Harnessing the power of enzymes for environmental stewardship

JBA-06412; No of Pages 21
Contents lists available at
Biotechnology Advances
Research review paper
Harnessing the power of enzymes for environmental stewardship
Philippe Demarche, Charles Junghanns, Rakesh R. Nair, Spiros N. Agathos
Earth & Life Institute, Laboratory of Bioengineering, Université Catholique de Louvain, Place Croix du Sud 2/19, 1348 Louvain-la-Neuve, Belgium
Available online xxxx
Enzymes are versatile catalysts with a growing number of applications in biotechnology. Their propertiesrender them also attractive for waste/pollutant treatment processes and their use might be advantageous
over conventional treatments. This review highlights enzymes that are suitable for waste treatment, with a
Pollutant biodegradation
focus on cell-free applications or processes with extracellular and immobilized enzymes. Biological wastes are
treated with hydrolases, primarily to degrade biological polymers in a pre-treatment step. Oxidoreductases
and lyases are used to biotransform specific pollutants of various nature. Examples from pulp and paper,
textile, food and beverage as well as water and chemical industries illustrate the state of the art of enzymatic
pollution treatment. Research directions in enzyme technology and their importance for future development
Directed evolution
in environmental biotechnology are elaborated. Beside biological and biochemical approaches, i.e. enzyme
Enzyme immobilization
prospection and the design of enzymes, the review also covers efforts in adjacent research fields such as
insolubilization of enzymes, reactor design and the use of additives. The effectiveness of enzymatic processes,
especially when combined with established technologies, is evident. However, only a limited number ofenzymatic field applications exist. Factors like cost and stability of biocatalysts need to be addressed and thecollaboration and exchange between academia and industry should be further strengthened to achieve thegoal of sustainability.
2011 Elsevier Inc. All rights reserved.
Pollution control via enzyme technology in industries . . . . . . . . . . . . . . . . . . . . . .
Abbreviations: 4-CP, 4-chlorophenol; ABTS, 2,2′-azino-bis-3-ethylbenzothiozoline-6-sulphonic acid; AOP, Advanced oxidation process; BOD, Biochemical oxygen demand; BPA,
Bisphenol A; CDH, Cellobiose dehydrogenase; CLEA®, Crosslinked enzyme aggregate; COD, Chemical oxygen demand; CYP450, Cytochrome P450; CSTR, Continuous stirred-tankreactor; DEHP, Di-(2-ethylhexyl)-phthalate; DHA, Haloalkane dehalogenase; DMP, Dimethyl phthalate; EDC, Endocrine disrupting chemical; EE2, 17α-ethinylestradiol; E1, Estrone;E2, 17β-estradiol; E3, Estriol; FBR, Fluidized bed reactor; FMR, Flat membrane reactor; HAA, Haloacetic acids; Hb, Hemoglobin; HBT, N-hydroxybenzotriazole; HRP, Horseradishperoxidase; LME, Lignin-modifying enzyme; MBR, Membrane bioreactor; MnP, Manganese peroxidase; MOW, Mixed office waste; NHase, Nitrile hydratase; NP, Nonylphenol; OP,Organophosphorus compound; OPAA, Organophosphorus acid anhydrolase; OPH, Organophosphorus hydrolase; O&G, Oil and grease; PAH, Polycyclic aromatic hydrocarbons; PBR,Packed bed reactor; PEG, Polyethylene glycol; PeMP, Pentyl methyl phthalate; PTT, Poly(trimethylene terephthalate); RT, Residence time; TAH, Tannase; TCS, Triclosan; Tyr,Tyrosinase; U, Unit of enzyme activity; WRF, White rot fungi; WWTP, Wastewater treatment plant.
⁎ Corresponding author at: Group of Bioengineering, Earth & Life Institute, Université Catholique de Louvain, Croix du Sud 2/19, 1348 Louvain-la-Neuve, Belgium. Tel.: +32
10473644; fax: + 32 10473062.
E-mail addresses: (P. Demarche), (C. Junghanns), (R.R. Nair),
(S.N. Agathos).
0734-9750/$ – see front matter 2011 Elsevier Inc. All rights reserved.
doi:
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),
P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
Additives: redox mediators, cosubstrates and protecting agents
Reactor and process design
side-reactions can occur) and energy consuming. Chemical processesoften use harsh chemicals which are unsafe for workers and the
Over the past century, there has been an increased awareness of
environment. Oxidoreductases may represent an alternative for
the effects of pollution, and public pressure has influenced both
pollution control through oxidation of organic contaminants.
industries and governments. Environmental pollution is no longer
Two types of copper-containing enzymes, laccase and tyrosinase
unavoidable. There are increasing demands to replace traditional
(Tyr), and three kinds of heme enzymes, namely peroxidases,
industrial processes by less- or non-polluting ones. During this
hemoglobin (Hb) and cellobiose dehydrogenase (CDH) are intro-
gradual shift which is a major challenge for our and the coming
duced below. In particular, fungal lignin modifying enzymes (LME),
generations, the treatment of wastes from current human activities
laccases and peroxidases, have been extensively investigated for
and as heritage of our industrial history remains a problem to be
potential biotechnological applications and are hence well repre-
solved. Only as these treatments will gradually merge with environ-
sented in this review ( ). In addition, the new basic and applied
mentally benign industrial processes, a truly sustainable economy will
knowledge represented by publications and patents on these enzymes
become a reality.
has an upward trend (). Lignin, a highly complex, stable and
The use of enzymes in industrial processes is usually linked to a
irregular polymer, requires enzymes with the ability to nonspecifi-
reduced consumption of energy as well as chemicals and thus
cally oxidize substrates with high redox potential (
beneficial for the environment. The enzyme world market grew at a
double-digit rate in the last seven years and was about $5.1 billion in
Laccases EC 1.10.3.2 [benzenediol:oxygen oxidoreductases] are
2009 ). Enzymes catalyze specific re-
widely distributed multi-copper proteins capable of oxidizing a
actions and mostly act under moderate conditions (temperature, pH,
variety of phenolic and non-phenolic compounds
solvents and ionic strength). Hence enzymes represent a promising
. They are extensively studied since the mid-
tool for the selective removal of pollutants from waste streams.
seventies of the last century (Laccases have
Enzyme specificity also precludes undesired side-reactions, which
various physiological functions depending on the producing species
would otherwise increase reactant consumption and correspondingly
and their cellular location. They are involved in virulence processes
raise the cost of treatment — a great advantage over conventional
(yeasts, bacteria, pathogenic fungi), lignin degradation (white rot
chemical treatment processes. The application of enzymes to waste
fungi) or deposition (plants), pigment synthesis (fungi, bacteria) and
treatment was already proposed in the 1930s (however
cuticle sclerotization in insects ). Laccases catalyze one-
the degradation of a pollutant (parathion) by enzymes was first
electron oxidations by transferring one electron from four substrate
illustrated in the late 1970s ().
molecules to one molecule of molecular oxygen which is reduced to
The purpose of this review is to highlight enzymes that are
water (Oxidized substrate radicals can
suitable for waste treatment applications, to summarize waste
undergo nonspecific polymerization reactions.
treatment situations in which the use of enzymes is feasible, and todefine the issues/perspectives and potential bottlenecks relevant
to the development of enzyme-based waste treatment processes.
In this review, only pollutant transformations mediated by cell-free or immobilized enzymes are considered. Processes with whole
cells have been included only when extracellular enzymes areinvolved.
1.1. Oxidoreductases EC 1
Laccases contain four copper atoms in different sites which are
classified according to their spectroscopic and functional properties:
Oxidoreductases catalyze the electron transfer from one substrate
Type 1 (blue), Type 2 (normal) and Type 3 (coupled binuclear). Type 2
to another, where the oxidized substrate is referred to as electron
and 3 represent the active site for the binding and the reduction of
donor in contrast to the reduced substrate, the electron acceptor. The
oxygen. For more detailed information on reaction mechanism and
recommended name according to the EC classification system is
physiological role, readers can refer to reviews (e.g.
"donor:acceptor oxidoreductase" (Nomenclature Committee of the
International Union of Biochemistry and Molecular Biology (NC-
Tyrosinase EC 1.10.3.1, EC 1.14.18.1 [catechol:oxygen oxidoreduc-
IUBMB)). Commonly used names are "donor dehydrogenase",
tase, monophenol:oxygen oxidoreductase] is another copper-contain-
"acceptor reductase" and, when molecular oxygen is the electron
ing protein known as a monophenol oxidase or catecholase,
acceptor, "donor oxidase".
ubiquitously distributed in organisms
Oxidation reactions are essential in current waste treatment
It shares most of its substrates with
strategies. Most of them are based on physicochemical principles,
laccases, but the ability to oxidize tyrosine is exclusive to Tyr. In
having the typical disadvantages of being nonspecific (i.e. undesirable
animals, fungi and plants, this trans-membrane protein is involved in
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),doi:
P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
Oxidoreductases Laccase (EC 1.10.3.2)
Phenols and halogenated phenols
Emerging pollutants; drugs, hormones,
personal care products, plasticizers,
Polycyclic aromatic hydrocarbons
(EC 1.10.3.1/1.14.18.1)
Peroxidase (EC 1.11.x)
Phenols and halogenated phenols
Emerging pollutants drugs, hormones,
personal care products, plasticizers,
micropollutants, etc.
Kraft pulping effluent
Aromatic amines, polycyclic aromatic
Cellobiose dehydrogenase
Pulp mill effluent
Cutinase (EC 3.1.1.74)
Phthalate derivatives
Malathion (pesticide)
Lipase (EC 3.1.1.3)
Phthalate derivatives
Pet food processing effluent
Waste cooking oil
Tannase (EC 3.1.1.20)
Olive mill wastes
Tannery effluents
Phosphoric triester hydrolases
Pesticides and nerve agents
Pulp and paper sludge
Amylases (EC 3.2.x)
Cassava starch detoxification
Paper recycling (deinking)
Alcohol distillery effluent
Biodegradable plastics
Chitinases (EC 3.2.1.x)
Shellfish wastes
Citrus processing waste
(EC 3.2.1.x + EC 4.2.2.x)
Protease (EC 3.4); keratinases,
Silk manufacturing effluent
collagenases, pepsins and papain Keratin-rich wastes (feathers, skins)
Seafood processing effluent
Alcohol distillery effluent
Mercury abatement
Nitrile degrading enzymes (EC
3.5); nitrilase, aliphatic nitrilaseand amidaseHaloalkane dehalogenase (EC
Nitrile hydratases (EC 4.2.1.84)
Cyanide hydratase (EC 4.2.1.66)
lyase (EC 4.2.2.x)
Citrus processing waste
Refer to pectinases (EC 3.2.1.x + EC 4.2.2.x)
a Pectinases and pectin lyases are often co-expressed by organisms and/or used in combination for the treatment of pectic substances.
the synthesis of melanin and other pigments (e.g. browning of sliced
subsequently oxidized to o-diquinones and the oxidation of o-
fruits and vegetables). Tyr contains a Type 3 copper center and
diphenols to o-quinones. The latter can undergo non-enzymatic
catalyzes two distinct reactions using molecular oxygen; the
polymerizations. Thus, Tyr is an oxidase with a monophenolase
hydroxylation of monophenols to form o-diphenols which are
activity (cresolase) and diphenolase activity (catecholase) (
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),

P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
), leading to the
The ferriprotoporphyrin IX prosthetic group is a common feature
assignment of two EC-numbers.
of all heme peroxidases. Ferriprotoporphyrin consists of four pyrrolerings linked by methylene bridges with iron (III) as central atom
(The catalytic cycle of peroxidases is a three-stepreaction. First, the native enzyme (Fe3+) is oxidized by hydrogen
peroxide. This produces water and the oxidized form of peroxidase,
called Compound I (Fe4+–R+•). Second, Compound I oxidizes onemolecule of substrate, resulting in a substrate radical and CompoundII (Fe4+). Compound II oxidizes a second substrate molecule, leading
to a second substrate radical and the native peroxidase (reducedstate Fe3+) (. The balance of the catalytic cycle is given in.
→ 2 oxidized donors þ H
Broad substrate specificity of different peroxidases is related to
their high redox potential (1000 mV) and structural properties.
Mainly non-animal heme-peroxidases were intensively investigated
as potential biocatalysts for the removal of various pollutants, among
While tyrosinase exerts hydroxylase activity on monophenols,
them horseradish peroxidase (HRP, Class III) and manganese
laccase oxidizes them through a radical mechanism
peroxidase (MnP, Class II). In recent years, efforts have been made
). Both enzymes are thus classified as phenol oxidases.
to obtain cheap peroxidases from diverse sources such as bitter gourd,
The redox potentials of laccases and Tyr vary between 400 and
turnip and soybean (
Hemoglobin (Hb) can exert peroxidase activity under specific
Peroxidases EC 1.11.x [donor: hydrogen peroxide oxidoreductases]
redox conditions, and was examined as a potential and inexpensive
are predominantly heme proteins which utilize hydrogen peroxide
(when derived from slaughterhouse wastes) substitute for main-
stream (heme) peroxidases. Hb is a macromolecular heme-protein
2O2) or organic hydroperoxides as cosubstrate to oxidize a variety of
organic and inorganic substrates. They are found in all five kingdoms of
present in bacteria, plants and animals (
life. Based on the EC nomenclature, peroxidases are divided into two
). Unlike heme peroxidases, Hb contains a
subclasses, peroxidases (EC 1.11.1) with currently 19 entries and
ferroprotoporphyrin IX with an iron(II)-center which is responsible
peroxygenases (EC 1.11.2) with 4 entries. This review will concentrate
for its ability to bind molecular oxygen, oxygen transport being its
on "non-animal heme-peroxidases" including intracellular peroxi-
physiological role. Peroxidase can bind molecular oxygen when the
dases of bacterial and eukaryotic origin (Class I, e.g. yeast cytochrome c
iron (III) is reduced to iron (II) and Hb likewise exhibits an intrinsic
peroxidase), secreted fungal peroxidases (Class II, e.g. lignin and
peroxidase activity ().
manganese peroxidases), and classic secretory plant peroxidases
Cellobiose dehydrogenase (EC.1.1.99.18) is an extracellular fungal
(Class III, e.g. horseradish and bitter gourd peroxidases). As the
enzyme of interest because of its ability to produce radicals. CDH is an
classification of peroxidases is currently a highly discussed topic, the
enzyme containing both flavin and b-type heme (ferriprotoporphyrin IX).
reader may follow dedicated literature (
It is secreted by wood-degrading fungi, including white-rot and soft-rot
fungi. Its probable physiological function is cellulose and lignindepolymerization and prevention of cellulose repolymerization Cellobiose dehydrogenaseoxidizes, in a ping-pong mechanism, various substrates such as cellobiose,cellodextrins, mannodextrins and lactose, to their corresponding lactoneswith the concomitant reduction of the flavin (FAD to FADH2). The flavin issubsequently reoxidized by the heme group ).
Quinones, Fe3+, Cu2+ and I−
are suitable final electron acceptors
(). Hydroxyl radicals are indirectly generatedthrough the reduction of Fe3+ to Fe2+ and O2 to peroxide (Fentontype reaction), even if oxygen is a poor substrate for CDH. Thoseradicals can react subsequently with diverse compounds (
Oxidation of various substrates by the above-mentioned oxidative
enzymes generates intermediates which can undergo nonenzymaticreactions like polymerization (i.e. intermediates react with each otherand/or with parent compounds), depolymerization (i.e. cleavage ofexisting polymers) and ring cleavage of aromatics (nitroaromatics,synthetic dyes, aromatic structures in lignin). All of these reactions canbe valuable in biotechnological processes, from biodegradation to foodprocessing, wood pulp biobleaching and biosensors
Fig. 1. Publications and patents on enzymes used in "waste treatment and disposal"
although there are
(Chemical Abstract section title in Scifinder®) as representation of the interest in
only a few applications of Tyr described, attributed to difficulties in
enzymes for pollution management strategies. Publications and patents are drawn in
production of the enzyme from eukaryotic sources (
black and blue, respectively. The dotted lines show publications and patents for all
Likewise, development of CDH applications has been hampered
enzymes listed in (excluding hemoglobin), plain lines represent oxidoreduc-tases and dashed lines represent hydrolases.
by the high cost of its production. Among them, amperometric
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),doi:
P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
biosensors for lactose and diphenol detection are the most tangible
1.2. Hydrolases EC 3
).
These sensors can be operated for considerable periods of time due to
Hydrolases are classified as EC 3 and further divided into 13
pH tolerance and stability of the constitutive CDH
subcategories according to the type of bonds hydrolyzed (NC-IUBMB).
). proposed to exploit CDH under
They catalyze the hydrolysis of various compounds according to the
particular reductive conditions for biodegradation purposes. They
showed that oxalate, when it serves as iron chelator, is oxidized byhydroxyl radicals to form the strongly reducing carboxylate anion
A−B þ H2O→A−OH þ B−H
radical. With this cascade, CDH could catalyze the reduction ofbromotrichloromethane to trichloromethyl radicals.
The systematic name is always formed by adding "hydrolase" to the
One of the largest research fields for applications of phenol oxidases
substrate (substrate hydrolase). The accepted name in most cases is
and heme proteins is the treatment of wastewaters containing phenolic
formed by the name of the substrate with the suffix -ase. Many
compounds. Examples of phenolic compounds treated with peroxidases
hydrolases, regardless of their phylogenetic origin or catalytic function,
under model wastewater conditions are phenol
share an α/β sheet of eight β-sheets connected by α-helices as first
described by This structural element is well conserved
), p-bromophenol ), α-naphthol
in order to keep three catalytic residues in a functional position.
phenylenediamines, catechol, resorcinol, hydroqui-
Exemplified on a trypsin-like protease, this so called catalytic triad is
none (and chlorocatechols
formed by an aspartate, a histidine and a serine. The serine hydroxyl
recently reviewed potential
group is deprotonated within the triad forming a very nucleophilic
peroxidase applications. Systems developed using synthetic wastewaters
alkoxide group (―O−) which may attack a suitably positioned substrate
are potentially transferable to the treatment of phenol-rich effluent
carbonyl carbon atom to form an acyl intermediate which is subse-
streams from e.g. pulp and paper plants, olive mills, petroleum refining
quently attacked by water to release the products.
facilities, resins and plastics manufacturing. A drop in the toxicity of
classified hydrolases based on their catalytic site,
phenolics is reported to mainly occur through polymerization processes
i.e. the residue forming a covalent bond with the substrate. Beside the
above mentioned serine-hydrolases, metal-dependant hydrolases and
). Oxidation of phenols
carboxyl (aspartyl and glutamyl) hydrolases are major groups based on
leads to phenoxy radicals which undergo non-enzymatic polymerization.
this classification scheme. Hydrolases are involved in virtually all
The insoluble polymer can be easily removed from the effluent
biochemical processes. Hydrolase-catalyzed reactions take part in
afterwards (filtration or sedimentation).
metabolism, signal transduction, development, etc. However, this review
Among the relevant lab-scale systems for the treatment of model
will focus on catabolic hydrolases, as they are of outstanding industrial
effluents are continuously operated membrane bioreactors (MBR)
interest – approximately 75% of the industrial enzymes are hydrolases
and packed bed bioreactors
) – and already have found numerous applications, including
(PBR) (These configurations save space
environmental ones (Catabolic hydrolases depolymerize
and energy, compared to e.g. continuously stirred tank reactors
macromolecules such as proteins, carbohydrates and nucleic acids for
(CSTR) in series with filtration or sedimentation units.
further metabolism. Because of the need to break down a wide range of
operated a packed-bed reactor, filled with white
nutrients, hydrolases usually have broad substrate specificity.
radish peroxidase immobilized on diethylaminoethyl cellulose, for the
Esterases (EC 3.1) act on ester bonds. Selected representatives in
treatment of 0.5 mM α-naphtol-spiked water over one month (57%
this review are cutinases, lipases, tannases and organophosphate
hydrolases. Esterases such as fungal cutinases (EC 3.1.1.74) can
Other potential applications of peroxidases in water treatment
hydrolyze xenobiotic polymers, e.g. polyamides, polyesters and poly
include oil removal in case of oil leakage in process water (
(lactic acid), due to analogies to their natural substrate, cutin.
and polymerization of dissolved organic matter (humic and
Molecular aspects of microbial degradation of xenobiotic polymers
fulvic acids) which acts as precursor of disinfection by-products in
and possible implications of microorganisms, enzymes and genes in
water supplies ().
environmental biotechnology are developed by .
Degradation studies are often conducted on synthetic wastewaters.
Lipases (triacylglycerol acylhydrolases, EC 3.1.1.3) are widely
However, assessment in relevant matrices should also be systematically
distributed and catalyze hydrolysis and synthesis of ester bonds in
considered at lab-scale. In a study of peroxidase-based removal of
long chain triglycerides (Their physiological role
estrogens ), a complete elimination was observed in
involves the breakdown and mobilization of lipids inside cells as well as
distilled and ultra-filtered water (Milli-Q system) with a few units of
transfers of lipids between organisms . Microbial
peroxidase, while 250-fold more enzyme activity was necessary to
lipases have biotechnological applications in organic synthesis, food and
achieve similar results in filtered wastewater.
flavor industries, detergent manufacturing, removal of oil and grease
Taking only redox potential and substrate specificity into considera-
(O&G) in wastewaters and biodiesel refining. Aspects of lipase
tion, a vast number of oxidoreductases are eligible for an application in
production, characterization, immobilization and applications are
environmental biotechnology. More than 2700 enzymes of the
comprehensively examined by
cytochrome P450 (CYP450) superfamily were identified in all domains
of life and have the ability to oxidize a large variety of organic
technologically interesting dimension of lipases is their tolerance
substances, often through their monooxygenase activity, i.e. insertion of
against organic solvents (
an oxygen atom into a carbon–hydrogen bond leading to a hydroxyl
Tannase (tannin acyl hydrolase (TAH), EC 3.1.1.20) is involved in the
group However, industrial
modification of complex tannins (e.g. during fruit ripening). Tannic
scale enzyme production, the requirement for cofactors (e.g. FAD, NAD),
substances are the most important plant phenolics after lignin-related
turnover number, and stability of multicomponent enzymes are criteria
phenols and the main chemical group of natural anti-microbial
which limit the number of suitable enzymes (
substances produced by plants ). Tannins are
These decisive factors can also be seen as severe
polyphenols of varying molecular weight able to form complexes with
constraints to be eliminated before envisaging cost-effective applica-
proteins and with others macromolecules such as sugars and cellulose,
tions. Possibilities to overcome such constraints are discussed hereafter
thereby precipitating them. TAHs transform tannic acid, methyl gallate,
(refer to ).
ethyl gallate, n-propylgallate, and isoamyl gallate to gallic acid and
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),
P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
glucose. Therefore, they may be used to degrade tannins contained in
100,000 tons per year, for the production of enzymes could decrease
industrial waste streams, such as tanneries TAHs
the cost of enzyme production and solve an environmental issue
can be obtained from plants, animals and microbes, and commercial
TAH is produced using filamentous fungi, mainly Aspergillus sp., in
Pectinases include polygalacturonases (EC 3.2.1.15), pectinesterases
submerged cultures The first
(EC 3.1.1.11), pectin lyases (EC 4.2.2.10) and pectate lyases (EC 4.2.2.2),
evidence of bacterial strains isolated from olive mill waste and using
based on their mode of action on the heterogeneous structure of pectic
tannic acid as sole source of carbon and energy was recently reported
substances. Pectins are polymers of D-galactopyranosyluronic acids
(. The highest tannase activity was detected in
joined by α-D-(1→4) glycosidic linkages. The main chain can be
Pantoea species.
modified in various ways (ramification with neutral sugars, esterifica-
Hydrolysis of organophosphorus compounds (OP), used as pesti-
tion, acetylation) (). The main-chain-degrading enzymes
cides and warfare agents, can be achieved using phosphoric triester
are esterases and depolymerases ). The
hydrolases. Aryldialkylphosphatase (EC 3.1.8.1) – also known as
latter group is further divided into hydrolases and lyases.
organophosphorus hydrolase (OPH) or phosphotriesterase – and
Pectic substances are ubiquitous in plants and are the major
diisopropyl-fluorophosphatase (EC 3.1.8.2) – commonly called organo-
components of the middle lamella, thus constituting the majority of
phosphorus acid anhydrolase (OPAA) – are the most studied subgroups.
fruit processing wastes (especially from citrus fruits) and represent-
OPH hydrolyzes esters of phosphoric acid (P―O bond), while OPAA
ing a potential field of application for pectinases.
acts preferably on P―F or P―CN bonds. Biotechnological applications
Peptidases (EC 3.4) constitute another group of enzymes with great
of OP enzymes for environmental cleaning and personal protection
economical implication. Proteases, peptidases or proteinases are
materials have been reviewed in
synonyms for enzymes conducting proteolysis, or cleavage of peptide
addressed hydrolases involved in the detoxifica-
bonds. Numerous proteases are commercially produced and used in
tion of OP but also carbamate and pyrethroid insecticides. Catabolic
(laundry) detergent formulations, protein synthesis, brewing, food
pathways for some OPs with emphasis on biochemical and molecular
industry, leather and dairy processing
aspects of OP degradation by microbes, along with the evolution and
Biodegradation/biotransformation applications include treat-
distribution of related genes/enzymes were deeply reviewed by
ment of various protein-rich effluents from food and beverage
There, they also report putative
industries. Keratinases, collagenases and pepsins are promising tools
applications of OP-degrading microbes and their enzymes for
to enhance the low biodegradability of structural animal proteins like
bioremediation and treatment of OP poisoning.
keratin and collagen. Keratin for example is compact and strongly
Glycosylases (EC 3.2) are economically the most important
stabilized by hydrogen bonds, hydrophobic interactions and disulfide
hydrolases. They hydrolyze bonds between a carbohydrate and
bonds which render it resistant to proteolytic degradation (
another group (including other carbohydrates), and are thus essential
Papain or cysteine protease (EC 3.4.22.2) is an enzyme found in
for breaking down carbohydrate polymers, such as cellulose,
papaya having numerous current applications (e.g. cell culture
hemicellulose and starch as well as chitin. Glycosylases – amylases,
preparations, meat tenderizer, medical debridement) which may
cellulases, xylanases and chitinases as prominent representatives –
substitute more expensive proteases.
are used in food, brewery and wine, animal feed, textile and laundry,
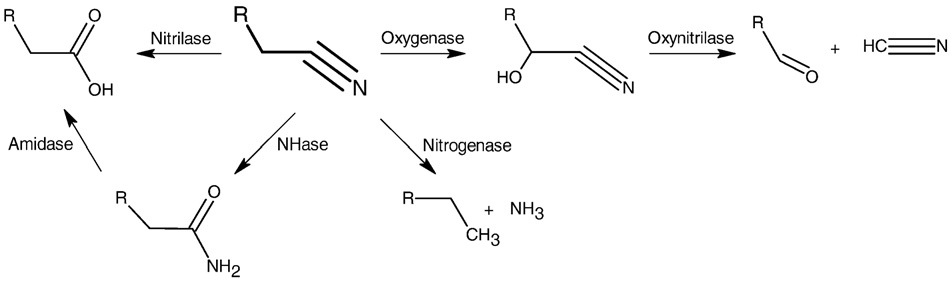
Nitrile degrading enzymes (EC 3.5) act on carbon–nitrogen bonds
pulp and paper industries, as well as in agriculture and for research
other than peptide bonds.
purposes. Their applications are diverse; treatment/recycling of
Hydrolysis is the common way for the microbial catabolism of nitriles
wastes, production of fermentable products, production of renewable
(. The biological degradation of nitriles
fuels (bioethanol) and fine chemicals. For a general overview on the
proceeds through two enzymatic routes Nitrilases (EC 3.5.5.1)
use of carbohydrolases please refer to Glycosylases are
and aliphatic nitrilases (EC 3.5.5.7) catalyze the direct hydrolysis of nitriles
rarely used for a direct biotransformation of pollutants, but are very
to the corresponding carboxylic acids, forming ammonia without free
useful to pre-treat polymeric wastes in order to decrease the waste
amide intermediates (Nitrilases exhibit a
volume, to increase the bioavailability of attached or linked pollutants
selectivity that cannot be achieved with acid- or base-catalyzed chemical
or to improve the digestibility of wastes for subsequent treatment
hydrolysis in addition to enantioselectivity, producing single stereoiso-
processes, exemplified by studies on the saccharification of municipal
mers, and regioselectivity, hydrolyzing a single nitrile group in a
waste leading to an increased conversion to ethanol after hydrolysis
compound with two or more nitrile groups Typically,
they are moderately thermostable enzymes with bacterial nitrilases
Glycosylases are also important in integrated waste management
possessing a higher temperature stability compared to fungal nitrilases
strategies, for example when the life cycle of biodegradable plastics is
(Amidases (EC 3.5.1.4) sequentially hydrolyze
considered (Amylase can degrade
nitriles in a two step reaction with amides as intermediate product
starch blend polyethylene (and recently a thermo-
(Amidases are often co-expressed along with nitrile hydratases
stable α-amylase from a marine bacterium was described as a
which are classified as lyases (NHase, EC 4.2.1.84). Oxygenases (EC 1.13)
powerful biocatalyst for the treatment of synthetic biodegradable
oxidize nitriles to hydroxyl nitriles which then decompose either by
spontaneous hydrolysis or by the action of hydroxylnitrilases (EC 4.1.2.37)
Chitin is one of the most abundant compounds produced by marine
to yield cyanide and the corresponding aldehydes or ketones. The further
invertebrates, insects, fungi and algae. It is a polymer of β-1,4-N-
degradation of cyanide is described in (Lyase).
acetylglucosamine, whereas chitosan is the deacetylated counterpart.
Dehalogenases (EC 3.8) act on halide bonds. Volatile halogenated
Chitin is seldom found pure in nature, most of producing organisms
organic compounds are widely used as pesticides, solvents and organic
modify chitin by linking it to other polymers, proteins, etc.
synthesis intermediates. Haloalkane dehalogenase (DHA, E.C. 3.8.1.5) is
). Chitinolytic enzymes or chitinases (EC 3.2.1.x) perform the
an enzyme found in e.g. Rhodococcus erythropolis, which can directly
hydrolysis of chitin to its residues N-acetylglucosamine, exo- and
hydrolyze short chained 1-haloalkanes (C2 to C8) to primary alcohols
endochitinases acting on different moieties of chitin (
without requiring a coenzyme ).
). Chitinase-producing strains usually use chitin/chitosan as carbonsources. () selected an Aspergillus sp. strain among
220 isolates based on its chitinase production. The enzyme wasproduced only when the cultivation medium contained shrimp shells.
Lyases are enzymes that catalyze the cleavage of chemical bonds
The bioconversion of shellfish waste, with an estimated volume of
(C―C, C―O, C―N and others) by other means than hydrolysis and
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),doi:
P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
oxidation (NC-IUBMB). Furthermore, they are capable of forming
issue. A short treatment of cellulosic sludge with cellulases facilitates
multiple bonds or new ring structures. Lyases are divided into 7
the dewatering with belt presses because it shortens the fibers leading
classes according to the bond broken. The systematic name is formed
to a higher solid content in the resulting cakes ().
according to the pattern substrate group-lyase. Carbon–oxygen lyases
Subsequent treatments are therefore facilitated. It should be noted
(EC 4.2) catalyze the cleavage of carbon–oxygen bonds leading to
that already in proposed the ethanol fermentation of
primary clarifier sludge produced in sulfite pulping operations to
Class EC 4.2.1 contains hydro-lyases or dehydratases that cleave
reduce the amount of waste. Simultaneous cellulase hydrolysis and
carbon–oxygen bonds by elimination of water molecules. Nitrile
yeast fermentation could reduce the sludge by 60% wt and release
hydratases (NHase, EC 4.2.1.84) are metalloenzymes that contain
fermentable sugar for ethanol production without end-product
either a non-heme Fe(III)-ion or non-corrin Co(III)-ion, leading to a
inhibition of the cellulases.
classification into ferric and cobalt NHases. They catalyze the
Wood pulping – the separation of cellulose fibers from lignin
hydration of diverse nitriles to their corresponding amides ).
structures – is generally based on mechanical (cooking, grinding)
NHase is a key enzyme in the enzymatic pathway for the
and/or chemicals technologies (acid sulfite or Kraft process).
conversion of nitriles to acids. A large number of microorganisms
Cholesteryl esters may form pitch deposits in pulp processing. Sterol
were isolated expressing NHases which have been purified and
esterase covalently immobilized on polyacrylate-based epoxy-activated
characterized ). Although the structural
carriers (Dilbeads™) was tested for the continuous hydrolysis
characterization of NHases has provided some insights, the exact
catalytic mechanism of NHases is still not fully elucidated. Cyanide
By the end of the last century, an alternative was introduced to
hydratase or formamide dehydratase or formamide hydro-lyase (EC
reduce the use of chemicals: the laccase-mediator-system applied to
4.2.1.66) is a primarily fungal enzyme which participates in
wood pulping (Lignozym®-process), similar to wood delignification
cyanoamino acid metabolism. HCN, the best substrate for this group
in nature, namely the white-rot of wood and compatible with the pulp
of enzymes, is transformed into formamide. Cyanide hydratase was
technologies at that time (
first partially purified from Stemphylium loti
Oxidoreductases and hydrolases were considered for the manage-
Class EC 4.2.2 contains lyases which cleave C―O bonds by the
ment of liquid and solid residues (). For instance, horseradish
elimination of an alcohol from a polysaccharide. Pectate lyase (EC
peroxidase was used for the efficient detoxification of phenol-
4.2.2.2) requires the presence of calcium ions and catalyzes the
containing condensates of evaporators from Kraft pulping
cleavage of (1 → 4)-α-D-galacturonan to oligosaccharides with 4-
deoxy-α-D-galacto-4-enuronosyl groups at their non-reducing ends.
For waste water treatment, an acidic and a basic CDH were
It is not only involved in the maceration and soft-rotting of plant
assessed for their ability to decolorize the acidic and the caustic pulp
tissue but also in the activation of defense systems presumably
mill effluents from a bleach plant Both
through the mechanism of elicitation ).
enzymes were reasonably effective in color removal at their
Pectate lyase activity was first discovered in cultures of Erwinia
respective optimum pH. In addition, CDH-treatment is relevant for
carotovora and Bacillus polymyxa
softwood and hardwood processing effluents. Even though technical
give an overview of so far identified bacterial species
feasibility was shown, the high cost of CDHs discourages their
producing pectate lyase. Pectate exo-lyase (EC 4.2.2.9) catalyzes the
exploitation for pulp bleaching at industrial scale.
Paper recycling is the cornerstone of further developments in paper
from the reducing end of pectate, i.e. de-esterified pectin, and endo-
industry. Recycling includes sorting, dissolving, deinking, etc. before the
pectin lyase (EC 4.2.2.10) catalyzes the cleavage of (1 → 4)-α-D-
paper making process takes place. The major contaminants in "mixed
galacturonan methyl ester to give oligosaccharides with 4-deoxy-6-O-
office wastes" (MOW) and photocopy prints are inks and the most
methyl-α-D-galacto-4-enuronosyl groups at their non-reducing ends
difficult step in recycling is the deinking. Physicochemical treatments
are not always successful to remove ink. Alkaline cellulase is believed todeink paper by releasing short fibers of 20 to 100 μm from the surface of
2. Pollution control via enzyme technology in industries
paper substrates examinedenzymatic (cellulase/hemicellulase) versus chemical deinking for MOW
2.1. Pulp and paper industry
and photocopy prints. Their conclusions were ambivalent. Althoughenzymes are suitable for deinking, their efficacy critically depends on
Pulp and paper industries convert wood and recycled cellulose
feed characteristics. Moreover, their action can affect paper strength
fibers into pulp and various forms of paper. Pulp and paper mills use
properties, which is consistent with the assumed mechanism.
significant volumes of water and strong chemicals and generate
In the paper making process, starch from corn, maize or potato –
enormous amounts of hazardous wastes containing e.g. phenolics and
depending on local resources – is used to improve strength and
chlorinated compounds. Millions of tons of dried sludge are annually
printing properties of paper. Cassava is an important source of starch
generated by pulp and paper mills in the US alone. Beside
in Africa and might be exploited in paper manufacturing. However,
environmental aspects, their handling represents an economical
cassava leaves and roots contain a cyanogenic glucoside called
Fig. 2. Different pathways of nitrile metabolism modified from Banerjee et al. (2002).
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),
P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
linamarin and by action of naturally present linamarases, poisonous
dione ). Laccases were shown to act on a variety of
hydrogen cyanide is released. Recently, a mixture of cellulase and
synthetic dyes; Acid Blue 62 ), Acid Blue 81,
xylanase were shown to detoxify cassava starch by releasing
Reactive Blue 19 (, Malachite Green (
), Reactive Black 5, Reactive Yellow 15, Reactive
The authors recommend two books which comprehensively cover
Red 239 (), Reactive Red 120 ),
enzyme technology in pulp and paper industry:
methyl orange crystal violet are
some examples. Peroxidases were also assayed for dye degradation, e.g.
methylene blue (),
2.2. Textile industry
bromophenol blue and methyl orange ). evaluated the potential of a cheap peroxidase from bitter gourd
Production of textiles can be divided in two parts; the fiber and
for dye decolorization over a broad range of 21 industrial dyes with
fabric manufacturing and the dyeing process. Only a few possible
various chemical structures. The tested peroxidase was able to degrade
applications of environmental biotechnology were found for the first,
nine of the dyes without mediators, and all of them when catalysis was
in connection with the low number and amounts of byproducts
mediated with 1-hydroxybenzotriazole (HBT). Beside peroxidases,
CDHs participate synergistically with laccase in the decolorization of
described a protease-based bioprocess for the
the anthraquinonic dye Acid Blue 62 ). In a
production of bioactive peptides derived from sericin found in silk
study of , culture broth containing laccase and
industry effluent. Sericin hydrolysate produced with a commercial
CDH was used to degrade synthetic dyes. They showed that CDH could
protease exhibited good antioxidant activity and tyrosinase-inhibiting
be used instead of HBT to enhance decolorization of the dyes Direct
activity particularly appreciated in food, cosmetic and pharmaceutical
Violet 51 and Ponceau xylidine due to hydroxyl radicals generated (refer
industries. Another prospective application refers to poly(trimethylene
terephthalate) (PTT), a linear aromatic polyester used in textile industry
Coupling reactions lead to less soluble polymers of azo and
as fiber, but also in films, filaments and plastics manufacturing. A higher
anthraquinonic dyes and often occur upon application of LMEs
hydrophilicity of such a material is appreciated for further processing
(e.g. dyeing) and generally obtained through alkaline and plasma
The predominance of those
treatments. Enzymatic hydrolysis may represent an alternative for
chromophores in industry, together with a reaction mechanism
surface modifications. Polymers and oligomers of PTT were treated
involving free radical cascades often leading to polymers, is a clear
using diverse polyesterases (cutinases and lipases) ).
justification why membrane technologies associated with an enzyme
The ability of polyesterases to functionalize PTT, which is poorly
treatment are extensively studied, and considered as a promising
biodegradable, might also be of interest to increase its bioavailability
textile effluent treatment process. For instance,
during waste processing. Finally, biotechnological degumming of bast
combined filtration and enzymatic treatment by efficiently immobi-
fibers of ramie and sunn hemp (found in East India) using pectinases
lizing and stabilizing laccases on a chitosan membrane. More than 95%
and xylanases after a mild chemical treatment presents an option to
of an azo dye (Acid Black 10 BX, 20 ppm) could be degraded in 21
manufacture textile materials ).
cycles of operation. Enzyme immobilization is an effective way to
The dyeing process – conferring color to textile materials – is a major
decrease the treatment cost since it allows several reactor
activity in textile industries which requires large amounts of water fordyeing, rinsing and cleaning. The removal of dyes from generatedeffluents is a matter of concern considering the progressively stringentenvironmental legislations. Indeed, textile effluents are not only
unaesthetic (colored) but toxic and partly carcinogenic. Dyes used intannery and textile manufacturing are mainly synthetic and categorized
according to physical and chemical properties (e.g. solubility andcharge) as acidic, basic, direct, disperse, reactive, sulfur ). As these properties are diverse, and because dyes were originallydesigned to be resistant to chemicals, water, light and microbialdegradation, effluents containing dyes are inherently difficult to clean.
Consequently, existing chemical processes, e.g. coagulation, advanced
oxidation processes (AOPs), adsorption, chlorination, are partiallyineffective and not economical for the treatment of dye mixtures().
Furthermore, textile effluents exhibit bacterial toxicity and a lowbiochemical oxygen demand (BOD)/chemical oxygen demand (COD)
ratio (N0.1), i.e. low biodegradability. As a result, a combination ofchemical coagulation and oxidation followed by aerobic biologicaloxidation is an often employed process ), which may be
fused in an integrated biochemical treatment. So far, white rot fungi
(WRF) with their lignin-modifying system are the most efficient aerobicorganisms to break down colored pollutants. reviewed the decolorization and detoxification capacity of WRF.
However, drawbacks associated with whole cell reactors like uncon-trolled biomass production are difficult to overcome. Here again, theapplication of suitable enzymes might be beneficial.
Azo and anthraquinonic structures account for more than 80% of all
textile dyestuff produced ). Azodyes are characterized by nitrogen–nitrogen double bonds (phenyla-
Fig. 3. Simplified process diagram of enzyme technology as applied to the pulp and
zobenzene) and anthraquinonic dyes are based on anthracene-9,10-
paper industry.
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),doi:
P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
configurations together with catalyst reuse. used
be retained when lipase-based hydrolysis was applied beforehand.
laccases immobilized on silanized alumina pellets activated with
Lipase immobilized on alginate beads was also used to hydrolyze O&G
glutaraldehyde and achieved the complete degradation of the resistant
in pet food industrial effluents.
diazo dye Reactive Black 5 (500 ppm) in a fluidized bed reactor (FBR)
evaluated the anaerobic treatability of such effluents with a hybrid
and different CSTRs as well as in a continuously operated tubular reactor
reactor system consisting of a packed bed reactor with immobilized
with this biocatalyst. exploited turnip
lipase followed by an upflow anaerobic sludge bioreactor. The system
peroxidase immobilized on wood shavings for the decolorization of a
was successfully operated over a period of 100 days with higher COD,
direct dye, Direct Red 23. Their continuously operated packed bed
fat removal and methane production rates as compared to a control
reactor, in series with an activated silica filter, was operated over
reactor without enzymatic pretreatment.
4 months with satisfactory results (64% removal efficiency retained).
Waste cooking oil (e.g. from potato processing plants) can be treated
Hematin – a hydroxylated form of heme – has been considered as an
with lipase to produce biodiesel of superior quality not competing with
economical alternative for some peroxidase-based catalysis.
food resources. Enzymatic transesterification of free fatty acids
studied the catalytic degradation of model dyes Eriochrome Blue
contained in waste oil (methanolysis) using immobilized lipase has
Black R and Fluorescein with HRP/H2O2 in comparison with a mimetic
led to a significant titer of fatty acid methyl esters
system consisting in hematin/H2O2. More than 85% elimination of both
Due to the high interest in lipases for biodiesel refining, it is
dyes was achieved with HRP in contrast to 30% of Eriochrome Blue Black
expected that their cost will decrease significantly, making it feasible to
R with only hematin.
apply them to a wider array of degradation processes.
Recently, compared the efficiency of
nanofiltration, coagulation/flocculation and commercial laccases
2.3.2. Protein transformation
(Denilite®IIS, Novozyme) for the treatment of a model effluent —
Wastes generated by the meat industry are mostly bones, organs and
Blue Bezaktiv S-GLD 150 and Black Novacron R plus typical salts.
hard tissues containing animal proteins of poor biodegradability. The
Beside the very effective nanofiltration (N99% color removal),
majority of hard-to-degrade proteins are extracellular matrix proteins
enzymatic catalysis was shown to be technically competitive since
(collagen, elastin, proteoglycans) and keratins. Keratin-rich wastes
better results (N98% color removal) were obtained compared to the
including feathers, hair, nails, horns, etc. are typical byproducts of
most efficient coagulants and flocculants (N93% color removal).
poultry slaughtering and the leather industry. Currently, incineration is
Enzyme inhibition by high salt concentrations and metal ions may
the main route for the disposal of such wastes.
potentially hamper the feasibility of an enzymatic treatment for
investigated thermophilic proteases (keratinase, collagenase, elastase)
textile effluents. Marine fungi and their laccases were considered for
with broad specificity and high activity for their use in the treatment of
their ability to decolorize black liquor and textile dyes in salty
hardly biodegradable proteins. treated porcine
effluents containing carbonates, sulfides, sulfates, chlorides, etc.
skin, as an in vitro model of human skin, with fungal keratinase. Their
results suggest that keratinase specificity renders it suitable for leather
studied the influence of Ca2+, Co2+, Zn2+, Cr6+, Cu2+ and Fe2+ on the
processing or in vivo applications as cosmetics. Despite numerous
laccase redox-mediated decolorization of Remazol Black-B and
potential applications of microbial keratinases (animal feed and
Remazol Brilliant Blue R (50 ppm). Except for Fe2+ that highly
fertilizers production, leather and textile processing, in detergent
inhibited enzyme activity, their presence did not exert much effect.
formulations, adjuvant in cosmetics, etc.), industrial usage and market
In textile effluents, a bacterial reductive cleavage of the azo dye
demand are still in their infancy (A promising
bonds may occur and release colorless amines ). Aromatic
process is the keratinase-based conversion of agroindustrial wastes into
amines are highly toxic pollutants also released from other anthropo-
amino acids and soluble proteins of nutritional value for animal feed. For
genic activities like rubber manufacturing, chemical, plastic and paper
instance, observed a production of more
processing. have investigated the potential of
than 5 g L−1 of soluble proteins along with 90% wt reduction of the
bitter gourd peroxidase for the degradation of aniline, m-chloroaniline,
initial feather content (0.7% feather waste as a sole carbon and nitrogen
N,N-dimethylaniline, diphenylamine, m-toluidine and p-aminobenzoic
source) after 72 h of incubation with protease-producing actinomycete
acid. All tested aromatic amines were recalcitrant to enzymatic
treatment but when peroxidase activity was mediated by o-dianisidine,
Seafood industry effluent streams are usually discarded directly in
satisfactory results were obtained with most of them (50 to 95% of
the sea without any treatment or valorization.
proposed to valorize them for the production of media for
Alongside oxidoreductases, a hydrolase was evaluated for the
microorganism cultivation through enzymatic hydrolysis. Peptones
remediation of tannery effluents. Tannase from Aspergillus candidus
obtained from papain, pepsin and trypsin pretreatments of industrial
entrapped in alginate beads efficiently removed effluent color and
octopus processing effluents could efficiently promote the growth of
reduced tannin concentrations below the discharge limit (
lactic acid bacteria and the production of bacteriocins. Marine
. Unlike whole cell treatments with A.
peptones produced after 4 to 10 h of protease treatment may compete
candidus, enzymatic treatment did not affect other physicochemical
with expensive commercial media and partially solve this pollution
properties of raw effluents, nor did it decrease the BOD. Cell-free
tannase may thus be applied in a pre-treatment step for effluentdetoxification and decolorization before a conventional biological
2.3.3. Sugar transformation
Various processes have been investigated for the treatment of
pectin-rich effluents from vegetable and fruit processing industries:
2.3. Food and beverage industry
physical dewatering, chemical coagulation, activated sludge, sprayirrigation onto lands, etc. These have several disadvantages such as the
2.3.1. Oil and grease transformation
low efficiency due to the recalcitrance of pectins, environmental
Wastewaters from food and feed processing manufacturing may
pollution from the use of chemicals and long treatment times in
have a high COD arising from fats and may require a pretreatment
addition to high costs (A cost-effective and
before discharge in sewer systems. demonstrated
environmentally friendly method is the (pre)treatment with pectinases
the effectiveness of a lipase-based hydrolysis of dairy effluents before
from bacteria, which selectively remove pectic substances from the
an activated sludge treatment. At 800 mg L−1 O&G, the COD removal
wastewater Alkaline pectinase and alkalophilic
efficiency of activated sludge fell to zero while a removal of 82% could
pectinolytic microbes facilitate the removal of pectinaceous material
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),
P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
and render it suitable for decomposition by activated sludge (
represent around 50% and carbohydrates and humic substances up to
In citrus processing industries, peels
20%. The solubilized material may be re-fed into the wastewater
and centrifugation pulp (semi-solid residue after fruit centrifugation)
stream and favor nitrogen and phosphorus removal as mentioned
are important byproducts. These fermentable wastes are an economic
and environmental problem ). Pectin can be
So-called "emerging pollutants" are a heterogeneous group of
extracted from citrus peel using pectinase as was shown with
substances which are causing increasing concern. Emerging pollutants
polygalacturonase secreted by Kluveromyces fragilis yeasts
can be defined as organic compounds in the aquatic environment –
). The treatment of centrifugation pulp with
surface, ground- and waste-waters – that are not covered by
commercial pectinolytic enzymes (Citrozym-LS; 80% polygalacturonase
established water quality regulations. They have direct or indirect
and 20% pectinesterase) was very efficient in improving pulp pressing
adverse effects for humans and wildlife at environmentally relevant
and drying of residues. The end-product obtained showed good in vitro
concentrations (ng/L–μg/L range). These contaminants include
digestibility and its protein content was comparable to agro-industrial
pharmaceuticals, personal care products, fragrances, plasticizers,
waste products currently used as animal feed (
steroids and hormones, illicit drugs, gasoline additives, flame
Disposal of liquid residues (e.g. spent wash, pot ale) remaining
retardants, etc. (
after alcohol distillation is of concern for distilleries. Cellulase is
A comprehensive study about their presence in
attractive for the pre-treatment of alcohol distillery effluents.
wastewaters was conducted between 1999 and 2000, by sampling
Enzymatic hydrolysis was shown to substantially increase the rate
139 wastewater streams all over the United States (
of subsequent biological oxidation (). An
). Eighty percent of the samples were tested positive for the
anaerobic digestion of yeast cells from Scotch distilleries is not
presence of at least one of the ninety-five monitored organic
feasible. The hydrolysis of intact cells was found to be the rate-limiting
pollutants. Frequency of detection (f) was 41% for bisphenol A
step ). Protease and β-glucanase exhibited a
(BPA), 51% for 4-nonylphenol (NP), 58% for triclosan (TCS) and
synergistic activity in cell disruption, and papain was also appropriate
between 6 and 21% for sex hormones (estrone, estriol, estradiols) to
for cell digestion in pot ale (N90% disruption). Subsequent anaerobic
highlight compounds used in below mentioned studies of enzymatic
digestion of pretreated yeast residue (pot ale) showed 87% COD
degradation. Urban wastewater samples were collected on the
reduction compared to 13% without enzyme treatment.
eastern shore of San Francisco Bay and analyzed for their endocrine
Chitinase can be applied to chitin-containing wastes from aquatic
disrupting chemical (EDC) content ). 95%
food industry, leading to a release of bioactive products (e.g.
of the samples contained at least one of the monitored EDCs.
antioxidants, carotenoids) from the chitin alongside with its hydrolysis
Phthalates (f = 91%) were widely detected but also triclosan
products These soluble
(f = 43%) and bisphenol A (f = 24%). Conventional wastewater and
sugars (N-glucosamine) can also promote bacterial fermentation to
drinking water systems were found relatively inefficient for the
produce lactic acid as demonstrated by .
removal of pharmaceuticals and hormones ). Theseresults are a call for prevention and for new remediation strategies.
2.3.4. Detoxification
Enzymes may be exploited in a tertiary – polishing – treatment in
studied the detoxification of a cyanide-
WWTP. The ability of laccases and peroxidases to degrade EDCs was
containing extract from dibittering apricot seeds by cyanidase™.
reviewed by . Studies have shown that natural
Cyanidase™ (Novo Nordisk, Denmark) is a preparation of immobi-
and synthetic hormones are major contributors to estrogenicity of
lized cyanide dihydratase from Alcaligenes denitrificans capable of
wastewaters. Among them, estrone (E1), 17β-estradiol (E2), 17α-
converting cyanide to ammonia and formate in a single-step reaction
ethinylestradiol (EE2) and estriol (E3) can be efficiently degraded by
(It is characterized by a high affinity toward
peroxidases (). Commercial laccases
cyanide and a high stability. It is able to remove this anion down to
were also able to transform these hormones and the common anti-
very low levels, i.e. b0.02 mg L−1 CN−. The diffusional-type flat
inflammatory diclofenac ).
membrane reactor (FMR) operated with immobilized cyanidase™
Even though peroxidases and laccases have similar affinity for the
had superior performance compared to stirred tank reactor and fixed
targeted estrogens, HRP for instance seems to be more affected by
bed reactor configurations. The advantage of employing a FMR is a
wastewater constituents (unidentified) than laccase ).
protection of the enzyme and the immobilization support from shear
Genistein is an isoflavonoid present in bleached wood pulp mill
damage. Cyanide diffuses through a semi-permeable membrane to
effluent and classified as an endocrine disruptor. Apparently, it is also
react with the entrapped enzyme and reaction products diffuse across
widely distributed in sewage effluents and very resistant to
the membrane to the solution.
wastewater treatment ). completely removed genistein from a synthetic solution
2.4. Water industry
using either MnP or laccase. Beside natural and synthetic hormones,laccase is also able to degrade BPA and NP. used
Biological nutrient removal (nitrogen and phosphorus) facilities
enzymes from lignin-degrading basidiomycetes for the removal of
installed in modern WWTP have a low efficiency when wastewater is
BPA and NP. They showed a decrease of estrogenic activity and the
deficient in organic matter (i.e. low BOD/nitrogen ratio). Addition of
production of stable polymers of BPA and NP after laccase treatment.
cheap external carbon sources might solve the problem.
This was confirmed by the results of
assayed the feasibility to reuse food waste, acid fermented
. Cabana et al. also developed a reactor
after an enzymatic pretreatment using a mixture of commercial
system for BPA and NP treatment. They applied cross-linked laccase
glycosylase, protease and lipase. Despite a high content of carbohy-
aggregates in a continuously operated perfusion basket reactor. With
drates, they noticed that protease among the three enzymes was the
this system, 85% of tested EDCs could be continuously eliminated over
most efficient for solubilization and the production of volatile fatty
a 7-day period ). The enzymatic degradation of
acids. They hypothesized that proteases can release carbohydrates
TCS and a substantial decrease of bacterial toxicity in the treated
from complex biopolymers stabilized by lectin-like proteins. A similar
solution were shown by several teams (
enzyme mixture also offers opportunities in sludge dewatering
). TCS is a broad-spectrum antimicrobial agent
processes by improving sewage sludge settling and reducing
used in personal care products and frequently found in surface water.
disposable solid content (). Indeed, organic
Under exposure to sunlight, it is transformed into chlorinated, highly
materials account for 60% of solid content in sludge where proteins
toxic and persistent products (Recently, laccases were
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),doi:
P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
conjugated with chitosan in order to improve their stability and
industrialization has left a legacy of polluted land, water and air.
reusability, and the conjugates were applied to TCS degradation and
Petrochemical, agrochemical, smelting, mineral extraction, polymers
detoxification In the same study, not only
and rubber as well as explosives industries are linked to a long list of
polymerization but also dechlorination of TCS by laccase was evidenced,
polluted sites due to the release of chemicals during processing, use and
as previously reported by upon use of laccase
accidental spills.
supplemented with mediators. applied Pd–nFe
Dimethyl phthalate (DMP) is one of numerous phthalic esters used
nanoparticles as a catalyst for TCS dechlorination under anaerobic
as plasticizers in plastics manufacturing. Its degradation intermediates
conditions and polymerized the resulting 2-phenoxyphenol using
are toxic, mutagenic and potential endocrine disruptors (
laccase. Another example of a biotransformation by laccases, not only
Some Bacillus species have the ability to grow on DMP as a sole
leading to polymerization products, was reported by
source of carbon. Isoesterases present in the cell-free extract of a Bacillus
Here, the laccase-catalyzed formation of a lactone from the
sp. culture are responsible for the initial de-esterification (
polycyclic musk fragrance galaxolide (HHCB) was shown.
while a cocktail of enzymes containing proteases, esterases,
The peroxidase-mediated degradation of BPA was studied and
amylases and tyrosinases are collectively involved in the degradation
optimized with respect to critical reaction parameters such as
pathway of DMP by growing bacteria When purified
temperature, pH, protective agents and peroxide concentrations by
cutinase and purified yeast esterase were compared regarding their
In addition, thirteen other BPA derivatives, also
efficiency of dipentyl phthalate degradation, cutinase exhibited a
extensively used in plastic synthesis, were nearly completely removed
remarkable activity The degradation products were
under the same conditions. Resulting oligomer precipitates were
mainly 1,3-isobenzofurandione when using cutinase and a mixture of 1,3-
easily removed from the aqueous solution by filtration. In another
isobensofurandione with pentyl methyl phthalate (PeMP) when using the
recent study, observed a reduced
esterase. Toxicity monitoring showed that PeMP caused inhibition of
genotoxicity of a 0.5 mM BPA solution after its continuous treatment
bacterial growth and cellular stress. evaluated cutinase
in a lab-scale fixed-bed reactor using bitter gourd peroxidase
and yeast esterase for the degradation of di-(2-ethylhexyl)-phthalate
immobilized on fly ash together with guaiacol as a mediator
(DEHP). Consistent with the above mentioned results, fungal cutinase was
(0.3 mM). At a flow rate of 20 mL h−1, 76% removal was retained
highly efficient in the degradation of DEHP and esterase treatment led to
after 30 days. In an attempt to overcome the inherent drawbacks of
toxic degradation products.
peroxidases in wastewater processing, i.e. their cost and stability,
Polyurethanes are widely used synthetic polymers with poor
commercially available Hb from bovine blood was assessed for the
biodegradability. These polymers result from condensation of
removal of BPA in the presence of electrochemically generated H2O2
polyisocyanates and polyalcohols. They are used in medical, automo-
Hb was immobilized on carbon fibers, which
tive and industrial applications. Esterases which hydrolyze ester bonds
served as a cathode, and immersed in a jacketed beaker (35 mL) along
of polyester polyurethanes are putative key enzymes in their microbial
with the stainless steel anode. Under optimized conditions, 51% of
100 mg L−1 BPA was removed after 2 h, as compared to a biochemical
Cyanides, inorganic compounds with a ―C N group, are potent
removal of 35% (Hb + H2O2 supplemented), and an electrochemical
respiratory inhibitors. An estimated 3 million tons of cyanide per year
removal of less than 5%.
are used in industrial processes including the production of chemical
Haloacetic acids (HAA), especially chloroacetic acids, are toxic
intermediates, synthetic fibers, rubber and pharmaceuticals, as well as
byproducts formed upon disinfection by addition of chlorine to
ore leaching, coal processing and metal plating ).
drinking and other process waters. As an alternative to activated
Cyanides are produced by certain bacteria, fungi, algae and plants only
carbon, electroenzymatic degradation of trichloroacetic acid and
in small concentrations; therefore their presence in the environment
bromoacetic acid was proposed using Hb immobilized on carbon
is mainly attributed to human activities ). Cyanide
nanotubes The results proved that reduced
waste treatment, if done, is usually a two-stage alkaline chlorination–
heme can be effectively regenerated by the electrode and that Hb
oxidation process, resulting in problematic sludge with high chlorine
exhibits high and stable activity. Tri-, di-, and monochloroacetic acids
content. Biological degradation of cyanide has been suggested as an
were converted sequentially and could be completely dechlorinated
environmentally friendly and inexpensive alternative to conventional
at a redox potential of −200 mV. The concept was finally illustrated in
processes ). used
an electroenzymatic packed-bed reactor where the average current
immobilized S. loti, a cyanide hydratase producer, to degrade cyanide
efficiency was almost 100%, i.e. HAA were the only electron acceptors
wastes to formamide in a continuous reactor system. Up to 100 mM of
cyanide was degraded in 2 h and the immobilized mycelia were more
Adsorption capacity of papain instead of its catalytic properties
stable than the free mycelia retaining about 55% and 15% activity after 3
was exploited for mercury abatement in water. Papain, immobilized on
and 6 days at 24 °C respectively. High level of cyanide hydratase activity
activated charcoal and alginate beads, was used in a finishing step to
was also reported in Gloeocercospora sorghi and Helminthosporium
remove low ppm to ppb levels of mercury remaining after
turcicum. Immobilized G. sorghi completely converted cyanide
conventional bulk unit operations (i.e. filtration or precipitation)
(70 mmol added continuously at 7.5 mL h−1) into formamide for
However, this process
30 days and the enzyme stability was dramatically enhanced by the
is not based on enzymatic catalysis, as papain is characterized by a
addition of glucose in the feed ).
strong tendency to bind metals due to the presence of four sulfhydryl
designed a packed-bed reactor based on immobilized Fusarium
groups in its active center. With both supports, results were
oxysporum (cyanide hydratase producer) and Methylobacterium sp. for
satisfactory since 99% of the mercury could be removed from
the integrated degradation of cyanide and formamide (refer to Section
solutions and the adsorption was characterized by fast kinetics.
Nitriles are organic cyanide (R―C N) compounds which are
2.5. Chemical industry
present in the environment due to either natural or industrialsyntheses. Xenobiotic nitrile compounds are used as solvents,
The chemical industry is a pillar of the modern economy, converting
pharmaceuticals, intermediates in organic synthesis, pesticides, etc.
raw materials (oil, natural gas, air, water, metals, and minerals) into tens
). Most of them are toxic, carcinogenic and
of thousands of different products. Chemicals are used in the
mutagenic () and thus their release into the
manufacturing of a wide variety of consumer goods and other products
environment needs to be limited. Researchers have described various
that are essential for agriculture and industry. However, a long history of
organisms, which possess different pathways for cyanide and nitrile
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),
P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
soil and sediments which contribute to their low biodegradability and
persistence in the environment. They represent a potential health
A mixed culture of bacteria producing
risk due to their mutagenicity and carcinogenicity. Bioremediation
different nitrile hydrolyzing enzymes (including NHase, nitrilase and
technology using microorganisms has been extensively evaluated for
amidase) was grown in batch and continuous culture and metabolized
PAH-contaminated soils ().
acrylonitrile, fumaronitrile, succinonitrile, etc. (
showed that the laccase from Trametes
Using this consortium, the authors reported the remediation of
versicolor could oxidize 11 PAHs (acenaphthylene, anthracene, benzo[a]
an effluent from acrylonitrile manufacturing industries with a 75%
pyrene, acenaphthene, fluoranthene, pyrene, benzo[a]anthracene,
reduction in COD and 99% reduction in toxicity.
chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene) to corre-
have also reported that acrylonitrile decontamination of polymer
sponding quinones, plus 3 others (naphthalene, fluorene, and
emulsions (aqueous latexes) can be efficiently achieved by NHase
phenanthrene) when HBT was added (refer to The
treatment. used a solvent-tolerant nitrile hydratase
same laccase was immobilized on kaolinite and tested for the removal
for the biotransformation of nitriles under mild conditions. A
of anthracene and benzo[a]pyrene in water in presence of 2,2′-azino-
combination of NHase and amidase-producing microorganisms
bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS)
resulted in an efficient conversion (N90%) of 6 M acetonitrile to acetic
). used free fungal laccases for the remediation
acid in 10 h. This procedure could be suitable for the treatment of
of a PAH contaminated soil. Reasonable transformation of PAH was
acetonitrile containing wastes on site or in treatment plants
achieved without addition of mediators. The authors hypothesized that
(Nitrilases are easily inactivated and their
phenol-like compounds in the soil may enhance the enzymatic
non-covalent binding onto carriers (hydrophobic, ionic interactions)
transformation of PAHs. A priori, the treatment of hydrophobic
is more promising than other immobilization methods due to the
pollutants adsorbed on organic matters in soil by enzymes in aqueous
mildness of this technique
solution seems paradoxical. However the addition of miscible organic
Emissions of volatile halogenated organic compounds threaten
solvents or surfactants in aqueous reaction media (to desorb PAHs)
environmental integrity. demonstrated the ability
coupled to stabilized enzymatic catalysis in such matrices could solve
of lyophilized R. erythropolis cells to continuously transform 1-
the problem. Recently, designed a spiral-bed
chlorobutane through haloalkane dehalogenase (DHA) activity in a
reactor using bitter gourd peroxidase immobilized on the surface of
solid–gas biofilter. Solid–gas systems have some advantages over
calcium-alginate beads for the continuous treatment of an anthracene-
liquid–solid systems, e.g. no solubility and diffusion problems, no need
containing model effluent. The reactor was operated over a month (40%
for additional solvents and simplified biocatalyst recovery. Cells treated
retained removal efficiency), for the treatment of 0.5 mM anthracene in
with lysozyme were more active (higher initial rate of degradation) but
organic mixture (acetone/N-N dimethylformamide, 35% v/v in water)
DHA activity declined with time due to a pH decrease caused by HCl
supplemented with 0.1 mM guaiacol as mediator. Further development
generation. The addition of volatile triethylamine (Lewis base) was
and scale-up of enzymatic remediation of PAHs are clearly restricted at
highly beneficial in maintaining the activity. Later,
the moment by the use of solvents and mediators as well as the cost of
pretreated the biocatalysts with ammonia vapor to further stabilize the
DHA (75% residual activity after 60 h) and extended degradation studiesto C5 and C6 halogenated compounds (chloro- and bromo-substituted).
3. Research directions in enzyme technology
A DHA from Bradyrhizobium japonicum was cloned and heterologouslyexpressed in Escherichia coli (The recombinant
Enzyme catalysis has been exploited in food and beverage processing
enzyme displayed broad substrate specificity toward haloalkanes and
for centuries. Nowadays scientists and engineers are aware of the
was able to hydrolyze longer compounds than DHA from Rhodococcus.
enormous potential and versatility of enzymatic reactions and are facing
For detailed information on aerobic pathways of the bacterial
the challenge of their exploitation. This challenge is significant as the
degradation of halogenated compounds and enzymes involved, the
cheap supply of highly active and stable biocatalysts has yet to become a
reader can refer to .
reality. Very recently, tools to improve existing biocatalysts, whole cells
Recently, the Defense Treaty Reduction Agency has published a call
or enzymes, used in established processes have been partially reviewed
for new biocatalysts to find an environmentally friendly way to remove
(). Here, we present examples for the use of these
OPs OPs present in some pesticides (e.g.
tools in connection with above-mentioned enzymes which also have
parathion, coumaphos, paraoxon) and nerve agents (e.g. sarin, VX, sulfur
potential environmental applications ).
mustard) display neurotoxicity in insects and mammals because of theirability to inactivate esterases in the central nervous system, leading tothe accumulation of neurotransmitters and receptor overstimulation.
3.1. Discovery of new enzymes
Severe poisonings may cause respiratory failure, convulsions and death(). OPs leaking from crop fields and warfare
Better enzymes are needed to meet the requirements of process
agent depots accumulate in ground- and municipal waters. In a
engineering and to enable sustainable bioprocesses to compete
comparative study, fungal cutinase and yeast esterase were evaluated
equitably with "older" processes. The search for new enzyme activities
for the degradation of malathion . Cutinase was more
among known or previously unknown organisms is called bioprospect-
suitable than esterase and had the advantage of transforming malathion
ing. The classic culture-dependent way of screening organisms for
into the non-toxic malathion diacid. Indeed, production of monoacid
interesting metabolic capabilities or finding them "accidentally" and
malathion by esterase appeared to severely inhibit protein synthesis in
subsequently identifying, isolating and characterizing responsible
bacterial cells. An esterase from a metagenomic library of bovine rumen
enzymes is time-consuming, laborious and seldom systematic, yet it
bacteria was successfully expressed in Pichia pastoris at a high expression
represents an established and proven methodology. Even in the era of "-
level of 4.0 g L−1 The results revealed a good
omics", this methodology has its value in prospecting enzymes and
OP hydrolytic activity since cadusafos, coumaphos, diazinon, dyfonate,
remains important in combination with molecular biological methods,
ethoprophos, fenamiphos, parathion and methylparathion could be
like cloning, heterologous expression, etc. Examples of its persisting
relevance include the discovery of novel secreted fungal peroxidases,
Polycyclic aromatic hydrocarbons (PAH) are ubiquitous pollutants
like aromatic peroxygenases and dye-decolorizing peroxidases, exhibit-
mainly resulting from incomplete combustion of organic matter. Because
ing surprising catalytic properties as reviewed by
of their hydrophobicity, they are easily adsorbed onto organic matter,
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),doi:
P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
For several decades, extremophiles have more than excited the
approach does not have a high success rate (i.e. percentage of mutants
interest of researchers. The first commercialized enzyme from such
with desired properties) since it introduces random point mutations
organisms was the DNA-polymerase of Thermus aquaticus (
while substantial changes in enzyme functions may require changes
Nevertheless, key drawbacks associated with the culture of
of not only single amino acids but modifications of larger portions of
extremophiles are the harsh conditions imposed on the equipment
the amino acid sequence (insertion, deletion, inversion, etc.),
during fermentations and downstream processing, e.g. high salt
occurring naturally during recombination events. Nevertheless,
concentrations (halophiles), high temperatures (thermophiles) or
directed evolution represents an approach yielding impressive
pressures (piezophiles)
results, especially when mutations affect the active site
Advances in molecular biology and analytical techniques coupled
). This was shown for one variant of OPH which exerted a 25-fold
with automation and computerized data processing enable a modern
higher activity against methyl parathion compared to the native
lab to have a high-throughput approach in enzyme prospection.
enzyme ). In a review, also
Genome sequence databases, or even more powerful metagenome
reported that directed evolution using DNA shuffling had produced
databases, allow an in silico screening of the whole diversity of enzymes,
bacterial mutants expressing OPH with a 725-fold higher hydrolytic
not limited by their expression or the culturing of the respective
activity against the pesticide chlorpyrifos. Stabilizing mutations often
affect the protein surface by adding electrostatic and hydrogen bonds
Whereas genome-mining is suitable for prokaryotic enzymes, introns in
). stated that mutations at
eukaryotic genes currently hamper a broad exploitation of fungal,
the surface rather than in the active site of laccase improved its
animal and plant genomes, thus calling for the application of (meta)
stability in solvents. The laccase mutant they obtained after five
transcriptomic approaches. This is illustrated by
rounds of directed evolution exhibited 19.2-fold higher stability than
who combined metatranscriptomics with a functional screening to
the native enzyme in 50% (v/v) water–ethanol mixtures.
arrive at expressed, functional fungal phosphatases. The next major
The choice between rational design and directed evolution
breakthrough can be expected, as proteomics is starting to provide
depends on the level of knowledge – including mechanistic
comprehensive libraries. Thus, in combination with the above men-
understanding – and on the existence of a selection scheme for a
tioned methods, proteomics offers the possibility to directly derive
given enzyme For instance, directed evolution applied
specific enzymes based on metabolic capabilities of microbial commu-
to cellulase is hampered by the development of an efficient selection
nities. Furthermore, it is able to overcome limitations of nucleic acid
or screening method for mutant cellulases due to the insoluble and
based approaches like post-translational modifications or alternative
heterogeneous nature of the cellulose substrate ).
splicing which can alter catalytic properties significantly
On the other hand, proposed that directed
evolution would be particularly attractive for the improvement ofcytochromes P450, as one-step detoxifying enzymes. Mutants
3.2. Designer enzymes
producing active, detoxifying P450-enzymes would be capable ofgrowing with high concentrations of substrates toxic to the host cells
Industrial applications of enzymes often require conditions (pH,
and may be easily selected. This is especially interesting within the
ionic strength, organic solvents) that are not congruent with optimal
scope of the present review, as the selection of enzymes for
activities and stability as suggested by an enzyme's physiological
environmental applications is often based on their detoxification
context. To fulfill the requirements of industry, protein engineers can
abilities and single enzyme-systems rather than degradation path-
use directed evolution and rational design to tailor catalytic efficiency
ways are preferred, thus allowing for the construction of such
(Km, Kcat), extend stability (pH, T, solvents) and even modify the
selection systems.
reaction mechanisms (). The impact of
Both approaches have already successfully produced stable and
these two methodologies on enzyme technology and bioprocess
highly active biocatalysts. Following a directed evolution methodology,
engineering is growing in importance. Rational design introduces
reported the heterologous expression of laccase
defined changes in the amino acid sequence using site-directed
which exhibited a 22-fold higher Kcat for ABTS, a common substrate for
mutagenesis based on the knowledge of three-dimensional structure
laccases. obtained a mutant of CYP450 from
of enzymes, functions and mechanisms. Molecular modeling predicts
Pseudomonas putida with an enhanced activity against PAHs (phenan-
how mutations would affect enzyme properties such as selectivity,
threne, fluoranthene, pyrene and benzo[a]pyrene) after two directed
activity and stability, using databases and structures of homologous
mutations in the active site. The reader may also refer to
enzymes as a model if the structure of the studied protein is unknown
for successful constructions of other heme enzymes such as
). , for example,
were able to increase nitrile hydratase activity of papain by 4 orders of
Directed evolution and rational design appear to be complemen-
magnitude. highlighted new computational tools for
tary and their combination increases the chance of obtaining
protein design dedicated to small libraries built on knowledge of
advanced biocatalysts (). In a two-step optimiza-
protein sequence, structure and function. Rational design might still
tion, computer modeling is used to identify key amino acids
be in its infancy, but an increasing understanding of structure–
responsible of enzyme activity and directed evolution is subsequent-
function relationships and post-translational processing of proteins
ly applied for mutagenesis of the DNA sequence of the "hot spot"
alongside with steadily increasing computational power for simula-
residues and selection of mutants. The process may be iterative and
tions and modeling, will soon make this methodology one of the most
after selection of evolved mutants in a first round, another rational
valuable tools for protein engineers.
design step may be carried out. followed a
In contrast, a popular method mimicking natural evolution called
combined approach in an attempt to enhance stability and activity of
directed evolution requires much less information. Indeed using
a fungal peroxidase for its use as dye-transfer inhibitor in laundry
random mutagenesis, it is possible to improve catalytic properties
detergents. They obtained a mutant enzyme with 174 times the stability
without information about protein spatial structure. However, for
and 100 times the oxidative activity of the wild-type enzyme. And
directed evolution one still needs to have the gene coding for the
recently, the total laccase activity (including secretion by Saccharomyces
enzyme of interest cloned and ready for expression. In earlier
cerevisiae and kinetics) was enhanced 34,000 times after eight rounds of
approaches mutations were introduced in whole bacterial genomes.
molecular evolution (). This is one tangible demon-
In addition, suitable methods for the screening of the obtained
stration of the possibilities offered by the above tools and concepts of
mutants for desired properties must be at hand. By its nature, this
protein engineering toward tailoring laccases (and, by extension, other
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),

P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
Fig. 4. Overview of research directions in enzyme technology aiming at an efficient bioprocess.
industrially relevant enzymes) to different applications (
expression of native P450 sequence, slight changes in the sequence
led to a high level of expression. In addition, an approach to overcome
The drop in stability caused by the accumulation of mutations was
the lack of P450 reductase activity would be the co-expression or the
identified and redressed by rational design.
construction of P450:P450 reductase fusion proteins (
followed an unusual path to improve a haloalkane dehalogenase from
Rhodococcus rhodochrous. They have redesigned residues in the
A recent thoughtful analysis of the combined role of structural
channel connecting the bulk and the buried active site instead of
biology and genetic engineering interventions for heterologous
concentrating on amino acids from the active site or its vicinity. After
expression shows that novel, flexible and highly efficient fungal
directed evolution, they obtained a mutant enzyme with a 32-fold
enzymes like laccases are possible Among other
enhanced activity toward 1,2,3-trichloropropane.
recommendations, the authors underline the possibility of exploiting
Heterologous enzyme expression has several advantages over
less studied aspects such as the enzyme's glycosylation status: the
cultivation of wild-type strains for enzyme production. Microbial
structure of glycans could have a decisive impact not only on catalytic
fermentations performed with improved strains are usually short,
efficiency (including redox potential range) but also on stability in
economical and allow the overproduction of tightly regulated
harsh environments ().
enzymes. Conversely, exploitation of e.g. wild-type white rot fungifor laccase production in bioreactors is still limited for two main
3.3. Insoluble biocatalysts
reasons: first, an advanced downstream processing is required torecover the target enzymes; second, fermentations of wild-type
The development of insoluble biocatalysts is beneficial for all kinds of
strains are inherently linked to secondary metabolism and associated
enzyme applications but decisively important in a low added value sector
process instability, due to protease activity, polysaccharide produc-
of the bioeconomy such as environmental services. Bioremediation/bio-
tion, uncontrolled growth, etc. ().
degradation with free enzymes is hardly feasible as long as the enzymes
have listed 49 laccases heterologously
are the major cost-determining factor of the treatment. Heterogeneous
expressed with varying degrees of success.
biocatalysis allows biocatalyst recycling in addition to a better process
CYP450 from mammalian cells may offer some advantages over
control, in turn leading to reduced treatment costs.
their bacterial counterparts and their catalytic properties are better
Immobilized enzymes consist of at least two elements; (a) the non-
investigated. They have been expressed in yeast (
catalytic part which aims at aiding the separation of the biocatalyst from
but expression in bacteria offered other advantages. Although it
its surroundings and (b) the catalytic function The dilution
was hampered by proteolysis of the NADPH-P450 reductase and
of specific enzyme activity (U enzyme g−1 support) and additional
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),doi:
P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
diffusion constraints are drawbacks of carrier-bound immobilization.
the reaction process could be followed by colorimetry and the shells'
Carrier-free immobilization approaches like the crosslinking of enzyme
magnetic properties allowed an easy separation. Using a different layer-
aggregates (CLEA®, enzyme precipitation followed by chemical reticu-
by-layer approach, spherical biopolymer particles made of 80% HRP
lation) are possible alternatives, e.g. laccase-CLEA®
were fabricated by co-precipitating calcium carbonate and the enzyme
and peroxidase-CLEA® ). However, the pure-
followed by cross-linking and dissolution of the inorganic matrix
protein nature of the biocatalysts thus obtained limits the choice of
). Up to three enzymes – β-glucosidase,
reactor types, e.g. an application in packed bed reactors is not readily
glucose oxidase and HRP – were co-immobilized in separate and defined
feasible until problems of fragility and deformability of the aggregates
compartments, still expressing their respective activities. Multicom-
are overcome.
partment systems may be desirable in drug delivery, bioreactors and
Reversible soluble–insoluble supports have been developed to solve
sensors when more than one function is required in a controlled
diffusion constraints encountered in heterogeneous biocatalysis.
Covalent immobilization of chitinase and protease on reversiblysoluble polymeric supports (hydroxypropyl methylcellulose acetate
3.4. Additives: redox mediators, cosubstrates and protecting agents
succinate) was demonstrated ).
These biocatalysts were water-soluble at pH above 5.5 and insoluble
Some oxidoreductases such as laccase and peroxidase have a broad
below pH 4.5. Chitin hydrolysis was exemplified in repeated batch
substrate spectrum. The range of applications, however, can be further
operations with pH cycles which facilitated the enzyme recovery.
expanded to complex or high redox-potential molecules with redox-
Recently, developed a protein engineering approach
mediators. These are chemicals which act as "intermediate substrates"
consisting in genetically fusing amino acids (α-helical leucine zipper
for enzymes, because their oxidized radical forms react with the target
domain, hexa-histidine tags, etc.) with OPH to produce OPH mutants
compounds They not only broaden the enzyme spectrum
able to self-assemble in hydrogels beyond a given concentration.
but commonly enhance the degradation rate. Typical redox mediators
Nanobiocatalysis is a growing research area which may reconcile
for laccases are HBT, ABTS, guaiacol and syringaldazine. The laccase
high specific activity and rigidity of biocatalysts. Advances in nanotech-
mediated oxidation of the PAHs anthracene and benzo[a]pyrene using
nology have opened new possibilities in catalysis for environmental
ABTS and HBT was reported ().
applications. Nanoporous/nanostructured matrices, carbon nanotubes,
achieved a degradation of naproxen, an anti-
nanofibers and nanoparticles are novel supports which allow the
inflammatory drug, by including HBT in the reaction medium. Although
manipulation of an enzyme environment at the nm-scale
mediators broaden the substrate range and expand the applicability of
Above all, the high specific surface area of these materials allows
laccases, their use is hampered by limitations such as high/extra costs
superior enzyme payloads, leading to high volumetric activity and
and additional pollution generated by the mediator itself. Their
eventually intensified processes. For instance, laccase was immobilized
utilization at industrial or field scale will probably stay limited until an
on silica nanoparticles produced with the Stöber method
effective technique allows their recovery or alternative mediators,
). A loss of 60% of laccase activity occurred following the
cheap and eco-friendly, are available e.g. from lignocellulose residues
immobilization but the laccase-nanoparticle conjugates could still
In the field of wood pulp bleaching,
efficiently degrade BPA. OPH-carbon nanotubes conjugates were
progress is made in the research of natural mediators and syringalde-
conceived for decontamination of nerve agents. OPH was irreversibly
hyde seems to be a promising alternative as delignifying agent regarding
immobilized onto carbon nanotubes, then incorporated in commercial
its toxicity and efficacy (
water-based paint and air-dried to obtain a coating of 450 μm
Some enzymes need cosubstrates (e.g. P450: NADP(H), MnP:
thickness ). The biocatalytic composites, containing
H2O2/Mn2+) for their catalytic cycle. One major drawback in the
only 0.07% (w/w) OPH, were more stable than native free enzyme and
exploitation of peroxidases is their need for peroxides, which have to
could decontaminate N99% of 10 g m−2 of paraoxon within 30 min and
be delivered in a controlled manner, keeping the concentration high
more than 95% of diisopropylfluorophosphate in 45 min.
enough to avoid rate limitations and low enough to keep suicide-
Biomimetic mineralization has opened a new range of possibilities to
inactivation of the enzymes at a minimum. To overcome this problem,
entrap enzymes under mild conditions
co-immobilized MnP and glucose oxidase on
). Biomineralization is the formation of
silica beads to create a catalytic cascade where H2O2 is produced by
hierarchically structured biological organic–inorganic materials such
glucose oxidase and readily consumed by MnP. Further investigations
as bones, shells and teeth. The field was expanded to the production of
on enzyme loading and kinetic constants would allow a fine-tuned
synthetic materials and called bioinspired/biomimetic material synthe-
system with a stoichiometric delivery of H2O2. Another option is the
sis (Papain was entrapped by biomimetic silication which
combination of enzymatic catalysis with in situ electrochemical
is the condensation of orthosilicates catalyzed by cationic polymers
generation of H2O2, which is exemplified in
). The immobilized enzymes exhibit enhanced pH and
For cosubstrates that need to be regenerated, such as NAD(H), the
thermal stability compared to free enzymes. have
constraints outlined above for mediators also apply. Immobilization of
reported the silica deposition onto a gold surface mediated by lysozyme
cosubstrates can prevent a washing out from a continuously operated
with concomitant immobilization of active OPH. This enzyme could
reactor. reported the immobilization of NAD(H) onto
degrade paraoxon over 2 days, but its activity decreased over time along
nanoparticles. They mixed glutamate dehydrogenase, lactate dehydro-
with the loss of silica. have described the
genase and NAD(H), all immobilized separately on silica particles, and
entrapment of horseradish peroxidase using biosilication methodology
demonstrated a successful production of α-ketoglutarate and lactate
for an amperometric sensing application. The bioactive silica matrix was
with a cyclic regeneration of NAD(H).
formed by the condensation of tetramethyl orthosilicate precursors in
Numerous studies reported the use of additives, e.g. synthetic
the presence of polyethyleneimine and peroxidases. With entrapment,
polymers, salts or sugars as protecting agents for various enzymes (
more than one enzyme or functionality can be simultaneously
Enzyme activation and stabilization using
immobilized Multifunctional capsules were produced by
synthetic polymers like polyethylene glycol (PEG) provide a stable and
and applied to BPA and phenol degradation. Based on
fixed enzyme conformation with high activity compared to their free
Tyr entrapped in a chitosan core, these workers produced a stable
form without polymer addition Likewise,
millimetric platform including various functions incorporated through a
stabilization as CLEA®s partly results from a stable enzyme conforma-
layer-by-layer technique. Beside the main oxidative activity of Tyr, the
tion obtained after addition of a precipitant (e.g. PEG) and locked in
capsules also bound oxidized products (quinones) on the core material,
place by the crosslinking. Such additives can be applied for the storage of
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),
P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
biocatalysts but pose problems in processes because high concentra-
have combined an electro-enzymatic (peroxidase
tions are generally needed to obtain a substantial stabilization effect.
immobilized on Celite and electrogenerated H2O2) and electrochemical(electrogenerated OCl−) process for the remediation of petrochemical
3.5. Reactor and process design
wastewater at pilot scale (40 L). The reactor was equally divided intotwo sections allowing enzymatic and chemical reactions in series. The
An efficient kinetic modeling is mandatory to operate and control
prototype exhibited impressive results with a removal efficiency of 85 to
enzyme reactors. The most significant factors affecting a continuous
93% over a 30-day period. In comparison with solely the electrochemical
enzymatic treatment of aqueous effluents are the residence time (RT),
process (OCl−), the COD removal efficiency of the integrated system
the biocatalyst loading (amount of enzymes) and the environmental
was two-fold higher, with a power consumption reduced by 30%
conditions (temperature, pH, composition of aqueous phase). The
(1.99 W g−1 COD). The complex aromatic compounds (methylethyl
choice of RT is related to the desired conversion yield and therefore
benzene, α-methyl styrene, acetophenone, benzaldehyde, etc.) in the
directly linked to pollutant load. The catalyst loading is calculated
effluent were transformed mainly into aliphatic compounds (dodecene,
with respect to conversion yield (and thus RT), and the catalyst
hexadecene, heptadecanal, etc.) by ring cleavage.
recycling rate is linked to the enzyme inactivation rate, dependent on
Enzymes are putative tools for the treatment of micropollutants
environmental conditions (). Complexity can arise
(BPA, NP, hormones, etc.). Substrate affinity of the enzyme, charac-
from multi-substrate reactions and substrate/product inhibitions. The
terized by Km, is a crucial factor since micropollutant concentrations
reactor design is linked to the biocatalyst formulation and vice-versa,
are typically much lower than the Km values (up to 6 orders of
e.g. suspended solids in water may hamper biocatalyst recovery if its
magnitude), limiting the reaction rate. Non-isothermal conditions
formulation is inadequate. Also, diffusion-limited catalysis generally
might represent a potential tool to increase the efficiency of
occurs with enzymes immobilized on porous materials with pore sizes
enzymatic treatments against pollutants at low concentrations.
below 8 to 10 times the enzyme diameter (). In this case, a
assayed tyrosinases and
diffusion term may be included in the kinetic modeling of the process.
laccases immobilized on nylon and modified polypropylene mem-
Low energy and space demanding processes such as fixed or
branes in a thermodialysis process for the degradation of BPA and
fluidized bed reactors and membrane bioreactors are favored. In
phenol derivatives. Thermodialysis is the selective transport across a
domestic wastewaters (i.e. complex effluents), enzymatic treatment is
hydrophobic membrane separating two solutions at different tem-
generally foreseen as a polishing step (or tertiary treatment), while it
peratures. Compared to the same system operated under isothermal
is often carried out as pretreatment in more defined industrial
conditions, a higher increase of enzymatic activity was shown for the
effluents. Nowadays, there is a trend toward integration of physical/-
lowest concentrations tested (50–1000 μM) and for substrates having
chemical treatments with enzyme reactors such as CSTR plus (ultra)
the lowest degradation rate in isothermal conditions. Overall, an
filtration, immobilized enzymes on membranes, CSTR plus electro-
increase in the enzyme activity was found, proportional to the applied
chemistry, etc.
transmembrane temperature difference. In a similar device,
have reported the
have also observed a lower apparent Km of immobilized lipase
use of membrane reactors (ultrafiltration, 30 kDa) for the oxidative
for dimethyl phthalate. The percentage of increase of the reaction rate
treatment of phenol-containing synthetic wastewater with free
for an actual temperature difference of 1 °C between the membrane
tyrosinase and peroxidase, respectively. The enzymes were main-
sides rises when DMP concentration decreases (up to 24% °C−1 of
tained in the reactor by recycling of the retentate, and the polymer
increase). Future studies applying substrate concentrations in the
products were gradually adsorbed on the membrane. Under real
submicromolar range should validate if non-isothermal conditions are
conditions, rather fast clogging of the membrane may occur in such
a promising approach to enhance the degradation rate of
systems. On the other hand, the use of immobilized tyrosinase on
siliceous supports or cross-linkedtyrosinase aggregates () would allow the use of
membranes with a higher cut-off (microfiltration) and would reduceclogging and fouling. In addition, superior stability achieved through
A number of waste/pollutant treatments where the use of enzymes
immobilization reduces the overall cost of operation. For further
might be beneficial have been identified. Biological wastes were
process intensification, tyrosinase can be efficiently immobilized onto
preferentially handled by hydrolases while oxidoreductases and
the membrane itself Instead of a
lyases are used to tackle various chemical pollutants. Hydrolases
membrane technology to recover the biocatalyst,
render wastes more amenable to conventional biological treatments
covalently immobilized HRP on magnetic beads (79%
(i.e. activated sludge) and to bioconversions to value-added sugars,
immobilization yield) and applied them in a lab-scale magnetically
proteins and lipids which have various outlets such as animal feed,
stabilized fluidized bed reactor for continuous transformation of 4-
culture media and biodiesel. Oxidoreductases are valuable for the
chlorophenol (4-CP). For the treatment of the 4-CP,
detoxification of textile effluent streams and waters containing
have compared soybean peroxidase and UV treatments. Better
phenols, drugs and hormones, often in association with filtration
results in terms of removal efficiency for concentrated solutions (up to
0.5 g L−1 4-CP) were obtained with the UV treatment at high intensity
The need for environmental sustainability is the key driving force for
(10 J cm−2), but the generation of hazardous soluble benzoquinone
the development of enzyme technology for environmental stewardship.
and hydroquinone was mentioned. While peroxidase treatment
This review shows that equivalent outcomes have been obtained with
required high enzyme concentration to be effective, it produced
enzyme technology and with existing technologies, and that better
insoluble and thus easily removable polymers. The design of a novel
results can be achieved when enzyme-based and classical technologies
photobioreactor where photodegradation would be followed by
are combined. However, the limited number of field applications
enzymatic polymerization was proposed.
indicates a gap between academia and industry. As the use of enzymes
combined ultrasound and enzymatic treatment, and showed an
in environmental applications often yields only limited added value, this
enhanced removal of 2-chlorophenol compared to the single
collaboration is crucial. An integrated approach is needed to efficiently
treatments. It was alleged that sonication can act physically through
transfer scientific findings to the market and make it a reality within the
accelerated diffusion and enzyme structure modifications and
emerging bioeconomy. The development of a biocatalytic process and
chemically through elimination of inhibiting intermediates via
the path to its application need input from diverse disciplines. A
hydroxyl radical production.
comprehensive identification of the target environmental application
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),doi:
P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
Fig. 5. Integrated development of an enzyme-based process in environmental technology.
involves not only physicochemical data but current and expected
Arica MY, Bayramoglu G. Reversible immobilization of tyrosinase onto polyethyleneimine-
grafted and Cu(II) chelated poly(HEMA-co-GMA) reactive membranes. J Mol Catal B:
legislation along with evaluation of potential markets for the technology
to be developed. Together with life cycle assessment and profitability
Arica MY, Altintas B, Bayramoglu G. Immobilization of laccase onto spacer-arm attached
studies, this should lead to a holistic decision making process to pursue
non-porous poly(GMA/EGDMA) beads: application for textile dye degradation.
Bioresour Technol 2009;100:665–9.
the development of a given technology ). While government
Arnold FH. Fancy footwork in the sequence space shuffle. Nat Biotechnol 2006;24:
bodies are promoting such activities (e.g. by dedicated funding
programs) the final question remains how to efficiently stimulate a
Ashraf H, Husain Q. Studies on bitter gourd peroxidase catalyzed removal of p-bromophenol
true interdisciplinary collaboration between industry and academia for
from wastewater. Desalination 2010a;262:267–72.
Ashraf H, Husain Q. Use of DEAE cellulose adsorbed and crosslinked white radish
the benefit of society.
(Raphanus sativus) peroxidase for the removal of [alpha]-naphthol in batch andcontinuous process. Int Biodet Biodeg 2010b;64:27–31.
Auriol M, Filali-Meknassi Y, Adams CD, Tyagi RD. Natural and synthetic hormone
removal using the horseradish peroxidase enzyme: temperature and pH effects.
Water Res 2006;40:2847–56.
Auriol M, Filali-Meknassi Y, Tyagi RD, Adams CD. Laccase-catalyzed conversion of
The authors gratefully acknowledge Belgium's Walloon Region
natural and synthetic hormones from a municipal wastewater. Water Res 2007;41:
(Contract No. 917011, ERA-NET MATERA LANCE; Contract No. 71/6662
Auriol M, Filali-Meknassi Y, Adams CD, Tyagi RD, Noguerol T-N, Piña B. Removal of
WINNOMAT 2 BIOVAMAT) and the European Commision (Contract No.
estrogenic activity of natural and synthetic hormones from a municipal
KBBE-4-265946 MINOTAURUS) Brussels-Capital Region (Contract IP
wastewater: efficiency of horseradish peroxidase and laccase from Trametes
ENVI 10 OXEROM) which support their research.
versicolor. Chemosphere 2008;70:445–52.
Aytar BS, Bakir U. Preparation of cross-linked tyrosinase aggregates. Process Biochem
Baldrian P. Fungal laccases — occurrence and properties. FEMS Microbiol Rev 2006;30:
Banci L. Structural properties of peroxidases. J Biotechnol 1997;53:253–63.
Aguilar CN, Gutierrez-Sanchez G. Review: sources, properties, applications and
Banerjee, Sharma. The nitrile-degrading enzymes: current status and future prospects.
potential uses of tannin acyl hydrolase. Food Sci Technol Int 2001;7:373–82.
Appl Microbiol Biotechnol 2002;60:33–44.
Aguilar C, Rodríguez R, Gutiérrez-Sánchez G, Augur C, Favela-Torres E, Prado-Barragan
Basheer S, Kut ÖM, Prenosil JE, Bourne JR. Development of an enzyme membrane
L, et al. Microbial tannases: advances and perspectives. Appl Microbiol Biotechnol
reactor for treatment of cyanide-containing wastewaters from the food industry.
Biotechnol Bioeng 1993;41:465–73.
Ahn J-Y, Kim Y-H, Min J, Lee J. Accelerated degradation of dipentyl phthalate by
Battistel E, Bernardi A, Maestri P. Enzymatic decontamination of aqueous polymer
Fusarium oxysporum f. sp. pisi cutinase and toxicity evaluation of its degradation
emulsions containing acrylonitrile. Biotechnol Lett 1997;19:131–4.
products using bioluminescent bacteria. Curr Microbiol 2006;52:340–4.
Battistuzzi G, Bellei M, Bortolotti CA, Sola M. Redox properties of heme peroxidases.
Aitken MD. Waste treatment applications of enzymes: opportunities and obstacles.
Arch Biochem Biophys 2010;500:21–36.
Chem Eng J 1993;52:B49–58.
Baumler H, Georgieva R. Coupled enzyme reactions in multicompartment micropar-
Akhtar S, Khan AA, Husain Q. Partially purified bitter gourd (Momordica charantia)
ticles. Biomacromolecules 2010;11:1480–7.
peroxidase catalyzed decolorization of textile and other industrially important
Bayramoglu G, Arica MY. Enzymatic removal of phenol and p-chlorophenol in enzyme
dyes. Bioresour Technol 2005;96:1804–11.
reactor: horseradish peroxidase immobilized on magnetic beads. J Hazard Mater
Alam MZ, Mansor MF, Jalal KCA. Optimization of decolorization of methylene blue by
lignin peroxidase enzyme produced from sewage sludge with Phanerochaete
Bayramoglu G, Arica MY. Immobilization of laccase onto poly(glycidylmethacrylate)
chrysosporium. J Hazard Mater 2009;162:708–15.
brush grafted poly(hydroxyethylmethacrylate) films: enzymatic oxidation of
Al-Ansari MM, Steevensz A, Al-Aasm N, Taylor KE, Bewtra JK, Biswas N. Soybean
phenolic compounds. Mat Sci Eng C 2009;29:1990–7.
peroxidase-catalyzed removal of phenylenediamines and benzenediols from
BeMiller JN. An introduction to pectins — structure and properties. In: Fishman ML, Jen
water. Enzyme Microb Technol 2009;45:253–60.
JJ, editors. Chemistry and function of pectins. ACS Symposium SeriesWashington,
Anwar A, Saleemuddin M. Alkaline proteases: a review. Bioresour Technol 1998;64:175–83.
DC: American Chemical Society; 1986. p. 2-12.
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),
P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
Betancor L, Luckarift HR. Bioinspired enzyme encapsulation for biocatalysis. Trends
Cherry JR, Lamsa MH, Schneider P, Vind J, Svendsen A, Jones A, et al. Directed evolution
of a fungal peroxidase. Nat Biotechnol 1999;17:379–84.
Bhat MK. Cellulases and related enzymes in biotechnology. Biotechnol Adv 2000;18:
Chien A, Edgar DB, Trela JM. Deoxyribonucleic acid polymerase from the extreme
thermophile Thermus aquaticus. J Bacteriol 1976;127:1550–7.
Bhattacharyya A, Dutta S, De P, Ray P, Basu S. Removal of mercury (II) from aqueous
Cho CMH, Mulchandani A, Chen W. Functional analysis of organophosphorus hydrolase
solution using papain immobilized on alginate bead: optimization of immobilization
variants with high degradation activity towards organophosphate pesticides.
condition and modeling of removal study. Bioresour Technol 2010;101:9421–8.
Protein Eng Des Sel 2006;19:99-105.
Bistolas N, Wollenberger U, Jung C, Scheller FW. Cytochrome P450 biosensors—a
Cho S-H, Lee H-J, Moon S-H. Integrated electroenzymatic and electrochemical
review. Biosens Bioelectron 2005;20:2408–23.
treatment of petrochemical wastewater using a pilot scale membraneless system.
Black GW, Gregson T, McPake CB, Perry JJ, Zhang M. Biotransformation of nitriles using
Process Biochem 2008;43:1371–6.
the solvent-tolerant nitrile hydratase from Rhodopseudomonas palustris CGA009.
Clanet M, Durand H, Tiraby G. Enzymatic saccharification of municipal wastes.
Tetrahedron Lett 2010;51:1639–41.
Biotechnol Bioeng 1988;32:930–4.
Bokare V, Murugesan K, Kim Y-M, Jeon J-R, Kim E-J, Chang YS. Degradation of triclosan
Claus H. Laccases: structure, reactions, distribution. Micron 2004;35:93–6.
by an integrated nano-bio redox process. Bioresour Technol 2010;101:6354–60.
Claus H, Decker H. Bacterial tyrosinases. Syst Appl Microbiol 2006;29:3-14.
Bollag J, Chu H, Rao M, Gianfreda L. Enzymatic oxidative transformation of chlorophenol
Cohen S, Belinky PA, Hadar Y, Dosoretz CG. Characterization of catechol derivative
mixtures. J Environ Qual 2003;32:63–9.
removal by lignin peroxidase in aqueous mixture. Bioresour Technol 2009;100:
Bolon DN, Voigt CA, Mayo SL. De novo design of biocatalysts. Curr Opin Chem Biol
Conesa A, Punt PJ, van den Hondel CAMJJ. Fungal peroxidases: molecular aspects and
Borkar IV, Dinu CZ, Zhu G, Kane RS, Dordick JS. Bionanoconjugate-based composites for
applications. J Biotechnol 2002;93:143–58.
decontamination of nerve agents. Biotechnol Prog 2010;26:1622–8.
Contesini FJ, Lopes DB, Macedo GA, Nascimento MdG, Carvalho PdO. Aspergillus sp.
Bornscheuer UT, Pohl M. Improved biocatalysts by directed evolution and rational
lipase: potential biocatalyst for industrial use. J Mol Catal B: Enzym 2010;67:
protein design. Curr Opin Chem Biol 2001;5:137–43.
Brandelli A. Bacterial keratinases: useful enzymes for bioprocessing agroindustrial
Couto S, Toca-Herrera J. Laccase production at reactor scale by filamentous fungi.
wastes and beyond. Food Bioprocess Technol 2008;1:105–16.
Biotechnol Adv 2007;25:558–69.
Brandelli A, Daroit D, Riffel A. Biochemical features of microbial keratinases and their
Cristóvão RO, Tavares APM, Ferreira LA, Loureiro JM, Boaventura RAR, Macedo EA.
production and applications. Appl Microbiol Biotechnol 2010;85:1735–50.
Modeling the discoloration of a mixture of reactive textile dyes by commercial
Brar SK, Verma M, Tyagi RD, Valéro JR, Surampalli RY. Concurrent degradation of
laccase. Bioresour Technol 2009;100:1094–9.
dimethyl phthalate (DMP) during production of Bacillus thuringiensis based
Dahiya N, Tewari R, Hoondal G. Biotechnological aspects of chitinolytic enzymes: a
biopesticides. J Hazard Mater 2009;171:1016–23.
review. Appl Microbiol Biotechnol 2006;71:773–82.
Bulter T, Alcalde M, Sieber V, Meinhold P, Schlachtbauer C, Arnold F. Functional
Dai Y, Niu J, Liu J, Yin L, Xu J. In situ encapsulation of laccase in microfibers by emulsion
expression of a fungal laccase in Saccharomyces cerevisiae by directed evolution.
electrospinning: preparation, characterization, and application. Bioresour Technol
Appl Environ Microbiol 2003;69:987–95.
Cabana H, Jiwan J-LH, Rozenberg R, Elisashvili V, Penninckx M, Agathos SN, et al.
Daniel R. The metagenomics of soil. Nat Rev Microbiol 2005;3:470–8.
Elimination of endocrine disrupting chemicals nonylphenol and bisphenol A and
D'Annibale A, Stazi SR, Vinciguerra V, Di Mattia E, Giovannozzi Sermanni G.
personal care product ingredient triclosan using enzyme preparation from the
Characterization of immobilized laccase from Lentinula edodes and its use in
white rot fungus Coriolopsis polyzona. Chemosphere 2007a;67:770–8.
olive-mill wastewater treatment. Process Biochem 1999;34:697–706.
Cabana H, Jones JP, Agathos SN. Elimination of endocrine disrupting chemicals using
Dasgupta S, Taylor KE, Bewtra JK, Biswas N. Inactivation of enzyme laccase and role of
white rot fungi and their lignin modifying enzymes: a review. Eng Life Sci 2007b;7:
cosubstrate oxygen in enzymatic removal of phenol from water. Water Environ Res
Cabana H, Jones JP, Agathos SN. Preparation and characterization of cross-linked laccase
Dash RR, Gaur A, Balomajumder C. Cyanide in industrial wastewaters and its removal: a
aggregates and their application to the elimination of endocrine disrupting
review on biotreatment. J Hazard Mater 2009;163:1-11.
chemicals. J Biotechnol 2007c;132:23–31.
de Carvalho CCCR. Enzymatic and whole cell catalysis: finding new strategies for old
Cabana H, Alexandre C, Agathos SN, Jones JP. Immobilization of laccase from the white
processes. Biotechnol Adv 2011;29:75–83.
rot fungus Coriolopsis polyzona and use of the immobilized biocatalyst for the
Dhingra V, Gupta M, Andacht T, Fu ZF. New frontiers in proteomics research: a
continuous elimination of endocrine disrupting chemicals. Bioresour Technol
perspective. Int J Pharm 2005;299:1-18.
Dodor DE, Hwang H-M, Ekunwe SIN. Oxidation of anthracene and benzo[a]pyrene by
Cabana H, Jones JP, Agathos SN. Utilization of cross-linked laccase aggregates in a
immobilized laccase from Trametes versicolor. Enzyme Microb Technol 2004;35:
perfusion basket reactor for the continuous elimination of endocrine-disrupting
chemicals. Biotechnol Bioeng 2009b;102:1582–92.
Donaghy JA, McKay AM. Pectin extraction from citrus peel by polygalacturonase
Cabana H, Ahamed A, Leduc R. Conjugation of laccase from the white rot fungus
produced on whey. Bioresour Technol 1994;47:25–8.
Trametes versicolor to chitosan and its utilization for the elimination of triclosan.
Doukyu N, Ogino H. Organic solvent-tolerant enzymes. Biochem Eng J 2010;48:270–82.
Bioresour Technol 2011;2:1656–62.
D'Souza DT, Tiwari R, Sah AK, Raghukumar C. Enhanced production of laccase by a
Calabrò V, Curcio S, De Paola MG, Iorio G. Optimization of membrane bioreactor
marine fungus during treatment of colored effluents and synthetic dyes. Enzyme
performances during enzymatic oxidation of waste bio-polyphenols. Desalination
Microb Technol 2006;38:504–11.
Duff SJB, Moritz JW, Andersen KL. Simultaneous hydrolysis and fermentation of pulp
Call HP, Mücke I. History, overview and applications of mediated lignolytic systems,
mill primary clarifier sludge. Can J Chem Eng 1994;72:1013–20.
especially laccase-mediator-systems (Lignozym®-process). J Biotechnol 1997;53:
Dunford HB. Heme peroxidases. New York: Wiley-VCH; 1999.
Durán N, Esposito E. Potential applications of oxidative enzymes and phenoloxidase-like
Cameron MD, Aust SD. Degradation of chemicals by reactive radicals produced by
compounds in wastewater and soil treatment: a review. Appl Catal B 2000;28:83–99.
cellobiose dehydrogenase from Phanerochaete chrysosporium. Arch Biochem
Dutta S, Bhattacharyya A, De P, Ray P, Basu S. Removal of mercury from its aqueous
solution using charcoal-immobilized papain (CIP). J Hazard Mater 2009;172:
Cameron MD, Aust SD. Cellobiose dehydrogenase — an extracellular fungal flavocytochrome.
Enzyme Microb Technol 2001;28:129–38.
Eberl A, Heumann S, Kotek R, Kaufmann F, Mitsche S, Cavaco-Paulo A, et al. Enzymatic
Cammarota MC, Freire DMG. A review on hydrolytic enzymes in the treatment of
hydrolysis of PTT polymers and oligomers. J Biotechnol 2008;135:45–51.
wastewater with high oil and grease content. Bioresour Technol 2006;97:
Eibes G, López C, Moreira MT, Feijoo G, Lema JM. Strategies for the design and operation
of enzymatic reactors for the degradation of highly and poorly soluble recalcitrant
Campos MG, Pereira P, Roseiro JC. Packed-bed reactor for the integrated biodegradation
compounds. Biocatal Biotransform 2007;25:260–8.
of cyanide and formamide by immobilized Fusarium oxysporum CCMI 876 and
Eijsink VGH, Bjørk A, Gåseidnes S, Sirevåg R, Synstad B, van den Burg B, et al. Rational
Methylobacterium sp. RXM CCMI 908. Enzyme Microb Technol 2006;38:848–54.
engineering of enzyme stability. J Biotechnol 2004;113:105–20.
Cañas AI, Camarero S. Laccases and their natural mediators: biotechnological tools for
El-Naggar MMA, Farag MG. Physical and biological treatments of polyethylene-rice
sustainable eco-friendly processes. Biotechnol Adv 2010;28:694–705.
starch plastic films. J Hazard Mater 2010;176:878–83.
Cantarella M, Cantarella L, Gallifuoco A, Spera A. Nitrile bioconversion by Microbacterium
Enaud E, Trovaslet M, Bruyneel F, Billottet L, Karaaslan R, Sener ME, et al. A novel
imperiale CBS 498-74 resting cells in batch and ultrafiltration membrane bioreactors. J
azoanthraquinone dye made through innovative enzymatic process. Dyes Pigment
Ind Microbiol Biotechnol 2006;33:208–14.
Cao L. Carrier-bound immobilized enzymes: principles, applications and design.
Enayatzamir K, Tabandeh F, Yakhchali B, Alikhani HA, Rodríguez Couto S. Assessment of
Weinheim: Wiley-VCH; New York; 2005.
the joint effect of laccase and cellobiose dehydrogenase on the decolouration of
Cedrone F, Ménez A, Quéméneur E. Tailoring new enzyme functions by rational
different synthetic dyes. J Hazard Mater 2009;169:176–81.
redesign. Curr Opin Struct Biol 2000;10:405–10.
Entezari MH, Mostafai M, Sarafraz-yazdi A. A combination of ultrasound and a bio-catalyst:
Cerniglia CE. Biodegradation of polycyclic aromatic hydrocarbons. Curr Opin Biotechnol
removal of 2-chlorophenol from aqueous solution. Ultrason Sonochem 2006;13:
Chen RD. Enzyme engineering: rational redesign versus directed evolution. Trends
Erable B, Maugard T, Goubet I, Lamare S, Legoy MD. Biotransformation of halogenated
compounds by lyophilized cells of Rhodococcus erythropolis in a continuous solid–gas
Chen JP, Chang KC. Immobilization of chitinase on a reversibly soluble–insoluble
biofilter. Process Biochem 2005;40:45–51.
polymer for chitin hydrolysis. J Chem Technol Biotechnol 1994;60:133–40.
Erable B, Goubet I, Seltana A, Maugard T. Non-conventional gas phase remediation of
Chen Y, Xiao B, Chang J, Fu Y, Lv P, Wang X. Synthesis of biodiesel from waste cooking oil using
volatile halogenated compounds by dehydrated bacteria. J Environ Manage
immobilized lipase in fixed bed reactor. Energy Convers Manage 2009;50:668–73.
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),doi:
P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
Estroff LA. Introduction: biomineralization. Chem Rev 2008;108:4329–31.
Hu XK, Wang P, Hwang HM. Oxidation of anthracene by immobilized laccase from
Farré Ml, Pérez S, Kantiani L, Barceló D. Fate and toxicity of emerging pollutants, their
Trametes versicolor. Bioresour Technol 2009;100:4963–8.
metabolites and transformation products in the aquatic environment. Trends Anal
Hublik G, Schinner F. Characterization and immobilization of the laccase from Pleurotus
ostreatus and its use for the continuous elimination of phenolic pollutants. Enzyme
Ferreira-Leitão VS, de Carvalho MEA, Bon EPS. Lignin peroxidase efficiency for
Microb Technol 2000;27:330–6.
methylene blue decolouration: comparison to reported methods. Dyes Pigm
Iamarino G, Rao MA, Gianfreda L. Dephenolization and detoxification of olive-mill
wastewater (OMW) by purified biotic and abiotic oxidative catalysts. Chemosphere
Fetzner S. Aerobic degradation of halogenated aliphatics. In: Timmis KN, editor.
Handbook of hydrocarbon and lipid microbiology. Berlin Heidelberg: Springer;
Ingvorsen K, Godtfredsen SE, Hojer-Pedersen B. Microbial cyanide converting enzymes,
2010. p. 865–85.
their production and use. US patent 5,116,744. (May 26, 1992).
Fillat A, Colom JF, Vidal T. A new approach to the biobleaching of flax pulp with laccase
Ispas CR, Ravalli MT, Steere A, Andreescu S. Multifunctional biomagnetic capsules for
using natural mediators. Bioresour Technol 2010;101:4104–10.
easy removal of phenol and bisphenol A. Water Res 2010;44:1961–9.
Franciscon E, Piubeli F, Fantinatti-Garboggini F, Ragagnin de Menezes C, Serrano Silva I,
Iyer PV, Ananthanarayan L. Enzyme stability and stabilization—aqueous and non-aqueous
Cavaco-Paulo A, et al. Polymerization study of the aromatic amines generated by
environment. Process Biochem 2008;43:1019–32.
the biodegradation of azo dyes using the laccase enzyme. Enzyme Microb Technol
Jackson J, Sutton R. Sources of endocrine-disrupting chemicals in urban wastewater,
Oakland, CA. Sci Total Environ 2008;405:153–60.
Friedrich J, Gradisar H, Vrecl M, Pogacnik A. In vitro degradation of porcine skin
Jayani RS, Saxena S, Gupta R. Microbial pectinolytic enzymes: a review. Process
epidermis by a fungal keratinase of Doratomyces microsporus. Enzyme Microb
Jee SH, Kim YJ, Ko SO. Transformation of dissolved organic matter by oxidative
Fry WE, Millar RL. Cyanide degradion by an enzyme from Stemphylium loti. Arch
polymerization with horseradish peroxidase. Water Sci Technol 2010;62:340–6.
Biochem Biophys 1972;151:468–74.
Jeffries TW, Viikari L, editors. Enzymes for pulp and paper processing. ACS Symposium
Galliker P, Hommes G, Schlosser D, Corvini PFX, Shahgaldian P. Laccase-modified silica
SeriesWashington, DC: American Chemical Society; 1996.
nanoparticles efficiently catalyze the transformation of phenolic compounds. J
Jeganathan J, Bassi A, Nakhla G. Pre-treatment of high oil and grease pet food industrial
Colloid Interface Sci 2010;349:98-105.
wastewaters using immobilized lipase hydrolyzation. J Hazard Mater 2006;137:121–8.
Gariev IA, Varfolomeev SD. Hierarchical classification of hydrolases catalytic sites.
Jeganathan J, Nakhla G, Bassi A. Hydrolytic pretreatment of oily wastewater by
immobilized lipase. J Hazard Mater 2007;145:127–35.
Georgieva S, Godjevargova T, Mita DG, Diano N, Menale C, Nicolucci C, et al.
Jensen ON. Modification-specific proteomics: characterization of post-translational
Non-isothermal bioremediation of waters polluted by phenol and some of its
modifications by mass spectrometry. Curr Opin Chem Biol 2004;8:33–41.
derivatives by laccase covalently immobilized on polypropylene membranes. J Mol
Jung F, Cammarota MC, Freire DMG. Impact of enzymatic pre-hydrolysis on batch
Catal B: Enzym 2010;66:210–8.
activated sludge systems dealing with oily wastewaters. Biotechnol Lett 2002;24:
Gianfreda L, Rao MA. Potential of extra cellular enzymes in remediation of polluted
soils: a review. Enzyme Microb Technol 2004;35:339–54.
Junghanns C, Moeder M, Krauss G, Martin C, Schlosser D. Degradation of the xenoestrogen
Gianfreda L, Xu F, Bollag J. Laccases: a useful group of oxidoreductive enzymes.
nonylphenol by aquatic fungi and their laccases. Microbiology 2005;151:45–57.
Bioremed J 1999;3:1-25.
Kakar S, Hoffman FG, Storz JF, Fabian M, Hargrove MS. Structure and reactivity of
Gill I, Ballesteros A. Bioencapsulation within synthetic polymers (part 1): sol gel
hexacoordinate hemoglobins. Biophys Chem 2010;152:1-14.
encapsulated biologicals. Trends Biotechnol 2000;18:282–96.
Kambiranda DM, Asraful-Islam SM, Cho KM, Math RK, Lee YH, Kim H, et al. Expression
Gómez JL, Bódalo A, Gómez E, Bastida J, Hidalgo AM, Gómez M. Immobilization of
of esterase gene in yeast for organophosphates biodegradation. Pestic Biochem
peroxidases on glass beads: an improved alternative for phenol removal. Enzyme
Microb Technol 2006;39:1016–22.
Kapoor M, Beg QK, Bhushan B, Singh K, Dadhich KS, Hoondal GS. Application of an
Gómez JL, Gómez E, Bastida J, Hidalgo AM, Gómez M, Murcia MD. Experimental behavior
alkaline and thermostable polygalacturonase from Bacillus sp. MG-cp-2 in
and design model of a continuous tank reactor for removing 4-chlorophenol with
degumming of ramie (Boehmeria nivea) and sunn hemp (Crotalaria juncea) bast
soybean peroxidase. Chem Eng Process: Process Intensif 2008;47:1786–92.
fibres. Process Biochem 2001;36:803–7.
Gomez M, Matafonova G, Gomez JL, Batoev V, Christofi N. Comparison of alternative
Karim Z, Husain Q. Redox-mediated oxidation and removal of aromatic amines from
treatments for 4-chlorophenol removal from aqueous solutions: use of free and
polluted water by partially purified bitter gourd (Momordica charantia) peroxidase.
immobilized soybean peroxidase and KrCl excilamp. J Hazard Mater 2009;169:46–51.
Int Biodet Biodeg 2009;63:587–93.
Guengerich FP. Cytochrome P450 proteins and potential utilization in biodegradation.
Karim Z, Husain Q. Application of fly ash adsorbed peroxidase for the removal of
Environ Health Perspect 1995;103(Suppl 5):25–8.
bisphenol A in batch process and continuous reactor: assessment of genotoxicity of
Guengerich FP, Gillam EMJ, Ohmori S, Sandhu P, Brain WR, Sari M-A, et al. Expression of
its product. Food Chem Toxicol 2010a:3385–90.
human cytochrome P450 enzymes in yeast and bacteria and relevance to studies on
Karim Z, Husain Q. Removal of anthracene from model wastewater by immobilized
catalytic specificity. Toxicology 1993;82:21–37.
peroxidase from Momordica charantia in batch process as well as in a continuous
Haghighi B, Gorton L, Ruzgas T, Jönsson LJ. Characterization of graphite electrodes
spiral-bed reactor. J Mol Catal B: Enzym 2010b;66:302–10.
modified with laccase from Trametes versicolor and their use for bioelectrochemical
Karpouzas DG, Singh BK. Microbial degradation of organophosphorus xenobiotics:
monitoring of phenolic compounds in flow injection analysis. Anal Chim Acta
metabolic pathways and molecular basis. Adv Microb Physiol 2006;51:119–85.
Kashyap DR, Vohra PK, Chopra S, Tewari R. Applications of pectinases in the commercial
Hamid M, Khalil-ur-Rehman. Potential applications of peroxidases. Food Chem
sector: a review. Bioresour Technol 2001;77:215–27.
Katuri KP, Venkata Mohan S, Sridhar S, Pati BR, Sarma PN. Laccase-membrane reactors
Hao OJ, Kim H, Chiang PC. Decolorization of wastewater. Crit Rev Environ Sci Technol
for decolorization of an acid azo dye in aqueous phase: process optimization. Water
Harford-Cross CF, Carmichael AB, Allan FK, England PA, Rouch DA, Wong L-L. Protein
Kawai F. The biochemistry and molecular biology of xenobiotic polymer degradation by
engineering of cytochrome P450cam (CYP101) for the oxidation of polycyclic
microorganisms. Biosci Biotechnol Biochem 2010;74:1743–59.
aromatic hydrocarbons. Protein Eng Des Sel 2000;13:121–8.
Kellner H, Luis P, Portetelle D, Vandenbol M. Screening of a soil metatranscriptomic
Haritash AK, Kaushik CP. Biodegradation aspects of polycyclic aromatic hydrocarbons
library by functional complementation of Saccharomyces cerevisiae mutants.
(PAHs): a review. J Hazard Mater 2009;169:1-15.
Microbiol Res in Press, Corrected Proof,
Hasan F, Shah AA, Hameed A. Industrial applications of microbial lipases. Enzyme
Khouni I, Marrot B, Ben Amar R. Decolorization of the reconstituted dye bath effluent by
Microb Technol 2006;39:235–51.
commercial laccase treatment: optimization through response surface methodology.
Hatakka A. Lignin-modifying enzymes from selected white-rot fungi: production and
Chem Eng J 2010;156:121–33.
role in lignin degradation. FEMS Microbiol Rev 1994;13:125–35.
Khouni I, Marrot B, Moulin P, Ben Amar R. Decolorization of the reconstituted textile
Henriksson G, Johansson G, Pettersson G. A critical review of cellobiose dehydroge-
effluent by different process treatments: enzymatic catalysis, coagulation/flocculation
nases. J Biotechnol 2000a;78:93-113.
and nanofiltration processes. Desalination 2011;268:27–37.
Henriksson G, Zhang L, Li J, Ljungquist P, Reitberger T, Pettersson G, et al. Is cellobiose
Kim YH, Lee J, Moon SH. Degradation of an endocrine disrupting chemical, DEHP [di-(2-
dehydrogenase from Phanerochaete chrysosporium a lignin degrading enzyme?
ethylhexyl)-phthalate], by Fusarium oxysporum f. sp. pisi cutinase. Appl Microbiol
Biochim Biophys Acta, Prot Struct Mol Enzymol 2000b;1480:83–91.
Hoenicke R, Oros DR, Oram JJ, Taberski KM. Adapting an ambient monitoring program
Kim G-Y, Lee K-B, Cho S-H, Shim J, Moon S-H. Electroenzymatic degradation of azo dye
to the challenge of managing emerging pollutants in the San Francisco Estuary.
using an immobilized peroxidase enzyme. J Hazard Mater 2005a;126:183–8.
Environ Res 2007;105:132–44.
Kim Y-H, Ahn J-Y, Moon S-H, Lee J. Biodegradation and detoxification of organophosphate
Hofrichter M. Review: lignin conversion by manganese peroxidase (MnP). Enzyme
insecticide, malathion by Fusarium oxysporum f. sp. pisi cutinase. Chemosphere
Microb Technol 2002;30:454–66.
Hofrichter M, Ullrich R, Pecyna M, Liers C, Lundell T. New and classic families of secreted
Kim HJ, Kim SH, Choi YG, Kim GD, Chung TH. Effect of enzymatic pretreatment on acid
fungal heme peroxidases. Appl Microbiol Biotechnol 2010;87:871–97.
fermentation of food waste. J Chem Technol Biotechnol 2006;81:974–80.
Horikoshi K. Enzymes of alkalophiles. In: Fogarty WM, Kelly CT, editors. Microbial enzymes
Kim EY, Chae HJ, Chu KH. Enzymatic oxidation of aqueous pentachlorophenol. J Environ
and biotechnology. 2nd ed. London: Elsevier Applied Science; 1990. p. 275–94.
Howard GT. Biodegradation of polyurethane: a review. Int Biodet Biodeg 2002;49:245–52.
Kim SD, Cho J, Kim IS, Vanderford BJ, Snyder SA. Occurrence and removal of
Hoy JA, Hargrove MS. The structure and function of plant hemoglobins. Plant Physiol
pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and
waste waters. Water Res 2007b;41:1013–21.
Hu XK, Zhao XH, Hwang HM. Comparative study of immobilized Trametes versicolor
Kim J, Grate JW, Wang P. Nanobiocatalysis and its potential applications. Trends
laccase on nanoparticles and kaolinite. Chemosphere 2007;66:1618–26.
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),
P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
Kim E-Y, Oh K-H, Lee M-H, Kang C-H, Oh T-K, Yoon J-H. Novel cold-adapted alkaline
Martínez AT. Molecular biology and structure–function of lignin-degrading heme
lipase from an intertidal flat metagenome and proposal for a new family of bacterial
peroxidases. Enzyme Microb Technol 2002;30:425–44.
lipases. Appl Environ Microbiol 2009;75:257–60.
Martínez-Trujillo A, Aranda JS, Gómez-Sánchez C, Trejo-Aguilar B, Aguilar-Osorio G.
Kirk TK, Farrell RL. Enzymatic "combustion": the microbial degradation of lignin. Annu
Constitutive and inducible pectinolytic enzymes from Aspergillus flavipes FP-500
Rev Microbiol 1987;41:465–505.
and their modulation by pH and carbon source. Braz J Microbiol 2009;40:40–7.
Ko C-H, Chen S-S. Enhanced removal of three phenols by laccase polymerization with
Martínková L, Kren V. Biotransformations with nitrilases. Curr Opin Chem Biol 2010;14:
MF/UF membranes. Bioresour Technol 2008;99:2293–8.
Kobayashi M, Shimizu S. Nitrile hydrolases. Curr Opin Chem Biol 2000;4:95-102.
Martínková L, Vejvoda V, Kaplan O, Kubác D, Malandra A, Cantarella M, et al. Fungal
Kohyama E, Yoshimura A, Aoshima D, Yoshida T, Kawamoto H, Nagasawa T. Convenient
nitrilases as biocatalysts: recent developments. Biotechnol Adv 2009;27:661–70.
treatment of acetonitrile-containing wastes using the tandem combination of nitrile
Maté D, García-Burgos C, García-Ruiz E, Ballesteros AO, Camarero S, Alcalde M.
hydratase and amidase-producing microorganisms. Appl Microbiol Biotechnol
Laboratory evolution of high-redox potential laccases. Chem Biol 2010;17:
Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, et al. Pharmaceuticals,
Matijosyte I, Arends IWCE, de Vries S, Sheldon RA. Preparation and use of cross-linked
hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: a
enzyme aggregates (CLEAs) of laccases. J Mol Catal B: Enzymatic 2010;62:142–8.
national reconnaissance. Environ Sci Technol 2002;36:1202–11.
Matto M, Husain Q. Decolorization of direct dyes by immobilized turnip peroxidase in
Kubác D, Cejková A, Masák J, Jirku V, Lemaire M, Gallienne E, et al. Biotransformation of
batch and continuous processes. Ecotoxicol Environ Saf 2009;72:965–71.
nitriles by Rhodococcus equi A4 immobilized in LentiKats®. J Mol Catal B: Enzym
Mello LD, Kubota LT. Biosensors as a tool for the antioxidant status evaluation. Talanta
Kudanga T, Nyanhongo GS, Guebitz GM, Burton S. Potential applications of laccase-mediated
Mita DG, Diano N, Grano V, Portaccio M, Rossi S, Bencivenga U, et al. The process of
coupling and grafting reactions: a review. Enzyme Microb Technol 2011;48:195–208.
thermodialysis in bioremediation of waters polluted by endocrine disruptors. J Mol
Kunamneni A, Camarero S, Garcia-Burgos C, Plou F, Ballesteros A, Alcalde M.
Catal B: Enzymatic 2009;58:199–207.
Engineering and applications of fungal laccases for organic synthesis. Microb Cell
Mita L, Sica V, Guida M, Nicolucci C, Grimaldi T, Caputo L, et al. Employment of
Fact 2008;7:32.
immobilised lipase from Candida rugosa for the bioremediation of waters polluted
Lai JK, Chuang TH, Jan JS, Wang SSS. Efficient and stable enzyme immobilization in a
by dimethylphthalate, as a model of endocrine disruptors. J Mol Catal B: Enzym
block copolypeptide vesicle-templated biomimetic silica support. Colloids Surf B
Mohammadi A, Moghaddam AB, Dinarvand R, Badraghi J, Atyabi F, Saboury AA.
Lante A, Crapisi A, Krastanov A, Spettoli P. Biodegradation of phenols by laccase
Bioelectrocatalysis of methyldopa by adsorbed tyrosinase on the surface of
immobilised in a membrane reactor. Process Biochem 2000;36:51–8.
modified glassy carbon with carbon nanotubes. Int J Electrochem Sci 2008;3:
Latch DE, Packer JL, Arnold WA, McNeill K. Photochemical conversion of triclosan to 2,8-
dichlorodibenzo-p-dioxin in aqueous solution. J Photochem Photobiol A 2003;158:63–6.
Mowat CG, Gazur B, Campbell LP, Chapman SK. Flavin-containing heme enzymes. Arch
LeJeune KE, Mesiano AJ, Bower SB, Grimsley JK, Wild JR, Russell AJ. Dramatically
Biochem Biophys 2010;493:37–52.
stabilized phosphotriesterase-polymers for nerve agent degradation. Biotechnol
Munnecke DM. Enzymatic hydrolysis of organophosphate insecticides, a possible
pesticide disposal method. Appl Environ Microbiol 1976;32:7-13.
LeJeune KE, Russell AJ. Biocatalytic nerve agent detoxification in fire fighting foams.
Murcia MD, Gomez M, Gomez E, Bodalo A, Gomez JL, Hidalgo AM. Assessing
Biotechnol Bioeng 1999;62:659–65.
combination treatment, enzymatic oxidation and ultrafiltration in a membrane
Lee SY, Choi J-i. Production and degradation of polyhydroxyalkanoates in waste
bioreactor, for 4-chlorophenol removal: experimental and modeling. J Membr Sci
environment. Waste Manag 1999;19:133–9.
Lewis DFV, Wiseman A. A selective review of bacterial forms of cytochrome P450
Murugan K, Al-Sohaibani SA. Biocompatible removal of tannin and associated color
enzymes. Enzyme Microb Technol 2005;36:377–84.
from tannery effluent using the biomass and tannin acyl hydrolase (E.C.3.1.1.20)
Li Y-P, Cao H-B, Zhang Y. Electrochemical dechlorination of chloroacetic acids (CAAs) using
enzymes of mango industry solid waste isolate Aspergillus candidus MTTC 9628. Res
hemoglobin-loaded carbon nanotube electrode. Chemosphere 2006;63:359–64.
J Microbiol 2010;5:262–71.
Li Y-P, Cao H-B, Zhang Y. Reductive dehalogenation of haloacetic acids by hemoglobin-loaded
Murugesan K, Kim Y-M, Jeon J-R, Chang Y-S. Effect of metal ions on reactive dye
carbon nanotube electrode. Water Res 2007;41:197–205.
decolorization by laccase from Ganoderma lucidum. J Hazard Mater 2009;168:
Li N-W, Zong M-H, Wu H. Highly efficient transformation of waste oil to biodiesel by
immobilized lipase from Penicillium expansum. Process Biochem 2009a;44:
Murugesan K, Chang Y-Y, Kim Y-M, Jeon J-R, Kim E-J, Chang Y-S. Enhanced
transformation of triclosan by laccase in the presence of redox mediators. Water
Li ZL, Liu W, Chen XF, Shang WL. Research on the application of horseradish peroxidase
and hydrogen peroxide to the oil removal of oily water. Water Sci Technol
Nazly N, Knowles CJ. Cyanide degradation by immobilised fungi. Biotechnol Lett
Lindgren A, Ruzgas T, Gorton L, Stoica L, Ciucu A. Development of a cellobiose
Nazly N, Knowles CJ, Beardsmore AJ, Naylor WT, Corcoran EG. Detoxification of cyanide
dehydrogenase modified electrode for amperometric detection of diphenols. Analyst
by immobilised fungi. J Chem Technol Biotechnol 1983;33:119–26.
Niazi JH, Prasad DT, Karegoudar TB. Initial degradation of dimethylphthalate by
Liu J-Z, Wang T-L, Ji L-N. Enhanced dye decolorization efficiency by citraconic
esterases from Bacillus species. FEMS Microbiol Lett 2001;196:201–5.
anhydride-modified horseradish peroxidase. J Mol Catal B: Enzym 2006;41:81–6.
Obinger C. Heme peroxidase biochemistry — facts and perspectives. Arch Biochem
Liu W, Zhang S, Wang P. Nanoparticle-supported multi-enzyme biocatalysis with in situ
cofactor regeneration. J Biotechnol 2009;139:102–7.
Oh Y-S, Shih I-L, Tzeng Y-M, Wang S-L. Protease produced by Pseudomonas aeruginosa
Lloret L, Eibes G, Lú-Chau TA, Moreira MT, Feijoo G, Lema JM. Laccase-catalyzed
K-187 and its application in the deproteinization of shrimp and crab shell wastes.
degradation of anti-inflammatories and estrogens. Biochem Eng J 2010;51:124–31.
Enzyme Microb Technol 2000;27:3-10.
Lu HD, Wheeldon IR, Banta S. Catalytic biomaterials: engineering organophosphate
Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, et al. The α/β hydrolase
hydrolase to form self-assembling enzymatic hydrogels. Protein Eng Des Sel
fold. Protein Eng Des Sel 1992;5:197–211.
O'Reilly C, Turner P. The nitrilase family of CN hydrolysing enzymes — a comparative
Lu J, Rao S, Le T, Mora S, Banerjee S. Increasing cake solids of cellulosic sludge through
study. J Appl Microbiol 2003;95:1161–74.
enzyme-assisted dewatering. Process Biochem 2011;46:353–7.
Osma JF, Toca-Herrera JL, Rodríguez-Couto S. Biodegradation of a simulated textile
Luckarift HR, Balasubramanian S, Paliwal S, Johnson GR, Simonian AL. Enzyme-
effluent by immobilised-coated laccase in laboratory-scale reactors. Appl Catal A
encapsulated silica monolayers for rapid functionalization of a gold surface.
Colloids Surf B 2007;58:28–33.
Özel YK, Gedikli S, Aytar P, Ünal A, Yamaç M, Çabuk A, et al. New fungal biomasses for
Lutz S. Beyond directed evolution — semi-rational protein engineering and design. Curr
cyanide biodegradation. J Biosci Bioeng 2010;110:431–5.
Opin Biotechnol 2010;21:734–43.
Pala H, Mota M, Gama FM. Enzymatic versus chemical deinking of non-impact ink
Maalej-Kammoun M, Zouari-Mechichi H, Belbahri L, Woodward S, Mechichi T.
printed paper. J Biotechnol 2004;108:79–89.
Malachite green decolourization and detoxification by the laccase from a newly
Parmar N, Singh A, Ward OP. Enzyme treatment to reduce solids and improve settling of
isolated strain of Trametes sp. Int Biodet Biodeg 2009;63:600–6.
sewage sludge. J Ind Microbiol Biotechnol 2001;26:383–6.
Majcherczyk A, Johannes C, Hüttermann A. Oxidation of polycyclic aromatic
Patil RS, Ghormade V, Deshpande MV. Chitinolytic enzymes: an exploration. Enzyme
hydrocarbons (PAH) by laccase of Trametes versicolor. Enzyme Microb Technol
Microb Technol 2000;26:473–83.
Pavlova M, Klvana M, Prokop Z, Chaloupkova R, Banas P, Otyepka M, et al. Redesigning
Makino N, McMahill P, Mason HS, Moss TH. The oxidation state of copper in resting
dehalogenase access tunnels as a strategy for degrading an anthropogenic
tyrosinase. J Biol Chem 1974;249:6062–6.
substrate. Nat Chem Biol 2009;5:727–33.
Mallick P, Akunna JC, Walker GM. Anaerobic digestion of distillery spent wash:
Payasi A, Sanwal R, Sanwal G. Microbial pectate lyases: characterization and
influence of enzymatic pre-treatment of intact yeast cells. Bioresour Technol
enzymological properties. World J Microbiol Biotechnol 2009;25:1-14.
Pepi M, Lampariello LR, Altieri R, Esposito A, Perra G, Renzi M, et al. Tannic acid
Mao L, Huang Q, Lu J, Gao S. Ligninase-mediated removal of natural and synthetic
degradation by bacterial strains Serratia spp. and Pantoea sp. isolated from olive
estrogens from water: I. Reaction behaviors. Environ Sci Technol 2008;43:374–9.
mill waste mixtures. Int Biodet Biodeg 2010;64:73–80.
Marín-Rodríguez MC, Orchard J, Seymour GB. Pectate lyases, cell wall degradation and
Pirillo S, Einschlag FSG, Ferreira ML, Rueda EH. Eriochrome Blue Black R and Fluorescein
fruit softening. J Exp Bot 2002;53:2115–9.
degradation by hydrogen peroxide oxidation with horseradish peroxidase and
Martin C, Moeder M, Daniel X, Krauss G, Schlosser D. Biotransformation of the
hematin as biocatalysts. J Mol Catal B: Enzym 2010;66:63–71.
polycyclic musks HHCB and AHTN and metabolite formation by fungi occurring in
Prasad S, Bhalla TC. Nitrile hydratases (NHases): at the interface of academia and
freshwater environments. Environ Sci Technol 2007;41:5395.
industry. Biotechnol Adv 2010;28:725–41.
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),doi:
P. Demarche et al. / Biotechnology Advances xxx (2011) xxx–xxx
Prince RC. Haloalkane dehalogenase caught in the act. Trends Biochem Sci 1994;19:3–4.
Tang T, Hou J, Ai S, Qiu Y, Ma Q, Han R. Electroenzymatic oxidation of bisphenol A (BPA) based
Raghukumar C. Fungi from marine habitats: an application in bioremediation. Mycol
on the hemoglobin (Hb) film in a membraneless electrochemical reactor. J Hazard Mater
Rattanakit N, Plikomol A, Yano S, Wakayama M, Tachiki T. Utilization of shrimp shellfish
Theriot C, Grunden A. Hydrolysis of organophosphorus compounds by microbial
waste as a substrate for solid-state cultivation of Aspergillus sp. S1–13: evaluation of
enzymes. Appl Microbiol Biotechnol 2010:1–9.
a culture based on chitinase formation which is necessary for chitin-assimilation. J
Thurston CF. The structure and function of fungal laccases. Microbiology 1994;140:19–26.
Biosci Bioeng 2002;93:550–6.
Torres P, Datla A, Rajasekar VW, Zambre S, Ashar T, Yates M, et al. Characterization and
Rattanakit N, Yang S, Wakayama M, Plikomol A, Tachiki T. Saccharification of chitin
application of a sterol esterase immobilized on polyacrylate epoxy-activated
using solid-state culture of Aspergillus sp. S1–13 with shellfish waste as a substrate.
carriers (Dilbeads™). Catal Commun 2008;9:539–45.
J Biosci Bioeng 2003;95:391–6.
Tripodo MM, Lanuzza F, Micali G, Coppolino R, Nucita F. Citrus waste recovery: a new
Raybuck SA. Microbes and microbial enzymes for cyanide degradation. Biodegradation
environmentally friendly procedure to obtain animal feed. Bioresour Technol
Riva S. Laccases: blue enzyme for green chemistry. Trends Biotechnol 2006;24:219–26.
Tsutsumi Y, Haneda T, Nishida T. Removal of estrogenic activities of bisphenol A and
Rivers DB, Emert GH. Factors affecting the enzymatic hydrolysis of municipal-solid-
nonylphenol by oxidative enzymes from lignin-degrading basidiomycetes. Chemo-
waste components. Biotechnol Bioeng 1988;31:278–81.
Rodgers CJ, Blanford CF, Giddens SR, Skamnioti P, Armstrong FA, Gurr SJ. Designer
Van Aken B, Ledent P, Naveau H, Agathos SN. Co-immobilization of manganese
laccases: a vogue for high-potential fungal enzymes? Trends Biotechnol 2010;28:
peroxidase from Phlebia radiata and glucose oxidase from Aspergillus niger on
porous silica beads. Biotechnol Lett 2000;22:641–6.
Rodríguez Couto S, Toca Herrera JL. Industrial and biotechnological applications of
Vanhulle S, Enaud E, Trovaslet M, Nouaimeh N, Bols C-M, Keshavarz T, et al. Overlap of
laccases: a review. Biotechnol Adv 2006;24:500–13.
laccases/cellobiose dehydrogenase activities during the decolourisation of
Sanchez S, Demain AL. Enzymes and bioconversions of industrial, pharmaceutical, and
anthraquinonic dyes with close chemical structures by Pycnoporus strains. Enzyme
biotechnological significance. Org Process Res Dev 2010;15:224–30.
Microb Technol 2007;40:1723–31.
Sangave PC, Pandit AB. Enhancement in biodegradability of distillery wastewater using
Vanhulle S, Enaud E, Trovaslet M, Billottet L, Kneipe L, Jiwan J-LH, et al. Coupling occurs
enzymatic pretreatment. J Environ Manage 2006;78:77–85.
before breakdown during biotransformation of Acid Blue 62 by white rot fungi.
Seetharam GB, Saville BA. Degradation of phenol using tyrosinase immobilized on
siliceous supports. Water Res 2003;37:436–40.
Vasileva-Tonkova E, Gousterova A, Neshev G. Ecologically safe method for improved
Sfetsas CC, Milios L, Skopelitou K, Venieraki A, Todou R, Flemetakis E, et al.
feather wastes biodegradation. Int Biodet Biodeg 2009;63:1008–12.
Characterization of 1,2-dibromoethane-degrading haloalkane dehalogenase from
Vázquez JA, Murado MA. Enzymatic hydrolysates from food wastewater as a source of
Bradyrhizobium japonicum USDA110. Enzyme Microb Technol 2009;45:397–404.
peptones for lactic acid bacteria productions. Enzyme Microb Technol 2008;43:66–72.
Shah AA, Hasan F, Hameed A, Ahmed S. Biological degradation of plastics: a
Vejvoda V, Kaplan O, Klozová J, Masák J, Čejková A, Jirků V, et al. Mild hydrolysis of
comprehensive review. Biotechnol Adv 2008;26:246–65.
nitriles by Fusarium solani strain O1. Folia Microbiol 2006;51:251–6.
Sharma R, Chisti Y, Banerjee UC. Production, purification, characterization, and
Verlicchi P, Galletti A, Petrovic M, Barceló D. Hospital effluents as a source of emerging
applications of lipases. Biotechnol Adv 2001;19:627–62.
pollutants: an overview of micropollutants and sustainable treatment options. J
Shimao M. Biodegradation of plastics. Curr Opin Biotechnol 2001;12:242–7.
Shin-ya Y, Aye HN, Hong K-J, Kajiuchi T. Efficacy of amphiphile-modified laccase in
Biotechnology in the Pulp and Paper Industry — 8th ICBPPI Meeting (8th International
enzymatic oxidation and removal of phenolics in aqueous solution. Enzyme Microb
Conference on Biotechnology in the Pulp and Paper Industry). In: Viikari L, Lantto R,
editors. Progress in Biotechnology, Vol. 21. Amsterdam: Elsevier; 2002.
Singh R, Sharma R, Tewari N, Geetanjali, Rawat DS. Nitrilase and its application as a
Vyas S, Lachke A. Biodeinking of mixed office waste paper by alkaline active cellulases
‘green' catalyst. Chem Biodivers 2006;3:1279–87.
from alkalotolerant Fusarium sp. Enzyme Microb Technol 2003;32:236–45.
Singh S, Kang SH, Mulchandani A, Chen W. Bioremediation: environmental clean-up
Wagner M, Nicell JA. Treatment of a foul condensate from kraft pulping with
through pathway engineering. Curr Opin Biotechnol 2008;19:437–44.
horseradish peroxidase and hydrogen peroxide. Water Res 2001;35:485–95.
Sogorb MA, Vilanova E. Enzymes involved in the detoxification of organophosphorus,
Wang SL, Liang TW, Yen YH. Bioconversion of chitin-containing wastes for the
carbamate and pyrethroid insecticides through hydrolysis. Toxicol Lett 2002;128:
production of enzymes and bioactive materials. Carbohydr Polym 2011;84:732–42.
Watanabe Y. Construction of heme enzymes: four approaches. Curr Opin Chem Biol
Songsiriritthigul C, Lapboonrueng S, Pechsrichuang P, Pesatcha P, Yamabhai M.
Expression and characterization of Bacillus licheniformis chitinase (ChiA), suitable
Wesenberg D, Kyriakides I, Agathos SN. White-rot fungi and their enzymes for the
for bioconversion of chitin waste. Bioresour Technol 2010;101:4096–103.
treatment of industrial dye effluents. Biotechnol Adv 2003;22:161–87.
SOPHIED — European FP6 project. Novel sustainable bioprocess for European color industries.
Wingate KG, Stuthridge T, Mansfield SD. Colour remediation of pulp mill effluent using
February 17.
purified fungal cellobiose dehydrogenase: reaction optimisation and mechanism of
Sornyotha S, Kyu KL, Ratanakhanokchai K. An efficient treatment for detoxification
degradation. Biotechnol Bioeng 2005;90:95-106.
process of cassava starch by plant cell wall-degrading enzymes. J Biosci Bioeng
Wu JH, Wang Z, Xu SY. Enzymatic production of bioactive peptides from sericin
recovered from silk industry wastewater. Process Biochem 2008a;43:480–7.
Spengler P, Körner W, Metzger JW. Substances with estrogenic activity in effluents of
Wu Y, Teng Y, Li Z, Liao X, Luo Y. Potential role of polycyclic aromatic hydrocarbons
sewage treatment plants in southwestern Germany. 1. Chemical analysis. Environ
(PAHs) oxidation by fungal laccase in the remediation of an aged contaminated soil.
Toxicol Chem 2001;20:2133–41.
Soil Biol Biochem 2008b;40:789–96.
Starr MP, Moran F. Eliminative split of pectic substances by phytopathogenic soft-rot
Wyatt JM, Knowles CJ. Microbial degradation of acrylonitrile waste effluents: the
bacteria. Science 1962;16:920–1.
degradation of effluents and condensates from the manufacture of acrylonitrile. Int
Steele HL, Jaeger KE, Daniel R, Streit WR. Advances in recovery of novel biocatalysts
Biodet Biodeg 1995;35:227–48.
from metagenomes. J Mol Microbiol Biotechnol 2009;16:25–37.
Yamada K, Ikeda N, Takano Y, Kashiwada A, Matsuda K, Hirata M. Determination of
Stolz A. Basic and applied aspects in the microbial degradation of azo dyes. Appl
optimum process parameters for peroxidase-catalysed treatment of bisphenol A and
Microbiol Biotechnol 2001;56:69–80.
application to the removal of bisphenol derivatives. Environ Technol 2010;31:243–56.
Sulek F, Fernández DP, Knez Z, Habulin M, Sheldon RA. Immobilization of horseradish
Yoon S-H, Robyt JF. Activation and stabilization of 10 starch-degrading enzymes by
peroxidase as crosslinked enzyme aggregates (CLEAs). Process Biochem 2011;46:765–9.
Triton X-100, polyethylene glycols, and polyvinyl alcohols. Enzyme Microb Technol
Suzuki Y, Tsujimoto Y, Matsui H, Watanabe K. Decomposition of extremely hard-to-
degrade animal proteins by thermophilic bacteria. J Biosci Bioeng 2006;102:73–81.
Zamora P, Narváez A, Domínguez E. Enzyme-modified nanoparticles using biomimetically
Tamagawa Y, Hirai H, Kawai S, Nishida T. Removal of estrogenic activity of endocrine-
synthesized silica. Bioelectrochemistry 2009;76:100–6.
disrupting genistein by ligninolytic enzymes from white rot fungi. FEMS Microbiol
Zhang YHP, Himmel ME, Mielenz JR. Outlook for cellulase improvement: screening and
selection strategies. Biotechnol Adv 2006;24:452–81.
Tanabe H, Kobayashi Y, Akamatsu I. Pretreatment of pectic wastewater from orange
Zille A, Munteanu F-D, Gübitz GM, Cavaco-Paulo A. Laccase kinetics of degradation and
canning by soft-rot Erwinia corotovora. J Ferment Technol 1986;64:265–8.
coupling reactions. J Mol Catal B: Enzym 2005;33:23–8.
Tanabe H, Yoshihara K, Tamura K, Kobayashi Y, Akamatsu I, Niyomwan N, et al.
Zumárraga M, Bulter T, Shleev S, Polaina J, Martínez-Arias A, Plou FJ, et al. In vitro
Pretreatment of pectic wastewater from orange canning process by an alkalophilic
evolution of a fungal laccase in high concentrations of organic cosolvents. Chem
Bacillus sp. J Ferment Technol 1987;65:243–6.
Please cite this article as: Demarche P, et al, Harnessing the power of enzymes for environmental stewardship, Biotechnol Adv (2011),
Source: http://food.inahosting.co.kr/board/download.php?path=board_private2&fv=35_0_13207359400.pdf:Demarche%202011%20BA%20(review)%20enzymes%20for%20environments.pdf
IN THE HIGH COURT OF DELHI AT NEW DELHI Ex.P. No. 81/2005 11th August, 2015 LILLY ICOS LLC. Mr. Chander M. Lall, Ms. Nancy Ray and Mr. Anuj Nain, Advs. AJANTA PHARMA LIMITED Mr. Rajiv Nayar, Sr. Adv. with Mr. Yogender Nath Bhardwaj, Adv. HON'BLE MR. JUSTICE VALMIKI J.MEHTA To be referred to the Reporter or not?
Im Auftrag der Stadtgemeinde Saalfelden seit 1996 Kinder & Jugendzentrum Saalfelden TREFFPUNKT · Berglandstraße 28 Gefördert durch die Stadtgemeinde Saalfelden und Mitteln des Land Salzburg Im Auftrag der Stadtgemeinde Saalfelden seit 1996 Im Wandel der Zeit! Das Kinder und Jugendzentrum Saalfelden „Treffpunkt", befi ndet sich ständig im Wandel. Viele der „älteren" Jugendlichen in der Altersgruppe von 15 bis 18 Jah-ren, haben nicht nur neue Interessen, sondern stehen auch vor neuen Herausfor-derungen wie dem Schulabschluss, den Beginn einer Lehre oder weiterführende Schule, der Führerscheinprüfung, dem Bundesheer oder Zivildienst und so wei-ter. Sie treffen sich im Jugendzentrum um sich diesbezüglich bei gleichaltrigen Alexander Houtman BEdauszutauschen und auch um Erfahrungswerte von BetreuerInnen anzuhören. Leiter des Kinder & Auffallend sind dabei die „kurzen" Besuche im Jugendzentrum - die Gespräche Jugendzentrum Saalfeldenmit den BetreuerInnen sind dafür aber intensiver.



