study. Lancet Neurol 2006;5:228–34.
ination: a comprehensive review. J Am Geriatr Soc 1992;
171. Kawarabayashi T, Shoji M. Plasma biomarkers of Alz-
heimer's disease. Curr Opin Psychiatry 2008;21:260–7.
150. Boise L, Camicioli R, Morgan DL, et al. Diagnosing
172. Andreasen N, Minthon L, Davidsson P, et al. Evaluation of
dementia: perspectives of primary care physicians. Geron-
CSF-tau and CSF-Abeta42 as diagnostic markers for
151. Kilada S, Gamaldo A, Grant EA, et al. Brief screening tests
Alzheimer disease in clinical practice. Arch Neurol 2001;58:
for the diagnosis of dementia: comparison with the mini-
mental state exam. Alzheimer Dis Assoc Disord 2005;19:
173. Kanai M, Matsubara E, Isoe K, et al. Longitudinal study of
cerebrospinal fluid levels of tau, A beta1-40, and A beta1
152. Schmand B, Lindeboom J, Launer L, et al. What is
-42(43) in Alzheimer's disease: a study in Japan. Ann Neurol
a significant score change on the mini-mental state exami-
nation? Int J Geriatr Psychiatry 1995;10:411–14.
174. Ertekin-Taner N, Younkin LH, Yager DM, et al. Plasma
153. Tombaugh TN. Test-retest reliable coefficients and 5-year
amyloid beta protein is elevated in late-onset Alzheimer
change scores for the MMSE and 3MS. Arch Clin Neuro-
disease families. Neurology 2008;70:596–606.
175. Skoog I, Davidsson P, Aevarsson O, et al. Cerebrospinal fluid
154. Feher EP, Mahurin RK, Doody RS, et al. Establishing the
beta-amyloid 42 is reduced before the onset of sporadic
limits of the Mini-Mental State. Examination of ‘subtests'.
dementia: a population-based study in 85-year-olds. Dement
Arch Neurol 1992;49:87–92.
Geriatr Cogn Disord 2003;15:169–76.
155. Teng EL, Chui HC. The Modified Mini-Mental State (3MS)
176. Moonis M, Swearer JM, Dayaw MP, et al. Familial
examination. J Clin Psychiatry 1987;48:314–18.
Alzheimer disease: decreases in CSF Abeta42 levels precede
156. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal
cognitive decline. Neurology 2005;65:323–5.
Cognitive Assessment, MoCA: a brief screening tool for mild
177. Sunderland T, Mirza N, Putnam KT, et al. Cerebrospinal fluid
cognitive impairment. J Am Geriatr Soc 2005;53:695–9.
beta-amyloid1-42 and tau in control subjects at risk for
157. Buschke H, Kuslansky G, Katz M, et al. Screening for
Alzheimer's disease: the effect of APOE epsilon4 allele. Biol
dementia with the memory impairment screen. Neurology
178. Vanderstichele H, Van Kerschaver E, Hesse C, et al. Stan-
158. Blessed G, Tomlinson BE, Roth M. The association between
dardization of measurement of beta-amyloid(1-42) in cere-
quantitative measures of dementia and of senile change in the
brospinal fluid and plasma. Amyloid 2000;7:245–58.
cerebral grey matter of elderly subjects. Br J Psychiatry
179. Kosaka T, Imagawa M, Seki K, et al. The beta APP717
Alzheimer mutation increases the percentage of plasma
159. Katzman R, Brown T, Fuld P, et al. Validation of a short
amyloid-beta protein ending at A beta42(43). Neurology
Orientation-Memory-Concentration Test of cognitive im-
pairment. Am J Psychiatry 1983;140:734–9.
180. Fukumoto H, Tennis M, Locascio JJ, et al. Age but not
160. Critchley M. The parietal lobes. New York, NY: Hafner, 1953.
diagnosis is the main predictor of plasma amyloid beta-
161. Rosen WG, Mohs RC, Davis KL. A new rating scale for
Alzheimer's disease. Am J Psychiatry 1984;141:1356–64.
protein levels. Arch Neurol 2003;60:958–64.
162. Mohs RC. Neuropsychological assessment of patients with
181. Freeman SH, Raju S, Hyman BT, et al. Plasma Abeta levels
Alzheimer's disease. In: Psychopharmacology: the fourth
do not reflect brain Abeta levels. J Neuropathol Exp Neurol
generation of progress. 4th ed. Philadelphia, PA: Lippincott
Williams & Wilkins, 1994.
182. Licastro F, Pedrini S, Caputo L, et al. Increased plasma levels
163. Morris JC, Heyman A, Mohs RC, et al. The Consortium to
of interleukin-1, interleukin-6 and alpha-1-antichymotrypsin
Establish a Registry for Alzheimer's Disease (CERAD). Part
in patients with Alzheimer's disease: peripheral inflammation
I. Clinical and neuropsychological assessment of Alz-
or signals from the brain? J Neuroimmunol 2000;103:97–102.
heimer's disease. Neurology 1989;39:1159–65.
183. Kalman J, Juhasz A, Laird G, et al. Serum interleukin-6 levels
164. Erzigkeit H. Manual zum SKT Formen A-E (4. Auflage).
correlate with the severity of dementia in Down syndrome and
(In German). Beltz, Weinheim, Germany, 1989.
in Alzheimer's disease. Acta Neurol Scand 1997;96:236–40.
165. Solomon PR, Hirschoff A, Kelly B, et al. A 7 minute
184. van Duijn CM, Hofman A, Nagelkerken L. Serum levels of
neurocognitive screening battery highly sensitive to
interleukin-6 are not elevated in patients with Alzheimer's
Alzheimer's disease. Arch Neurol 1998;55:349–55.
disease. Neurosci Lett 1990;108:350–4.
Epidemiol Rev 2008;30:15–34
Dementia of the Alzheimer Type
185. Angelis P, Scharf S, Mander A, et al. Serum interleukin-6
204. Price JC, Klunk WE, Lopresti BJ, et al. Kinetic modeling of
and interleukin-6 soluble receptor in Alzheimer's disease.
amyloid binding in humans using PET imaging and Pitts-
Neurosci Lett 1998;244:106–8.
burgh Compound-B. J Cereb Blood Flow Metab 2005;25:
186. Chao CC, Ala TA, Hu S, et al. Serum cytokine levels in
patients with Alzheimer's disease. Clin Diagn Lab Immunol
205. Klunk WE, Engler H, Nordberg A, et al. Imaging brain
amyloid in Alzheimer's disease with Pittsburgh Compound-
187. Strohmeyer R, Rogers J. Molecular and cellular mediators of
B. Ann Neurol 2004;55:306–19.
Alzheimer's disease inflammation. J Alzheimers Dis 2001;3:
206. Ikonomovic MD, Klunk WE, Abrahamson EE, et al. Post-
mortem correlates of in vivo PiB-PET amyloid imaging in
188. Irizarry MC. Biomarkers of Alzheimer disease in plasma.
a typical case of Alzheimer's disease. Brain 2008;131:1630–45.
207. Rubin CD. The primary care of Alzheimer disease. Am J
189. Scheltens P, Korf ES. Contribution of neuroimaging in the
Med Sci 2006;332:314–33.
diagnosis of Alzheimer's disease and other dementias. Curr
208. Doody RS, Stevens JC, Beck C, et al. Practice parameter:
Opin Neurol 2000;13:391–6.
management of dementia (an evidence-based review). Report
190. Jack CR Jr, Dickson DW, Parisi JE, et al. Antemortem MRI
of the Quality Standards Subcommittee of the American
findings correlate with hippocampal neuropathology in
Academy of Neurology. Neurology 2001;56:1154–66.
typical aging and dementia. Neurology 2002;58:750–7.
209. Cohen-Mansfield J. Nonpharmacologic interventions for
191. Scheltens P, Leys D, Barkhof F, et al. Atrophy of medial
inappropriate behaviors in dementia: a review, summary, and
temporal lobes on MRI in ‘‘probable'' Alzheimer's disease
critique. Am J Geriatr Psychiatry 2001;9:361–81.
and normal ageing: diagnostic value and neuropsychological
210. Dietch JT, Hewett LJ, Jones S. Adverse effects of reality
correlates. J Neurol Neurosurg Psychiatry 1992;55:967–72.
orientation. J Am Geriatr Soc 1989;37:974–6.
192. Jack CR Jr, Petersen RC, Xu YC, et al. Medial temporal
211. APA Work Group on Alzheimer's Disease and Other
atrophy on MRI in normal aging and very mild Alzheimer's
Dementias, Rabins PV, Blacker D, et al. American Psychi-
disease. Neurology 1997;49:786–94.
atric Association practice guideline for the treatment of
193. Barber R, Gholkar A, Scheltens P, et al. Medial temporal lobe
patients with Alzheimer's disease and other dementias.
atrophy on MRI in dementia with Lewy bodies. Neurology
Second edition. Am J Psychiatry 2007 (suppl);164:5–56.
212. Requena C, Lopez Ibor MI, Maestu F, et al. Effects of
194. Golebiowski M, Barcikowska M, Pfeffer A. Magnetic
cholinergic drugs and cognitive training on dementia.
resonance imaging-based hippocampal volumetry in patients
Dement Geriatr Cogn Disord 2004;18:50–4.
with dementia of the Alzheimer type. Dement Geriatr Cogn
213. Matsuda O. Cognitive stimulation therapy for Alzheimer's
disease: the effect of cognitive stimulation therapy on the
195. Frisoni GB, Laakso MP, Beltramello A, et al. Hippocampal
progression of mild Alzheimer's disease in patients treated
and entorhinal cortex atrophy in frontotemporal dementia and
with donepezil. Int Psychogeriatr 2007;19:241–52.
Alzheimer's disease. Neurology 1999;52:91–100.
214. Acevedo A, Loewenstein DA. Nonpharmacological cognitive
196. Ashford JW, Shih WJ, Coupal J, et al. Single SPECT
interventions in aging and dementia. J Geriatr Psychiatry
measures of cerebral cortical perfusion reflect time-index
estimation of dementia severity in Alzheimer's disease.
215. Spira AP, Edelstein BA. Behavioral interventions for agita-
J Nucl Med 2000;41:57–64.
tion in older adults with dementia: an evaluative review. Int
197. Rodriguez G, Nobili F, Copello F, et al. 99mTc-HMPAO
regional cerebral blood flow and quantitative electroenceph-
216. Livingston G, Johnston K, Katona C, et al. Systematic review
alography in Alzheimer's disease: a correlative study. J NuclMed 1999;40:522–9.
of psychological approaches to the management of neuro-
198. Claus JJ, Walstra GJ, Hijdra A, et al. Measurement of
psychiatric symptoms of dementia. Am J Psychiatry 2005;
temporal regional cerebral perfusion with single-photon
emission tomography predicts rate of decline in language
217. Mittelman MS, Ferris SH, Shulman E, et al. A family
function and survival in early Alzheimer's disease. Eur J Nucl
intervention to delay nursing home placement of patients
with Alzheimer disease. A randomized controlled trial.
199. Dougall NJ, Bruggink S, Ebmeier KP. Systematic review of
the diagnostic accuracy of 99mTc-HMPAO-SPECT in
218. Vickrey BG, Mittman BS, Connor KI, et al. The effect of
dementia. Am J Geriatr Psychiatry 2004;12:554–70.
a disease management intervention on quality and outcomes
200. Chaves ML, Ilha D, Maia AL, et al. Diagnosing dementia and
of dementia care: a randomized, controlled trial. Ann Intern
normal aging: clinical relevance of brain ratios and cognitive
performance in a Brazilian sample. Braz J Med Biol Res
219. Doody RS, Geldmacher DS, Gordon B, et al. Open-label,
multicenter, phase 3 extension study of the safety and
201. Dickerson BC, Sperling RA. Neuroimaging biomarkers for
efficacy of donepezil in patients with Alzheimer disease.
clinical trials of disease-modifying therapies in Alzheimer's
Arch Neurol 2001;58:427–33.
disease. NeuroRx 2005;2:348–60.
220. Reisberg B, Doody R, Stoffler A, et al. A 24-week open-label
202. Silverman DH, Gambhir SS, Huang HW, et al. Evaluating
extension study of memantine in moderate to severe
early dementia with and without assessment of regional
Alzheimer disease. Arch Neurol 2006;63:49–54.
cerebral metabolism by PET: a comparison of predicted costs
221. Cummings JL, Doody R, Clark C. Disease-modifying
and benefits. J Nucl Med 2002;43:253–66.
therapies for Alzheimer disease: challenges to early in-
203. Klunk WE, Engler H, Nordberg A, et al. Imaging the
tervention. Neurology 2007;69:1622–34.
pathology of Alzheimer's disease: amyloid-imaging with
222. Cummings JL. Treatment of Alzheimer's disease: the role of
positron emission tomography. Neuroimaging Clin N Am
symptomatic agents in an era of disease-modifying therapies.
Rev Neurol Dis 2007;4:57–62.
Epidemiol Rev 2008;30:15–34
Jalbert et al.
223. Doody RS. Current treatments for Alzheimer's disease:
242. Schneider LS, Dagerman KS, Insel P. Risk of death with
cholinesterase inhibitors. J Clin Psychiatry 2003;64:11–17.
atypical antipsychotic drug treatment for dementia: meta-
224. Geldmacher DS. Treatment guidelines for Alzheimer's
analysis of randomized placebo-controlled trials. JAMA
disease: redefining perceptions in primary care. Prim Care
Companion J Clin Psychiatry 2007;9:113–21.
243. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death
225. Wilkinson DG, Francis PT, Schwam E, et al. Cholinesterase
associated with the use of conventional versus atypical
inhibitors used in the treatment of Alzheimer's disease: the
antipsychotic drugs among elderly patients. CMAJ 2007;176:
relationship between pharmacological effects and clinical
efficacy. Drugs Aging 2004;21:453–78.
244. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug
226. Maelicke A, Samochocki M, Jostock R, et al. Allosteric
use and mortality in older adults with dementia. Ann Intern
sensitization of nicotinic receptors by galantamine, a new
treatment strategy for Alzheimer's disease. Biol Psychiatry
245. Kryzhanovskaya LA, Jeste DV, Young CA, et al. A review of
treatment-emergent adverse events during olanzapine clinical
227. Birks J. Cholinesterase inhibitors for Alzheimer's disease.
trials in elderly patients with dementia. J Clin Psychiatry
Cochrane Database Syst Rev 2006:CD005593.
228. Hansen RA, Gartlehner G, Kaufer DJ, et al. Drug class
246. Cummings JL, Schneider E, Tariot PN, et al. Behavioral
review on Alzheimer's drugs: final report. June 2006.
effects of memantine in Alzheimer disease patients receiving
donepezil treatment. Neurology 2006;67:57–63.
247. Bhasin M, Rowan E, Edwards K, et al. Cholinesterase
229. Bullock R, Touchon J, Bergman H, et al. Rivastigmine and
inhibitors in dementia with Lewy bodies: a comparative
donepezil treatment in moderate to moderately-severe
analysis. Int J Geriatr Psychiatry 2007;22:890–5.
Alzheimer's disease over a 2-year period. Curr Med Res Opin
248. Steinberg M, Tschanz JT, Corcoran C, et al. The persistence
of neuropsychiatric symptoms in dementia: the Cache
230. Chohan MO, Iqbal K. From tau to toxicity: emerging roles of
County Study. Int J Geriatr Psychiatry 2004;19:19–26.
NMDA receptor in Alzheimer's disease. J Alzheimers Dis
249. Feldman HH, Van Baelen B, Kavanagh SM, et al. Cognition,
function, and caregiving time patterns in patients with mild-
231. Peskind ER, Potkin SG, Pomara N, et al. Memantine
to-moderate Alzheimer disease: a 12-month analysis.
treatment in mild to moderate Alzheimer disease: a 24-week
Alzheimer Dis Assoc Disord 2005;19:29–36.
randomized, controlled trial. Am J Geriatr Psychiatry 2006;
250. Families care: Alzheimer's caregiving in the United States
2004. Alzheimer's Association and National Alliance for
232. Bakchine S, Loft H. Memantine treatment in patients with
Caregiving, 2004.
mild to moderate Alzheimer's disease: results of a rando-
mised, double-blind, placebo-controlled 6-month study.
251. Alzheimer's disease facts and figures 2007. Alzheimer's
J Alzheimers Dis 2007;11:471–9.
Association, 2007.
233. Reisberg B, Doody R, Stoffler A, et al. Memantine in
moderate-to-severe Alzheimer's disease. N Engl J Med 2003;
252. Schulz R, Martire LM. Family caregiving of persons with
dementia: prevalence, health effects, and support strategies.
234. Tariot PN, Farlow MR, Grossberg GT, et al. Memantine
Am J Geriatr Psychiatry 2004;12:240–9.
treatment in patients with moderate to severe Alzheimer
253. Aneshensel CS, Pearlin LI, Mullan JT, et al. Profiles in
disease already receiving donepezil: a randomized controlled
caregiving: the unexpected career. San Diego, CA: Academic
trial. JAMA 2004;291:317–24.
Press, 1995.
235. Bird SM, Matthews F, Muniz G. NICE judgment: good law
254. Langa KM, Chernew ME, Kabeto MU, et al. National
risks bad science. Lancet Neurol 2007;6:843–4.
estimates of the quantity and cost of informal caregiving for
236. Chibnall JT, Tait RC, Harman B, et al. Effect of acetamin-
the elderly with dementia. J Gen Intern Med 2001;16:770–8.
ophen on behavior, well-being, and psychotropic medication
255. Teri L. Behavior and caregiver burden: behavioral problems
use in nursing home residents with moderate-to-severe
in patients with Alzheimer disease and its association with
dementia. J Am Geriatr Soc 2005;53:1921–9.
caregiver distress. Alzheimer Dis Assoc Disord 1997;
237. Jewart RD, Green J, Lu CJ, et al. Cognitive, behavioral, and
physiological changes in Alzheimer disease patients as
256. Burns A. The burden of Alzheimer's disease. Int J Neuro-
a function of incontinence medications. Am J Geriatr
257. Schulz R, O'Brien AT, Bookwala J, et al. Psychiatric and
238. Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness
physical morbidity effects of dementia caregiving: preva-
of atypical antipsychotic drugs in patients with Alzheimer's
lence, correlates, and causes. Gerontologist 1995;35:
disease. N Engl J Med 2006;355:1525–38.
239. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of
258. Ory MG, Hoffman RR 3rd, Yee JL, et al. Prevalence and
neuropsychiatric symptoms of dementia: a review of the
impact of caregiving: a detailed comparison between de-
evidence. JAMA 2005;293:596–608.
mentia and nondementia caregivers. Gerontologist 1999;39:
240. Ballard C, Waite J. The effectiveness of atypical antipsy-
chotics for the treatment of aggression and psychosis in
259. Rabow MW, Hauser JM, Adams J. Supporting family care-
Alzheimer's disease. Cochrane Database Syst Rev 2006:
givers at the end of life: ‘‘they don't know what they don't
know''. JAMA 2004;291:483–91.
241. Brodaty H, Ames D, Snowdon J, et al. A randomized
260. Haley WE, West CA, Wadley VG, et al. Psychological,
placebo-controlled trial of risperidone for the treatment of
social, and health impact of caregiving: a comparison of
aggression, agitation, and psychosis of dementia. J Clin
black and white dementia family caregivers and noncare-
givers. Psychol Aging 1995;10:540–52.
Epidemiol Rev 2008;30:15–34
Dementia of the Alzheimer Type
261. Schulz R, Newsom J, Mittelmark M, et al. Health effects of
282. Gruneir A. Dementia special care units in US nursing homes:
caregiving: the caregiver health effects study: an ancillary
a current description of distribution and major features.
study of the Cardiovascular Health Study. Ann Behav Med
Presented at the Gerontological Society of America, Dallas,
Texas, November 2006.
262. Wu H, Wang J, Cacioppo JT, et al. Chronic stress associated
283. Leon J, Cheng C, Alvarez RJ. Trends in special care: changes
with spousal caregiving of patients with Alzheimer's de-
in SCU from 1991 to 1995 ('95/96 TSC). J Ment Health
mentia is associated with downregulation of B-lymphocyte
GH mRNA. J Gerontol A Biol Sci Med Sci 1999;54:
284. Coleman EA, Barbaccia JC, Croughan-Minihane MS. Hos-
pitalization rates in nursing home residents with dementia. A
263. Shaw WS, Patterson TL, Semple SJ, et al. Longitudinal
pilot study of the impact of a special care unit. J Am Geriatr
analysis of multiple indicators of health decline among
spousal caregivers. Ann Behav Med 1997;19:101–9.
285. Chappell NL, Reid RC. Dimensions of care for dementia
264. Shaw WS, Patterson TL, Ziegler MG, et al. Accelerated risk
sufferers in long-term care institutions: are they related to
of hypertensive blood pressure recordings among Alzheimer
outcomes? J Gerontol B Psychol Sci Soc Sci 2000;55:
caregivers. J Psychosom Res 1999;46:215–27.
265. Scharlach AE, Runkle MC, Midanik LT, et al. Health
286. Phillips CD, Sloane PD, Hawes C, et al. Effects of residence
conditions and service utilization of adults with elder care
in Alzheimer disease special care units on functional out-
responsibilities. J Aging Health 1994;6:336–52.
comes. JAMA 1997;278:1340–4.
266. Schulz R, Beach SR. Caregiving as a risk factor for mortality:
287. Holmes D, Teresi J, Kong J. Service inputs and costs of care
the Caregiver Health Effects Study. JAMA 1999;282:2215–19.
related to outcomes among cognitively impaired nursing
267. Covinsky KE, Goldman L, Cook EF, et al. The impact of
home residents. J Ment Health Policy Econ 2000;3:121–7.
serious illness on patients' families. SUPPORT Investigators.
288. Phillips CD, Spry KM, Sloane PD, et al. Use of physical
Study to Understand Prognoses and Preferences for Out-
restraints and psychotropic medications in Alzheimer special
comes and Risks of Treatment. JAMA 1994;272:1839–44.
care units in nursing homes. Am J Public Health 2000;90:
268. Scharlach AE. Caregiving and employment: competing or
complementary roles? Gerontologist 1994;34:378–85.
289. Mehr DR, Fries BE. Resource use on Alzheimer's special
269. Stephens MA, Franks MM, Townsend AL. Stress and
care units. Gerontologist 1995;35:179–84.
rewards in women's multiple roles: the case of women in the
290. Gruneir A, Lapane KL, Miller SC, et al. Is dementia special
middle. Psychol Aging 1994;9:45–52.
care really special? A new look at an old question. J Am
270. Tarlow BJ, Wisniewski SR, Belle SH, et al. Positive aspects
Geriatr Soc 2008;56:199–205.
of caregiving. Res Aging 2004;26:429–53.
291. The incidence of dementia in Canada. The Canadian Study of
271. Kramer BJ. Gain in the caregiving experience: where are we?
Health and Aging Working Group. Neurology 2000;55:
What next? Gerontologist 1997;37:218–32.
272. Motenko AK. The frustrations, gratifications, and well-being
292. Miech RA, Breitner JC, Zandi PP, et al. Incidence of AD may
of dementia caregivers. Gerontologist 1989;29:166–72.
decline in the early 90s for men, later for women: the Cache
273. Gold DP, Reis MF, Markiewicz D, et al. When home
County study. Neurology 2002;58:209–18.
caregiving ends: a longitudinal study of outcomes for care-
293. Ganguli M, Dodge HH, Chen P, et al. Ten-year incidence of
givers of relatives with dementia. J Am Geriatr Soc 1995;
dementia in a rural elderly US community population: the
MoVIES Project. Neurology 2000;54:1109–16.
274. Hux MJ, O'Brien BJ, Iskedjian M, et al. Relation between
294. Hendrie HC, Ogunniyi A, Hall KS, et al. Incidence of
severity of Alzheimer's disease and costs of caring. CMAJ
dementia and Alzheimer disease in 2 communities: Yoruba
residing in Ibadan, Nigeria, and African Americans residing
275. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver
in Indianapolis, Indiana. JAMA 2001;285:739–47.
characteristics and nursing home placement in patients with
295. Fratiglioni L, Launer LJ, Andersen K, et al. Incidence of
dementia. JAMA 2002;287:2090–7.
dementia and major subtypes in Europe: a collaborative study
276. Bullock R. The needs of the caregiver in the long-term
of population-based cohorts. Neurologic Diseases in the
treatment of Alzheimer disease. Alzheimer Dis Assoc Disord
Elderly Research Group. Neurology 2000;54(suppl):S10–15.
296. Bermejo-Pareja F, Benito-Leon J, Vega S, et al. Incidence and
277. Smith GE, Kokmen E, O'Brien PC. Risk factors for nursing
subtypes of dementia in three elderly populations of central
home placement in a population-based dementia cohort.
Spain. J Neurol Sci 2008;264:63–72.
J Am Geriatr Soc 2000;48:519–25.
297. Ravaglia G, Forti P, Maioli F, et al. Incidence and etiology of
278. Rovner BW, German PS, Broadhead J, et al. The prevalence
and management of dementia and other psychiatric disorders
dementia in a large elderly Italian population. Neurology
in nursing homes. Int Psychogeriatr 1990;2:13–24.
279. Jones A. The National Nursing Home Survey: 1999 sum-
298. Ruitenberg A, Ott A, van Swieten JC, et al. Incidence of
mary. National Center for Health Statistics. Vital Health Stat
dementia: does gender make a difference? Neurobiol Aging
299. Li S, Yan F, Li G, et al. Is the dementia rate increasing in
280. Maas ML, Swanson E, Specht J, et al. Alzheimer's special
Beijing? Prevalence and incidence of dementia 10 years later
care units. Nurs Clin North Am 1994;29:173–94.
in an urban elderly population. Acta Psychiatr Scand
281. Special care units for people with Alzheimer's and other
dementias: consumer, education, research, regulatory, and
300. Chandra V, Pandav R, Dodge HH, et al. Incidence of
reimbursement issues. Washington, DC: Office of Technol-
Alzheimer's disease in a rural community in India: the Indo-
ogy Assessment, 1992.
US study. Neurology 2001;57:985–9.
Epidemiol Rev 2008;30:15–34
Jalbert et al.
301. Ferri CP, Prince M, Brayne C, et al. Global prevalence of
320. Hock C, Konietzko U, Papassotiropoulos A, et al. Generation
dementia: a Delphi consensus study. Lancet 2005;366:
of antibodies specific for beta-amyloid by vaccination of
patients with Alzheimer disease. Nat Med 2002;8:1270–5.
302. Plassman BL, Langa KM, Fisher GG, et al. Prevalence of
321. Hock C, Konietzko U, Streffer JR, et al. Antibodies against
dementia in the United States: the aging, demographics, and
beta-amyloid slow cognitive decline in Alzheimer's disease.
memory study. Neuroepidemiology 2007;29:125–32.
303. Bachman DL, Wolf PA, Linn R, et al. Prevalence of dementia
322. Du Y, Dodel R, Hampel H, et al. Reduced levels of amyloid
and probable senile dementia of the Alzheimer type in the
beta-peptide antibody in Alzheimer disease. Neurology 2001;
Framingham Study. Neurology 1992;42:115–19.
304. Hendrie HC, Osuntokun BO, Hall KS, et al. Prevalence of
323. Hyman BT, Smith C, Buldyrev I, et al. Autoantibodies to
Alzheimer's disease and dementia in two communities:
amyloid-beta and Alzheimer's disease. Ann Neurol 2001;49:
Nigerian Africans and African Americans. Am J Psychiatry
324. Saunders AM, Strittmatter WJ, Schmechel D, et al. Associ-
305. Farrag A, Farwiz HM, Khedr EH, et al. Prevalence of
ation of apolipoprotein E allele epsilon 4 with late-onset
Alzheimer's disease and other dementing disorders: Assiut-
familial and sporadic Alzheimer's disease. Neurology 1993;
Upper Egypt study. Dement Geriatr Cogn Disord 1998;9:
325. Gomez-Isla T, West HL, Rebeck GW, et al. Clinical and
306. Ott A, Breteler MM, van Harskamp F, et al. Prevalence of
pathological correlates of apolipoprotein E epsilon 4 in
Alzheimer's disease and vascular dementia: association with
Alzheimer's disease. Ann Neurol 1996;39:62–70.
education. The Rotterdam study. BMJ 1995;310:970–3.
326. Mann DM, Iwatsubo T, Pickering-Brown SM, et al. Prefer-
307. Gurvit H, Emre M, Tinaz S, et al. The prevalence of dementia
ential deposition of amyloid beta protein (Abeta) in the form
in an urban Turkish population. Am J Alzheimers Dis Other
Abeta40 in Alzheimer's disease is associated with a gene
dosage effect of the apolipoprotein E E4 allele. Neurosci Lett
308. Dong MJ, Peng B, Lin XT, et al. The prevalence of dementia
327. Schiele F, De Bacquer D, Vincent-Viry M, et al. Apolipo-
in the People's Republic of China: a systematic analysis of
protein E serum concentration and polymorphism in six
1980–2004 studies. Age Ageing 2007;36:619–24.
European countries: the ApoEurope Project. Atherosclerosis
309. Zhang ZX, Zahner GE, Roman GC, et al. Dementia subtypes
in China: prevalence in Beijing, Xian, Shanghai, and
328. Taddei K, Clarnette R, Gandy SE, et al. Increased plasma
Chengdu. Arch Neurol 2005;62:447–53.
apolipoprotein E (apoE) levels in Alzheimer's disease.
310. Cullen B, O'Neill B, Evans JJ, et al. A review of screening
Neurosci Lett 1997;223:29–32.
tests for cognitive impairment. J Neurol Neurosurg Psychi-
329. Panza F, Solfrizzi V, Colacicco AM, et al. Apolipoprotein E
(APOE) polymorphism influences serum APOE levels in
311. O'Connor DW, Pollitt PA, Hyde JB, et al. The reliability and
Alzheimer's disease patients and centenarians. Neuroreport
validity of the Mini-Mental State in a British community
survey. J Psychiatr Res 1989;23:87–96.
330. Scacchi R, Gambina G, Ruggeri M, et al. Plasma levels of
312. McDowell I, Kristjansson B, Hill GB, et al. Community
apolipoprotein E and genetic markers in elderly patients with
screening for dementia: the Mini Mental State Exam
Alzheimer's disease. Neurosci Lett 1999;259:33–6.
(MMSE) and Modified Mini-Mental State Exam (3MS)
331. Slooter AJ, de Knijff P, Hofman A, et al. Serum apolipo-
compared. J Clin Epidemiol 1997;50:377–83.
protein E level is not increased in Alzheimer's disease: the
313. Lorentz WJ, Scanlan JM, Borson S. Brief screening tests for
Rotterdam study. Neurosci Lett 1998;248:21–4.
dementia. Can J Psychiatry 2002;47:723–33.
332. Siest G, Bertrand P, Qin B, et al. Apolipoprotein E poly-
314. Fillenbaum GG, Heyman A, Wilkinson WE, et al. Compar-
morphism and serum concentration in Alzheimer's disease in
ison of two screening tests in Alzheimer's disease. The
nine European centres: the ApoEurope study. ApoEurope
correlation and reliability of the Mini-Mental State Exami-
group. Clin Chem Lab Med 2000;38:721–30.
nation and the modified Blessed test. Arch Neurol 1987;44:
333. Lehtimaki T, Pirttila T, Mehta PD, et al. Apolipoprotein E
(apoE) polymorphism and its influence on ApoE concen-
315. Stuss DT, Meiran N, Guzman DA, et al. Do long tests yield
trations in the cerebrospinal fluid in Finnish patients with
a more accurate diagnosis of dementia than short tests? A
Alzheimer's disease. Hum Genet 1995;95:39–42.
comparison of 5 neuropsychological tests. Arch Neurol 1996;
334. Pratico D, Clark CM, Lee VM, et al. Increased 8,12-iso-
iPF2alpha-VI in Alzheimer's disease: correlation of a non-
316. Cerhan JH, Ivnik RJ, Smith GE, et al. Diagnostic utility of
invasive index of lipid peroxidation with disease severity.
letter fluency, category fluency, and fluency difference scores
Ann Neurol 2000;48:809–12.
in Alzheimer's disease. Clin Neuropsychol 2002;16:35–42.
335. Montine TJ, Quinn J, Kaye J, et al. F(2)-isoprostanes as
317. Lin KN, Wang PN, Chen C, et al. The three-item clock-
biomarkers of late-onset Alzheimer's disease. J Mol Neurosci
drawing test: a simplified screening test for Alzheimer's
disease. Eur Neurol 2003;49:53–8.
336. Weiner HL, Selkoe DJ. Inflammation and therapeutic vacci-
318. Sunderland T, Hampel H, Takeda M, et al. Biomarkers in the
nation in CNS diseases. Nature 2002;420:879–84.
diagnosis of Alzheimer's disease: are we ready? J Geriatr
337. Tarkowski E, Blennow K, Wallin A, et al. Intracerebral
Psychiatry Neurol 2006;19:172–9.
production of tumor necrosis factor-alpha, a local neuro-
319. Zlokovic BV. Clearing amyloid through the blood-brain
protective agent, in Alzheimer disease and vascular de-
barrier. J Neurochem 2004;89:807–11.
mentia. J Clin Immunol 1999;19:223–30.
Epidemiol Rev 2008;30:15–34
Source: http://www.epi.msu.edu/janthony/EpidemiologyReviewsPathopsychology/Jalbert2008.pdf
Food Chemistry 115 (2009) 279–284 Contents lists available at Isolation and characterisation of a novel angiotensin I-converting enzyme(ACE) inhibitory peptide from the algae protein waste I.-Chuan Sheih a, Tony J. Fang a,1, Tung-Kung Wu b,* a Department of Food Science and Biotechnology, National Chung Hsing University, 250 Kuokuang, Taichung 40227, Taiwan, ROCb Department of Biological Science and Technology, National Chiao Tung University,75 Po-Ai Street, Hsin-Chu 30068, Taiwan, ROC
20 Medizin Medical Tribune · 45. Jahrgang · Nr. 6 · 12. Februar 2010 Westerwälder Hausärzte verschreiben sich dem RennsportSieg im Cockpit – darüber entscheidet auch der Doktor MONTABAUR – Extreme Ge- Herzfrequenz und auch Luftschad- falls gibt es ein Nickerchen schwindigkeiten, massive Er- stoffe, wie sie etwa beim Abrieb der zwischendurch, da wo ge-
