General guidelines for agitated patients in palliative care


Symptom
Management
Pocket Guides:
DELIRIUM
DYSPNEA
NAUSEA & VOMITING
PAIN
LOSS OF APPETITE
BOWEL CARE
ORAL CARE
August 2010/July 2012
Table of Contents
ESAS…………………………….………….97 Click on hyperlink to go to specific symptom.
Delirium
Considerations ……………………….…… 1
Assessment ………………………………… 2
Diagnosis ……………………………………. 3
Non-Pharmacological treatment 3
Pharmacological treatment …….… 5
Mild Delirium………………………….…. 6
Moderate Delirium………………….… 6
Severe Delirium ……………………….… 7
Adverse Effects …………………………. 7
Selected References …………………. 9
The underlying etiology needs to be
identified in order to intervene.
Delirium may interfere with the patient‟s
ability to report other symptom experiences.
Provide explanation and reassure the
family that the symptoms of delirium will fluctuate, are caused by the illness, are not within the patient‟s control, and the patient is not going „insane‟.
Some hallucinations, nightmares, and
misperceptions may reflect unresolved fears, anxiety or spiritual passage.
Include the family in decision making,
emphasizing the shared goals of care; support caregivers.
CCO‟s Symptom Management Pocket Guide: Delirium 1
Correct reversible factors – infection,
constipation, pain, withdrawal, drug toxicity.
Review medications; consider opioid
rotation to reverse opioid neurotoxicity; discontinue unnecessary drugs or prolong dosing interval for necessary drugs. Anticipate the need to change treatment
options if agitation develops, particularly in cases where patient, family and staff safety may become threatened.
Misinterpreting symptoms of agitation/
restlessness, moaning and/or grimacing as poorly controlled pain, with subsequent administration of more opioids, can potentially aggravate the symptom and cause opioid neurotoxicity.
Assessment
Ongoing comprehensive assessment is the
foundation of effective management of delirium and restlessness including interview, physical assessment, medication review, medical and surgical review, psychosocial review, review of physical environment and appropriate diagnostics.
Delirium may interfere with optimal pain
and symptom expression (self-reporting), assessment and management.
In situations where a patient is not able to
complete an assessment by self reporting, then the health professional and/or the caregiver may act as a surrogate.
2 CCO‟s Symptom Management Pocket Guide: Delirium
Diagnosis
Identifying the underlying etiology of the
delirium or restlessness is essential in determining the interventions required.
The causes of delirium are usually multi-
factorial (See table below, adapted from Capital Health).
Determining the underlying etiology,
education/reassuring the patient/family and treating the symptoms should occur simultaneously.
D Drugs, drugs, drugs, dehydration, depression
Electrolyte, endocrine dysfunction (thyroid, adrenal),
E ETOH (alcohol) and/or drug use, abuse or
L Liver failure
I Infection (urinary tract infection, pneumonia, sepsis)
R Respiratory problems (hypoxia), retention of urine or
stool (constipation)
I Increased intracranial pressure;
U Uremia (renal failure), under treated pain
Metabolic disease, metastasis to brain, medication
M errors/omissions, malnutrition (thiamine, folate or
Non-Pharmacological Treatment
Report hallucinations that become
Instruct the family to provide gentle,
repeated reassurance and avoid arguing with the patient.
CCO‟s Symptom Management Pocket Guide: Delirium 3
Watch for the "sun downing" effect
(nocturnal confusion) , as it may be the first symptom of early delirium.
Provide a calm, quiet environment and
help the patient reorient to time, place and person (visible clock, calendar, well known or familiar objects).
Presence of a well known family
member is preferred.
Provide a well lit, quiet environment.
Provide night light.
To prevent over-stimulation, keep
visitors to a minimum, and minimize staff changes and room changes.
Correct reversible factors – dehydration,
nutrition, alteration in visual or auditory acuity (provide aids), sleep deprivation.
Avoid the use of physical restraints and
other impediments to ambulation. Avoid catheterization unless urinary retention is present.
Encourage activity if patient is
physically able.
When mildly restless provide
observation and relaxation techniques (massage, tub baths, gentle music) as applicable.
Encourage the family to be present in a
4 CCO‟s Symptom Management Pocket Guide: Delirium
Pharmacological Treatment
Review medications; consider opioid rotation
to reverse opioid neurotoxicity
Consider psychotropic drugs for patients
developing "sun downing" effect (confusion in the evening).
Anticipate the need to change treatment
options if agitation develops – particularly in cases where patient, family and staff safety may become threatened.
Benzodiazepines may paradoxically excite
some patients and should be avoided unless the source of delirium is alcohol or sedative drug withdrawal, or when severe agitation is not controlled by the neuroleptic.
If patient has known or suspected brain
metastases a trial of corticosteroids is worthwhile. o Dexamethasone 16 - 32 mg po daily in the
morning may be used (Suggestion is based on expert opinion and doses may vary from region to region).
Misinterpreting symptoms of agitation/
restlessness, moaning and/or grimacing as poorly controlled pain, with subsequent administration of more opioids, can potentially aggravate the symptom and cause opioid neurotoxicity.
Titrate starting dose to optimal effect.
CCO‟s Symptom Management Pocket Guide: Delirium 5
Mild Delirium
Orient patient as per non-pharmacological
recommendations.
Pharmacological
Haloperidol is the gold standard for
management of delirium.
If titration with haloperidol is not effective
consider using methotrimeprazine.
Haloperidol 0.5-1 mg po / subcut bid-tid Alternate agents:
o Risperidone 0.5-1 mg po bid o Olanzapine 2.5–15 mg po daily o Quetiapine fumarate 50-100 mg po bid o Methotrimeprazine 5-12.5 mg po OR
6.25-12.5 mg subcut q4-6h prn
o Chlorpromazine 25-50 mg po q4-6h prn
Moderate Delirium
Pharmacological
Haloperidol 0.5-2 mg subcut q1h prn until
episode under control; may require a starting dose of 5 mg subcut
Alternate agents:
o Risperidone 0.5-1 mg po bid o Olanzapine 2.5-15 mg po daily o Quetiapine fumarate 50-100 mg po bid
Benzodiazepines may paradoxically excite some
patients and should be avoided unless the source
6 CCO‟s Symptom Management Pocket Guide: Delirium
of delirium is alcohol or sedative drug withdrawal, or when severe agitation is not controlled by the neuroleptic
Severe Delirium
Titrate starting dose(s) to optimal effect.
If agitation is refractory to high doses of
neuroleptics, (as outlined in moderate delirium) consider adding lorazepam 0.5-2 mg subcut q4-6h prn or midazolam 2.5-5 mg subcut q1-2h prn in conjunction with the neuroleptic.
Alternate agents to consider:
o Methotrimeprazine 12.5–25 mg subcut
q8-12h and q1h prn OR
o Chlorpromazine 25-50 mg po/subcut
If above not effective consider:
o Haloperidol 10 mg subcut. Typically,
in palliative care the maximum dose of haloperidol is 20 mg/day OR
o Methotrimeprazine 25-50 mg subcut
q6-8h and q1h prn.
Adverse Effects of Medications
Used to Treat Delirium
Extrapyramidal side effects (EPS) are
common adverse events of neuroleptics, with the newer atypical neuroleptics having a lower risk of EPS than the older typical neuroleptics.
CCO‟s Symptom Management Pocket Guide: Delirium 7
Potentially all dopamine antagonists can
cause EPS, to varying degrees, due to the D2 central antagonist actions.
Manifestations of EPS are usually dose
dependent. Extrapyramidal side effects may include: acute dystonia, akathisia, and Parkinson-like signs/symptoms.
Akathisia and acute dystonias tend to
resolve with discontinuation of the offending drug.
For the treatment of mild cases one should
consider discontinuation of the drug or switching to a less antidopaminergic agent if possible.
If pharmacologic management is needed,
then consider benztropine (1st line) 1-2 mg po/subcut bid (or 2mg IM/IV for acute dystonic reactions). Alternative medications include biperiden 2 mg po bid or diphenhydramine 25-50 mg po/subcut bid to qid (or 25-50 mg IV/IM for acute dystonia).
8 CCO‟s Symptom Management Pocket Guide: Delirium
Selected References
1) Fraser Health. Hospice Palliative Care Program
Symptom Guidelines: Delirium/Restlessness. Fraser Health Website, 2006. Accessed: August 2008. Available from:
2) Common Questions by Capital Health, Regional
Palliative Care Program Edmonton, 3rd Edition, 2006, Pg. 60.
3) Adapted with permission from the National
Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology Palliative Care. V.2.2011. Website:
For full references and more information please refer to
Disclaimer:
Care has been taken by Cancer Care Ontario‟s
Symptom Management Group in the preparation of the
information contained in this pocket guide.
Nonetheless, any person seeking to apply or consult the
pocket guide is expected to use independent clinical judgment
and skills in the context of individual clinical circumstances
or seek out the supervision of a qualified specialist clinician.
CCO makes no representation or warranties of any kind
whatsoever regarding their content or use or application and
disclaims any responsibility for their application or use in any
CCO‟s Symptom Management Pocket Guide: Delirium 9
10 CCO‟s Symptom Management Pocket Guide: Delirium
Non-Pharmacological treatment… 12
Pharmacological treatment …….……. 13
Mild Dyspnea …………………….……………. 13
Moderate Dyspnea …………….………… 15
Severe Dyspnea ……………………….……. 17
Oxygen Cost Diagram………………………. 22
Selected References ………………………. 23
Because dyspnea is subjective, the patient‟s
self report of symptoms should be acknowledged and accepted.
Identify and treat common exacerbating
medical conditions underlying dyspnea or shortness of breath, e.g. COPD, CHF, pneumonia (link to table in guide).
Evaluate impact of anxiety and fear on
dyspnea and treat appropriately.
Use Edmonton Symptom Assessment
System (ESAS) and Oxygen Cost
Diagram (OCD) (See OCD) to measure
outcome.
CCO‟s Symptom Management Pocket Guide: Dyspnea 11
Non-Pharmacological Treatments
Ambient air flow can be achieved by
opening a window, using a fan, or administering air through nasal prongs.
Cool temperatures can be applied to the
brow or upper cheek bones by applying a cool cloth or opening a window to let cooler air in.
A program of cognitive behavioural
interventions involving the following 6
interventions for a time period of 3 to 8
weeks is recommended:
1) Assessment of breathlessness – what
improves and what worsens it
2) Provision of information and support
for patients and families in the management of breathlessness
3) Exploration of the significance of
breathlessness with patients, their disease, and their future
4) Instruction on breathing control,
relaxation and distraction techniques and breathing exercises
5) Goal setting to enhance breathing and
relaxation techniques as well as to enhance function, enable participation in social activities and develop coping skills
6) Identification of early signs of
problems that need medical or pharmacotherapy intervention
These suggestions should be taught as preventative strategies, when patients are not dyspneic, and regular practice should be encouraged.
12 CCO‟s Symptom Management Pocket Guide: Dyspnea
Pharmacological Treatments
Mild Dyspnea ESAS 1 to 3
Supplemental oxygen is recommended for
hypoxic patients experiencing dyspnea.
Supplemental oxygen is not recommended
for non-hypoxic, dyspneic patients.
Non-hypoxic Patients (>90% O2 saturation) 1
For patients with PPS 100% - 10%:
Use a fan or humidified ambient air via nasal
prongs (as per patient preference and
availability). This is not covered by the Ontario
Ministry of Health and Long-Term Care
(MOHLTC)
If effective and tolerated, then utilize one or
If not effective or not tolerated, consider a
trial of humidified, supplemental oxygen via nasal prongs – assess benefits over a few days and discontinue if no benefit reported for dyspnea (covered by MOHLTC on the Home Oxygen program for up to 3 months if the "palliative care" indication is used).
1 ≤88% oxygen saturation at rest or on exertion is the
threshold for MOHLTC approval of funding for home
oxygen for palliative care patients beyond 3 months; for
some patients ≤90% oxygen saturation may be a more
appropriate threshold for introducing home oxygen therapy.
CCO‟s Symptom Management Pocket Guide: Dyspnea 13
Hypoxic Patients (≤90% O2 saturation at rest
or on exertion)
For Patients with PPS 100% - 10%:
Use humidified, supplemental oxygen via nasal
prongs, continuously or as-needed, at flow rates
between 1 and 7 litres per minute, aiming for
oxygen saturations over 90% or improvement in
dyspnea at tolerated flow rates.
Continue this therapy if it is effective at
improving dyspnea and is tolerated.
If dyspnea and low oxygen saturation persist despite maximum-tolerated flow of humidified, oxygen by nasal prongs, consider offering a trial of supplemental oxygen by oxymizer (nasal cannulae with reservoir), ventimask or non-rebreathing mask to deliver a more predictable fraction of inspired oxygen to the lungs. If this is not tolerated, the patient can return to the best-tolerated flow of humidified oxygen by nasal prongs or discontinue supplemental oxygen altogether.
Systemic opioids, by the oral or
parenteral routes, can be used to manage
dyspnea in advanced cancer patients.
For patients with PPS 100-10%: Other pharmacological treatments are not generally needed for patients with mild dyspnea, regardless of their PPS; however, systemic opioids (oral or parenteral) may be considered if non-pharmacological approaches result in inadequate relief of dyspnea. Consider systemic opioids for mild,
continuous dyspnea, not for dyspnea that is mild and intermittent (eg. on exertion) since
14 CCO‟s Symptom Management Pocket Guide: Dyspnea
any benefit is limited by the time to onset of effect.
If systemic opioids are considered, weigh
their potential risks and benefits and reassess the severity of the dyspnea and the effect the dyspnea has on the patient‟s function.
If the patient is already taking a systemic
opioid for another indication, such as pain o titrate the dose of the same opioid, if it is
well-tolerated, to improve the dyspnea
o switch to an alternate opioid, if the
current opioid is not tolerated, and titrate it to improve the dyspnea
If the patient is opioid naïve, introduce an
opioid to treat the dyspnea.
Properly titrated, systemic opioids do not
produce respiratory depression.
Moderate Dyspnea ESAS 4 to 6
For Patients with PPS 100% - 10%:
Non Opioids
May use benzodiazepines for anxiety. There is no evidence for the use of systemic
corticosteroids
Systemic Opioids
For opioid-naïve patients:
Morphine (or equivalent dose of alternate
immediate-release opioid) 5mg po q4h
CCO‟s Symptom Management Pocket Guide: Dyspnea 15
regularly and 2.5mg po q2h PRN for breakthrough dyspnea
If the oral route is not available or reliable,
morphine 3 mg subcut q4h regularly and 1.5
mg subcut q1h PRN for breakthrough
dyspnea.
For patients already taking systemic
opioids:
Increase the patient‟s regular dose by 25%,
guided by the total breakthrough doses used in the previous 24 hours
The breakthrough dose is 10% of the total
24-hour regular opioid dose, using the same opioid by the same route.
o oral breakthrough doses q2 hrs as needed o subcutaneous breakthrough doses q1hr as
needed, due to more rapid peak effect.
Do not use nebulized opioids, nebulized
furosemide, nebulized lidocaine or benzodiazepines.
For Patients with PPS 100% - 20%
If patient has or may have COPD, consider a
5-day trial of a corticosteroid
o Dexamethasone 8 mg/day po or subcut or
o Prednisone 50 mg/day po o Discontinue corticosteroid if there is no
obvious benefit after 5 days
If the patient does not have COPD, but has
known or suspected lung involvement by the cancer, weigh the risks before commencing a 5-day trial
o Other potential benefits, such as for
appetite stimulation or pain management, may justify a 5-day trial of a corticosteroid
16 CCO‟s Symptom Management Pocket Guide: Dyspnea
Do not start prophylactic gastric mucosal
protection therapy during a 5-day trial of a corticosteroid, but consider such therapy if the corticosteroid is continued past the trial
Prochlorperazine is not recommended as a
therapy for managing dyspnea.
No comparative trials are available to support
or refute the use of other phenothiazines, such as chlorpromazine and methotrimeprazine.
For Patients with PPS 30% - 10%:
Consider a trial of chlorpromazine or
methotrimeprazine, if dyspnea persists despite other therapies
o Methotrimeprazine 2.5-10 mg po or subcut
q6-8h regularly or as needed
o Chlorpromazine 7.5-25 mg po q6-8h
regularly or as needed
Anxiety, nausea or agitation, may justify a
trial of chlorpromazine or methotrimeprazine
Severe Dyspnea ESAS 7 to 10
For Patients with PPS 100% - 10%:
Systemic Opioids
For opioid-naïve patients:
Give a subcut bolus of morphine 2.5 mg (or
an equivalent dose of an alternate opioid). o If tolerated, repeat dose every 30 minutes if
o Consider doubling dose if 2 doses fail to
produce an adequate reduction in dyspnea and are tolerated
CCO‟s Symptom Management Pocket Guide: Dyspnea 17
o Monitor the patient‟s respiratory rate
closely, since the time to peak effect of a sc dose of morphine may be longer than 30 minutes
If intravenous access is available, consider
giving an IV bolus of morphine 2.5 mg (or an equivalent dose of an alternate opioid) to achieve a more rapid effect.
o If tolerated, repeat dose every 30 minutes if
o Consider doubling dose if 2 doses fail to
produce an adequate reduction in dyspnea and are tolerated
o Monitor the patient‟s respiratory rate
closely, since IV boluses of morphine result in faster and higher peak effects.
Start a regular dose of an immediate-release
opioid, guided by the bolus doses used
o For the breakthrough opioid dose, consider
using the subcut route initially for severe dyspnea until the symptom comes under control.
For patients already taking systemic opioids:
Follow the same suggestions as above for
opioid naïve patients, with the following changes.
o Give a subcut bolus of the patient‟s current
opioid using a dose equal to 10% of the regular, 24-hour, parenteral-dose-equivalent of the patient‟s current opioid (a parenteral dose is equivalent to half the oral dose)
o Consider giving an IV bolus of the
patient‟s current opioid, using a dose equal to 10% of the regular, 24-hour, parenteral-
18 CCO‟s Symptom Management Pocket Guide: Dyspnea
dose-equivalent of the patient‟s current opioid
o Increase the regular opioid dose by 25%,
guided by the bolus doses used
Phenothiazines
Consider a trial of chlorpromazine or
methotrimeprazine, if severe dyspnea persists despite other therapies.
Methotrimeprazine 2.5-10 mg po or subcut
q6-8h regularly or as needed
Chlorpromazine 7.5-25 mg po or IV q6-8h
regularly or as needed
Consider benzodiazepine for co-existing
CCO‟s Symptom Management Pocket Guide: Dyspnea 19
Titration Guide
General principles:
1.
Calculate the total opioid dose taken by the patient in
24 h (regular q4h dose x 6 PLUS the total number of
breakthrough doses given x breakthrough dose).
Divide this 24 h total by 6 for the equivalent q4h dose.
Divide the newly calculated q4h dose by 2 for the breakthrough dose.
Use clinical judgment regarding symptom control as to whether to round up or down the obtained result (both breakthrough and regular dosing). Remember to consider available doses (in the case of PO medications especially).
If the patient is very symptomatic, a review of how many breakthrough doses have been given in the past few hours might be more representative of his/her needs.
Example:
A patient is ordered morphine 20 mg q4h PO and 10 mg
PO q2h PRN, and has taken 3 breakthrough doses in the
past 24 h.
1.
Add up the amount of morphine taken in the past 24 h:
6 x 20 mg of regular dosing, plus 3 x 10 mg PRN doses equals a total of 150 mg morphine in 24 h
Divide this total by 6 to obtain the new q4h dose:
150 divided by 6 = 25 mg q4h
Divide the newly calculated q4h dose by 2 to obtain the new breakthrough dose: 25 mg divided by 2 = 12.5 mg q1 - 2h PRN
If this dose provided reasonable symptom control, then order 25 mg PO q4h, with 12.5 mg PO q1 - 2h PRN. (It would also be reasonable to order 10 mg or 15 mg PO q2h for breakthrough.)
20 CCO‟s Symptom Management Pocket Guide: Dyspnea
Conversion Guide
(To convert from long-acting preparations to short-
acting preparations)
General principles in converting from sustained
release to immediate release preparations (for the
same drug):
1. Add up the total amount of opioid used in the past
24 h, including breakthrough dosing.
2. Divide this total by 6 to obtain equivalent q4h
3. Divide the q4h dose by 2 to obtain breakthrough
4. Use clinical judgment to adjust this dose up or
down depending on symptom control.
5. Consider available tablet sizes when calculating
Example:
A patient is ordered a sustained release morphine
preparation at a dose of 60 mg PO q12h, with 20 mg
PO q4h for breakthrough, and has taken 4
breakthrough doses in 24 h.
1. Add up the amount of opioid taken in 24 h: 2 x 60
mg of sustained release morphine plus 4 x 20 mg of breakthrough is 200 mg of morphine in 24 h
2. Divide this total by 6 to obtain the equivalent q4h
dosing: 200 divided by 6 is approximately 33 mg PO q4h
3. Divide this q4h dose by 2 for the breakthrough
dose 33 mg divided by 2 is 16.5 mg
If the patient had reasonable symptom control with the previous regimen, then a reasonable order would be: 30 mg PO q4h and 15 mg q1 - 2h PO PRN
CCO‟s Symptom Management Pocket Guide: Dyspnea 21
Oxygen Cost Diagram (OCD)
The Oxygen Cost Diagram is a 100 mm vertical lines along
which every day activities are placed which correspond to
different activity levels and oxygen cost. The activities
range from "brisk walking uphill" to "sleeping". Patients are
asked to mark the activity that will make them breathless.
McGavin et al found that the patient‟s ratings of their
breathlessness with this scale were correlated r = 0.68
(p<0.001) with the 12 minute walking test. (McGavin et al,
1978) Others have found that the OCD correlated
significantly with lung function and respiratory muscle
strength. (Mahler et al, 1988)
EQUIANALGESIC CONVERSION TABLE
12:1 (PO codeine
oxycodone to PO morphine)
hydromorphone to PO morphine)
22 CCO‟s Symptom Management Pocket Guide: Dyspnea
Selected References
1) Fraser Health (2009) „Hospice Palliative Care
Program Symptom Guidelines: Dyspnea‟ Fraser Health Website [Internet]. Available from: (Accessed: 23 July 2009).
2) Oncology Nursing Society (2008) „Putting
Evidence Into Practice: Evidence-Based Interventions for Cancer-Related Dyspnea‟ Oncology Nursing Society Website [Internet]. Available from:
3) Viola, R., Kiteley, C., Lloyd, N., Mackay, J. A.,
Wilson, J., Wong, R. and the Supportive Care Guidelines Group (2006) „The Management of Dyspnea in Cancer Patients: A Clinical Practice Guideline‟ Program in Evidence Based Care Series 13-5, Cancer Care Ontario.
For full references and more information please refer t
Disclaimer:
Care has been taken by Cancer Care Ontario‟s
Symptom Management Group in the preparation of the
information contained in this pocket guide.
Nonetheless, any person seeking to apply or consult the
pocket guide is expected to use independent clinical judgment
and skills in the context of individual clinical circumstances
or seek out the supervision of a qualified specialist clinician.
CCO makes no representation or warranties of any kind
whatsoever regarding their content or use or application and
disclaims any responsibility for their application or use in any
CCO‟s Symptom Management Pocket Guide: Dyspnea 23
Nausea & Vomiting
Assessment ………………………………… 25
Diagnosis ……………………………………. 25
Non-Pharmacological treatment. 26
Pharmacological Treatment ……… 28
Selected References …………………. 33
Assessment
Comprehensive assessment includes: interview, physical assessment, nutrition assessment, medication review, medical and surgical review, psychosocial and physical environment review and appropriate diagnostics.
Diagnosis
Nausea and vomiting is common and
has multiple etiologies, several of which
may be present at the same time, hence
identifying the underlying causes is
essential.
CCO‟s Symptom Management Pocket Guide: Nausea & Vomiting 25
Non-Pharmacological Treatments
Providing information and education is
recommended as it is fundamental to
enhance the patient and family's ability
to cope.
Consult with the inter-professional team
members (e.g., social worker, spiritual practitioner, physiotherapist, occupational therapist, counselor for psychosocial care and anxiety reduction).
Explain to the patient/family what is understood about the multiple triggers of nausea and/or vomiting and that it may take a number of strategies to make a difference
Consult with a Clinical Dietitian and have
them provide dietary/nutritional advice
Limit spicy, fatty and excessively salty or
sweet foods, foods with strong odours and foods not well tolerated.
Use small, frequent, bland meals and
snacks throughout the day. Suggest small amounts of food every few hours. (Hunger can make feelings of nausea stronger).
Hard candies, such as peppermints or
lemon drops may be helpful.
Sip water and other fluids (fruit juice, flat
pop, sports drinks, broth and herbal teas such as ginger tea) and suck on ice chips, popsicles or frozen fruit. It is important to try and drink fluids throughout the day even when not feeling thirsty.
26 CCO‟s Symptom Management Pocket Guide: Nausea & Vomiting
Limit the use of caffeine, including colas
and other caffeinated soft drinks, such as coffee drinks, and tea (both hot and cold).
Reduce meal size when gastric distension is
Ingest liquids and solids separately. It is
often helpful to drink fluids after and/or in between meals.
Consume food/liquids cold or at room
temperature to decrease odours.
Sit upright or recline with head elevated for
30-60 minutes after meals.
If vomiting, limit all food and drink until
vomiting stops; wait for 30-60 minutes after vomiting, then initiate sips of clear fluid.
When clear fluids are tolerated, add dry
starchy foods (crackers, dry toast, dry cereal, pretzels)
When starchy foods are tolerated, increase
diet to include protein rich foods (eggs, chicken, fish) and lastly incorporate dairy products into the diet.
Environmental modification
(where possible)
Eliminate strong smells and sights.
Optimize oral hygiene, especially after episodes of vomiting. Rinse with ½ tsp baking soda, ½ tsp salt in 2 cups water.
Try rinsing mouth before eating to remove thick oral mucus and help clean and moisten mouth.
Wear loose clothing.
If possible try to create a peaceful eating place with a relaxed, calm atmosphere. A well ventilated room may also be helpful.
CCO‟s Symptom Management Pocket Guide: Nausea & Vomiting 27
Complementary Therapies
Acupuncture or acupressure points. Visualization, hypnosis, distraction.
Pharmacological Treatments
Selection of antiemetics should be based on the
most likely etiology of nausea and vomiting and site of action of medication.
Any unnecessary medications that may be
contributing to nausea and vomiting should be discontinued.
Constipation may be a factor contributing to
nausea and vomiting and requires treatment.
It is necessary to rule out bowel obstruction and
if present, appropriate treatment should be undertaken.
Choosing an antiemetic
Metoclopramide is recommended as the drug of
first choice to control chronic nausea/vomiting in patients with advanced cancer.
Titrate metoclopramide to maximum benefit and
tolerance. If not effective add/switch to another dopamine antagonist (e.g. haloperidol).
Domperidone may be substituted for patients
who can swallow medications and who have difficulties with extrapyramidal reactions.
Titrate antiemetics to their full dose, unless
patient develops undesirable effects, before adding another drug.
If nausea is not controlled with a specific
antiemetic within 48h, add another antiemetic
28 CCO‟s Symptom Management Pocket Guide: Nausea & Vomiting
from another group, but do not stop the initial agent.
Consider combinations but monitor overlapping
toxicities.
Use regular dosing of antiemetics if
experiencing constant nausea and/or vomiting.
For persistent nausea and/or vomiting
antiemetics should be prescribed on a
regular dosing schedule with a breakthrough
dose available.
All medications need to be individually titrated
to the smallest effective dose or until undesirable side effects occur.
Treatment and Management
1. Treat the cause, if possible. 2. Symptomatic management:
Fluid and electrolyte replacement as
Nutritional advice – consider making
patient NPO if obstructed or until emesis has resolved for several hours; if not obstructed, change diet as appropriate, depending on the cause of nausea.
Treat gastrointestinal obstruction
(may need to consider interventions such as nasogastric tube (NGT), venting gastrostomy tube (PEG), stents, ostomies, possible surgical resection).
Pharmacological treatment of
CCO‟s Symptom Management Pocket Guide: Nausea & Vomiting 29
Pharmacological Treatment of
Symptoms: Step 1
The choice of antiemetic depends on the cause and the receptors and neurotransmitters involved:
For delayed gastric emptying or
abdominal causes (excluding bowel
obstruction, see above):
o Metoclopramide 5-20 mg po/subcut/IV
q6h (or tid AC meals plus qhs); may be used q4h if needed; 40-100 mg/24 h subcut/IV continuous infusion.
o Alternative (if metoclopramide is not
well tolerated): domperidone 10mg TID to QID (The risk of serious abnormal heart rhythms or sudden death (cardiac arrest) may be higher in patients taking domperidone at doses greater than 30mg a day or in patients who are more than 60 years old).
For patients treated with palliative
o For symptoms that occur within 24
hours of administration of radiotherapy: Ondansetron 8 mg po/subcut/IV q8 – 24h; Granisetron 1 mg po q12h or 1 mg IV once daily
o For anticipatory nausea or vomiting:
lorazepam 1-2 mg po/sl/IV/subcut
o The above agents are also best given
prior to radiation for optimal effect.
For opioid-induced nausea:
o Metoclopramide 10-20 mg
po/subcut/IV q6h
30 CCO‟s Symptom Management Pocket Guide: Nausea & Vomiting
o Alternative: haloperidol 0.5-2.5 mg
For other chemical/metabolic causes:
o Haloperidol 0.5-2.5 mg po/subcut q12h o Alternative: metoclopramide 10-20 mg
po/subcut/IV q6h
For brain metastases:
o Dexamethasone 4-8 mg po/subcut/IV bid (0800
and 1300 h); for brain metastases that do not respond to dexamethasone or for leptomeningeal carcinomatosis:
o Haloperidol 1-2 mg po/subcut q12h
For vestibular causes:
o Scopolamine (transdermal patch) one or two 1.5
o Alternate: Dimenhydrinate 25-50 mg po/
If psychogenic factors play a role:
o Oxazepam 10 mg po tid or lorazepam 1-2 mg
po/sl/subcut/IV tid
o Psychological techniques (particularly for
chemotherapy-induced nausea and vomiting)
Pharmacological Treatment of Symptoms:
A combination of different antiemetics is required in approximately 30% of cases. Combination therapy is only beneficial if different neurotransmitters are targeted.
CCO‟s Symptom Management Pocket Guide: Nausea & Vomiting 31
If the response to monotherapy is inadequate, the following combinations may be considered:
Metoclopramide po/subcut/IV +
dexamethasone po/subcut/IV.
Haloperidol po/subcut + dexamethasone
Pharmacological Treatment of Symptoms:
If dexamethasone combined with either metoclopramide or haloperidol yields insufficient results, the following approaches may be considered:
Serotonin (5HT3) antagonists (ondansetron
4 - 8 mg po/subcut/IV q8-12h; granisetron 1 mg po q12h/1mg IV once daily; or dolasetron 100 mg po/IV once daily); in principle, combine with dexamethasone 4 mg po/subcut/IV once daily. Disadvantages of the serotonin antagonists: high costs; side effects include constipation, headaches.
Methotrimeprazine monotherapy using a
starting dose of 5 – 10 mg po q8h PRN or 6.25-12.5 mg subcut q8h PRN. Increase as needed to maximum of 25 mg per dose.
Olanzapine monotherapy 2.5 – 5 mg
po/sl/subcut once daily or bid.
Diphenhydramine may be used for the treatment of akathesias secondary to increased doses of metoclopramide.
32 CCO‟s Symptom Management Pocket Guide: Nausea & Vomiting
Selected References
1) Cancer Care Nova Scotia. Guidelines for the
Management of Nausea and Vomiting In Cancer Patients, Jan 2004.
2) Fraser Health. Hospice Palliative Care Program
Symptom Guidelines. Fraser Health, 2006.
3) Editorial Board Palliative Care: Practice
Guidelines. Nausea and vomiting. Utrecht, The Netherlands: Association of Comprehensive Cancer Centres (ACCC), National Guideline Clearinghouse, 2006.
4) Adapted with permission from the National
Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology Palliative Care. V.2.2011. Website:
For full references and more information please refer to
Disclaimer:
Care has been taken by Cancer Care Ontario‟s
Symptom Management Group in the preparation of the
information contained in this pocket guide.
Nonetheless, any person seeking to apply or consult the
pocket guide is expected to use independent clinical judgment
and skills in the context of individual clinical circumstances
or seek out the supervision of a qualified specialist clinician.
CCO makes no representation or warranties of any kind
whatsoever regarding their content or use or application and
disclaims any responsibility for their application or use in any
CCO‟s Symptom Management Pocket Guide: Nausea & Vomiting 33
Assessment ……………………………………… 35
Non-Pharmacological Treatment …. 37
Pharmacological Treatment …………. 38
Mild Pain ……………….………………………. 41
Moderate Pain ………………………………. 42
Severe Pain ……………….………………… 44
Severe Pain Crisis ………………………… 45
Conversion Ratios….……………………… 46
Selected References …….……………… 51
Assessment
Prior to treatment an accurate assessment
should be done to determine the cause(s), type(s) and severity of pain and its impact.
A comprehensive assessment of pain
should consider the following domains:
o physical effects/manifestations of pain o functional effects (interference with
activities of daily living)
o spiritual aspects o psychosocial factors (level of anxiety,
mood) cultural influences, fears, effects on interpersonal relationships, factors affecting pain tolerance.
CCO‟s Symptom Management Pocket Guide 35
Self assessment pain scales should be used
by patients with no cognitive impairment.
Observational pain rating scales should
be used in patients who cannot complete a self assessment scale.
The frequency of the review depends
upon the severity of the pain and associated distress.
General Principles of Cancer Pain
Assessment
1. Perform an adequate pain history. 2. Use tools valid for the patient‟s age
and cognitive abilities, with additional attention to the language needs of the patient (e.g., Brief Pain Inventory (BPI), Edmonton Symptom Assessment System (ESAS), Palliative Performance Scale (PPS)).
3. Record medications currently taken as
well as those used in the past, including efficacy and any adverse effect.
4. Classify the pain – nociceptive,
neuropathic or mixed?
5. Consider common cancer pain
syndromes while conducting the history and physical examination.
6. Assess for functional impairment and
the need for safety measures.
7. Incorporate a psychosocial evaluation
into the assessment, including determination of the patient‟s/family‟s goals of care
8. Use a pain diary to track the
effectiveness of therapies and evaluate changes in pain.
36 CCO‟s Symptom Management Pocket Guide: Pain
9. Review current diagnostic tests for clues
to the origin of the pain. Order a diagnostic test (e.g., MRI, CT, laboratory testing) when warranted for new pain or increasing pain, and only if it will contribute to the treatment plan.
10. Evaluate for the presence of other
symptoms, as pain is highly correlated with fatigue, constipation, mood disturbances, and other symptoms.
11. Assess for risk if opioids are being
Non-Pharmacological Treatment
Radiation Therapy
All patients with pain from bone
metastases which is proving difficult to control by pharmacological means should be referred to a radiation oncologist for consideration of external beam radiotherapy
Vertebroplasty
Vertebroplasty or percutaneous
cementoplasty should be considered in patients with pharmacologically difficult to control bone pain from malignant vertebral collapse or pelvic metastases.
Surgery
Removal of tumours or stabilization of
bones may remove localized pain.
CCO‟s Symptom Management Pocket Guide 37
Anesthetic Interventions
Interventions such as coeliac plexus
block and neuraxial opioids should be considered to improve pain control and quality of life in patients with difficult to control cancer pain.
Other Therapies
Consider role for physiotherapy or
occupational therapy
Complementary therapies (e.g. massage,
aromatherapy, music therapy, acupuncture, transcutaneous electrical nerve stimulation, reflexology, Reiki, hypnotherapy) may be considered.
Psycho-social-spiritual interventions
Psycho-social-spiritual interventions
(patient education, counseling, recreational activities, relaxation therapy imagery, social interaction, spiritual counseling) should be considered.
Pharmacological Treatment
General Principles in Using
Adjuvants
The type and cause of the pain will influence
the choice of adjuvant analgesic (e.g. nociceptive, neuropathic, bone metastases).
The choice of antidepressant or
anticonvulsant should be based on concomitant disease, drug therapy and drug side effects and interactions.
38 CCO‟s Symptom Management Pocket Guide: Pain
Patients with neuropathic pain should be
given either a tricyclic antidepressant (eg amitriptyline, desipramine, nortriptyline or imipramine) or an anticonvulsant (eg gabapentin or pregabalin) with careful monitoring for adverse effects.
Cannabinoids may have a role in refractory
pain, particularly refractory neuropathic pain.
General Principles in Using Opioids
Educate the patient and/or family about the use of opioids and the expected outcomes.
Anticipate adverse effects like sedation and educate patients about the fact that they will quickly tolerate most adverse effects except for constipation.
In opioid-naïve patients and the frail
elderly, start low and go slow with
titration. Transdermal fentanyl is not
recommended in opioid-naïve patients.
In patients already on opioids, titrate them fairly quickly to the point where they are getting adequate pain control without intolerable adverse effects.
Immediate release or sustained release products can both be used for titration and maintenance.
Give opioids regularly, around the clock for constant pain, not „as required‟.
Always prescribe breakthrough doses.
Prevent adverse effects e.g., for constipation prescribe laxatives right from the initiation of therapy and decide on a plan for the management of constipation.
CCO‟s Symptom Management Pocket Guide 39
Monitor patients closely as you are titrating opioids.
10. Use universal precautions where a risk for
abuse is identified.
11. Specialist pain or palliative care advice
should be considered for the appropriate choice, dosage and route of opioids in patients with reduced kidney function or in patients with difficult to control pain.
All patients with moderate to severe cancer
pain, regardless of etiology, should receive a trial of opioid analgesia.
In the presence of reduced kidney function
all opioids should be used with caution and at reduced doses and/or frequency.
Fentanyl, methadone and oxycodone are the
safest opioids of choice in patients with chronic kidney disease.
Methadone requires an experienced
Check for significant drug interactions
before prescribing any drug to a patient
on methadone.
When using a transmucosal fentanyl
formulation for breakthrough pain the effective dose should be found by upward titration independent of the regular opioid dose.
For those with stabilized severe pain and on
a stable opioid dose or those with swallowing difficulties or intractable nausea and vomiting, fentanyl transdermal patches may be appropriate.
40 CCO‟s Symptom Management Pocket Guide: Pain
Adverse Effects of Opioids
Many opioid-naïve patients will develop
nausea or vomiting when starting opioids, tolerance usually occurs within 5-10 days. Patients commencing an opioid for moderate to severe pain should have access to an antiemetic to be taken if required.
The majority of patients taking opioids will
develop constipation. Little or no tolerance develops. The commonest prophylactic treatment for preventing opioid-induced constipation is a combination of stimulant (senna or bisacodyl)and osmotic laxatives (lactulose or PEG 3350)
Patient Education should include:
Taking routine and breakthrough analgesics,
adverse effect management, non pharmacologic measures that can be used in conjunction with pharmacologic treatment.
Mild Pain ESAS 1 to 3
TREATMENT WITH NON-OPIOIDS
Acetaminophen and NSAIDS
Acetaminophen and NSAIDS including
COX-2 inhibitors should be considered at the lowest effective dose.
The need for ongoing or long term
treatment should be reviewed periodically, if no significant response in one week drugs should be stopped.
CCO‟s Symptom Management Pocket Guide 41
Long term use of NSAIDs should require
gastric mucosa protection.
Bisphosphonates
There is insufficient evidence to recommend
bisphosphonates for first line therapy for pain management.
TREATMENT WITH OPIOIDS
For mild to moderate pain, weak opioids
such as codeine or tramadol could be given in combination with a non-opioid analgesic.
If pain is not controlled with these
combinations go to "Moderate Pain" re: initiation and treatment with opioids
Moderate Pain ESAS 4 to 6
TREATMENT WITH OPIOIDS
If the person is opioid naïve:
o Morphine starting dose is usually 5mg
Q4h with 2.5-5mg Q1H PRN for breakthrough pain. For elderly or debilitated patients consider a starting dose of 2.5mg Q4h.
o Hydromorphone starting dose is 1mg
Q4h with 0.5-1mg Q1h PRN for breakthrough pain. For elderly or debilitated patients consider a starting dose of 0.5 mg Q4h.
42 CCO‟s Symptom Management Pocket Guide: Pain
o Oxycodone starting dose is 2.5 mg or
one half tablet Q4H with 2.5 mg or one half tablet Q2H PRN for breakthrough. (The lowest dose oxycodone tablets available, either in combination with acetaminophen or alone, contain 5mg of oxycodone, equivalent to 5-10mg of morphine).
If the person is taking an opioid:
o As an immediate release preparation
with q4h dosing, increase the regular and breakthrough doses by 25%.
o As a sustained release opioid, increase
this dose by 25%. Change the breakthrough dose to 10% of the regular 24h dose, either q1-2h PRN PO or q30 min PRN subcut.
o Patients with stable pain and analgesic
usage, receiving oral morphine, oxycodone or hydromorphone should have the drug converted to a sustained or controlled release formulation given q12h for ease of administration. The short acting breakthrough dose is usually 10% of the total daily dose.
o The frequency of breakthrough doses
for oral opioids is Q1-2h PRN. After conversion to a long acting preparation, if pain is not well controlled, reassess the patient and consider why multiple breakthrough doses are being used and the effectiveness of the breakthrough doses.
CCO‟s Symptom Management Pocket Guide 43
o If indicated after proper assessment,
the daily dose can be titrated by adding 20 to 30% of the breakthrough doses used in the preceding 24 hrs to the daily sustained release formulation.
Make frequent assessments and adjustments to the opioid dose until the pain is better controlled.
Severe Pain ESAS 7 to 10
TREATMENT WITH STRONG OPIOIDS
If the person is opioid naïve: Oral:
Morphine 5-10 mg PO q4h and 5mg PO q1h
PRN OR hydromorphine 1.0-2.0 mg PO q4h
and 1.0 mg PO q1h PRN OR Subcutaneous:
Morphine 2.5 - 5 mg subcut q4h & 2.5 mg
subcut q30min PRN OR hydromorphone 0.5
- 1.0 mg subcut q4h & 0.5 mg subcut
q30min PRN.
If the patient is taking an opioid with q4h
dosing, increase the regular and breakthrough doses by 25%. Change frequency of the breakthrough to q1h PRN if PO and q30min PRN if subcut.
If the patient is taking a sustained release
opioid, increase this dose by 25%. Change the breakthrough dose to 10-15% of the regular 24h dose, either q1h PRN PO or q30 min PRN subcut.
Titrate the dose every 24h to reflect the
previous 24h total dose received
If unmanageable opioid-limiting adverse
effects are present (e.g. nausea, drowsiness,
44 CCO‟s Symptom Management Pocket Guide: Pain
myoclonus), consider switching to another opioid and re-titrate or consult palliative care.
For patients with severe uncontrolled pain
consider switching back to an equivalent daily dose of immediate release morphine to allow more rapid titration of dose or switch to a sc preparation/infusion.
Meperidine and pentazocine should
generally not be used in cancer patients with chronic or acute pain.
If there is difficulty getting the pain under
control consider a consultation to palliative care.
Severe Pain Crisis
1. A severe pain crisis requires prompt
use of analgesics, adjuvant therapies, reassurance and a calm atmosphere.
2. Consider a consultation to palliative
care or a cancer pain specialist.
3. If IV access is present, and the person
is opioid naïve give stat morphine 5-
10 mg IV q10min until pain is
relieved; if the person is on opioids
give the po PRN dose IV q10min until
pain is relieved. Monitor carefully.
4. If no IV access available, and the
person is opioid naïve give stat
morphine 5-10 mg subcut q20-30min
until pain is relieved; if the person is
on opioids give the po PRN dose
subcut q20-30min until pain is
relieved.
CCO‟s Symptom Management Pocket Guide 45
5. Titrate dose by 25% every 1 - 2 doses
until pain is relieved.
6. When pain is controlled: If the patient
is taking a sustained release opioid
increase this dose by 25% and change
to q4h dosing po or subcut. Do Not try
to manage a severe pain crisis with a
long-acting opioid. Change the
breakthrough dose to half of the
regular dose, either q1h PRN PO or
q30 min PRN subcut.
CONVERSION RATIOS
It should be noted that these conversion
ratios, based on available evidence, are conservative in the direction specified; if converting in the reverse direction, a reduction in dose of one third should be used following conversion, or specialist advice sought.
46 CCO‟s Symptom Management Pocket Guide: Pain
Approximate Equivalent
Parenteral
Fentanyl
Morphine
Oxycodone
Pethidine
Sufentanil
Tramadol
Methadone
a. From single dose studies using immediate-release dosage
forms. These approximate analgesic equivalences should be used only as a guide for estimating equivalent doses when switching from one opioid to another. Additional references should be consulted to verify appropriate dosing of individual agents.
b. Route of administration not applicable. c. With repeated dosing. d. Tramadol's precise analgesic potency relative to morphine
is not established. Consult the product monograph for dosing recommendations.
e. For methadone, se
CCO‟s Symptom Management Pocket Guide 47
Conversion doses from oral morphine to
transdermal fentanyl
Oral 24-hour
Transdermal Fentanyl
morphine (mg/day)
37 (if available, otherwise 25)
62 (if available, otherwise 50)
48 CCO‟s Symptom Management Pocket Guide: Pain
TITRATION GUIDE
General principles:
6.
Calculate the total opioid dose taken by the patient
in 24 h (regular q4h dose x 6 PLUS the total
number of breakthrough doses given x
breakthrough dose).
Divide this 24 h total by 6 for the equivalent q4h dose.
Divide the newly calculated q4h dose by 2 for the breakthrough dose.
Use clinical judgment regarding symptom control as to whether to round up or down the obtained result (both breakthrough and regular dosing). Remember to consider available dosage forms (in the case of PO medications especially).
10. If the patient is very symptomatic a review of how
many breakthrough doses have been given in the past few hours might be more representative of his/her needs.
Example:
A patient is ordered morphine 20 mg q4h PO and 10
mg PO q2h PRN, and has taken 3 breakthrough
doses in the past 24 h.
1.
Add up the amount of morphine taken in the past 24 h:
6 x 20 mg of regular dosing, plus 3 x 10 mg PRN doses equals a total of 150 mg morphine in 24 hours
Divide this total by 6 to obtain the new q4h dose:
150 divided by 6 = 25 mg q4h
Divide the newly calculated q4h dose by 2 to obtain the new breakthrough dose: 25 mg divided by 2 = 12.5 mg q1 - 2h PRN
If this dose provided reasonable symptom control, then order 25 mg PO q4h, with 12.5 mg PO q1 - 2h PRN. (It would also be reasonable to order 10 mg or 15 mg PO q2h for breakthrough.)
CCO‟s Symptom Management Pocket Guide 49
CONVERSION GUIDE
(To convert from long-acting preparations to
short-acting preparations)
General principles in converting from sustained
release to immediate release formulations (for the
same drug):
1. Add up the total amount of opioid used in the
past 24 h, including breakthrough dosing.
2. Divide this total by 6 to obtain equivalent q4h
3. Divide the q4h dose by 2 to obtain breakthrough
4. Use clinical judgment to adjust this dose up or
down depending on symptom control.
5. Consider available tablet sizes when calculating
Example:
A patient is ordered a long-acting morphine
preparation at a dose of 60 mg PO q12h, with 20
mg PO q4h for breakthrough, and has taken 4
breakthrough doses in 24 h.
1. Add up the amount of opioid taken in 24 h: 2 x
60 mg of long-acting morphine plus 4 x 20 mg of breakthrough is 200 mg of morphine in 24 h
2. Divide this total by 6 to obtain the equivalent q4h
dosing: 200 divided by 6 is approximately 33 mg PO q4h
3. Divide this q4h dose by 2 for the breakthrough
dose 33 mg divided by 2 is 16.5 mg
If the patient had reasonable symptom control with the previous regimen, then a reasonable order would be: 30 mg PO q4h and 15 mg q1 - 2h PO PRN
50 CCO‟s Symptom Management Pocket Guide: Pain
Selected References:
1. Scottish Intercollegiate Guidelines Network SIGN
106, „Control of Cancer Pain in Adults with Cancer: A National Clinical Guideline‟ November 2008.
For full references and more information please refer to
Disclaimer:
Care has been taken by Cancer Care Ontario‟s Symptom
Management Group in the preparation of the information
contained in this pocket guide.
Nonetheless, any person seeking to apply or consult the
guide to practice is expected to use independent clinical
judgment and skills in the context of individual clinical
circumstances or seek out the supervision of a qualified
specialist clinician.
CCO makes no representation or warranties of any kind
whatsoever regarding their content or use or application and
disclaims any responsibility for their application or use in
CCO‟s Symptom Management Pocket Guide 51
52 CCO‟s Symptom Management Pocket Guide
Loss of Appetite
Definition of Terms
Diagnosis
Assessment
Treatment
Pharmacological Treatment
Selected References
Definition of Terms
The first step in managing this symptom will be validation of Edmonton Symptom Assessment System (ESAS) score with patient. An understanding of primary cachexia and how it differs from anorexia is needed to establish whether you are dealing with anorexia, secondary cachexia or primary cachexia.
Definitions Anorexia is the loss of appetite or the desire to eat. Cancer Cachexia is a multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutrition support and leads to progressive functional impairment. Weight loss is evident. Losses associated with cancer cachexia are in excess of that explained by anorexia
CCO‟s Symptom Management Pocket Guide 53
alone; however anorexia can hasten the course of cachexia.
Secondary Cachexia: is characterized by potentially correctable causes that could explain the syndrome. Once identified, prompt intervention can greatly impact the patient‟s quality of life and overall
prognosis. Primary Cachexia should only be considered when all secondary causes have been identified and treated.
Sarcopenia is a condition characterized by loss of muscle mass and muscle strength. Patients presenting with loss of muscle mass, but no weight loss, no anorexia, and no measureable systemic inflammatory response may well be sarcopenic. Recent literature encourages the staging of primary cachexia to support patients and potentially improve the type and timing of treatment modalities (Figure 1).
54 CCO‟s Symptom Management Pocket Guide
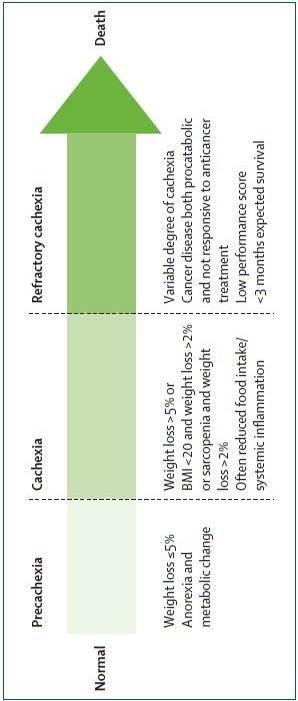
xia: hecac r . er
t e ighon ra opyr
ia
xehcac yra
s oegtaS : 1erugFi
CCO‟s Symptom Management Pocket Guide 55
Diagnosis
Establish whether loss of appetite is related to
treatment side effects (e.g. radiation therapy,
chemotherapy, or surgical treatment), other
medication and/ or psychosocial factors. If these
factors are not deemed to be causative then tumor
related factors may be at work and determination of
the physical vs. metabolic factors should be further
considered. See table 1 for causes of anorexia and
secondary cachexia.
Description
Factors secreted by tumour (e.g.
tumour necrosis factor/cachectin,
interleukin-6, lipid-mobilizing factor, proteolysis-inducing factor) Metabolic and hormonal
abnormalities (e.g. alterations in
carbohydrate, lipid and protein
utilization synthesis and breakdown)
Taste and smell abnormalities or
Fatigue / malaise and asthenia (cycle
can occur in which decreased intake
leads to lethargy and weakness,
leading to a further decrease in oral intake) Gut involvement (e.g. intraluminal
gastrointestinal malignancy, gut
atrophy, partial bowel obstruction,
decreased production of digestive secretions, decreased peristalsis, constipation) Malabsorption Syndrome (fats and
carbohydrates not metabolized/
56 CCO‟s Symptom Management Pocket Guide
Description
Infection (e.g. low grade sepsis)
Diarrhea (e.g. cytotoxic effects on
the gut mucosa/ radiation enteritis/
short bowel syndrome)
Taste and Smell abnormalities
Xerostomia (e.g. mucositis,
infection, poor hygiene,
dehydration, medication, taste bud alternation) Palliative gastrectomy
Systemic antineoplastic drugs (e.g.
chemotherapy, targeted therapy,
Antimicrobial agents
cide Antidepressants (e.g. selective
serotonin reuptake inhibitors such as
fluoxetine, sertraline, escitalopram,
paroxetine; atypicals such as bupropion)
Fear of eating because of possibility
of making symptoms worse (e.g.
pain, incontinence, diarrhea,
constipation) or because of certain beliefs that eating will make the
CCO‟s Symptom Management Pocket Guide 57
Description
cancer, symptoms, or health worse.
Lack of emotional support
Lack of functional
support/independence Lack of financial resources/support
The causes of primary cachexia are also tumour-
related causes of anorexia:
factors secreted by tumour (e.g. tumour necrosis factor/cachectin, interleukin-6, lipid-mobilizing factor, proteolysis-inducing factor) , and
metabolic and hormonal abnormalities (e.g. alterations in carbohydrate, lipid and protein utilization synthesis and breakdown).
Assessment
Ongoing comprehensive assessment is the foundation of effective anorexia and cachexia management. An in-depth assessment should include:
review of medical history with current medication(s),
review of treatment plan/effects and clinical goals of care,
weight and diet history,
physical assessment,
available laboratory investigations, and
review of psychosocial and physical environment.
Consider the following validated tools for further screening and in-depth assessment:
58 CCO‟s Symptom Management Pocket Guide
Malnutrition Screening Tool (MST)
Patient Generated Subjective Global Assessment (PG-SGA)
Percentage of weight loss over time evaluates malnutrition:
> or equal 5% loss of usual body weight in one month.
> or equal 7.5% loss of usual body weight in 3 months.
> or equal 10% loss of usual body weight in 6 months.
Non Pharmacological Treatment
Stage of disease, progression of disease and Palliative
Performance Scale (PPS), or functional status, should
be considered when determining goals of care and
treatment plans.
Psychosocial Strategies
Provide emotional support to patient and family.
Consider importance of food in the social context and impact on quality of life.
Consider cultural issues
Consider patient's accessibility to food.
Referral to other health care professionals where appropriate.
Nutrition Education Strategies
Provide nutrition-focused patient education for self-management early in symptom trajectory with a goal to improve or maintain nutritional and functional status via oral nutrition. •
Suggest eating small, frequent meals and choosing high energy, high protein foods. See Patient Education tools below.
Ensure adequate hydration, preferably through energy and protein containing liquids.
Suggest making mealtimes as relaxing and enjoyable as possible.
CCO‟s Symptom Management Pocket Guide 59
Suggest convenience foods, deli or take-out foods, Meals on Wheels® or catering services, Home Making services, or asking friends/family to help out.
Taking medication with a high calorie / protein fluid such as milkshakes or nutrition supplements can also increase nutritional intake. This should be reviewed by a dietitian and/or pharmacist because of potential drug/nutrient interaction(s).
Nutritional supplements, as recommended by a dietitian and/or pharmacist.
Refer to a registered dietitian. See section below.
Patient Education tools:
•
Healthy Eating Using High Energy, High Protein Foods
High Energy and High Protein Menu items
Food ideas to help with poor appetite
Increasing Fluid Intake
Suggestions for Increasing Calories and Protein
Eating Well When You Have Cancer
Canada‟s Food Guide
Exercise Strategies
Encourage exercise, as tolerated by patient. Walking fifteen minutes a day can help regulate appetite.
Patient should start the exercise regimen slowly, and gradually increase intensity.
Exercise can be initiated at most levels above PPS 30-40% but caution should be guiding principle, as well as presence of bony metastases and low blood counts.
60 CCO‟s Symptom Management Pocket Guide
Referral to a Registered Dietitian
Individualized dietary counseling has been shown to reduce incidence of anorexia and improve nutritional intake and body weight, as well as improve quality of life.
Non-Pharmacological Treatment specific to
Primary Cachexia: Refractory stage
Consider Palliative Performance Scale (PPS) scores, in conjunction with ESAS scores, to determine appropriateness and aggressiveness of interventions.
Assist families and caregivers to understand and accept benefits and limits of treatment interventions, and to look at alternate ways to nurture patient (oral care, massage, reading, conversing).
While underlying cause(s) may be evident, treatment may not be indicated.
Ice chips, small sips of beverages and good mouth care becomes norm.
Consider symbolic connection of food and eating with survival and life. Food may become a source of emotional distress experienced by both family and patient.
It is important to educate that a person may naturally stop eating and drinking as part of illness progression and dying process.
Focus should be on patient comfort and reducing patient and caregiver anxiety, as reversal of refractory cachexia is unlikely.
Recognize that discontinuation of nutrition is a value-laden issue. Consider consultation with
CCO‟s Symptom Management Pocket Guide 61
registered dietitian, spiritual counselor or bioethicist, to clarify clinical goals.
Referral to other health care professionals where appropriate.
Pharmacological Treatment
The following pharmacological treatments are
suggested to alleviate the symptom of loss of appetite
and may improve quality of life. They may affect
weight gain; however weight gain may be attributable
to water retention and/or fat, not muscle gain.
Appetite stimulants can be used in combination with or after failure of oral nutritional management.
Use of appetite stimulants is particularly warranted in patients with incurable disease. Appetite stimulants can be administered to patients with any type of tumour.
The optimal mode of administration for these products is not known.
Please refer to the drug table on next page.
62 CCO‟s Symptom Management Pocket Guide
can- g- h- i- cat- mu
Synthetic
Prokinetics
CCO‟s Symptom Management Pocket Guide 63
Selected References:
BC Cancer Agency. Nutritional Guidelines for Symptom Management: Anorexia [Internet]. 1996, updated 2005. [cited 21/12/2010] Available from:
Desport JC, Gory-Delabaere G, Blanc-Vincent MP, Bachmann P, Be´ al J, Benamouzig R, Colomb V, Kere D, Melchior JC, Nitenberg G, Raynard B, Schneider S, and Senesse P. FNCLCC Standards, Options and Recommendations for the use of appetite stimulants in oncology. British Journal of Cancer; 2000:89(Suppl 1):S98 – S100, doi:10.1038/sj.bjc.6601090.
Fraser Health Hospice Palliative Care Program. Symptom Guidelines: Nutrition and cachexia [Internet]. 2006. [cited 21/12/2010] Available from:
Fearon K, Strasser F, Anker S, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, Macdonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch P, Walsh D, Wilcock A, Kaasa S, & Baracos V. Definition and classification of cancer cachexia: an international consensus . The Lancet; 2011; 12: 489-495.
For full references, links to tools, and more
information please refer to CCO's Symptom
Management Guide-to-Practice: Loss of Appetite
document (www.cancercare.on.ca/symptools).
64 CCO‟s Symptom Management Pocket Guide
CCO‟s Symptom Management Pocket Guide 65
66 CCO‟s Symptom Management Pocket Guide
Bowel Care
Assessment …………………………………. 67
Diagnosis ………………………………………. 68
Non-Pharmacological Treatment… 68
Pharmacological Treatment …….… 72
Selected References …………………. 79
Assessment
Obtaining a detailed history, including assessment of functional status and goals of care, is an important step in identifying etiologic factors and appropriate management strategies for constipation and diarrhea.
Physical assessment should include vital signs, functional ability, hydration status, cognitive status, abdominal exam, rectal exam and neurological exam if a spinal cord or cauda equine lesion is suspected. Consider abdominal x-rays if bowel obstruction or severe stool loading of the colon is suspected.
CCO‟s Symptom Management Pocket Guide 67
Diagnosis
Identifying the etiology of constipation and diarrhea is essential in determining the interventions required.
Non-Pharmacological Interventions
Constipation
Consider performance status, fluid intake,
diet, physical activity and lifestyle when
managing constipation.
General Education It is not necessary to have a bowel
movement every day. As long as stools are soft and easy to pass, every two days is generally adequate.
Avoid excessive straining.
In absence of oral intake, the body
continues to produce 1-2 ounces of stool per day.
PPS Stable, Transitional and End of Life (30-
100%)
Fluid Intake
Encourage intake of fluids throughout the
Aim for fluid intake between 1500-2000
For patients who are not able to drink large
volumes, encourage sips throughout the day.
Limit intake of caffeinated and alcoholic
beverages, as they may promote dehydration
68 CCO‟s Symptom Management Pocket Guide
Physical Activity Physical activity should be tailored to the
individual‟s physical ability, health condition and personal preference, to optimize adherence.
Frequency, intensity and duration of
exercise should be based on the patient‟s tolerance.
For PPS 60% and above, walking is
recommended (e.g., 15-20 minutes once or twice per day or 30-60 minutes daily, 3-5 times per week).
For PPS 30-50% exercises such as low
trunk rotation and single leg lifts, for up to 15 to 20 minutes per day, are encouraged, if able.
Personal Considerations Provide privacy during toileting. Attempts at defecating should be made 30
to 60 minutes following ingestion of a meal, to take advantage of the gastro-colic reflex.
PPS Stable and Transitional (40-100%)
Diet
The following dietary recommendations are
not applicable if bowel narrowing or obstruction is suspected.
Dietary fibre intake should be gradually
increased once the patient has a consistent fluid intake of at least 1500 ml per 24 hours.
Aim for dietary fibre intake of at least 25
grams per day (choose 7-10 servings per day of whole fruits and vegetables, instead of juices, choose 6-8 servings of grain
CCO‟s Symptom Management Pocket Guide 69
products per day, 100% whole grain breads and high fibre cereals, plant proteins daily as part of the 2-3 servings of meats and alternatives).
Fruit laxative (125 ml pitted dates, 310 ml
prune nectar, 125 ml figs, 200 ml raisins,125 ml pitted prunes).
Consult with a dietitian for specific
nutritional advice regarding fibre intake.
Personal Considerations Walking to the toilet, if possible, is
recommended. If walking is difficult, use a bedside commode.
Assuming the squat position on the toilet
can facilitate the defecation process.
o Sitting with feet on a stool may help
with defecation.
PPS End of Life (10-30%)
Raising the head of the bed may facilitate
the defecation process.
Simulate the squat position by placing the
patient in the left-lateral decubitus position, bending the knees and moving the legs toward the abdomen.
PPS End of Life (10-20%)
For patients with PPS 10-20%, consider the
burdens and benefits of regular bowel care, using good clinical judgment when making recommendations.
Diarrhea
Consider performance status, diet, fluid
intake, quality of life and lifestyle when
managing diarrhea.
70 CCO‟s Symptom Management Pocket Guide
PPS Stable, Transitional and End of Life (30-
100%)
Diet
Eat small frequent meals.
Limit consumption of caffeine, fried,
greasy foods and foods high in lactose.
Avoid sorbitol containing foods (e.g.,
sugar-free gum and sugar-free candy).
Limit/avoid foods high in insoluble fiber
(e.g., wheat bran, fruit skins and root vegetable skins, nuts and seeds, dark leafy greens and legumes such as dried peas).
Include foods high in soluble fibre (barley,
potatoes, bananas and applesauce).
Avoid hyper-osmotic liquids (fruit drinks
and sodas). Dilute fruit juices with water.
Fluid Intake Parenteral hydration may be required for
Provide fluids orally, if dehydration is not
An oral rehydration solution can be
prepared by mixing 1/2 teaspoon salt and 6 level teaspoons sugar in 1 litre of tap water.
Commercially available oral rehydration
solutions containing appropriate amounts of sodium, potassium and glucose can be used.
PPS Stable, Transitional and End of Life (10-
100%)
Quality of Life
Persistent diarrhea can have severe effects
on image, mood and relationships.
Attention must be paid to understanding the
emotional impact from the patient‟s perspective.
CCO‟s Symptom Management Pocket Guide 71
Offer practical strategies to assist with
coping: carefully plan all outings, carry a change of clothes, know the location of restrooms, use absorbent undergarments.
Life style Take steps to prevent skin excoriation:
o Use mild soap, consider sitz bath. o Apply a skin barrier product.
Hydrocolloid dressings may be used as a
physical barrier to protect excoriated skin.
PPS End of Life (10-20%)
Exercise good clinical judgment regarding the
burden and benefits of parenteral fluids for the
individual patient
Pharmacological Treatments
Ask patient whether using non-traditional
or alternative therapies for bowel management.
Consider the etiology of constipation or
diarrhea before initiating any pharmacological treatment.
Constipation
Consider the patient's preferences and
previous
experiences
management when determining a bowel
regimen.
Consider the patient's recent bowel function
and response to previous treatments to guide
appropriate selection and sequence of
pharmacological treatments.
72 CCO‟s Symptom Management Pocket Guide
Recommended first line agents
Oral colonic stimulant (sennosides or bisacodyl)
Oral colonic osmotic (lactulose or polyethylene
Recommended second line agents
Suppositories (glycerin or bisacodyl)
Enemas (phosphate enema)
Recommended third line (rescue) agents
Picosulfate sodium-magnesium oxide-citric acid
Methylnaltrexone (if the patient is taking regular
opioids)
Fecal Impaction If stool is impacted in the rectum, use a
glycerin suppository to soften the stool, followed 1 hour later by digital disimpaction, if necessary (after pretreatment with analgesic and sedative), and/or a phosphate enema.
If stool is higher in the left colon, use an oil
retention enema, followed by a large volume enema at least 1 hour later.
Constipation Management in Special Circumstances
Opioid-induced constipation is much easier
to prevent than to treat. Start a first line oral
laxative on a regular basis for all patients
taking opioids.
CCO‟s Symptom Management Pocket Guide 73
Initial 3-Day Trial of methylnaltrexone
If no bowel movement for 48 hours, give
methylnaltrexone subcutaneously - 8 mg if 38-62 kg
or 12 mg if 62-114 kg
ethylnaltrexone is considered effective if a bowel
ovement occurs within 4 hours after injection.
Effective
Effective
The same dose can be repeated every 24
hours for 2 days, if necessary, if a bowel
movement does not subsequently occur
NOT
Effective
Effective
Methylnaltrexone is
The same dose can be
unlikely to work for
offered in the future if no
this patient at this
bowel movement occurs for
time. No further
48 hrs. Doses should not be
given more frequently than
h lnaltre
74 CCO‟s Symptom Management Pocket Guide
Patients with a Colostomy
Use the same approach to bowel care as for
the patient without a colostomy. A patient with a very proximal colostomy may not benefit from colonic laxatives.
There is no role for suppositories since they
cannot be retained in a colostomy.
Enemas may be useful for patients with a
descending or sigmoid colostomy.
Paraplegic Patients A patient with paraplegia is unable to
voluntarily evacuate the rectum.
Passage of stool spontaneously may
represent overflow only.
As for patients without paraplegia, oral
laxatives may be needed to move stool to the rectum, but the paraplegic patient needs help to empty the rectum. o Schedule a rectal exam daily or every 2
days, depending on the patient‟s preference, followed, if necessary, by assistance emptying the rectum using one or more of the following: suppository enema digital emptying
Develop an effective, regular protocol that
is acceptable to the patient.
Table 1 and 2 below offer additional information on oral and rectal laxatives available in Canada.
CCO‟s Symptom Management Pocket Guide 75
Table 1. Oral Laxatives
Bisacodyl
increase up to 15 mg tid
Lactulose
ntly softening, secondarily stimulant
Picosulfate
citric acid
until good effect
ne glycol
Sennosides Colonic
to 4 tablets or 20 ml bid
Notes: bid = twice daily; gm = grams; mg = milligrams; ml = milliliter; qhs = every night at bedtime; tid = three times a day.
76 CCO‟s Symptom Management Pocket Guide
Table 2. Rectal Laxatives
Rectal or
Bisacodyl
suppository stimulating
Glycerin
dilation and Normal
stimulation; saline
(tap water
or saline)
retention
Phosphate
solution in pre-packed bottles
Notes: mg = milligrams; ml = milliliters; prn = as required;
Diarrhea
A single liquid or loose stool usually does not
require intervention.
A single drug should be used for diarrhea
and care should be taken to avoid sub-
therapeutic doses.
Loperamide (2 mg tablets; 2 mg/15 ml
solution) is the preferred first-line anti-diarrheal agent:
CCO‟s Symptom Management Pocket Guide 77
o Initially, use 2 mg orally after each
loose bowel movement, up to 16 mg per day.
o For chronic diarrhea, a regular bid dose
can be used, based on the 24-hour dose found to be effective, plus 2 mg after each loose bowel movement, up to 32 mg per day total.
Diphenoxylate/atropine (2.5/0.025 mg
tablets) o 1-2 tablets orally as needed, up to 4
times per day (maximum 20 mg diphenoxylate per day)
o Titrate dose down once diarrhea
control achieved, to determine the maintenance dose.
Opioids – consider if the patient is not
currently on an opioid for other indications.
Metronidazole 500 mg orally tid for 2
weeks for Clostridium difficile diarrhea.
Octreotide 50-600 mcg per day
subcutaneously (dosed bid or tid) can be considered for severe, refractory diarrhea.In cases of severe diarrhea, parenteral rehydration may be required.
If the perianal skin is already inflamed or
excoriated, use a topical corticosteroid cream for 1 to 2 days.
78 CCO‟s Symptom Management Pocket Guide
Selected References:
Fraser Health. Hospice palliative care program symptom guidelines: Bowel Care [Internet]. Surrey, BC: Fraser Health Website; 2006. Website:
Oncology Nursing Society. Putting Evidence Into Practice: Evidence-Based Interventions to Prevent, Manage, and Treat Chemotherapy- and Radiotherapy-Induced Diarrhea. Clinical Journal of Oncology Nursing 2009;13(3):336-341. Link to ONS PEP Diarrhea Quick View Resource:
Registered Nurses‟ Association of Ontario. Prevention of Constipation in the Older Adult Population. (Revised). Toronto, Canada: Registered Nurses‟ Association of Ontario; 2005. Website:
National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology Palliative Care. V.2.2011. Website:
For full references and more information please refer to CCO‟s
CCO‟s Symptom Management Pocket Guide 79
80 CCO‟s Symptom Management Pocket Guide
Oral Care
Assessment …………………………….…………………… 82
Diagnosis ……………………….……………………….… 82
General Oral Care
Non-Pharmacological Interventions……………. 82
Pharmacological Interventions ….………………. 84
Oral Mucositis Prevention
Non-Pharmacological Interventions……………. 85
Pharmacological Interventions……………………. 86
Oral Mucositis Management
Non-Pharmacological Interventions……………. 86
Pharmacological Interventions…………….……. 87
Xerostomia Prevention
Non-Pharmacological Interventions……………. 87
Pharmacological Interventions……………………. 87
Xerostomia Management
Non-Pharmacological Interventions……………. 87
Pharmacological Interventions……………………. 88
Dysgeusia Prevention
Non-Pharmacological Interventions………….…. 89
Pharmacological Interventions……………………. 89
Dysgeusia Management
Non-Pharmacological Interventions……………. 89
Pharmacological Interventions……………………. 90
Intra-Oral Infections Prevention
Non-Pharmacological Interventions…………. 90
Pharmacological Interventions……………………. 90
Intra-Oral Infections Management
Non-Pharmacological Interventions……………. 91
Pharmacological Interventions……………………. 91
Selected References …………………………….…. 94
CCO‟s Symptom Management Pocket Guide 81
Assessment
Common symptoms to screen for include oral
pain, dry mouth, taste changes and difficulty with opening/closing of the mouth.
Common signs to screen for include cavities,
bleeding, infections, ulcerations and abnormal lesions.
Diagnosis
Significant risk factors for the development of oral
complications include the type of cancer, type of cancer treatments, cumulative doses of chemotherapy or radiation treatment, method of delivery and duration of treatment.
Predisposing medical, dental, and lifestyle factors
may increase the severity of the complications.
Oral complications can significantly affect the
patient‟s morbidity, ability to tolerate treatment, and overall quality of life.
Rigorous assessment, diagnosis and early
intervention are important in preventing and decreasing oral complications.
General Oral Care
Non-Pharmacological Interventions
Principles of Oral Care
Good oral care is fundamental in preventing and
decreasing oral complications and has the potential to modify the acute and long term sequelae of therapy.
The major purposes of oral care are to maintain
normal function of the oral tissues, to maintain comfort, and to reduce the risk of bleeding, local infection and systemic infection.
82 CCO‟s Symptom Management Pocket Guide
A uniform systematic education plan for oral care
is recommended to help patients understand and cope with symptoms of oral complications.
An important component of oral care management is the assessment of nutritional status, including adequacy of oral solid and fluid intake.
It is important to keep oral mucosa and lips, clean, soft, moist and intact thus preventing infection.
Good dental care is encouraged.
The recommended rinsing solution is a bland rinse (1 teaspoon salt, 1 teaspoon baking soda in 1 liter/ 4 cups of water). The rinse should be prepared at least once daily and should not be refrigerated.
Following emesis, patients should be instructed to rinse mouth with the bland rinse to neutralize the mouth immediately, minimizing tooth enamel demineralization.
Patients may chew xylitol gum or suck on xylitol lozenges up to 6 grams a day.
While there is no evidence to recommend either for or against the use of club soda, the Oral Care SMG working group suggests it should be avoided due to the acidic pH, a result of the carbonic acid content found in carbonated soft drinks.
Factors to consider for oral care at the end of life; o
Discussions with patients/families should be done early and as often as necessary to explain the etiology of mouth complications, determine the goals of care, clarify the declining health status and determine desired levels of care pertaining to nutrition, hydration and interventions.
As patients approach the end of life, the objective of oral care is to avoid complications, treat potentially reversible conditions rapidly and/or provide relief of
CCO‟s Symptom Management Pocket Guide 83
symptoms caused by the offending oral complication.
Oral candidiasis is common in this patient population and therefore the oral cavity should be evaluated daily.
Pharmacological Intervention
Analgesics
With continuous pain (e.g., moderate to severe
oral mucositis) consider an oral analgesic
prescribed regularly to allow for more thorough
tooth brushing.
When appropriate, oral opioid analgesics are preferably given 60 minutes before brushing.
Topical anesthetics (e.g., viscous lidocaine 2% or viscous xylocaine 2%, 2-5 ml) may be applied 10 minutes before eating to provide enough comfort for the person to be able to eat or drink. As an alternative use an oral analgesic 1 hour prior to eating.
For cognitively intact head and neck cancer patients receiving radiation therapy, 2 to 5 ml of viscous lidocaine 2% may be swallowed, up to a maximum of 6 times per day, to allow for adequate hydration, nutrition and oral care. This advisement would be at the discretion and recommendation of the patient‟s most responsible physician.
If topical anesthetics are used only for rinsing, without swallowing, then the recommended maximum dose of viscous lidocaine 2% is 60 ml per day.
If patient is allergic to lidocaine, dyclonine 0.5 to 1% may be used (5 ml q6-8 hours, swish and swallow as needed).
Medications for Excessive Secretions
For excessive salivary secretions, tricyclic antidepressants (e.g., nortriptyline) are a consideration, starting at a low dose and titrating to effect.
84 CCO‟s Symptom Management Pocket Guide
Another possibility is scopolamine transdermal 1.5 mg patch changed every 72 hours.
At end of life, decreased cognitive ability, extreme fatigue and weakness may contribute to patient's inability to clear secretions from nose, mouth or throat. o Anticholinergic medications are often useful
for managing excessive secretions at end of life.
o Atropine 1% ophthalmic solution
administered sublingually, 1-2 drops (1 drop 0.5 mg) q4h prn.
o Ipratropium 0.03% Nasal Spray
administered intranasally or sublingually, 2 sprays at bedtime.
o Scopolamine 0.2 to 0.8 mg subcut q2-4h prn. o Glycopyrrolate 0.2 to 0.6 mg subcut q2-4h
o Buscopan (hyoscine butylbromide) 10 mg
o Glycopyrrolate is less sedating than
Oral Mucositis - Prevention
Non-Pharmacological Interventions
There is some evidence for the use of ice chips for the prevention of oral mucositis.
IMRT is currently the treatment of choice for head and neck patients to minimize intra-oral complications.
There is some evidence that Low Level Laser Therapy may reduce the incidence of oral mucositis and its associated pain, in patients receiving high-dose chemotherapy or chemo-radiotherapy before Hematopoietic Stem Cell Transplant (HSCT).
To prevent nutritional deficiencies a multivitamin may be considered.
CCO‟s Symptom Management Pocket Guide 85
Pharmacological Interventions
A systematic approach to oral care should be followed to reduce the amount of microbial flora, reduce pain and bleeding, prevent infection and reduce the risk of dental complications.
There is no evidence of benefit for the use of chlorhexidine for the prevention of oral mucositis when compared with placebo or no treatment.
Oral Mucositis - Management
Non-Pharmacological Interventions
Nutritional Care
Choose texture as tolerated and modify as required.
May need to start with soft, moist, smooth foods; if not tolerated try extra soft/pureed foods.
If only liquids are tolerated, choose high calorie, high protein fluids every 2 hours.
Choose foods high in calories and protein, 6-8 small meals/snacks daily.
Cook solid foods until tender, use moist sauces, choose soft, bland foods.
Avoid foods that irritate the mouth or throat.
Avoid eating foods which are abrasive, rough,
tart, salty, spicy, acidic, very hot or very cold.
Oral commercial nutritional supplements may be
There is insufficient evidence to support the use
of vitamin B12, beta-carotene calcium, chamomile, glutamine, or curcumin in the treatment of oral mucositis.
If oral intake is inadequate for a prolonged
period consider using a regular strength multivitamin.
Severe oral mucositis during cancer treatment
(grade 3 or 4) may be managed with an
86 CCO‟s Symptom Management Pocket Guide
appropriately placed feeding tube or total parenteral nutrition (TPN) depending on the patient‟s goals of care.
The type of tube (i.e., gastrostomy or
jejunostomy) and the method of placement (i.e., surgical or radiological) should be determined by the degree and extent of mucositis and the potential worsening of symptom due to planned cancer treatment.
Consult dietitian if possible.
Pharmacological Interventions
Systemic analgesia with morphine (or other
strong opioid) is the recommended treatment of choice for oral mucositis pain in patients undergoing HSCT.
Xerostomia - Prevention
Non-Pharmacological Interventions
The use of parotid sparing Intensity Modulated
Radiation Therapy (IMRT) is recommended for prevention of salivary gland hypofunction and xerostomia in head and neck cancer patients.
Pharmacological Interventions
Xerostomia – Management
Non-Pharmacological Interventions
Nutritional Care
Add extra moisture to foods, increase fluid consumption.
Oral rinses may improve swallowing/taste problems.
Soft, mild tasting food is often better tolerated.
CCO‟s Symptom Management Pocket Guide 87
Moisten food by adding sauces, gravy, butter, dressings, broth or another liquid.
Food and drinks should be cold or tepid.
Plain ice cubes, sugar-free popsicles, sugar-free gum, frequent sips of cold water or mouth sprays may increase fluid consumption and help cool and moisten mouth.
Avoid foods, fluids and other items which may dry or irritate mouth and teeth, including highly acidic foods and fluids, foods high in sugar, caffeine and alcohol.
To stimulate residual salivary secretion and to ameliorate the condition of the mucosa, regular use of fresh, lightly acidic fruits, slices of cold cucumber and tomato or thin slices of cold apples can be used as long as patient is not experiencing mucositis.
The use of milk, jello, sherbet, applesauce and ice cream is also suggested.
Acupuncture
Acupuncture treatment is a possible intervention for the treatment of radiation-induced xerostomia in patients with a residual functional capacity of the salivary glands and is a treatment modality without serious adverse effects.
Artificial saliva
Artificial saliva products may also be considered for a brief course to determine effectiveness and patient acceptability, followed by continuing therapy when warranted.
Pharmacological Interventions
Oral pilocarpine (sialogogue) 5mg tid following radiation therapy is recommended in head and
88 CCO‟s Symptom Management Pocket Guide
neck cancer patients for improvement of xerostomia.
Results for the use of pilocarpine HCl concomitantly with radiation therapy to reduce xerostomia and salivary gland hypofunction are inconsistent, however in some patients a beneficial effect has been shown on xerostomia.
Amifostine
No consensus could be reached regarding a recommendation as most clinical studies do not have the statistical power to evaluate the influence of amifostine on the therapeutic index.
Dysgeusia – Prevention
Non-Pharmacological Interventions
Excluding the tip of the tongue during radiation therapy may prevent dysgeusia. In one trial patients who had the tip of the tongue included in the radiation treatment field, reported marked increases in mean threshold values to the four taste qualities being tested (salt, sweet, sour, and bitter).
Pharmacological Interventions
Zinc gluconate is not recommended for the prevention of dysgeusia in head and neck cancer patients.
Amifostine is not recommended for the prevention of dysgeusia in head and neck cancer patients.
Dysgeusia – Management
Non-Pharmacological Interventions
Nutritional Care
As taste changes are unique to each person and can vary over time, an individualized approach
CCO‟s Symptom Management Pocket Guide 89
needs to be taken to identify tolerable foods. Ongoing follow up is recommended.
To prevent compromised food intake, patients may need encouragement and support to try foods again that may have resulted in food aversions secondary to taste changes.
Encourage patients to:
o Enjoy foods that taste good. o Experiment with food flavours to enhance
o Drink plenty of fluids. o Avoid strong smells.
Nutritional counseling is recommended.
Pharmacological Interventions
Intra-Oral Infections – Prevention
Non-Pharmacological Interventions
The best prevention for any intra-oral infections is non-pharmacological in nature.
It is necessary to follow meticulous oral care plans (Se.
Pharmacological Interventions
Fluconazole is found to be very effective in the prevention of clinical oral fungal infections and in reducing oral fungal colonization in patients receiving cancer therapy.
Prophylactic fluconazole 100 mg po daily (400 mg po daily for HSCT patients) may be considered for prevention of oral candidiasis in cancer patients.
90 CCO‟s Symptom Management Pocket Guide
Intra-Oral Infections – Management
Non-Pharmacological Interventions
Pharmacological Interventions
Topical agents are considered preferable to systemic
agents for the management of mild intra-oral fungal
infection due to the lower risk of side effects and
drug interactions (e.g., sugarless nystatin rinse).
Clotrimazole lozenges or sugarless nystatin suspension may be used as first-line therapy for the management of mild oropharyngeal candidiasis.
Sugarless nystatin suspension 100,000 units/ml may be used as follows: Swish around and hold in the mouth for at least one minute, then swallow; use 5 ml qid for 7-14 days (works by direct contact).
Soak dentures overnight in sugarless nystatin 100,000 units/ml solution or use sugarless nystatin 100,000 units/ml cream to treat dentures.
Use sugarless nystatin popsicles (for cooling relief).
Clotrimazole oral suspension (1mg/ml) may be used as follows: Swish around the mouth for one minute and then swallow; use 10 mL qid.
If topical agents are not well tolerated or the response
rate is poor, then it is advised to proceed with the use
of systemic agents.
The management of moderate to severe
oropharyngeal candidiasis, fluconazole 100 mg
daily as first-line therapy is equal or more
effective against oropharyngeal candidiasis in
cancer patients than nystatin or clotrimazole.
To prevent relapse after initial treatment, maintenance therapy using fluconazole 50 mg (up to 400 mg) daily may be considered.
CCO‟s Symptom Management Pocket Guide 91
For fluconazole refractory disease, itraconazole or posaconazole are recommended, with voriconazole and amphotericin B reserved for refractory cases.
Patients who cannot tolerate fluconazole (or other antifungals) may use sugarless nystatin suspension.
Additional systemic agents include the lipid formulations of amphotericin B, and the echinocandins (caspofungin, anidulafungin, and micafungin).
Use of these systemic agents may be limited by their side effects, especially for amphotericin B.
These agents are optimally used for short durations of treatment.
Bacterial Infections
First line: amoxicillin 500 mg po q8h for 7-10 days
Alternative: penicillin V 300-600 mg po q6h for 7-10
days
Alternative: clindamycin 300-450 mg po q6h for 7-
10 days
o Amoxicillin/ clavulanic acid (Clavulin®): 500
mg tablet (contains amoxicillin 500 mg and clavulanic acid 125 mg) po q8h OR the 875 mg tablet (contains amoxicillin 875 mg and clavulanic acid 125 mg) po q12h for 7-10 days.
If one is certain that the infection is periodontal in origin then the recommendation for first line therapy is metronidazole 500 mg po q8h for 7-10 days.
Viral infections
Herpes simplex
Topical acyclovir: apply to affected area q3-4 hours, for a total of 6 times/day for 7 days (apply a sufficient quantity to adequately cover all lesions).
Systemic acyclovir for larger lesions: o Primary Herpes Simplex Virus (HSV):
acyclovir 200 mg po q4 hours, 5 times/day for 10 days or 400 mg po tid for 7-10 days (in
92 CCO‟s Symptom Management Pocket Guide
immunocompromised patients, consider 400 mg po q4hours, 5 times/day for 10 days).
o Recurrent HSV: acyclovir 200 mg po q4
hours, 5 times/day for 5 days; Valacyclovir 500 mg po bid (twice daily) or q12h for 3 days. Adjust for renal dysfunction.
Varicella-zoster
Acyclovir 400 mg po 5 times/day for 7-10 days.
For severe infection, acyclovir 5 mg (base) per kg body weight IV (over at least 1 hour) q8 hours for 5-7 days.
Patients with acute or chronic renal impairment may require dose reduction (e.g., acyclovir 200 mg po q12 hours when CrCl is 0-10 mL/min).
Valacyclovir 1000 mg po tid for 7 days (superior to acyclovir for post-herpetic infections).
Cytomegalovirus
Ganciclovir: induction: 5mg/kg IV over 1 hour q12h, maintenance: 5 mg/kg IV over one hour once per day
Dose reductions are recommended for renal impairment.
Ganciclovir should not be administered in patients with severe neutropenia (ANC less than 500/μL) or severe thrombocytopenia (platelets less than 25,000/μL) or severe anemia (hemoglobin less than 80 g/L).
CCO‟s Symptom Management Pocket Guide 93
Selected References:
1. Broadfield L, Hamilton J., Best Practice Guidelines for the
Management of Oral Complications from Cancer Therapy. Supportive Care Cancer Site Team, Cancer Care Nova Scotia; 2006.
2. Harris DJ, Eilers J, Harriman A, Cashavelly BJ, Maxwell
C. Putting Evidence into Practice: Evidence-based interventions for the management of oral mucositis. Clinical Journal of Oncology Nursing. 2008; 12(1):141-152.
3. BC Cancer Agency. Professional Practice Nursing
Standards: Symptom Management Guidelines: Oral Mucositis. [Internet]. 2010.
4. BC Cancer Agency. Professional Practice Nursing
Standards: Symptom Management Guidelines: Xerostomia. [Internet]. 2010.
5. HealthPartners Dental Group and Clinics. Oral cancer
guideline. [Internet]. 2007.
6. Keefe DM, Schubert MN, Elting LS, Sonis ST, Epstein JB,
Raber-Durlacher JE, et al. Updated Clinical Practice Guidelines for the Prevention and Treatment of Mucositis. Cancer 2007;109:820–31.
7. Worthington HV, Clarkson JE, Bryan G, Furness S,
Glenny AM, Littlewood et al. Interventions for preventing oral mucositis for patients with cancer receiving treatment. Cochrane Database Systematic Review. 2011; Issue 4.
Meyer S. Interventions for treating oral mucositis for patients with cancer receiving treatment. 2010 Issue 10.
9. Jensen SB, Pedersen AML, Vissink A, Andersen E, Brown
CG, Davies AN, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. 010;18(8):1061-79.
et al. A systematic review of oral fungalinfections in patients receiving cancer therapy010;18(8):985-92.
et al. A systematic review of dysgeusia induced by cancer therapies2010;18(8):1081-7.
For full references and more information please refer to CCO's ocument.
94 CCO‟s Symptom Management Pocket Guide
CCO‟s Symptom Management Pocket Guide 95
96 CCO‟s Symptom Management Pocket Guide
Edmonton Symptom Assessment System
CCO‟s Symptom Management Pocket Guide 97
98 CCO‟s Symptom Management Pocket Guide
Disclaimer:
Care has been taken by Cancer Care Ontario‟s
Symptom Management Group in the preparation of the
information contained in these pocket guides.
Nonetheless, any person seeking to apply or consult
the pocket guide is expected to use independent
clinical judgment and skills in the context of
individual clinical circumstances or seek out the
supervision of a qualified specialist clinician.
CCO makes no representation or warranties of any
kind whatsoever regarding their content or use or
application and disclaims any responsibility for their
application or use in any way.
Dates of Publication:
August 2010 - Pain, Dyspnea, Delirium and Nausea & Vomiting
July 2012 – Loss of Appetite, Bowel Care, Oral Care
CCO‟s Symptom Management Pocket Guide 99
Source: http://hpcconnection.ca/wp-content/uploads/2014/07/SymptomManagementPocketGuides.pdf
Antibiotic resistance in antibiotic free environment Antibiotic resistance in the absence of An association between antibiotic use and the development of clinical resisitance has been clearly documented in several cases Use of macrolides and resistance in S. pyogenes Use of penicillins and resistance S. pneumoniae Use of floroquinolones and resistance in Enterobacteriacea and P. aeruginosa Use of third-generation cephalosporins and resistance in Enterobacteriacea Use of carbapenems and resistance in P. aeruginosa Use of linezolid and resistance in E. faecium
UNIVERSIDAD NACIONAL AUTÓNOMA DE HONDURAS CONSEJO UNIVERSITARIO SECRETARIA Página 1 de 46 Sesión Ordinaria Febrero Acta No.CU-O-002-02-2011 ACTA NÚMERO CU-O-002-02-2011 CONSEJO UNIVERSITARIO SESIÓN ORDINARIA 25, Febrero de 2011 En la Ciudad Universitaria, "José Trinidad Reyes", Tegucigalpa, Municipio del Distrito Central, en el Auditórium "José Oswaldo Ramos Soto", el viernes veinticinco de febrero de dos mil once, siendo las nueve y cincuenta de la mañana 09:50(Hrs), se dio inicio a la Sesión Ordinaria del Consejo Universitario con la asistencia de los siguientes Miembros: MSC. JULIETA CASTELLANOS RUIZ, Presidenta; FACULTAD DE CIENCIAS MÉDICAS: Dr. DR. MARCO TULIO MEDINA, Decano; DR. GUILLERMO EMILIO AYES CARIAS, Representante Propietario Claustro Docente; FACULTAD DE CIENCIAS ECONÓMICAS. MSC. BELINDA FLORES DE MENDOZA, Decana; LIC. ROSA MARÍA TRIMARCHI, Representante Suplente Claustro Docente; FACULTAD DE ODONTOLOGÍA: DRA. LOURDES MURCIA CARBAJAL, Decana; MSC. YOVANNY DUBÓN TROCHEZ, Representante Propietario Claustro Docente; DRA. CARMEN BEATRIZ GUTIÉRREZ, Representante Suplente Claustro Docente; FACULTAD DE INGENIERÍA: ING. JOSÉ MÓNICO OYUELA, Decano; ING. SAÚL ABELARDO JIMÉNEZ, Representante Propietario Claustro Docente; FACULTAD DE CIENCIAS SOCIALES: LIC. IMELDA VALLADARES, Decana; LIC. JUAN PABLO CARIAS, Representante Propietario Claustro Docente; LIC. MIRNA LIZETH FLORES GIRÓN, Representante Suplente Claustro Docente; FACULTAD DE HUMANIDADES Y ARTES: ARQ. ROSAMALIA ORDOÑEZ FERRERA, Decana; FACULTAD DE CIENCIAS ESPACIALES: MSC. MARÍA CRISTINA PINEDA, Decana; MSC. MARIBEL SUYAPA GUERRERO, Representante Propietario Claustro Docente; CENTRO REGIONAL UNIVERSITARIO DEL LITORAL ATLÁNTICO (CRULA): MSC. MAGDA ELSY HERNÁNDEZ. Directora; ING. MARIO CESAR MARTÍNEZ, Representante Propietario Claustro Docente; CENTRO REGIONAL UNIVERSITARIO DEL LITORAL PACÍFICO (CRULP): DR. WILFREDO DOMÍNGUEZ MEDINA, Director. LIC. MARIO MEDINA, Representante Propietario Claustro Docente; CENTRO REGIONAL UNIVERSITARIO NORORIENTAL (CRUNO): DR. JOSÉ ROBERTO BACA, Director; ING. JUAN GUILLERMO CÁLIX, Representante Propietario Claustro Docente; CENTRO REGIONAL UNIVERSITARIO DEL CENTRO (CRUC): ING. OSCAR MEZA PALMA, Director; ING. JOSÉ FRANCISCO RODRÍGUEZ, Representante Suplente Claustro Docente; CENTRO REGIONAL UNIVERSITARIO VALLE DEL AGUAN (CRUVA): ING. JOSÉ LEONEL CASTILLO, Director; ING. CAROLINA VENTURA DE PUERTO



