Report of the ices ospar workshop on lysosomal stability data quality and interpretation (wklys)
ICES/OSPAR WKLYS REPORT 2010
ICES ADVISORY COMMITTEE
ICES CM 2010/ACOM:61
REF. OSPAR, ACOM
Report of the ICES OSPAR Workshop on
Lysosomal Stability Data Quality and
Interpretation (WKLYS)
13–17 September 2010
Alessandria, Italy
International Council for the Exploration of the Sea
Conseil International pour l'Exploration de la Mer
H. C. Andersens Boulevard 44–46 DK-1553 Copenhagen V Denmark Telephone (+45) 33 38 67 00 Telefax (+45) 33 93 42 15 www.ices.dk
[email protected]
Recommended format for purposes of citation:
ICES. 2010. Report of the ICES OSPAR Workshop on Lysosomal Stability Data Qual-ity and Interpretation (WKLYS), 13–17 September 2010, Alessandria, Italy. ICES CM 2010/ACOM:61. 57 pp.
For permission to reproduce material from this publication, please apply to the Gen-eral Secretary.
The document is a report of an Expert Group under the auspices of the International Council for the Exploration of the Sea and does not necessarily represent the views of the Council.
2010 International Council for the Exploration of the Sea
ICES/OSPAR WKLYS REPORT 2010
ICES/OSPAR WKLYS REPORT 2010
ICES/OSPAR WKLYS REPORT 2010
Executive summary
ICES/OSPAR WKLYS on the quality and interpretation of lysosomal stability data met for five days (13–17 September) at the University of Piamonte Orientale "Amedeo Avogadro" (Alessandria, Italy). Part of WKLYS (16–17 September) was performed in association with a MEDPOL training workshop held on 13–17 September 2010. WKLYS was convened by C. Martínez-Gómez (Spain) and attended by seven partici-pants from three OSPAR/ICES member states (Spain, UK and Italy). The main tasks undertaken were to:
• Develop guidance on the interpretation of the results obtained from the
neutral red retention assay for lysosomal membrane stability in marine samples, giving particular attention to the variation in sample characteris-tics experienced through the ICES/OSPAR area in coordination with mem-bers of the parallel MED POL training course.
• Develop written and graphical material to elaborate, harmonize and clarify
criteria of interpretation and the standard analytical method for the neutral red retention assay of lysosomal membrane stability recommended to be used through the ICES/OSPAR area, in coordination with members of the parallel MED POL training course.
• Develop proposals for effective and repeatable external quality assurance
programmes for the measurement of lysosomal membrane stability (ICES/OSPAR).
The main outcomes of the meeting were:
A critical review and proposals for improving the standard analytical method ICES TIMES SERIES No 36 for the neutral red retention assay of ly-sosomal membrane stability recommended to be used through the ICES/OSPAR area. An illustrated draft document with proposals for improved consistency in the interpretation of the neutral red retention assay of lysosomal membrane stability recommended to be used through the ICES/OSPAR area. Identification of the main aspects of the operational procedure that need fur-ther discussion and consensus to harmonize the use of the Neutral Red Re-tention as-say, in terms of monitoring and intercomparison purposes to be used through the ICES/OSPAR and MED POL area. Proposal for a practical session to be held in conjunction with the WGBEC in mid February 2011 in Vigo (Spain) to further complete harmonization of ly-sosomal stability data quality and interpretation.
ICES/OSPAR WKLYS REPORT 2010
Opening of the meeting
The meeting was held at the University of Piamonte Orientale "Amedeo Avogadro" (Alessandria, Italy) and opened by C. Martínez-Gómez (IEO, ES) at 09.00 h on Thurs-day 16 September 2010. The meeting was co-chaired by C. Martínez-Gómez and J. Bignell (Cefas, UK). The list of participants is given in Annex 1.
ICES/OSPAR WKLYS REPORT 2010
Adoption of the agenda
The Agenda was adopted without amendment (Annex 2). The Terms of Reference for the meeting (see Annex 3) require WKLYS to report by 15 November 2010 to ACOM and OSPAR.
ICES/OSPAR WKLYS REPORT 2010
Preparation of the Report
The Report of WKLYS was drafted and adopted during the meeting, and will be completed and circulated by the co-chairs for comment. The complete Report would be sent to ICES before 22 November 2010.
ICES/OSPAR WKLYS REPORT 2010
Review progress with national/international monitoring activities of the use of the neutral red retention assay for lysosomal membrane stability in mussel samples/international monitoring activities within OSPAR/MEDPOL
C. Martínez-Gómez (ES) gave a presentation on the current state of the use of the neutral red retention assay for lysosomal membrane stability in mussels within the framework of the Spanish biomonitoring activities of the marine chemical contamina-tion. A survey was conducted on the Northern Spanish coast, measuring lysosomal mem-brane stability (LMS) in native mussels from eight sampling sites by using the Neu-tral Red Retention (NRR) assay. However, the LMS biomarker is only being routinely applied at 12–15 sampling sites along the Iberian Mediterranean coast as part of the biomonitoring programme since 2002 (Martínez-Gómez
et al., 2008). Between 2010–2012, the biomonitoring programme along the Spanish Mediterranean coast will be continued to meet the obligations to the Barcelona Convention and if possible, to con-tribute to the GES assessment for the Marine Strategy Framework Directive. All LMS data obtained so far in mussels from the Mediterranean coast will be sent to the MED POL database, under the responsibility of the Ministry of Rural and Marine Envi-ronment. LMS in mussels is monitored annually in native mussels in a coordinated way with chemical monitoring. Field sampling is conducted along the Iberian Mediterranean coast between May 15 and June 15 approximately every year (prespawning period). About 12–16 specimens from each site are taken for NRR assay, the number of mus-sels analysed was decided through cost/benefit considerations (Figure 1).
ICES/OSPAR WKLYS REPORT 2010
Figure 1. Examples of accumulated variance of NRR time values in relation with the number of
specimens analysed in three sampling sites located in the Mediterranean Coast of Spain.
Ambient water temperature and salinity are recorded at each sampling site. In order to reduce the possible effect of specimen size and sexual maturity on the response of this biomarker, mussels are randomly sampled within the size range of 35–45 mm shell length. Mussel samples are transferred by road on the day of collection to the Oceanographic Centre of Murcia (IEO, www.ieo.es), using refrigerated thermo-insulated and humid containers and arriving before 10:00 pm on next day. When the mussels arrived in the laboratory, they were kept overnight in aquaria with clean aerated seawater (with the same temperature and salinity conditions as the original sampling sites) before testing started. This procedure was followed during the first years that NRR was assayed (2002–2003 and 2004). However, the differences in temperature from the refrigerated thermo-insulated containers (about 6–10ºC) to the ambient water in the aquaria occasionally caused stress in mussels (for example, inducing spawning in the aquarium) and therefore, ruining the sample. For that rea-son, it was decided for subsequent surveys to keep mussels in thermo-insulated con-tainers until the test was starting. The time period between sampling and analysis of the mussels range between 24–30 hrs. The NRR assay used is according to the method described in Times ICES No 36, with minor modifications according MED POL methodology as described below:
• Poly-L-lysine is used as a coating agent to promote cell adhesion on slides. • 0.2 ml of haemolymph is withdrawn from the posterior adductor muscle
by using a 1–2 ml hypodermic syringe fitted with a 21-gauge needle and containing 0.2 ml of physiological saline.
• Humid chamber is introduced into an incubation chamber with a con-
trolled temperature of 18ºC for 30 min to allow cells to attach.
ICES/OSPAR WKLYS REPORT 2010
Two slides are prepared per sample (two replicates). Only one of the replicates (R1) is checked systematically under the microscope. If the sample is checked under direct microscope, a cover slide is added after the 15 min of dye incubation. The second rep-licate (R2) is kept inside the humidity chamber without a cover slide, and once the dye is lost to the remainder of the cytosol in most of the 50% of the cells of the R1 (time X min), R2 is checked. This second observation is used as confirmation or cor-rection: i.e. if R1 has been stressed by light and/or temperature during observations, R2 (non-stressed) is used (test continued with R2). So far, results have demonstrated a high natural variability between specimens from the same batch/sampling site and that mean retention times along the coast of Spain occasionally show values over 120 min (Figure 2 and 3).
ICES/OSPAR WKLYS REPORT 2010
Figure 2. Preliminary results of LMS over time in three sampling sites located in the Mediterra-
nean Coast of Spain.
ICES/OSPAR WKLYS REPORT 2010
Figure 3. Preliminary results of LMS in eight sampling sites located in the North coast of Spain at
2004.
4.2 United Kingdom
J. Bignell from Cefas described the current situation regarding the UK monitoring programme utilizing the neutral red retention (NRR) assay for lysosomal stability. Cefas are currently developing a fit for purpose inshore monitoring programme in the UK to measure anthropogenic chemicals and their biological effects on marine organisms. The programme aims to incorporate the integration of methods identified by the ICES Working Group for the Biological Effects of Contaminants [WGBEC (ICES, 2006)] and the ICES Workshop on Integrated Monitoring [WIKMON (ICES, 2007c)] in European flounder and Blue Mussels (
Mytilus sp.). This will provide an assessment of changes at the whole organism, tissue and sub cellular level in relation to tissue chemistry. The measurement of lysosomal stability in mussels is recognized as a prioritized core technique by WGBEC. As such, several field studies jointly implemented by Cefas and Marine Scotland between October 2009 and April 2010 have utilized this tech-nique with an aim to develop and optimize techniques for use in UK monitoring. Quality Assurance (QA) in biological effects monitoring is extremely important and there are several techniques that have now established a QA programme under Bio-logical Effects Quality Assurance in Monitoring Programmes (BEQUALM). It is im-portant to maintain standardized sampling and laboratory methods to ensure compa-rability of data between samples across geographical regions. This is particularly true for the measurement of lysosomal membrane stability due to its sensitive nature and ability to detect generic stress responses. Previous studies have indicated the poten-tial for air exposure to adversely affect the neutral red retention time in haemocyte lysosomes. Briefly, Song
et al. (2007) demonstrated that when Black Lipped Abalone
ICES/OSPAR WKLYS REPORT 2010
(
Haliotis rubra) were exposed to air at three different temperatures (7, 16 and 23°C ) for total duration of 12 hours, the mean neutral red retention (NRR) time decreased from approximately 110 min to 30 min. This occurred in all three replicates. Further-more, the mean NRR time of the 7 and 23°C replicates actually decreased to 30 min merely three h following removal from water. Another study (Zhang
et al., 2006) demonstrated a similar decline of the mean lysosomal NRR time in the haemocytes of Pacific oysters (
Crassostrea gigas). These observations have led to a sampling ethos at Cefas that aims to standardize time elapsed post field sampling. Wherever possible, these times are kept constant in order to reduce any potential variability of the data that may be encountered down-stream. Numerous studies conducted elsewhere have previously placed field col-lected mussels into aquarium facilities for 48 hours for acclimatization. However, the majority of the studies conducted by Cefas since October 2009 have not had access to such facilities due to the remote nature of the field sampling. Therefore all samples were simply kept in a cool insulated environment for 24 hours prior to sampling. Nonetheless, it would be interesting and perhaps timely to investigate the issue of air exposure further in
Mytilus sp. Since 2009, a number of field studies have been conducted incorporating several biomarkers for whole organism, tissue and subcellular changes. In October 2009, mussels were collected from Hvassahraun and Bjarnarhöfn, Iceland in order to serve as a reference for subsequent sampling events. In February 2010, mussels were col-lected from numerous sites on the River Clyde (Scotland) along a perceived contami-nation gradient. Passive samplers were also deployed as part of the programme. An additional sample from Southampton Water was also obtained. In April 2010, refer-ence mussels were obtained from Moreston and caged alongside passive samplers in the Humber and Mersey estuaries. Mussels were collected after five weeks. The tech-niques utilized during all of the above studies included histopathology, stress on stress, condition factor, COMET assay, gene expression, micronucleus formation and lysosomal stability (NRR). The NRR assay was carried out according to the ICES TIMES Series document No. 36 (ICES, 2004) and is presented below. Analysis of all other techniques is still ongoing. The mean NRR time for all mussels collected during this these sampling activities is presented in a table below. Mussels from Hvassahraun and Bjarnarhöfn were classi-fied as "healthy" according to the ICES/OSPAR assessment criteria (Table 1) whereas mussels from Great Harbour, Mersey and the two Humber sites were considered "stressed". All remaining sites were classified "stressed but compensating".
ICES/OSPAR WKLYS REPORT 2010
Table 1. Mean neutral red retention times for all sites examined by Cefas from the period October
2009 to April 2010. The current ICES/OSPAR assessment criteria is also highlighted: stressed
(red), stressed but compensating (amber) and healthy (green).
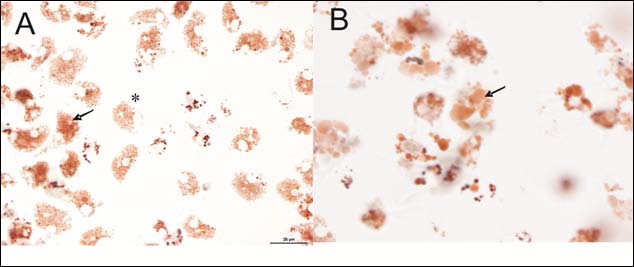
Figure 4. (A) Micrograph displaying haemocytes containing neutral red dye within the lysosomes
(*) and haemocytes exhibiting leakage of dye into the cytosol of the cell (arrow). (B) Micrograph
showing enlarged lysosomes within the haemocytes (arrow). Lysosomes were often of numerous
sizes and frequency.
Generally, the NRR assay performed well, however a large percentage of the total number of samples collected exhibited unusual phenomenon not depicted in the ICES TIMES Series document No. 36. These included enlarged lysosomes (Figure 4) within the haemocytes that developed as the NRR assay duration progressed. These characteristics were seen considerably more as opposed to the classic endpoint of the assay i.e. leakage of neutral red dye into the cytosol. This was also used as an end-point for the assay despite this not been well documented in the TIMES document. Due to the varying degree of this observation, it was often difficult to judge the end-point of the test.
P. Dymond (UK) presented the current state of the use of the neutral red retention assay for lysosomal membrane stability in mussels and the use of cytochemical assay for lysosomal stability in tissue sections of fish liver.
ICES/OSPAR WKLYS REPORT 2010
Marine Scotland Science (MSS) is currently in the second year of a three year research project that incorporates the use of lysosomal membrane stability (LMS) as one of the biomarkers incorporated in an integrated assessment plan. The project uses other biomarkers, chemical analysis and histopathology to put Scotland in a position to deliver integrated assessments of contaminants and biological effects for OSPAR, State of Scotland Seas report and to use these for assessing GES against descriptor 8 in the EU MSFD. The integrated project incorporates a trial monitoring programme of inshore mussels at a number of UK sites and is in collaboration with Cefas. There are approximately 6–8 sites proposed in Scotland and they include the Forth and Clyde estuaries. In Feb 2010 the first set of mussel samples were collected at sites from the Clyde and in-cluded Great Harbour and Lunderston Bay (Figure 5). Scientists from MSS and Cefas collected ten samples of the native blue mussel (Mytilus edulis) from each site. The mussels were collected from below the mid-tide line and were transported to a local laboratory in cool boxes. Within six hours of collection, blood samples were taken from the adductor muscle to assess LMS using the neutral red retention (NRR) assay. NRR methodology was directed from Cefas scientists and was in accordance with the description in the ICES Times No. 36 report.
Figure 5. Mussels sampled from LMS at Great Harbour and Lunderston Bay on the Clyde estuary
on the west coast of Scotland.
An initial set of result from the two Clyde sites was established using the NRR health status thresholds proposed by Moore (2006); animals are considered to be stressed, but compensating, if the NRR time is 50–120 minutes (such as at Lunderston Bay) and are classed severely stressed and probably exhibiting pathology if the NRR is <50 minutes, as was the case at the Great Harbour in February 2010.
ICES/OSPAR WKLYS REPORT 2010
Figure 7. Lysosomal membrane stability (neutral red retention time) of mussel haemocytes in
relation to the health status scheme proposed by Moore et al., 2006.
The current proposal is for further sites to be assessed in the Forth estuary on the east coast of Scotland in February 2011; collecting mussels from these sites and analysing them at a local laboratory using the NRR method used on the Clyde. The integrated assessment project also includes an inshore and offshore assessment of flatfish and incorporates lysosomal stability in fish liver tissue sections using the cytochemical cryostat method stated in the ICES Times No. 36 paper. Flatfish samples of dab (Limanda limanda) and flounder (Platicthys flesus) were caught during MSS scheduled cruises in 2009 and 2010; with a further set of sampling proposed for 2011. Samples were collected from a selection of inshore sites on the east and west coast of Scotland, including Clyde and Forth estuaries, and from offshore sites around Scot-land which stretched up to the Shetland Islands in the north. Around 200 samples of flatfish livers were collected in 2009 and 275 samples were collected in 2010; similar numbers of samples are proposed for 2011. The flatfish were trawled, sexed and euthanized on board the ship. A section of the livers were placed into cryovials and immediately frozen in liquid nitrogen; later transferring to -80°C freezers. Lysosomal stability of the liver tissue is still awaiting analysis. The use of these lysosomal stability techniques, as biomarkers included in a wider integrated marine assessment project, has further scope for expansion beyond the current project deadline. The continuation of these techniques into future Scottish monitoring programmes, which are driven at a high level by European Union Direc-tives and OSPAR Strategies, is an expected outcome.
4.4 MED POL Programme
A. Viarengo (IT) gave a presentation on current state of the use of the neutral red re-tention assay by the riparian Mediterranean countries to assess the lysosomal mem-brane stability in mussels. In the MED POL programme, both standard operating procedures to determine lysosomal membrane stability are described in UNEP/RAMOGE (1999). So far, four countries are using NRR assay to evaluate LMS
ICES/OSPAR WKLYS REPORT 2010
in mussels. Other Mediterranean countries are routinely using digestive gland tissue sections to determine the lysosomal labilization period.
References ICES. 2004. Biological effects of contaminants: measurements of lysosomal membrane stability.
By M.N. Moore, D. Lowe, and A. Köhler. ICES Techniques in Marine Environmental sci-ences, No. 36. 31 pp.
Martínez-Gómez C, Benedicto J., Campillo J.A. and Moore M.N. 2008. Application and evalua-
tion of the neutral red retention (NRR) assay for lysosomal stability in mussel populations along the Iberian Mediterranean coast. Journal of Environmental Monitoring, 10: 490–499.
Moore, M.N., Allen, J.I., and McVeigh, A. 2006. Environmental prognostics: an integrated
model supporting lysosomal stress responses as predictive biomarkers of animal health status. Marine Environmental Research, 61, 278–304.
Song, L., Li, X., Bott, K., Wang, T., Clarke, S., Zhao, W. 2007. Effects of air exposure on the ly-
sosomal membrane stability of haemocytes in blacklip abalone, Haliotis rubra (Leach). Aquaculture Research, 38 (3), pp. 239–245.
UNEP/RAMOGE: manual on the biomarkers Recommended for the MED POL Biomonitoring
Programme. UNEP, Athens. 1999.
Zhang, Z., Li, X., Vandepeer, M., Zhao, W. 2006. Effects of water temperature and air exposure
on the lysosomal membrane stability of haemocytes in pacific oysters, Crassostrea gigas (Thunberg). Aquaculture, 256 (1–4), pp. 502–509.
ICES/OSPAR WKLYS REPORT 2010
Review methodology recommended by ICES to assess Lysosomal membrane Stability in mussels by using Neutral Red Retention Assay
Agenda item Review and harmonize the standard analytical methods of NRR assay to measure lysosomal membrane stability in mussels currently recommended by ICES, 2004 and Mediterranean Action Plan (MAP) in UNEP/ RAMOGE, 1999 (Annex 5). WKLYS 2010 attended the theory and practical sessions of the MED POL training workshop on 13–14 September and received the current MED POL protocol to assess the LMS by using NRR assay. The analytical protocol of MEDPOL and ICES were found quite similar to procedures published by Lowe et al. (1992) and Lowe et al. (1995a, 1995b). However some differences were noted and discussed during the practical session in the MED POL training course. WKLYS jointly reviewed the MED POL and ICES protocols, together with comments made by MED POL participants, and made proposals for amendment of the ICES protocol. The main aspects re-viewed, discussed and/or amended are listed below.
5.1 The use of mussel physiological saline
The physiological saline solution described in both protocols is the same and salinity of this solution is = 30.5. Both protocols indicate that pH must be adjusted to 7.36 with 1M NaOH.
HEPES 4.77 g Sodium chloride 25.48 g Magnesium sulphate 13.06 g Potassium chloride 0.75 g Calcium chloride 1.47 g
In mussels, the salinity of haemolymph reflects the salinity of the ambient surround-ing seawater. Sea water salinity around OSPAR area ranges up to 36. In Mediterra-nean Sea are ranges up to 44. C. Martínez-Gómez reported that some laboratory experiments in Spain on the salin-ity of haemolymph had corroborated this in M. galloprovincialis (laboratory data, un-published results).
Table 2. Salinity measured in pooled samples (N=50) of haemolymph from M. galloprovincialis
immersed in seawater at different salinities (Author: C. Martínez-Gómez).
SALINITY AMBIENT SW
SALINITY HAEMOLYMPH
ICES/OSPAR WKLYS REPORT 2010
"Processing for neutral red retention (NRR) in samples of molluscs adapted to low salinity
environments should use either physiological saline adjusted to the equivalent ionic strength
or else use ambient filtered seawater". WKLYS found this recommendation described in
the Background Document on Biological Effects Monitoring Techniques, Chapter 4
"Lysosomal stability as a global health status indicator in biomonitoring", (OSPAR
2007). However, WKLYS noticed that the use of ambient filtered seawater is not
mentioned in the ICES TIMES series No. 36 (Moore et al., 2004) neither in MED POL.
However, Professor Aldo Viarengo clarified that most of the MED POL laboratories
are using ambient filtered seawater because it is even more convenient than the saline
solution and in fact, its use was fully recommended to the participants of the training
MED POL course. According Professor A. Viarengo, the adjustment of pH to 7.36
when ambient filtered seawater is used is not necessary though that should be vali-
dated. Plymouth laboratory also uses filtered seawater from the same sample sites as
the mussels in order to prevent problems related to salinity difference (e.g. can be
very problematical in some Baltic sites with very low salinity).
Action: Countries assessing LMS in monitoring programmes by using physiological
saline should validate the use of ambient filtered seawater and provided results to
WGBEC 2011 and when demonstrated the use of ambient filtered seawater should be
recommended in ICES TIMES protocol.
5.2 Haemolymph extraction
During the MED POL training course, Professors A. Viarengo and M. Moore indi-cated the critical importance of the needle used for haemolymph extraction. They indicated a 1–2 ml hypodermic syringe should be fitted with a 21-gauge needle, to reduce shearing forces during the extraction that may damage the cells. Recommendation: The use of a 21-gauge needle should be amended in ICES protocol as a hypodermic 25-gauge needle is currently recommended.
5.3 Slide preparations and dye incubation
The ICES protocol indicates that the slide preparations are kept cool throughout the
period of cell attachment and dye incubation. In the MED POL protocol, it is recom-
mended that the slides are maintained 15–16°C during the analysis.
Action: Countries assessing LMS in monitoring programmes should indicate what
temperature is used during the dye incubation and report to WGBEC in 2011 for fur-
ther discussion.
5.4 Determination of neutral red retention endpoint
WKLYS found that the determination of the neutral red retention endpoint is not harmonized among participants and that members of WKLYS and MED POL are us-ing different endpoints (ICES TIMES Series N0 36, UNEP, MEP (1999). This aspect is a critical point in terms of intercomparison exercises.
5.4.1 According ICES, 2004 "The endpoint is when 50% or more of the cells, based on a visual or a digital photographic determination (see below), exhibit lysosomal leakage or show abnormalities such as enlarge-ment (Figure 9)." WKLYS found this statement described in the ICES TIMES series N0. 36.
ICES/OSPAR WKLYS REPORT 2010
C. Martínez-Gómez (ES), J. Bignell (UK) and A. Viarengo (IT) agreed that they were interpreting and recording Retention Time data as the time when they observed that 50% or more of the cells exhibit lysosomal leakage. However, in the example pro-vided in "Data recording" ICES TIMES series N0. 36 is indicated: "The figure used for the calculation of the retention time corresponds to the last time period recorded when there was no evidence of dye loss or lysosomal abnormalities". WKLYS found the description of the endpoint in the ICES protocol rather confusing and participants indicated they were not assessing it accordingly this later statement (Table 3).
Table 3. ICES TIMES SERIES No. 36. (Moore et al., 2004).
Moore et al. (2006) demonstrated a significant linear relationship between the NRR
and cytochemical methods. For mussel digestive gland, timing intervals of 3, 5, 10, 15,
20, 30 and 40 minutes are normally utilized. When cytochemical assay using cryostat
sections is used, Lysosomal destabilization is measured as the increased permeability
of the substratum (naphthol AS-BI N-acetyl-β-glucosaminide) visualized by the reac-
tion with the enzyme into the lysosomes in the presence of diazonium salt. Time 0 it
is not considered in the evaluation of the maximal staining intensity peak. The end-
point recorded is the incubation time in the acid buffer that produces maximal stain-
ing reactivity (labilisation period (LP)). It has been stated that the absolute values for
measurement of lysosomal stability (NRR and cytochemical method; Lowe et al.,
1995b; Moore et al., 2006) are directly comparable (OSPAR, 2007) but WKLYS found
that differences in terms of RT data recording observed may affect substantially the
future intercomparison exercises and the assessment of results.
Professor M. Moore indicated that this point should be reviewed and is consulting
with the other authors of ICES TIMES Series No 36 (David Lowe and Angela
Koehler).
Action: OSPAR/ICES Member Countries that are applying NRR assay should indi-
cate how they are determining the NRR time endpoint and report to WGBEC in 2011.
5.4.2 According BEEP/Plymouth Marine Laboratory CMG indicated that in the protocol developed during BEEP project and Standard Operating procedure of the Plymouth Marine Laboratory (ECoH No:010 v.2.0), the endpoint is stated as: "The test for each slide is terminated when dye loss or lysosomal change as described above are evident in 50 % of the granular haemocytes and the time is recorded when this occurs. The mean and median retention time is then calculated for each sample set". Data sheet example
ICES/OSPAR WKLYS REPORT 2010
in the mentioned document comprises retentions time from 15 min to 180 min (no 0 min). However, about if it is not possible to determine if the RT to be used corre-sponds to the last time period recorded when there was no evidence of dye los, as it is clearly stated in ICES TIME Series No 36.
References Lowe D. and Wedderburn J. Standard Operating Procedure BEEP Project - Plymouth Marine
Laboratory. In vitro Neutral Red Retention Test for Lysosomal Stability in Blood Cells of Marine Mussels. ECoH No:010 v.2.0.
Lowe, D.M., Fossato, V.U., and Depledge, M.H. 1995a. Contaminant induced lysosomal mem-
brane damage in blood cells of mussels Mytilus galloprovincialis from the Venice Lagoon: an in vitro study. Marine Ecology Progress Series, 129: 189–196.
Lowe, D.M., Moore, M.N., and Evans, B.M. 1992. Contaminant impact on interactions of mo-
lecular probes with lysosomes in living hepatocytes from dab Limanda limanda. Marine Ecology Progress Series, 91: 135–140.
Lowe, D.M., Soverchia, C., and Moore, M.N. 1995b. Lysosomal membrane responses in the
blood and digestive cells of mussels experimentally exposed to fluoranthene. Aquatic Toxicology, 33: 105–112.
Moore, M.N., Allen, J.I. and McVeigh, A. 2006. Environmental prognostics: an integrated
model supporting lysosomal stress responses as predictive biomarkers of animal health status. Marine Environmental Research, 61, 278–304.
5.4.3 According UNEP/RAMOGE (1999) In the MED POL protocol, the definition of neutral red retention endpoint is the same although the example provided is quite different. According to the example, time 0 represents the time of the first inspection of the cells under the microscope (i.e. after 15 min of incubation with the neutral red dye). In terms of absolute values, according to MED POL protocol, value zero never occurs.
Table 4. UNEP/MAP Indicator fact sheet. Version 04-04-05 Biomarker of stress: Lysosomal mem-
brane stability in molluscs and fish cells.
5.4.4 According MED POL training course 2010 Determination of the LMS endpoint by using the NRR assay is established using an NR index. More information about this operational procedure is described briefly below.
ICES/OSPAR WKLYS REPORT 2010
Sampling size to assess to assess Lysosomal membrane Stability in mussels by using Neutral Red Retention Assay
WKLYS reviewed the sampling size recommended to be used to assess lysosomal
membrane stability in mussels by using NRR assay in ICES and MED POL protocols.
In the ICES protocol, it is indicated that samples should contain a minimum of 10
animals. In MED POL protocol any no detailed information was found regarding
recommended sample sizes. During the MED POL training course, the sampling size
recommended using the image analysis procedure is five mussels (though a larger
number of individuals is also recommended if pooling the haemolymph samples). C.
Martinez Gomez indicated that 12–16 mussels are currently used in Spanish Mediter-
ranean monitoring. J. Bignell (Cefas) and P. Dyamond (MSS) follow the ICES TIMES
series No. 36 (Moore et al., 2004) protocol whereby ten mussels are recommended.
In a recent study, Fang et al. (2008) were re-analysed published data on NRR time in
lysosomes to ascertain their statistical power to detect a minimum 20% spa-
tial/temporal change in field studies. Results revealed that the power analysis of NRR
time studies were <50%, suggesting that data from this biomarker may lead to erro-
neous conclusions if sample size is inadequate. They determined that to achieve sta-
tistically valid power, the optimal sample size for monitoring lysosomal NRR time
should be higher than 30 animals. However, if conducting the assay as detailed in the
ICES TIMES document, this number of samples would require the use of additional
scientists to conduct the assay in order to maintain quality assurance i.e. can 30 mus-
sels realistically be evaluated within the given time intervals without any overlap.
Action: Work should be done to harmonize a common and adequate sampling size
for lysosomal NRR time for ICES/MED POL. OSPAR/ICES member that are applying
NRR assay should indicate the sampling size used and report to WGBEC in 2011.
Reference J. K. H. Fang, R. S. S. Wu., C. K. M. Yip, P. K. S. Shin. 2008. Power analysis for biomarkers in
mussels for use in coastal pollution monitoring. Marine Pollution Bulletin, 58: 1152–1158.
ICES/OSPAR WKLYS REPORT 2010
Operating procedure recommended in the MED POL training course to assess Lysosomal membrane Stability in mussels by using Neutral Red Retention Assay
During the practical session of NRR assay in the MED POL training course, WKLYS
observed that a different operating procedure than that described in the MED POL
documentation (UNEP/RAMOGE, 1999). Professor A. Viarengo (IT) explained to
WKLYS that most of the laboratories participating in NRR assay intercomparison
exercise around the Mediterranean Sea are using image analysis, instead of the calcu-
lating the average Retention Time, to assess the lysosomal membrane stability end-
point. The image analysis protocol has not been yet made available through MED
POL programme, though it will be published soon. This protocol will be useful for
ICES/OSPAR member states.
Action: Future work should be done to harmonize a common protocol for image
analyses for ICES/MED POL. Professor A. Viarengo agreed to lead on this.
Briefly, the operating procedure was as follows. The assay was conducted on a con-
trol (mussels from the laboratory aquaria: n=5) and a study sample (mussel from
field/dosing study in laboratory aquaria: n=5). A larger number of samples can be
analysed by pooling haemolymph. The UNEP/MEDPOL protocol was similar to the
ICES/OSPAR method up until the point following application of the neutral red dye.
A working solution of neutral red was drained off samples after 15 min incubation
and replaced with filtered seawater (A. Viarengo explained that this was carried out
to prevent the neutral red dye from establishing equilibrium thus preventing red dye
from leaking into the cytosol). This time was considered time 0 (timer start to run at
that time for microscopic observations). At time 0, ten fields of view per mussel were
quickly photographed using a digital camera. This was done for both the control and
study samples. After 60 min, a further ten fields of view per mussel were photo-
graphed in a similar fashion to the time 0 samples.
Each photograph contained approximately 4 to 8 granulocytes. Generally, image
analysis was conducted on two granulocytes per field of view using the software
Scion Image www.scioncorp.com (obtained by free download). Each photograph was
opened as a grey scale image. Once opened the threshold of the lysosomes was "de-
fined". This was achieved by selecting the lysosomes and increasing the tolerance
until the lysosomes were overlaid with a binary threshold layer (Figure 8). Once in
place, the area of the lysosomes could therefore be measured (number of pixels). Fol-
lowing lysosomal area measurement, the total area of the granulocyte was also meas-
ured. These measurements were then used to calculate an index that would provide
an indication of dye loss from the lysosomes (presumably due to a decrease in the
lysosomal compartment although this needs to be clarified in a working document).
The neutral red index was calculated by:
Area of lysosomes (no of pixels)
Area of granulocyte (no pf pixels)
ICES/OSPAR WKLYS REPORT 2010
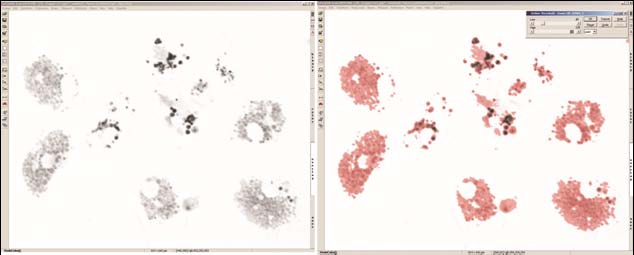
Figure 8. Typical thresholding process using the image analysis programme, Lim Lucia G Screen
Measurement (a) Grey scale micrograph of haemocytes prior to image analysis. (b) Grey scale
micrograph of haemocytes with threshold overlay (red) which is used for the actual quantifica-
tion of area.
This procedure does not detect absolute values such as the ICES TIMES document. Instead, the technique looks at the differential responses between the control and study samples. The result obtained from the control is always considered to be 100%. A >20% change in the study sample (compared with the control) is considered a sig-nificant difference and would be reported as "compromised". Study samples that exhibit <20% change is considered to be compromised. Clearly, this operational pro-cedure requires the use of control samples each time a set of study samples are tested. While this may be suitable for specific laboratory studies, WKLYS highlighted that this may not be suitable for large-scale monitoring because the number of samples to be analysed is duplicated and hence so are any associated costs. C. Martínez-Gómez and J. Bignell discussed possible limitations when utilizing im-age analysis. The image analysis technique described used grey scale images so ly-sosomes appeared grey and not red. In the demonstration provided to the WKLYS, when increasing the tolerance during thresholding, areas of the image background (as well as the lysosomes themselves) also began to threshold. This resulted in a false measurement therefore the tolerance was reduced. This in turn had a knock-on effect because the lysosomal compartment could not be fully defined resulting in an inaccu-rate measurement. John Bignell expressed his concerns over the use of image analysis of grey scale images in this manner as image analysis should be employed as a ‘quan-titative' tool. The potential inaccuracies introduced would result in a detection method that is no more quantitative than the method described in the ICES TIMES Series document No. 36. There is also potential that image analysis would not be able to distinguish those cells whereby fusion of the lysosomes has occurred as a result of membrane instability. However, this is only an issue if enlarged lysosomes are to be considered as an additional endpoint for the assay. S. Sforzini (Italy) indicated that in her laboratory only granulocytes with small lysosomes (BG) retaining NR are consid-ered for image analysis.
ICES/OSPAR WKLYS REPORT 2010
Harmonize and clarify interpretation criteria of sample observa-tions to assess Lysosomal membrane Stability in mussels by us-ing Neutral red retention Assay methodology recommended by ICES
C. Martínez-Gómez presented a set of images of haemolymph samples to be dis-cussed and harmonized in terms of interpretation criteria. C. Martínez-Gómez, J. Bignell, M. Moore and A. Viarengo participated in this item. An illustrated docu-ment with proposals for standardization of the interpretation of the neutral red reten-tion assay of lysosomal membrane was drafted (Annex 6). Part of the discussion was based on the identification of the different cell types that may appear in haemolymph samples such as granulocytes and hyalinocytes (agranu-locytes). This discussion arose because the ICES (2004) protocol for NRR assay as UNEP/RAMOGE (1999) indicates that "… blood cell lysosomes" or "haemolymph cells" and that "…the Retention Time of the NR probe by the lysosomes is recorded by estimating the proportion of cells displaying leakage from the lysosomes into the cytosol and/or exhibiting abnormalities in lysosomal size and colour". It was discussed that while under taking the neutral red assay, one should focus on the use of granulocytes during the technique. Granulocytes generally posses a low nuclear to cytoplasm ratio compared with hya-linocytes that posses a relatively larger nucleus to cytoplasm ratio. As the name sug-gests, granulocytes posses many cytoplasmic granules; however hyalinocytes can contain few or no granules. The granules within the cytoplasm contain acid hy-drolases are considered to be lysosomal in origin (Pipe, 1990b; Cajaraville, 1995; Hine, 1999). As described previously, there are occasions where the presence of enlarged lysosomes increases. Initially these can appear as numerous and slightly enlarged compared with those lysosomes that are considered normal. As the assay progresses, the frequency of these lysosomes decreases, however the general size increases. The result is fewer enlarged lysosomes as those shown in Figure 9. Mike Moore described how lysosomes may undergo fusion when lysosome membrane stability is compro-mised which may manifest as these enlarged lysosomes. This also fits with those ob-servations of C. Martínez-Gómez and John Bignell. Due to the very visual nature of the assay, WKLYS consider it important to discuss and address this issue in an up-date to the ICES TIMES document including the insertion of additional annotated images. This will further strengthen the existing document for use in future. It has been reported in the literature that the cellular composition of the haemolymph is different in impacted animals to that of clean animals. Dolcetti and Venier (2002) found significant increases in granular cells in haemolymph and gills when coupled comparison between two industrial sites and the related reference site were tested. Furthermore, they found that the granular cell fraction was substantially lower in the reference vs. industrial sites in gills and haemolymph. This is supported by the evi-dence of an increase in lysosomal size, suggesting an enhanced rate of fusion of pri-mary lysosomes with auto/hetero phagosomes. For pollutant-exposed cells, lysosomal enlargement and swelling usually indicates increased autophagy, which may greatly contribute to induction of cell dysfunctions, potentially leading to cell death (ICES, 2004; UNEP/RAMOGE, 1999). WKLYS pointed out that the definition of the number of haemocytes cell types and their nomenclature is still a subject of debate. A good number of authors (Moore and Lowe, 1977; Rasmussen et al., 1985; Renwrantz, 1990; Noël et al., 1993; Cajaraville and
ICES/OSPAR WKLYS REPORT 2010
Pal, 1995; Cajaraville et al., 1997) have described two types of haemocytes in M. edulis and M. galloprovincialis: hyalinocytes and granulocytes. On the basis of light micros-copy, Moore and Lowe (1977) subdivided the granular cells in M. edulis into baso-philic (or macrophages) and eosinophilic granulocytes. Pipe (1990) separated the granuclocytes of the mussel M. edulis in granulocytes with smaller granules (0.2 to 0.3 μm) and granulocytes with large granules (0.5 to 1.5 μm). N
öel et al. (1994) demon-
strated that the basophilic granulocytes of M. edulis observed by light microscopy correspond to the granulocytes with small granules and the acidophilic/eosinophilic granulocytes correspond to the granulocytes with large granules. Carballal et al., (1997) identified hyalynocytes and three different types of granulocytes according to the staining properties of their granules in M. galloprovincialis:
1 ) Hyalinocytes are smaller than granulocytes and have large nuclei and re-
duced basophilic cytoplasm, generally without granules. Hyalinocytes are less abundant than granulocytes in haemolymph and have a limited ability to spread on glass slides, thus demonstrating a round shape (cell diameter 8.73 ± 0.54 μm; nucleus diameter 5.27 ± 0.27 μm).
2 ) Granulocytes display less basophilia and spread more than cytoplasm of
hyalinocytes on glass slides. Ectoplasm and endoplasm can be easily dis-tinguished. Granulocytes are most abundant in haemolhymph than hyali-nocytes. Some granulocytes present abundant acidophilic/eosinophilics granules (AG) (cell diameter 49.08 ± 0.70 μm; nucleus diameter 6.86 ± 0.09 μm). These cells have the largest size and the highest density in the mussel hemolymph. Other granulocytes present only basophilic granules (BG) (cell diameter 43.70 ± 0.0.91 μm; nucleus diameter 7.71 ± 0.12 μm). Few granulocytes contain both types of granules (BAG) (cell diameter 44.70 ± 1.34 μm; nucleus diameter 7.54 ± 0.13 μm).
Moore and Elbe (1977) and Nöel et al. (1994) suggested that different granulocytes are maturing stages within a single cell line. Cheng (1981) proposed that granulocytes with large granules are the most mature cells, and probably arise from granulocytes with small granules. Cheng (1981) also proposed an ontogenic model with two cell lines, one for hyalinocytes and another for granulocytes, each originating in a differ-ent prohemocyte. For some other authors (Ottaviani et al., 1998), differences in cytol-ogy and number of haemocytes appear to be due to cellular aging and that only one cell type in two different stages, young or old, is present in haemolymph in mussels, being observed in various forms at various stages of maturation, rather than different cell types. Participants in WKLYS noted two main types of granulocytes (named Type 1 and Type 2 during the workshop) in most of their observations. Morphological character-istics of these smeared granulocytes on the glass slides indicate that type 1 would correspond to basophilic granulocytes and type 2 correspond to eosinophilic granu-locytes, according Nöel et al., (1994). However, according the nucleus and cell diame-ter described by Carballal et al., (1997), type 1 would correspond to eosinophilic granulocytes and type 2 corresponds to basophilic granulocytes.
ICES/OSPAR WKLYS REPORT 2010
GRANULOCYTES IN MUSSELS
RED ARROWS IN FIGURE
BLACK ARROWS IN FIG-URE
Number of granules
Size of granules
Nucleus diameter
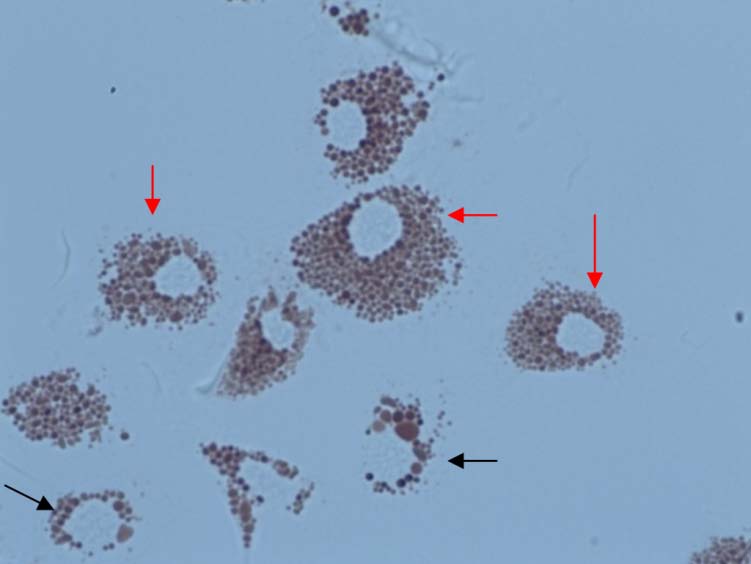
Figure 9. Main type of granulocyte cells frequently observed in haemolymph samples in Mytilus
sp. Red arrow = type 1; black arrow = type 2.
A recent laboratory study demonstrated that granulocytes in control animal typically appeared with the characteristic pseudopodial projection and that treated animal granulocytes clearly demonstrated an increase in their dimensions, cell rounding and a substantial loss of pseudopods (Calisi et al., 2008). Enlargement of granulocytes is considered an abnormality according to the ICES protocol. In fact, the endpoint stated is " …when 50% or more of the cells (…) exhibit lysosomal leakage or show abnor-malities such as enlargement (Figure 9)". WKLYS found the Figure 9 in ICES protocol (Figure 10) rather out of focus and confusing in terms of interpretation criteria (dis-playing an elongated granulocyte but with evidences of leakage of NR in the cytosol).
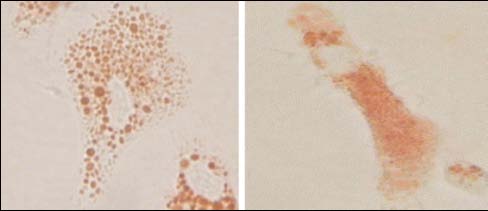
ICES/OSPAR WKLYS REPORT 2010
Figure 10. Example of the Figure 9 displayed in ICES Time Series Nº 36 (ICES, 2004).
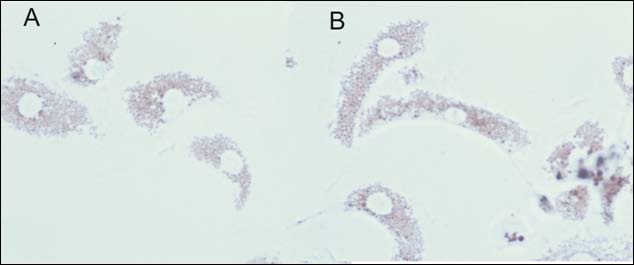
WKLYS discussed this aspect with A. Viarengo and M. Moore this aspect. It was agreed that elongated granulocytes, as far as they contain normal size lysosomes and they are able to retain the NR dye inside the lysosomes, should be considered as healthy cells in terms of the NRR assay protocol described by ICES (Figure 11). Pro-fessor A. Viarengo pointed out that, granulocytes with this characteristic are also de-scribed as healthy cell in the image analyses procedure presented during the MED POL training course.
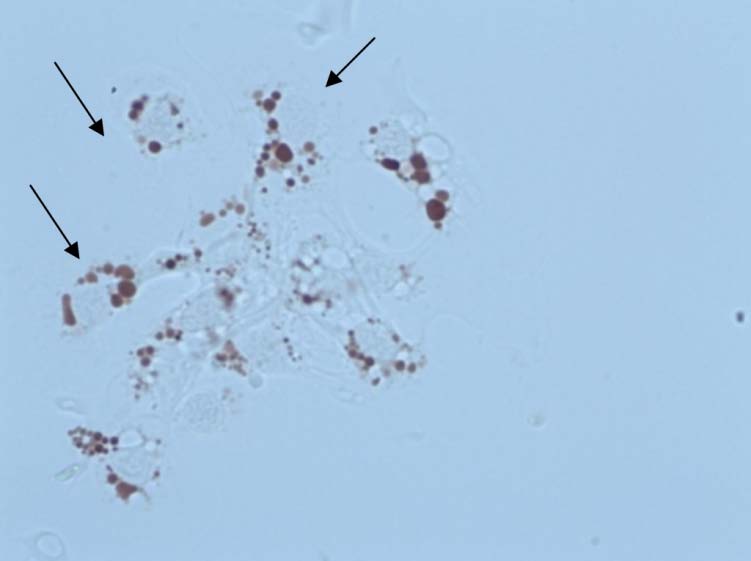
Figure 11. Example of granulocytes retaining NR dye inside the lysosomes (x600) (Mytilus gallo-
provincialis sampled in Spanish Mediterranean Waters). Cells usually show round (A) or elon-
gated shapes (B). That observation must be recorded in the data recording sheet, though in terms
of interpretation criteria elongated cells are considered normal cells retaining NR.
WKLYS and MED POL participants also discussed if all different types of granulo-cytes observed in a haemolymph sample should be considered. Different approaches were observed among participants. C. Martínez-Gómez (Spain) reported that in her laboratory, granulocytes with small granules (presumed BG) retaining NR are only being considered as healthy cells in terms of NRR time, as it was agreed by consulting Professor M. Moore. However, she indicated that in some samples she had observed granulocytes with large granules or granules of different size and red colour (presumed AG and BAGs) as the predomi-nant cell type in hemolymph. When this happens, the NRR assay is conducted on this granulocytes but test is terminated at time 90 min if they do not demonstrate evidence of dye loss (as usually it has been observed until the end of the test (180
ICES/OSPAR WKLYS REPORT 2010
min)) because their condition indicates lysosomal abnormalities (and these observa-
tions are registered in the data recording sheet).
J. Bignell (England) indicated that in his laboratory also granulocytes with small
granules (BG) retaining NR are also being considered as healthy cells in terms of NRR
time. Granulocytes with big granules or granules of different sizes are considered
stressed cells (presumed AG or BAG).
S. Sforzini (Italy) indicated that in her laboratory granulocytes with small granules
(BG) retaining NR are the only ones assessed in the image analyses assessment.
Action: Clarification is required to whether one or both types of granulocytes retain-
ing the NR in lysosomes should be assessed as healthy cells in the NRR assay proto-
cols (ICES, 2004; UNEP/RAMOGE 1999). Professor Moore proposed to contact Dr D.
Lowe to further clarify this point of discussion and will report back to WGBEC 2011.
J. Bignell and C. Martinez-Gómez noted that their experience of using NRR assay in
field samples is of three main of case-types of samples:
Case 1: Samples that demonstrate a predominance of small granulocytes retaining
NR in their lysosomes at the first microscope observation. These types of samples
evolve toward a) stationary situation b) swelling or enlargement of the lysosomal
compartment c) leakage of NR into the cytosol followed by a rounding process of the
cells.
Case 2: Samples containing a predominance of big granulocytes (usually demonstrat-
ing different sizes and strength of coloration) retaining NR inside their lysosomes at
the first microscope observation. This kind of samples normally evolves toward an
enhanced staining and/or enlargement of the lysosomal compartment but not to-
wards a leakage of NR into the cytosol.
Case 3: Samples that demonstrate a predominance of small stainless granulocytes in
their lysosomes at the first microscope observation. These types of samples normally
remain in this condition over the duration of the test, though in some cases they
evolve toward a weak enhancement of the lysosomal staining.
In case 1, NRR time is clearly established in the following observations, when 50% or
more of the granulocytes demonstrate evidences of leakage of NR into the cytosol. In
case 3, NRR Time is established at time 15 min. However in case 2, no clear agree-
ment was found. Anyhow, noted they have never observed a sample case 1 sample
evolving to-ward a case 3 sample.
References Cajaraville M. P., López M. C., Azevedo C., Villalba A. 1997. Menolymph cell types of the mus-
sel Mytilus galloprovincialis. Dis. Aquat. Org., 29:127–135.
Cajaraville M.P., Pal S.G. 1995. Mosphofunctional study of the haemocytes of the bivalve mol-
lusc Mytilus galloprovincialis with emphasis on the endolysosomal compartment. Cell. Strct. Funct., 20: 355–367.
Cajaraville, M.P., Pal, S.G. 1995. Morphofunctional study of the haemocytes of the bivalve mol-
lusc Mytilus galloprovincialis with emphasis on the endolysosomal compartment. Cell Structure and Function, 20 (5), pp. 355–367.
Carballal M.J., López, M.C., Azevedo, C., Villalba, A. 1997. Hemolymph cell type of the mussel
Mytilus galloprovincialis. Diseases of aquatic organisms, 29: 127–135.
Cheng T. C. 1981. Bivalves. In: Ratcliffe N. A, Rowley A. F. (Eds.). Invertebrate blood cells.
London, Academic press, pp 233–270.
ICES/OSPAR WKLYS REPORT 2010
Dolcetti L. and Venier P. 2002. Susceptibility to genetic damage and cell types in Mediterra-
nean mussels. Marine Environmental Research, 54: 487–491.
Hine, P.M. 1999. The inter-relationships of bivalve haemocytes. Fish and Shellfish Immunol-
ogy, 9 (5), pp. 367–385.
Moore M.N. and Lowe D.M. 1977. The cytology and cytochemistry of the haemocytes of Myti-
lus edulis and their responses to experimentally injected carbon particles. J. Invert. Path., 29: 18–30.
Nöel D., Pipe R., Elston R., Bachere E., Mialhe. 1994. Antigenic characterization of haemocyte
subpopulations in the mussel Mytilus edulis by means of monoclonal antibodies. Mar. Biol., 119:549–556.
Noël D., Bachère E., Mialhe E. 1993. Phagocytosis associated chemiluminiscence of haemocytes
in Mytilus edulis (Bivalvia). Dev. Comp. Immunol., 17: 483–493.
Ottaviani E., Franchini, A., Barbieri, D. and Kletsas D. 1998.Comparative and morphofunc-
tional studies on Mytilus galloprovincialis haemocytes: presence of two aging-related haemocyte stages. Ital. J. Zool, 65: 349–354.
Pipe R.K. 1990. Differential binding of lectins to haemocytes of the mussel Mytilus edulis. Histo-
chem J., 22:595–603.
Pipe, R.K. 1990b. Hydrolytic enzymes associated with the granular haemocytes of the marine
mussel Mytilus edulis. Histochemical Journal, 22 (11), pp. 595–603.
Rasmussen L. P. D., Hage E., Karlog O. 1985. An electron microscope study of the circulating
leucocytes of the marine mussel Mytilus edulis. J. Invertebr.Pathol, 45:158–167.
Renwrantz L. 1990. Internal defence system of Mytilus edulis. In: G.B. Stefano (Ed.), Neurobiol-
ogy of Mytilus edulis. Studies in neurosciences No. 10. Manchester University Press, Man-chester, pp. 256–275.
ICES/OSPAR WKLYS REPORT 2010
Review Assessment Criteria proposed by OSPAR to assess Lysosomal membrane stability in mussels by using NRR assay
"Assessment criteria for Lysosomal membrane stability in mussels, by applying the Neutral Red Retention Assay has been determined from data based on numerous studies and in all organisms tested to date as protozoans, annelids (terrestrial and marine), molluscs (freshwater and marine), crustaceans (terrestrial and aquatic), echinoderms and fish, the absolute values for measurement of lysosomal stability (NRR and cytochemical method) are directly comparable" (OSPAR, 2007).
Assessment criteria adopted currently by ICES.
WKLYS attempted to identify the dataset that had been used to calculate and estab-lish ACs for lysosomal membrane stability. However, this dataset is actually not available as conclusions were drawn from review of a large number of papers, and many years of practical experience (Professor M. Moore). Nonetheless, a paper de-scribing how assessment criteria were established would be a welcome addition to the scientific literature. A seasonal study of lysosomal membrane stability revealed a significant reduction of neutral red retention time in organisms sampled in July in a reference site from Adri-atic sea (Bochetti and Regoli, 2006), mean values ranging from 60 to 100 minutes. Overall, the NRR values observed in May–June in most of the Spanish sampling sites were lower than the minimum optimum (120 minutes) established for healthy mussel populations. Studies conducted along the Portuguese coast revealed similar NRR times (mean values ranging from 20 to 100 min) (Castro et al., 2004). In a recent study, mussels caged in a reference site used as control displayed also mean NRR times of about 90–100 min (Bocchetti et al., 2008). Though in some expo-sure studies, mean NRR times values in control groups are upon 120 min (e.g. Mamaca et al., 2005), values ranging from 54 to 74 min has been also reported in sea-water and solvent control groups (e.g. Fang et al., 2008).
ICES/OSPAR WKLYS REPORT 2010
10 Proposals for effective and repeatable external quality assurance
programmes for the measurement of lysosomal membrane sta-bility (ICES/OSPAR)
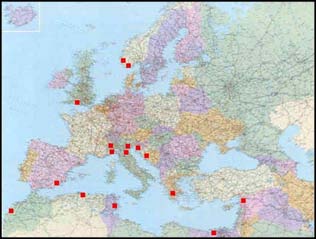
Quality assurance WKLYS made a review of the NRR assay intercalibration exercises for lysosomal sta-bility carried out in the past years. The first exercises were conducted in the ICES/UNESCO-IOC-GEEP Bremerhaven Research Workshop and UNEP-MEDPOL programme, and for the neutral red retention method in the GEF Black Sea Environ-ment Programme (Köhler et al., 1992; Lowe et al., 1992; Moore et al., 1997, 1998a, b; Viarengo et al., 2000). The results from these operations indicated that both tech-niques could be used in the participating laboratories in an effective manner with insignificant inter-laboratory variability. The standards used in these intercalibration involved digestive glands from marine mussels prepared at the University of Genoa/University of Eastern Piedmont, Alessandria (Italy). Comparisons of the cyto-chemical and the neutral red retention techniques have been performed in fish liver (ICES-IOC Bremerhaven Workshop, 1990) and in mussels experimentally exposed to PAHs (Lowe et al., 1995) as well in within the framework of the BEEP project. Results from the ring test undertaken within the framework of the BEEP project (Work Pack-age 4) were somewhat disappointing and there was a poor agreement in the results between the participating laboratories. This later intercomparison exercise involved transportation of live mussel samples to the partner laboratories (Final Report BEEP Project; http://beep.lptc.u-bordeaux.fr). The first intercalibration exercise combining MED POL and ICES a laboratory was carried out in 2009, and 16 laboratories participated (Figure 12). The results are ex-pected by November 2010.
Figure 12. Location of the laboratories that have participated in the NRR assay intercalibration
exercise carried out in 2009.
10.1 New proposal for NRR assay intercalibration exercise
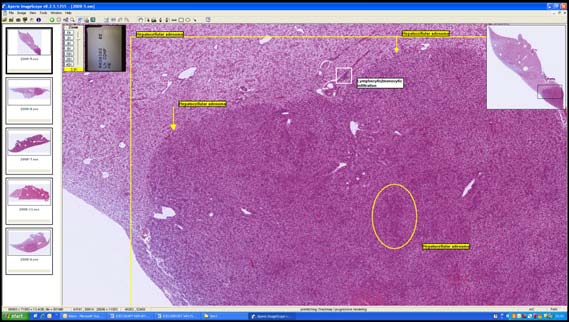
John Bignell described the use of virtual slides as a unique alternative method for undertaking intercalibration exercises incorporating microscope preparations. In es-sence, virtual slides are image scans of a microscope slide at a high magnification (generally 200–400x magnification) (Figure 13). These scans are stored as electronic
ICES/OSPAR WKLYS REPORT 2010
files which can then be opened on an individual's computer and viewed in a similar fashion to Google Earth. Furthermore, virtual slides can also be annotated. While specialist equipment is required in order to prepare the slides, no specialist equip-ment is required to view them. The BEQUALM Fish Disease Measurement pro-gramme has been utilizing virtual slides for three years and has proven to be a great success. At the end of the intercalibration, a number of small annotation files are e-mailed to participants who subsequently import them into their virtual slides. The annotation layer is then superimposed over the virtual slides describing any pathol-ogy and diagnostic criteria to the participant.
Figure 13. Typical example of a virtual slide showing slide box, slide identifier, slide overview
and main browser pain with annotation layer.
There is considerable potential for virtual slide intercalibrations to be used in the con-text of the neutral red retention assay. This would be achieved by scanning a set number of neutral red slides at specific time intervals and sending the produced vir-tual slides to participants. The benefit of this approach is that all participants would be analysing the same samples from the same mussels meaning that results are di-rectly comparable. The disadvantage of this approach is that the intercalibration would only assess the ability to interpret results and not test the ability for the par-ticipant to undertake the assay itself.
ICES/OSPAR WKLYS REPORT 2010
11 Closure of the meeting
C. Martínez-Gómez would lead on completion of the meeting report, with help from J. Bignell and A. Viarengo. The workshop was closed at 14:00 on 17 September 2010.
ICES/OSPAR WKLYS REPORT 2010
Annex 1: List of participants
Centre for Environment,
Fisheries and Aquaculture
Science (Cefas) Weymout
The Nothe Barrack Road DT4 8UB Weymouth, Dorset UK
Instituto Español de
Oceanografía (IEO)
Centro Oceanográfico de
Varadero 1 San Pedro del Pinatar 30740 Murcia Spain
Plymotuh PL 1 3DH
University of the Eastern
[email protected]
Via Bellini, 25G
IT-15100 Alessandria
University of Piemonte
Marine Scotland Science
[email protected]
Laboratorio de Biología Celular, +34(0)
[email protected]
fac. de Ciencias y tecnología.
Universdiad del Pais vasco.
Leioa Barrio sarriena s/n Spain
ICES/OSPAR WKLYS REPORT 2010
Draft agenda and tentative timetable WKLYS 16–2.17 September 2010
chair, tour the table
Adoption of agenda CMG, JB
tional/international
monitoring activities of the use
of the neutral red retention assay for lysosomal
membrane stability in mussel samples
/international monitoring
activities within OSPAR / MEDPOL
harmonize the standard analytical
methods of NRR assay to measure lysosomal
membrane stability in mussels
currently recom-mended by ICES
Collect and discuss
graphical material to elaborate,
harmo-nize and clarify criteria of
interpretation to assess Lysosomal membrane Stability
in mussels by using Neutral red
membrane Stability in mussels (NRR
assay) by using Image analysis:
Methodology recommended by MED POL
ICES/OSPAR WKLYS REPORT 2010
Review Assessment CMG, JB, MM?
Criteria used to assess Lysosomal membrane Stability
in mussels by using Neutral red
retention Assay proposed by ICES
proposals for effective and
repeatable exter-nal quality assurance
pro-grammes for the measurement of
lysosomal membrane stability (ICES/OSPAR).
ICES/OSPAR WKLYS REPORT 2010
Annex 3: Terms of reference for WKLYS
Terms of Reference for an ICES/OSPAR Workshop on 16–17 September on the ly-sosomal stability data quality and interpretation (WKLYS), in association with a MEDPOL training workshop to be held in Università del Piemonte Orientale "Amedeo Avogadro", Alessandria, Italy on 13–17 September 2010, and convened by Concepción Martínez-Gómez (Spain).
Objectives The objectives of the joint MEDPOL and ICES/OSPAR workshops are:
1 ) provide training in the measurement of lysosomal membrane stability in
2 ) provide training in other biological effects measurement techniques for
marine samples relevant to OSPAR and to MSFD, including of lipofuscin accumulation, micronuclei enumeration and Stress on Stress.
3 ) develop guidance on the interpretation of the results by using neutral red
retention assay for lysosomal membrane stability in marine samples, giv-ing particular attention to the variation in sample characteristics experi-enced through the ICES/OSPAR area;
4 ) develop written and graphical material to elaborate, harmonize and clarify
criteria of interpretation and the standard analytical method for the neutral red retention assay of lysosomal membrane stability (ICES/OSPAR);
5 ) develop proposals for effective and repeatable external quality assurance
programmes for the measurement of lysosomal membrane stability (ICES/OSPAR).
Activities The ICES/OSPAR element of the workshop will be organized around the following activities, building on the activities of SGIMC (and WKIMON):
Presentation and discussion of the background information
a ) existing experience of the use of the neutral red retention assay for ly-
sosomal membrane stability in mussel samples will be presented, re-viewed, and collated;
b ) the information will be reviewed in the light of experience during the
training elements of the MEDPOL Workshop.
Output from the Workshop
c ) enhanced methodological guidance for the neutral red retention assay for
lysosomal membrane stability in mussel samples;
d ) proposals for effective and repeatable external quality assurance pro-
grammes for the measurement of lysosomal membrane stability by the neutral red retention assay.
ICES/OSPAR WKLYS REPORT 2010
Organisation The workshop will be coordinated by Aldo Viarengo (Italy) for MEDPOL and by Concepción Martínez-Gómez (Spain) for ICES/OSPAR. Participants: the ICES/OSPAR component of the workshops should be attended by scientists with experience of the application of the neutral red retention assay for ly-sosomal membrane stability in marine samples and in the assessment of data, and in the development of QA schemes to ensure that the resulting report to ICES/OSPAR is of high quality.
Preparatory work The following existing documents are relevant to the Workshop and will be collated and distributed prior to the Workshop.
a ) Background documents in the OSPAR Strategy for Hazardous Substances; b ) OSPAR Background Documents concerning lysosomal membrane stability,
relevant biological effects;
c ) ICES TIMES and MEDPOL documents on the measurement of lysosomal
membrane stability by the neutral red retention assay in marine samples. Reports of relevant meetings of ICES/OSPAR WKIMON and SGIMC groups, and of ICES WGBEC;
d ) Assessment criteria for biological effects currently adopted by OSPAR and
Location The Workshop will be held at Università del Piemonte Orientale "Amedeo Avogadro", Alessandria, Italy.
DEVELOP EFFECTIVE WORKING RELATIONS WITH THE MEDPOL ORGANIZER AND DEVELOP PRO-
FEBRUARY–JUNE 2010
WORKSHOP ORGANIZER
GRAMME FOR THE WORKSHOP.
July–August 2010
Workshop organizer and host
Collate and distribute back-
13–18 September 2010
Report of the Workshop finalized and made available to ICES and
OSPAR Secretariats
Output from Workshop pro-
vide input to SGIMC.
ACOM to review Workshop
output as a contribution to the
further development of guidance on integrated moni-toring of chemicals and
biological effects (OSPAR request 8/2008).
ICES/OSPAR WKLYS REPORT 2010
Annex 4: Recommendations
FOR FOLLOW UP BY:
1. ICES and OSPAR to review the WKLYS report
2. Support the organization of a second
workshop on lysosomal stability data quality and
interpretation using neutral red retention assay) in mussels to be held in conjunction with the
WGBEC in mid February 2011 in Vigo (Spain) to further complete harmonization of lysosomal
stability data quality and interpre-tation. 1. To update the TIMES ICES document No 36
concerning NRR assay 2. To perform a comparative assessment on LMS
using NRR assays between new MED POL
endpoints (% change) and ICES/OSPAR endpoints (NR Retention Time) 3. To review and collate the calculation
WGBEC, CMG, JB, DL, AK
procedure of Retention Time endpoint by ICES countries monitoring programmes that are e
applying NRR assay. To clarify how NRRT it was recorded to establish current ACs proposed in the Background Document on Biological Effects
Monitoring Techniques, Chapter 4 "Lysosomal stability as a global health status indicator in
biomonitoring", (OSPAR 2007). (ACs for NRR assay and cytochemical assay on cryostat
ICES/OSPAR WKLYS REPORT 2010
Annex 5: UNEP/RAMOGE protocol to assess LMS by using the
Neutral red retention assay in mussels
MED POL UNEP/MAP Indicator fact sheet Biomarker of stress: Lysosomal membrane stability in molluscs and fish cells
VERSION 04-04-05
Key message A biomarker of stress is a biological parameter which value may change in relation to the toxic
effects of pollutants. A biomarker of stress is able to integrate the biological response to the total charge of pollutants bioavailable for the sentinel organisms. Lysosomal membrane stability is the best-acknowledged stress biomarker: it is extremely sensitive and the change of its value is extremely rapid in pollutant-exposed organisms.
Policy relevance Lysosomal membrane stability was adopted as stress biomarker in different interna-tional programmes such as the UNEP MAP Mediterranean Biomonitoring Pro-gramme. This stress biomarker was also employed in the framework of the RAMOGE activity and in the UNIDO activity of the Black Sea. In the BEEP (Biological Effects of Pollutants in Marine Coastal Ecosystems) UE Programme it was considered a "core" biomarker. It is a biomarker suggested by ICES and it is in the list of the biomarker intercalibration programme realized by Belquam.
Policy context UNEP MAP Mediterranean Biomonitoring Programme,. RAMOGE activity and UNIDO and different regional biomonitoring programmes have proposed the utiliza-tion of this biomarker to evaluate stress response in fish and molluscs.
Environmental context Lysosomes are cytoplasmic, single membrane organelles that display peculiar charac-teristics: they contain more than 40 different classes of hydrolytic enzymes (such as proteases, nucleases, lipases, etc.) with optimal activities at acidic pHs. These en-zymes are able to hydrolyze essentially all biological molecules, from proteins and nucleic acids and nucleotides to complex sugars and lipids. The lysosomal matrix has a pH of 4,5–5; this low pH is maintained by active proton pumping due to the activ-
ICES/OSPAR WKLYS REPORT 2010
ity of a H+-ATPase present in the membrane of the organelle and by the acidic pro-teins within the lysosomal matrix. In the cells, lysosomes display heterogeneous shapes; this is mainly due to the fact that, after their assembling in the Golgi apparatus, they have an initial size of about 0,5 μm: these vesicles, having a pH of about 6 and an increasing amount of hydrolytic enzymes, are not functionally active, and are usually named ‘primary lysosomes'. When mature lysosomes directly take up components from the cytoplasm or fuse with autophagosomes or hetero phagosomes, their size increases up to several μm and the organelles, whose pH is about 5, are actively involved in the digestion of bio-logical macromolecules (‘secondary lysosomes): at the final phase of their activity a residual body containing un-degradable material (mainly lipofuscin) and with mini-mal hydrolytic activity is formed (‘tertiary lysosomes', i.e. residual bodies). In those cells able to active exocytosis the content of the residual bodies is released into the extracellular fluids. The functions of lysosomes in different cell types and tissues of different organisms (from protozoa to mammals) may be specific and very different, but in all organisms the lysosomal vacuolar system is involved in the degradation of the material as-sumed in the cell by endocytosis, as well as in the regulation of the catabolic rate of cellular macromolecules, proteins in particular (Moore et al., 1985; Viarengo, 1989). It has long been known that lysosomes, despite their acidic internal pH, are also able to accumulate metal cations: this seems to be mainly due to the presence in the ly-sosomal matrix of lipofuscins, end-products of peroxidation processes that are able to trap inside their growing granules different metal cations (Viarengo, 1989). Ly-sosomes are also able to accumulate lipophilic organic compounds, such as aromatic hydrocarbons and PCBs (Viarengo, 1989; Viarengo and Nicotera, 1991). This fact is of particular importance in organisms, such as mussels, whose mixed function oxy-genase (MFO) activity is extremely low, and a typical cytocrome p450 is absent: in this case, most aromatic compounds are not metabolized but accumulated in these organelles, that thus represent one of the most important sites of accumulation of or-ganic pollutants, and therefore of their action at the cellular level. It is now widely accepted that the accumulation of contaminants within these organ-elles may represent the main mean by which toxic chemicals are able to alter ly-sosomal physiology, and consequently increase the protein catabolic rate stimulating the autophagic activity in the target cells. Moreover, these organelles reveal a high capacity of pollutant accumulation and are at the same time extremely sensitive to minimal concentrations of toxic chemicals that penetrate into the cells (in mussels, nanomolar concentration of both inorganic and organic pollutants are able to destabi-lize the lysosomal membranes and to activate protein catabolizm). Moreover, recent data obtained in mussels demonstrated that inorganic pollutants, such as Cu, Hg, Cd, can alter lysosomal activity by affecting Ca-dependent cell sig-nalling. It was demonstrated that the metal-induced increase in cytosolic [Ca2+] con-centrations is able to activate a Ca-dependent phospholipase A2 (PLA2). PLA2 binds to lysosomal membranes and activates the process of vacuole fusion with the associ-ated increase in protein catabolizm (Burlando et al., 2002). Moreover, organic con-taminants known as endocrine disrupters have been demonstrated to alter lysosomal membrane stability of mussel cells through activation of components of kinasemedi-ated cell signalling, in particular of the stress-activated Mitogen Activated Protein Kinase p38 and protein kinase C (PKC) (Canesi et al., 2004).
ICES/OSPAR WKLYS REPORT 2010
An increase in lysosomal size indicates an enhanced rate of fusion of primary ly-sosomes with auto/hetero phagosomes, but for pollutant-exposed cells, lysosomal enlargement usually indicates increased autophagy, which may greatly contribute to induction of cell dysfunctions and, as an ultimate consequence, to cell death. The evaluation of lysosomal membrane stability is a suitable parameter to quantify the changes in the lysosomal activity induced by pollutants. This parameter was found to be sensitive to the presence of minimal, nM concentrations of toxic chemi-cals in the marine environment and accumulated in the target cells of different organ-isms. In mussels, lysosomal membrane stability represents the simplest, most sensitive and low-cost biomarker to evaluate the physiological status of the organ-isms. Two methodologies have been developed to evaluate lysosomal membrane stability: the former is applied to living cells (such as haemolymph cells of mussels), the latter to cryostatic tissue sections. The in vivo lysosomal membrane stability assay utilizes the lipid-soluble dye Neutral Red (NR) that is rapidly taken up by lysosomes; once protonated, is it sequestered in the lysosomal matrix. The assay evaluates the lysosomal membrane stability on the basis of the NR retention time by the organelles: control cells, with intact lysosomal membranes, usually demonstrate retention times over 60 min. In the cells of organ-isms exposed to pollutants, the NR retention is reduced proportionally to lysosomal membrane damage induced by toxic chemicals present in the water and accumulated in the cells. The method is very simple, low-in-cost, extremely sensitive, and it only needs basic equipment for the analysis (a microscope in the simplest setting). The evaluation of lysosomal membrane stability in tissue cryostatic sections is also highly sensitive, but it needs more training for the histological analyses of the sam-ples, and a more complex instrumentation (at least a cryostat and a microscope). It must be said, however, that this method allows not only to collect data of ly-sosomal membrane stability, but also a good evaluation of the lysosomal size, a bio-marker often utilized in biomonitoring programmes; moreover, the analysis of the cells of mussel digestive tubules also permits to estimate histological changes due to the increased lysosomal activity (i.e. reduction in the volume of cell cytoplasm) and also to evaluate possible changes in the proportion of digestive/secreting cells in the tubules (Cajaraville, 1995). Both these parameters are of great importance in the integration of the biomarker data by the expert system to rank the stress syndrome of the animals.
Assessment of the indicator
Background It is very difficult to evaluate the molecular changes affecting the permeability of the lysosomal membrane. These analyses require extensive purified lysosomal mem-brane preparations and their examination at molecular level. An easier way to assess this parameter is to examine whether its normal physiological function has been al-tered or disrupted following exposure to pollutants. An approach that links the results of morphological and biochemical analysis to de-scribe pathological alterations is cytochemistry. In addition to the requirement of very small amounts of tissue samples, this technique is also ideal to detect changes in particular target cells and tissues.
ICES/OSPAR WKLYS REPORT 2010
Cytochemistry has been successfully applied to assess lysosomal integrity by visual-izing the hydrolytic enzymes within the lysosomes, and has proven to be a rapid and sensitive tool for evaluating the effects of organic xenobiotics and other injurious agents at very low intracellular concentrations. This generalized response occurs in all cell types ranging from fungi to vertebrates, so that such a cytochemical test can be applied on a fairly widespread basis.
Determination of lysosomal membrane stability in living cells: neutral red retention assay
Neutral red is a lipophilic dye and as such will freely permeate the cell membrane. Within cells the compound becomes trapped by protonisation in the lysosomes and accumulated in these organelles, where it can be visualized microscopically. The de-gree of trapping of this lysosomotropic marker depends on the pH of the lysosome as well as the efficiency of its membrane associated proton pump (Segien, 1983). The acidic environment of lysosomes is maintained by a membrane Mg2+- ATPase dependent H+ ion proton pump (Ohkuma et al., 1982); the neutral red retention assay reflects the efflux of the lysosomal contents into the cytosol following damage to the membrane and, possibly, impairment of the H+ ion pump (Lowe et al., 1992). So any impairment of this latter system will result in a reduction of the dye retention assay. Studies indicate that, similarly to the cytochemical method described above, the neu-tral red retention assay is sensitive to the main classes of chemical pollutants (Lohse, 1990). The following protocol has been specifically adapted to be used on mussels.
Chemicals and solutions
Physiological saline
20 mM (4.779) Hepes 436 mM (25.489) NaCl 53 mM (13.069) MgSO4 10 mM (0.759) KCl 10 mM (1.479) CaCl2 Dissolve these in 1 litre of distilled water. Gas for 10 minutes (95%02:5% CO2) and adjust to pH 7.3 with 1 M NaOH. Store solution in refrigerator, but use it at room temperature. B.
Prepare stock solution by dissolving 20 mg of neutral red powder in 1 ml di-methyl-sulfoxide (DMSO). Transfer 5 1.11 of stock solution into 995 1.11 of physiological saline (working solution). Keep neutral red in the dark and in fridge when not utilized. The working solution must be prepared freshly be-fore analysis.
Practical evaluation The following procedure is according to Lowe et al. (1992) and Lowe et al. (1995). Fill the eppendorf tubes with sigmacote (SIGMA) far 10–30 minutes, then return sig-macote to container (it is reusable). Put 2 μl of Poly-L-Lysine (SIGMA), diluted 1 to 10
ICES/OSPAR WKLYS REPORT 2010
with distilled water, on a microscope slide and spread out with a cover slip. Leave to dry in a humidity chamber. Insert scissors half way along the ventral surface of the mussel and partially disclose the valves to allow the insertion of the hypodermic syringe. Drain the water from the shell. Fill a hypodermic syringe with 0.5 ml of physiological saline then aspirate 0.5 ml of haemolymph from the posterior abductor muscle of the mussel. After obtaining the haemolymph sample, discard the needle and expel the content in a siliconised eppendorf tube. Dispense 40 μl of haemolymph-saline mixture on the slide, in the same position where the poly-l-lysine was added and incubate in humidity chamber for 30 minutes to allow the cells to attach. Carefully drain the excess solution from the slide by plac-ing the slide on its side and letting the liquid run-off. Add 40 μl of the neutral red working solution and leave in a humidity chamber for 15 min (maintained 15–16°C during the analysis). Apply a cover slip and inspect the preparation under a micro-scope. Look at the slides every 15 minutes for the first hour then every 30 minutes for the next two hours thereafter. Determine the time at which 50% of the lysosomes in the cells leaches out neutral red in the cytosol. Derive a mean value for each specimen then a global mean far all specimens pertaining to the same pool. Compare samples from monitored field sites with those taken from reference field sites and determine gradient of cytotoxicity. An increase in leaching rates would indicate cellular stress due to pollution.
more than 50% of the lysosomes in the cells retaining neutral red
less than 50% of the lysosomes in the cells retaining neutral red
Data source B. Burlando, B. Marchi, I. Panfoli, A. Viarengo. Essential role of Ca2+-dependent phospholipase
A2 in estradiol-induced lysosome activation. Am J Physiol Cell Physiol. 283(5):C1461-8 2002.
M.P. Cajaraville, Y. Robledo, M. Etxeberria, I. Marigomez. Cellular biomarkers as useful tools
in the biological monitoring of environmental pollution: molluscan digestive lysosomes. Cell Biology in Environmental Toxicology. M.P. Cajaraville editor pp. 29–55 1995.
L. Canesi, M. Betti, C. Ciacci, A. Scarpato, B. Citterio, C. Pruzzo, G. Gallo. Environmental es-
trogens can affect the function of mussel haemocytes through rapid modulation of kinase pathways. Gen. Comp. Endocrinol., 138:58–69, 2004.
A. Viarengo, A. Marro, B. Marchi, B. Burlando. Single and combined effects of heavy metals
and hormones on lysosomes of haemolymph cells from the mussel Mytilus galloprovin-cialis. Mar. Biol. 137: 907–912 2000.
A. Viarengo, L. Canesi, M. Pertica, D.R. Livingstone. Seasonal variations in the antioxidant
defence systems and lipid peroxidation of the digestive gland of mussels. Comp. Biochem. Physiol. 100C: 187–190 1991.
ICES/OSPAR WKLYS REPORT 2010
A. Viarengo, P. Nicotera. Possible role of Ca2+ in heavy metal cytotoxicity. Comp. Biochem.
Physiol. C 100:81–84 1991
A. Viarengo. Heavy metals in marine invertebrates: mechanisms of regulation and toxicity at
the cellular level. Aquatic Sciences Review 1(2): 295–317.1989.
M.N. Moore, J. Widdows, J.J. Cleary, R.K. Pipe, P.N. Salked, P. Donkin, S. Farrar, S.V. Evans,
P.E. Thomson. Responses of the mussel Mytilus edulis to copper and phenanthrene. Mar. Environ. Res. 14:167–183 1984.
M.N. Moore. Cellular responses to pollutants. Mar. Pollut. Bull. 16: 134–139 1985.
ICES/OSPAR WKLYS REPORT 2010
Annex 6: Illustrated guidance draft document for interpretation of
Lysosomal Membrane Stability observations by using NRR assay in mussels
(Author of the pictures C. Martínez-Gómez).
1. Evidence of lysosomal membrane stabilization
Image 1. Examples of normal granulocytes observed in M. edulis and M. galloprovincialis with
small lysosomes. Neutral red is well retained inside the lysosomes.
Image 2. Example of granulocytes retaining NR dye inside the lysosomes (x600) (Mytilus gallo-
provincialis sampled in Spanish Mediterranean Waters). Cells usually show round (A) or elon-
gated shapes (B). That observation must be recorded in the data recording sheet, though in terms
of interpretation criteria elongated cells are considered normal cells retaining NR.
ICES/OSPAR WKLYS REPORT 2010
Image 3. Example of a haemolymph sample (x600) in which more than 50% of the granulocytes
retain NR dye inside the lysosomes. (Mytilus galloprovincialis sampled in Spanish Mediterra-
nean Waters). In this image pseudopods are clearly well developed in most of the granulocytes.
Arrow indicates evidence of leakage of NR into the cytosol.
ICES/OSPAR WKLYS REPORT 2010
Image 4. Example of a haemolymph sample (x200) in which more than 50% of the granulocytes
retain NR dye inside the lysosomes. (Mytilus galloprovincialis sampled in Spanish Mediterra-
nean Waters).
2. Evidence of lysosomal membrane destabilization I
Image 5. Examples of granulocytes observed in M. edulis and M. galloprovincialis with small ly-
sosomes. Neutral red is not retained inside the lysosomes.
ICES/OSPAR WKLYS REPORT 2010
Image 6. Example of a haemolymph sample (x400) in which more than 50% of the granulocytes
are not able to retain Neutral Red dye inside the lysosomas (Mytilus galloprovincialis from Span-
ish Mediterranean Waters).
Image 7. Example of a haemolymph sample (x200) in which more than 50% of the granulocytes
are not able to retain Neutral Red dye inside the lysosomas (Mytilus galloprovincialis from Span-
ish Mediterranean Waters).
ICES/OSPAR WKLYS REPORT 2010
3. Evidence of lysosomal membrane destabilization II
Image 8. Sequential process observed by using NRR assay. Rounding-up of the cells usually fol-
lows the leakage of neutral red into the cytosol of the granulocytes with small lysosomes.
Image 9. Example of a haemolymph sample that demonstrated a predominance of granulocytes
with small lysosomes retaining NR at the first microscope observation. This sample evolved in
time toward (1) swelling or enlargement of the lysosomal compartment (2) leakage of NR into the
cytosol, followed in time by a rounding process of the cells (3).
4. Evidence of lysosomal abnormalities (I)
Image 10. Haemocytes showing lysosomal vacuolation, which generally occurs much later (e.g. 90
minutes) into the incubation, although once again in severely impacted animals is evident within
the first 15–30 minutes of incubation.
ICES/OSPAR WKLYS REPORT 2010
5. Evidence of lysosomal abnormalities (II)
Image 11. In cases where an animal is severely impacted lysosomal enlargement is evident at the
first microscopic inspection of the cells following the 15 minute incubation in neutral red. In
lesser impacted animals it will occur later during the incubation (after 45 min). Interpretation of
these results needs further discussion in terms of NRR time endpoint.
5. Evidence of lysosomal abnormalities (III)
Image 12. Example of two types of granulocytes with lysosomes retaining NR inside the ly-
sosomes. M. galloprovincialis from and Italian sampling site. A) Granulocyte with small ly-
sosomes B) Granulocyte showing lysosomal swelling or granulocyte belonging to a different
population of cell granulocytes (i.e. Basophilic granulocyte). Further discussion is necessary in
terms of criteria interpretation by using NRR assay.
ICES/OSPAR WKLYS REPORT 2010
Image 13. Example of granulocytes with vacuoles (lipid droplets?). M. edulis from Wadden Sea
(The Netherlands) sampling site. Further discussion is necessary in terms of criteria interpreta-
tion by using NRR assay.
Image 14. Example of granulocytes with vacuoles (lipid droplets?). M. galloprovincialis from and
Italian sampling site. Further discussion is necessary in terms of criteria interpretation by using
NRR assay.
ICES/OSPAR WKLYS REPORT 2010
Image 15. Example of granulocytes with vacuoles (lipid droplets?). M. galloprovincialis from and
Mediterranean Spanish sampling site.
Image 16. Example of granulocytes full of vacuoles (lipid droplets or lysosomes?). M. galloprovin-
cialis from a Mediterranean Spanish sampling site.
ICES/OSPAR WKLYS REPORT 2010
Image 17. Example of granulocytes retaining NR dye inside the lysosomes but showing vacuoles
(lipid droplets?) and small number of lisosomes with different staining colour. M. galloprovin-
cialis from a Mediterranean Spanish sampling site. Further discussion is necessary in terms of
criteria interpretation by using NRR assay.
Image 18. Example of granulocytes retaining NR dye inside the lysosomes but showing vacuoles
(lipid droplets?) and low number of lisosomes with different staining colour. M. galloprovin-
cialis from a Mediterranean Spanish sampling site. Further discussion is necessary in terms of
criteria interpretation by using NRR assay.
ICES/OSPAR WKLYS REPORT 2010
Image 19. Example of a macrogranulocyte found in a sample of haemolymph in M. galloprovin-
cialis from a Mediterranean Spanish sampling site. These cells are considered to be the result of a
fusion of granulocytes in some pathological conditions or rejection of grafts. Multinucleate giant
haemocyte have been reported in M. galloprovincialis heavily infected by a trematode, Proctoeces
maculates (Carballal et al., 1997).
Image 20. Example of a haemolymph sample in which granulocytes are showing different types
of lysosomal abnormalities. Arrow indicating some pathological conditions (infection by para-
sites?). Further research is necessary. M. galloprovincialis from a Spanish Mediterranean site.
Source: http://ices.dk/sites/pub/CM%20Doccuments/CM-2010/ACOM/ACOM6110.pdf
The Harry Benjamin International Gender Dysphoria Association's Standards Of Care For Gender Identity Disorders, Sixth Version Committee Members: Walter Meyer III M.D. (Chairperson), Walter O. Bockting Ph.D., Peggy Cohen-KettenisPh.D., Eli Coleman Ph.D., Domenico DiCeglie M.D., Holly Devor Ph.D., Louis Gooren M.D., Ph.D., J. Joris HageM.D., Sheila Kirk M.D., Bram Kuiper Ph.D., Donald Laub M.D., Anne Lawrence M.D., Yvon Menard M.D., StanMonstrey M.D., Jude Patton PA-C, Leah Schaefer Ed.D., Alice Webb D.H.S., Connie Christine Wheeler Ph.D.
Programa Nacional de Eliminación de la Oncocercosis de Colombia (PNEOC). Protocolo de Vigilancia en Salud Pública Fernando de la Hoz DOCUMENTO ELABORADO POR Director General INS Programa Nacional de Eliminación de la Mancel Enrique Martínez Duran Oncocercosis de Colombia (PNEOC) Director Vigilancia y Análisis del Riesgo en Salud Pública







