Doi:10.1016/s0140-6736(07)60669-2
Valsartan in a Japanese population with hypertension
and other cardiovascular disease (Jikei Heart Study):
a randomised, open-label, blinded endpoint
morbidity-mortality study
Seibu Mochizuki, Björn Dahlöf, Mitsuyuki Shimizu, Katsunori Ikewaki, Makoto Yoshikawa, Ikuo Taniguchi, Makoto Ohta, Taku Yamada, Kazuhiko Ogawa, Kiyoshi Kanae, Makoto Kawai, Shingo Seki, Fumiko Okazaki, Masayuki Taniguchi, Satoru Yoshida, Naoko Tajima, for the Jikei Heart Study group*
Summary
Background Drugs that inhibit the renin–angiotensin–aldosterone system benefi t patients at risk for or with existing Lancet 2007; 369: 1431–39
cardiovascular disease. However, evidence for this eff ect in Asian populations is scarce. We aimed to investigate See
Comment page 1407
whether addition of an angiotensin receptor blocker, valsartan, to conventional cardiovascular treatment was eff ective *Members listed at end of article
in Japanese patients with cardiovascular disease.
Division of Cardiology
(Prof S Mochizuki MD,
Methods We initiated a multicentre, prospective, randomised controlled trial of 3081 Japanese patients, aged Prof M Shimizu MD,
20–79 years, (mean 65 [SD 10] years) who were undergoing conventional treatment for hypertension, coronary heart K Ikewaki MD, M Yoshikawa MD,
I Taniguchi MD, M Ohta MD,
disease, heart failure, or a combination of these disorders. In addition to conventional treatment, patients were assigned T Yamada MD, K Ogawa MD,
either to valsartan (40–160 mg per day) or to other treatment without angiotensin receptor blockers. Our primary K Kanae MD, M Kawai MD,
endpoint was a composite of cardiovascular morbidity and mortality. Analysis was by intention to treat. The study was S Seki MD, F Okazaki MD,
M Taniguchi MD, S Yoshida MD),
registered at clintrials.gov with the identifi er NCT00133328.
and Division of Diabetes,
Metabolism, and
Findings After a median follow-up of 3·1 years (range 1–3·9) the primary endpoint was recorded in fewer individuals Endocrinology
given valsartan than in controls (92 vs 149; absolute risk 21·3 vs 34·5 per 1000 patient years; hazard ratio 0·61, 95% CI (Prof N Tajima MD)
,
Department of Internal
0·47–0·79, p=0·0002). This diff erence was mainly attributable to fewer incidences of stroke and transient ischaemic Medicine, Jikei University
attack (29 vs 48; 0·60, 0·38–0·95, p=0·028), angina pectoris (19 vs 53; 0·35, 0·20–0·58, p<0·0001), and heart failure School of Medicine, Tokyo,
(19 vs 36; 0·53, 0·31–0·94, p=0·029) in those given valsartan than in the control group. Mortality or tolerability did Japan;
and Institute of
not diff er between groups.
Medicine, Department of
Emergency and Cardiovascular
Medicine, Sahlgrenska
Interpretation The addition of valsartan to conventional treatment prevented more cardiovascular events than University Hospital/Östra,
supplementary conventional treatment. These benefi ts cannot be entirely explained by a diff erence in blood pressure Göteborg, Sweden control.
Correspondence to:
Prof Seibu Mochizuki, Division of
Direct implementation of available evidence into clinical Cardiology, Department of
Cardiovascular disorders are the leading cause of mortality
practice in Japan might not be warranted by the available Internal Medicine, The Jikei
worldwide,1 and are expected to continue to increase with
data, since responses to drug intervention and its clinical University School of Medicine,
general ageing of the world's population and rapid consequences might diff er between ethnic groups. Clinical 3-25-8 Nishi-shinbashi,
Minato-ku, Tokyo, Japan
socioeconomic changes in the developing world. Hence, trials of angiotensin receptor blockers on end-organ
[email protected] optimum pharmacotherapy for cardio vascular disease is damage in Japanese patients show cardiovascular benefi ts,
urgently needed, in addition to lifestyle changes, to but because of shortcomings such as small sample sizes
provide symptomatic relief and long-term protection. and observational data, these results are not conclusive
Hypertension is the most common cause of coronary and cannot be directly translated into clinical outcomes.17–21
heart disease and heart failure in Japan, and Thus, further large-scale Japanese clinical trials are
cerebrovascular disease is more prevalent in the Japanese
population than in western societies.2 Angiotensin II has
We aimed to implement a large-scale clinical trial to
a well defi ned role in the pathogenesis of hypertensive investigate the eff ect of control of blood pressure (to a left ventricular hyper
trophy, stroke, coronary heart target of less than 130/80 mm Hg) with an added
disease, and heart failure.3–5 Over the past decade, several
angiotensin receptor blocker, valsartan, compared with
clinical trials have shown the benefi ts of treatments that conventional treatment in a large Japanese population that specifi
cally block the renin–angio
tensin–aldosterone was representative of the cardiovascular continuum of
system. Angiotensin receptor blockers were originally disease.22 Our hypothesis was that treatment with valsartan targeted at hyper tension, but also benefi t patients with a would yield additional protective benefi ts, compared with range of diseases6–15 and reduce the incidence of new convent
ional treatment, beyond those attributable to
onset type II diabetes.7,11,16
control of blood pressure.
www.thelancet.com
Vol 369 April 28, 2007
generated list of random numbers to assign patients to
receive either valsartan or conventional treatment. We used
Our study design, organisation, clinical measurements, the minimisation method25 to adjust for baseline endpoint defi nitions, power calculations, and recruitment
characteristics. Investigators entered all patient data on a
rates have been published previously.23 Briefl y, between secure website. Electronic case report forms were then January, 2002, and December, 2004, we recruited patients transferred to the data centre in Kobe. A data management to an investigator-initiated, independent, investigator-led, team updated the database every month. All data were kept multicentre, controlled trial.23 Participating centres independently of the funding source. included the four hospitals of the Jikei University in Tokyo,
The primary endpoint was a composite of cardiovascular
which has some of the largest inpatient and outpatient mortality and morbidity. Components of the endpoint facilities in Japan, and 17 associated hospitals led by included the following: hospital admissions for stroke or physicians from Jikei University.23 We used a prospective transient ischaemic attack (neurological defi cit persisting randomised open blinded endpoint (PROBE) design.24
for less than 24 hours); myocardial infarction (chest pain,
We recruited patients with hypertension, coronary heart ECG-changes, and biomarkers for myocardial necrosis);
disease, heart failure, or a combination of these admission for congestive heart failure (clinical symptoms cardiovascular disorders. The study population was including dyspnoea, shortness of breath, and peripheral selected and stratifi ed to be representative of the range of oedema, together with left ventricular dysfunction by echo-cardiovascular disease in a Japanese population. cardiography, according to the guidelines of the American Participants could be 20–79 years of age, and of either sex.
College of Cardiology and American Heart Association
Patients with hypertension must have been diagnosed at [AHA/ACC]); admission because of angina pectoris least 3 months before enrolment, and have been under (diagnosed as ECG changes along with chest discomfort or treatment with antihypertensive drugs. Patients with pain, with documented coronary heart disease according coronary heart disease were enrolled if they had either a to AHA/ACC guidelines); dissecting aneurysm of the aorta history of the disease or had been newly diagnosed on the
(diagnosed by imaging technique); doubling of serum
basis of typical symptoms, with coronary angiography creatinine; or transition to dialysis. The fi rst of these events showing at least one coronary stenosis of more than 75%. to arise in any specifi c patient was noted as the primary Patients with heart failure (New York Heart Association event. [NYHA] class II–IV), diagnosed on the basis of a historically
Any component of composite primary endpoint for
low ejection fraction (echocardiography) or diastolic which a patient could be counted once in each category dysfunction, were enrolled if they had received standard was treated as a secondary endpoint. Death from any cause treatment (diuretics, angiotensin-converting enzyme [ACE]
was also designated a secondary endpoint. A cardiovascular
inhibitors, β blockers, or a combination of these) for at event was regarded as causal of death on the basis of the least 4 weeks before enrolment.
judgment of a participating physician, irrespective of the
Exclusion criteria included acute coronary syndrome or time between the event and death.
myocardial infarction within 6 months, any cerebrovascular
event within 3 months, serum creatinine higher than
Procedures
265 μmol/L, potassium higher than 5 mmol/L, treatment At enrolment we recorded patients' demographics and
with an angiotensin receptor blocker 4 weeks or less before
baseline characteristics, including sex, age, height,
randomisation, or judgment by the physician that bodyweight, symptoms and signs, and risk factors for participation was unwise on the basis of patient cardiovascular disease (smoking, hyperlipidaemia, and characteristics and drug safety.
diabetes mellitus). We assessed cardiac function, cardiac
We used good clinical practice guidelines in accordance remodelling, and renal function at baseline and at 6-month
with the Declaration of Helsinki. Institutional review intervals. The general clinical laboratory tests were boards at every participating hospital approved the protocol
urinalysis (proteinuria); blood chemistry (creatinine,
and subsequent amendments. At the fi rst hospital visit, sodium, potassium, total cholesterol, triglyceride, low 4 weeks before randomisation, we carefully explained the density lipoprotein cholesterol and high density lipoprotein trial objectives and study design, and the risks and benefi ts cholesterol, plasma glucose, and haemoglobin A )
of participation to all patients and obtained written measured in the fasting state after an overnight 12 h fast; informed consent. Patient privacy was strictly protected. electrocardiography (ECG); echocardiography (left The study was registered at register.clintrials.gov with the ventricular diastolic dimension, ventricular systolic identifi cation number NCT00133328.
dimension, ejection fraction, fractional shortening, intraventricular septum, and posterior wall thickness); and
Study design
chest radiogram. We assessed the quality of life of patients
Eligible patients with more than one cardiovascular with congestive heart failure with the modifi ed Minnesota disorder were stratifi ed into groups according to the living with heart failure and NYHA cardiac functional class following sequence of severity: heart failure, coronary heart
scales.26 Patients could be seen every 2–4 weeks, at least
disease, and hypertension. We then used a computer-
every 6 months for up to 3·5 years. At every visit, a skilled
www.thelancet.com
Vol 369 April 28, 2007
physician took standard blood pressure measure ments,
Non-ARB treatment
with the patient at rest (5–10 min) in the sitting position,
with a validated mercury sphygmomano meter. The mean
Non-ARB treatment
of three measurements was calculated and recorded. The
Non-ARB treatment
timing of blood pressure measurement was not constant in relation to patients' intake of medication.
We aimed to control blood pressure in both treatment
Valsartan (40–80 mg daily)
groups to less than 130/80 mm Hg. Figure 1 shows the
Valsartan (40–160 mg daily)
phases of treatment in our study protocol. Hypertensive patients in the valsartan group were initially given 80 mg
Non-ARB treatment
of valsartan orally, once daily in the morning, fl exibly adjusted to a dose of 40–160 mg per day, as needed to control blood pressure. Patients with heart failure or
coronary heart disease in the valsartan group were started
on 40 mg once daily and uptitrated as tolerated. Controls
Figure 1: Schematic of study protocol with treatment phases
were given either an increased dose of their existing Doses of valsartan were given once daily. ARB=angiotensin receptor blocker. *Both groups given conventional
treatment or an additional conventional treatment to non-ARB treatment.
achieve the blood pressure goal.
Diagnoses of endpoints were verifi ed automatically by and used Cox's proportional hazard regression analysis to
the computer system and by a data monitoring committee
compare the rate of event development. For primary
consisting of four expert cardiologists from Jikei analysis of intergroup diff erences in endpoints we used University. An independent endpoint committee of three inference testing (95% CIs) with signifi cance defi ned at an members who were not affi
liated with the University, all α level of less than 5%. Hazard ratios were calculated and
of whom were unaware of treatment allocation, also adjusted for sex, age, hypercholesterolaemia, diabetes adjudicated the diagnoses. The end
point committee mellitus, smoking, and concomitant antihypertensive
reviewed all available document ation, including patient treatment with Cox's proportional hazard model. To assess records. Endpoints were confi rmed only after agreement signifi cance, we compared categorical data between groups from all members of this committee.
with the χ² test or Fisher's exact test and compared quantitative data between groups with the
t test or analysis
Statistical analysis
of variance. We compared the total number and rate of
Few epidemiological data about cardiovascular risk adverse events for each group.
profi les in Japan were available. Information about the
Our data safety and monitoring board reviewed
prognosis of patients treated by specialist doctors at eff ectiveness and safety at regular intervals throughout the specialised hospitals was especially scarce. Although the study. This board did three interim analyses, with the cardiovascular event rate in the Japanese population is O'Brien–Fleming method,27 beginning 6 months after the low, the hospitals participating in our study undertake tertiary care of cardiovascular disease and therefore treat more severely ill patients than those seen in other
3085 patients assessed
hospitals. We estimated that the 3-year event rate for cardiac mortality and morbidity for patients with complicated cardiovascular disease would be about 12%.
4 withdrew consent
The fi ndings of a retrospective investigation of a few patients under treatment at our participating sites were
3081 randomly allocated
almost identical to this estimate.
Since our study was event-driven, we calculated that to
include 300 primary events, we would need a sample size of at least 3000 patients, followed up for an average of
1541 randomly allocated to
1540 randomly allocated to
valsartan treatment
non-ARB treatment
3 years. We assumed that the valsartan group would
achieve a 20% reduction of risk compared with the conventional treatment group, giving our study 80%
8 withdrew consent
statistical power and an α error of less than 5% if 10% of
6 withdrew consent
8 lost to follow-up
7 lost to follow-up
patients discontinued treatment or were lost to follow-up.
Analyses were based on intention to treat. The statistical
analysis group at Osaka City University, which was
1541 available for
1540 available for
independent of the study implementation group and the
funding source, did data analyses. We checked that patient characteristics were uniformly distributed between groups,
Figure 2: Trial profi le
www.thelancet.com
Vol 369 April 28, 2007
premature end of the study coincided with the planned
Valsartan treatment group
Non-ARB treatment group
duration of follow-up.
Role of the funding source
The sponsor had no role in study design, data collection,
data analysis, data interpretation or writing of the report.
Systolic blood pressure (mm Hg)
The executive committee had full access to all the data at
Diastolic blood pressure (mm Hg)
the end of the study, and had fi nal responsibility for the
Heart rate (beats per min)
decision to submit for publication.
Body-mass index (kg/cm2)
Electrocardiograph (S V1 wave and
R V5/V6 wave, mm)
Figure 2 shows the trial profi le and table 1 the baseline
characteristics for all the 3081 patients who were assigned
Total cholesterol (mmol/L)
to treatment. The two treatment groups were well matched
LDL cholesterol (mmol/L)
for baseline characteristics: all patients were Japanese, with
HDL cholesterol (mmol/L)
a mean age of 65 years, a mean body-mass index (BMI) of
Triglyceride (mmol/L)
24 kg/m2, and a blood pressure of 139/81 mm Hg. About a
Fasting plasma glucose (mmol/L)
third were female. Patients were censored at death or at
Serum creatinine (μmol/L)
last known visit, with a median follow-up of 3·1 years (SD
0·8, range 1–3·9). In total the study gathered information
Potassium (mmol/L)
for 8627 patient years (4326 in the valsartan group and 4321
in the control group). Figure 2 shows that 14 patients
(0·5%) withdrew consent after random allocation and
Coronary heart disease
15 patients (0·5%) were lost to follow-up. We obtained
complete endpoint information at the end of the study for
Diabetes mellitus
Table 1 shows that, at baseline, blood pressure in both
groups combined was at a mean of 139/81 mm Hg (SD
ARB=angiotensin receptor blocker. LDL=low-density lipoprotein. HDL=high-density lipoprotein. Hb=haemoglobin. Data are mean (SD) or number (%).
11/11). Throughout the study it fell to 131/77 mm Hg (12/8) in the valsartan group, and 132/78 (11/8) mm Hg
Table 1: Baseline characteristics
in controls. The changes in blood pressure were 8·2/4·7 mm Hg in the valsartan treatment group and
last person had been enrolled. In December, 2005, the data
7·2/3·7 mm Hg in controls. At the end of the trial only
safety and monitoring board recommended that the study
122 (4%) of patients in both groups had blood pressure
should be stopped for ethical reasons, because additional greater than 140/90 mm Hg. 1118 (75%) of patients given valsartan treatment was associated with a reduction in the
valsartan and 1033 (70%) in the control group achieved
primary endpoint (p<0·001, adjusted for three interim the target blood pressure of less than 130/80 mm Hg.
analyses). This recommendation was endorsed by the The Levene test for equality of variances showed no
executive and steering committees. In January, 2006, all diff erences between the groups. Blood pressure and heart
patients were recalled for fi nal visits. Since the event rate rate did not diff er between the valsartan regimen and the
was lower and the risk reduction larger than expected, the
control regimen throughout the trial (table 3, p=0·196 for
All patients
Patients with
Patients with coronary Patients with
Calcium-channel blocker
Antialdosterone agent
ARB=angiotensin receptor blocker. ACE=angiotensin-converting enzyme.
Table 2: Medication at baseline
www.thelancet.com
Vol 369 April 28, 2007
End of study
139·2 (11) 138·8 (11)
130·4 (14) 131·9 (14)
132·0 (14) 132·0 (14)
Pulse rate (bpm)
All antihypertensive drugs
SBP=systolic blood pressure. DBP=diastolic blood pressure. ACE=angiotensin-converting enzyme. CCB=calcium-channel blocker. bpm=beats per minute. *Doses of individual drugs adjusted as fractions of the standard dose of those drugs in Japan. For example, the standard dose of valsartan is 80 mg; if 90% of patients took an average dose that was 110% of this standard dose, the dose-adjusted fi gure would be 99% (0·9×1·1). For valsartan, the dose-adjusted fi gure was 95% at the end of the study, representing an average dose of 76 mg.
Table 3: Patient characteristics and medications throughout the study in the two treatment groups
Non-ARB treatment group
Hazard ratio
0·61 (0·47–0·79)
Stroke or transient ischaemic attack
0·60 (0·38–0·95)
New or recurrent acute
0·90 (0·47–1·74)
myocardial infection
New occurrence or exacerbation
0·35 (0·20–0·58)
of angina pectoris needing hospitalisation
New occurrence or exacerbation
0·53 (0·31–0·94)
of heart failure needing hospitalisation
Dissecting aneurysm of the aorta
0·19 (0·04–0·88)
Transition to dialysis, doubling of
0·93 (0·34–2·61)
serum creatinine levels
All-cause mortality
1·09 (0·64–1·85)
Cardiovascular mortality
1·03 (0·41–2·60)
Incidence of endpoint reduced
Incidence increased
Figure 3: Eff ect of treatment on all endpoints
Hazard ratios are adjusted for sex, age, hypercholesterolaemia, diabetes, smoking, and concomitant antihypertensive treatment. Diamonds and squares indicate the hazard ratio estimate for each type
of event; horizontal lines show 95% CIs.
systolic blood pressure and p=0·176 for diastolic blood (table 3). The average number of antihypertensive drugs pressure at end of study).
taken during the study was slightly higher in the valsartan
Table 2 shows patients on medication at baseline: about group than in controls. However, when doses for all drugs
two-thirds were receiving a calcium antagonist, a third an were adjusted to a standard dose, according to Japanese ACE-inhibitor, another third a β blocker, a tenth a diuretic,
clinical practice, the dose-adjusted numbers of drugs for all
and almost a third a statin. A webtable sets out all doses of
treatment groups were identical at the end of the study See
Online for webtable
antihypertenstive medications in more detail. The average
(table 3). Table 4 shows selected biochemical results.
additional dose of valsartan was 75 (SD 14) mg per day.
Figure 3 shows that the primary endpoint was recorded
Other additional treatments in both groups were mainly in fewer patients given valsartan (92, 6·0%) than in those calcium-channel blockers, ACE-inhibitors, and β blockers given additional non-ARB treatment (149, 9·7%); the hazard
www.thelancet.com
Vol 369 April 28, 2007
Valsartan treatment group (n=1541)
Non-ARB treatment group (n=1540)
comparison
Total cholesterol (mmol/L)
0·02 (−0·04–0·09)
0·03 (−0·03–0·08)
LDL cholesterol (mmol/L)
0·02 (−0·04–0·07)
0·03 (−0·02–0·08)
HDL cholesterol (mmol/L)
Triglyceride (mmol/L)
0·04 (−0·02–0·11)
0·02 (−0·04–0·08)
Fasting plasma glucose (mmol/L)
Serum creatinine (μmol/L)
0·35 (−1·1–1·8)
0·46 (−1·1–2·0)
0·05 (−0·03–0·13)
0·07 (0·01–0·13)
Potassium (mmol/L)
0·004 (-0·002–0·01)
eGFR (mL/min per 1·73 m2)
−2·4 (10·1·)
−0·84 (-2·4–0·7)
Values are mean (SD). Hb=haemoglobin. eGFR=estimated glomerular fi ltration rate (normal range of 90–130 mL/min per 1·73 m2).
Table 4: Biochemical variables
ratio was 0·61 (95% CI 0·47–0·79, p=0·0002). This transient ischaemic attacks in the valsartan group, and endpoint was a composite of several secondary endpoints 43 strokes and fi ve transient ischaemic attacks in the (fi gure 3). The diff erence in the number of primary conventional-treatment group.
endpoints was mainly attributable to reduced frequency of
Table 5 shows that only 2·5% of patients reported any
stroke and transient ischaemic attack, angina pectoris, and
adverse event during the study, with no signifi cant
heart failure. 29 patients given valsartan had stroke or diff erence between treatment groups. The only diff erence transient ischaemic attack, compared with 48 controls (HR
between the groups was a higher incidence of dizziness in
0·60, 95%CI 0·38–0·95, p=0·028); 19 patients given the valsartan group, with nine cases compared with three valsartan had angina pectoris compared with 53 controls in the control group.
(HR 0·35, 95%CI 0·20–0·58, p=0·0001); 19 patients given valsartan had heart failure, compared with 36 controls (HR
0·53, 95%CI 0·31–0·94, p=0·0293); and two patients given
Addition of the angiotensin receptor blocker valsartan to
valsartan had dissecting aneurysm of the aorta, compared standard cardiovascular treatment, compared with an with ten controls (HR 0·19, 95%CI 0·04–0·94, p=0·0293). increased dose or number of standard drugs, in Japanese Mortality, myocardial infarction, or progression of renal patients with cardiovascular disease, reduced the incidence disease did not diff er between groups.
of the primary composite endpoint, of heart, brain, and
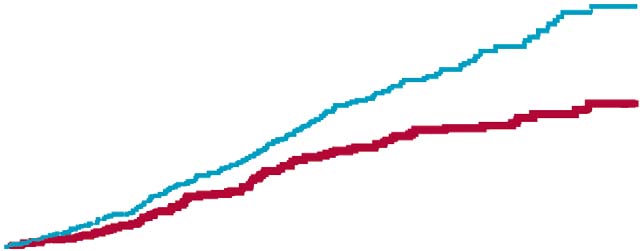
The event rate curves in fi gure 4 show that, excluding any
kidney complications. The main eff ect of addition of
of the components of the primary endpoint, the overall valsartan was to reduce stroke, angina pectoris, dissecting signifi cance of the primary endpoint was maintained in all
aortic aneurysm, and heart failure. These benefi ts were
cases. For the endpoint of stroke or transient ischaemic noted despite a short median follow-up of 3·1 years, and attack, nearly all events were strokes: 25 strokes and four were seen across various subgroups (data not shown).
Unfortunately, Asian patients have often been under-
represented in cardiovascular trials, including trials of
angiotensin receptor blockers. For example, Asians made
Non-ARB treatment group 149 events
Valsartan treatment group 92 events
up 2·8%, 3·5%, and less than 2% of the populations in the
Val-HeFT trial,6 the VALUE trial,16 and the LIFE trial,11
respectively. None of these trials included a Japanese
centre. One previous study on the eff ects of the angiotensin receptor blocker candesartan compared with standard treatment in a hypertensive Japanese population21 had
shortcomings such as defi ciencies in randomisation and quality control, and large numbers lost to follow-up.
Patients in both treatment groups showed a similar
degree of blood pressure control, achieving good control
Number at risk
of the same magnitude. Since this was an active-controlled
study, we could not ascertain to what degree regression-
to-the-mean or placebo eff ects might have contributed to
Figure 4: Kaplan-Meier curves of cumulative frequency of the primary endpoint
www.thelancet.com Vol 369 April 28, 2007
The mean dose of valsartan in this study (75 mg) might highly unlikely to account for diff erences between groups
seem low, but studies in Japanese people have shown that
of the magnitude we recorded.
80 mg of valsartan produced similar antihypertensive
The reduction in the risk of stroke with added valsartan
eff ects to those of nifedipine (20 mg)28 and amlodipine treatment was consistent with that reported with losartan (5 mg).29 Moreover, because the mean BMI in our study in the LIFE study.11 However, we recorded a much lower was low (24, compared with the VALUE trial, for which absolute risk than that reported by LIFE, which is probably mean BMI was 28),16 the doses we used would seem related to the lower mean blood pressure in our study suffi
cient. Doses of all antihypertensive drugs, including population. The stroke endpoint combines both stroke and
valsartan, were based on the guidelines of the Japanese transient ischaemic attack, but the rates of transient Hypertension Society.30
ischaemic attacks were very low in our study. Debate about
The Kaplan-Meier curves for the primary endpoint the degree to which reduction of stroke in the LIFE trial
diverged early and separated throughout the trial (fi gure 4), should be attributed to losartan and how much to a lack of indicating that the response to treatment was early and stroke benefi ts in the group given the comparator (atenolol) sustained. The overall reduction in the primary composite
is unresolved.31 In our study, atenolol was used in only 5%
endpoint was not driven by any one component, indicating
of patients in each group. In both studies, benefi ts for
a broad range of benefi t—ie, a reduction of the total burden
stroke reduction were noted at a similar degree of blood
of cardiovascular disease. The eff ects on myocardial pressure control in treatment and control groups. infarction and renal endpoints were neutral. However,
These fi ndings contrast with the VALUE trial,11 in which
event rates for secondary endpoints were low, and these valsartan treatment did not reduce the frequency of strokes results should not be overinterpreted.
compared with amlodipine. In the VALUE trial, blood
Some further comments are warranted. The reduction pressure diff ered much more between the groups,
in angina with valsartan treatment (65%) was not matched
consistent with the notion that stroke risk is mainly, but
by a similar reduction in myocardial infarction, although not entirely, related to blood pressure, especially in some underlying pathophysiological processes would be high-risk patients.32 Any benefi ts associated with valsartan similar. However, other large-scale trials such as LIFE11
treatment in the VALUE trial could possibly have been
and VALUE16 have also failed to show signifi cant masked by the early diff erences in blood pressure. The low diff erential eff
ects of myocardial infarction with blood pressures in our study, and the fact that they were
angiotensin receptor blockers compared with other similar in both treatment groups, suggest that blood treatments, despite other cardiac benefi ts. We could pressure was not a major determinant of outcomes. speculate that the renin–angiotensin–aldosterone system Furthermore, stroke rates did not cluster early, although has a larger role in the development of angina than in any (minor) blood pressure diff erences were only seen myocardial infarction, in which other factors more related
during the fi rst 12 months. The possible benefi ts of
to rupture of atheromas and thrombosis are major angiotensin receptor blockers indicated in our study are determinants. A possible caveat should be noted: the highly relevant to the Japanese population, in which stroke PROBE design used in our study carries a risk of causes four times more mortality and morbidity than does under-reporting, especially for softer endpoints such as coronary heart disease.33 angina. However, we believe that such a scenario would be
Our study participants represented a range of cardio-
vascular risk and disease. The range of patients was broader than in most other intervention studies, which
Adverse events (n≥2)
have focused on particular stages of cardiovascular disease.
Cancer or metastasis
Although limiting patient heterogeneity in that regard
would have simplifi ed interpretation of the results, such a
strategy could also have limited the clinical implications of
the fi ndings. The cardiovascular diseases represented in
our study population—hypertension, coronary heart
Stomach discomfort
disease, and heart failure—are all disorders in which
activation of the renin–angiotensin–aldosterone system is
thought to play a major part.22,34
Some further limitations of our study suggest possibilities
for further investigation. First, the use of aortic dissection
or peripheral arterial disease as a component of the primary
endpoint is uncommon, although not unique to this study.
Elevated serum potassium
Aortic dissection or lower limb arterial obstruction was
Any adverse event
reduced in the valsartan group, although the number of
events was very low. Since blood pressure was similar between the two treatment groups, the reduced aortic
Table 5: Adverse events occurring in more than one case
dissection could indicate that valsartan had a benefi cial
www.thelancet.com Vol 369 April 28, 2007
eff ect on the aortic wall. Second, neither transition to Statistics analysis organisation
dialysis nor doubling of creatinine concentrations were Clinical epidemiology, Osaka City University Graduate School—Nobuo
associated with cardio
vascular benefi ts from valsartan. Shirahashi.
These events are standard endpoints in trials to assess the
renal protection of angiotensin receptor blockers in Division of Cardiology, Jikei University School of Medicine—Seibu Mochizuki,
Ikuo Taniguchi, Katsunori Ikewaki, Makoto Ohta, Kenichi Sugimoto,
diabetic patients with nephropathy.14,35 However, since Kazuhiko Ogawa, Satoru Yoshida, Takahiro Shibata, Kenichi Hongo, numbers of participants with impaired renal function in Hideki Sasaki, Teiichi Yamane, Naofumi Aoyama, Makoto Kawai, Hidenori our study were low, our fi ndings lack suffi
cient power to Yagi, Kimiaki Komukai, Takayuki Ogawa, Fumiko Okazaki, Ryuko
draw any conclusions.
Anzawa, Taro Date, Sahachiro Nakae, Hisashi Takatsuka, Tadashi Tamura, Tsuneo Mizokami, Osamu Kurusu, Eriko Yokomizo, Yuji Higaki, Hidehiko
A third limitation of our study was that doses of ACE Kashiwagi, Koichi Marutani, Koshin Mizuniwa, Tomohisa Sakai, Tokuo
inhibitors given to some patients before the start of our Kasai, Keiji Iwano, Atsushi Seo; Division of Diabetes and Endocrinology, Jikei study were low by western standards, although consis-
University School of Medicine—Naoko Tajima, Yoichi Sakamoto, Hideaki Kurata; Division of Cardiology, Jikei University School of Medicine, Aoto
tent with clinical practice recommendations in Japan. Hospital—Shingo Seki, Masayuki Taniguchi, Toru Arino, Chikashi Sato,
Thus, we have no proof that the renin–angiotensin–
Satoshi Takeda, Hidekazu Miyazaki, Kiyoshi Kanae, Shuji Nakada, Makoto
aldosterone system had been adequately inhibited before
Miyairi, Akihiko Kagami, Kenji Noma, Izuru Nakamura; Division of
the trial, and we cannot exclude the possibility that the Cardiology, Jikei University School of Medicine, Daisan Hospital—
Makoto Yoshikawa, Kazutoshi Takigawa, Keiichi Chin, Yoshiyuki
results would have diff ered in patients who had already Hashizume, Yoshihisa Shimazu; Division of Cardiology, Jikei University
been given high doses of ACE inhibitors, or that School of Medicine, Kashiwa Hospital—Mitsuyuki Shimizu, Taku Yamada, increasing the ACE inhibitor dose would have provided Masafumi Kusaka, Toshio Hasuda, Yoshiki Uehara, Yoshiyuki Azuma, benefi ts in these patients. Last, our study was not Shinichiro Takizawa, Hiroshi Yoshida, Tomotake Suzuki, Mie Kawai,
Hiroyuki Okumura; Division of Cardiology, Atsugi Municipal Hospital—
adequately powered to detect changes in cardiovascular Kenichi Maie, Koichi Hashimoto, Takuya Okada, Nobunori Tominaga,
or all-cause mortality and our median follow-up of Kazuhiro Aoki; Division of Cardiology, Fuji City Metropolitan Central 3·1 years was short.
Hospital—Hidefumi Mikawa, Hiroshi Takeda, Satoshi Arase, Katsumi Ohnuki, Kosuke Minai; Division of Cardiology, Sakuragaoka
General Hospital—Takao Shimada, Tetsushi Ito, Ken Nogimura; Division of
SM and BD designed the study, wrote the protocol, supervised the
Cardiology, West-Saitama Central Hospital—Tatsuyuki Onodera,
implementation of the research, coordinated data collection, wrote the
Masao Kuwata, Yumi Nishibayashi; Division of Cardiology, Saitama
analysis plan, supervised the analyses, interpreted the results, and wrote
Cardiovascular and Respiratory Centre—Makoto Muto, Tetsuya Ishikawa,
the report. All members of the steering committee approved the protocol
Hiroshi Sakamoto, Tetsushi Tsurusaki, Satoru Onoda; Division of
and analysis plan, supervised the study and had input to the report. All
Cardiology, Shonan Hospital—Noriaki Yoshitake, Hideaki Suzuki, Kunihiko
authors have seen and approved the fi nal version.
Abe; Division of Cardiology, Oarai-kaigan Hospital—Osamu Aizawa,
Takehiko Izumi, Kazuaki Horikoshi, Shunichi Tamura; Division of
Executive committee
Cardiology, Machida Metropolitan Hospital—Syunrou Minami,
Jikei University School of Medicine—Seibu Mochizuki; Sahlgrenska
Satoshi Imamoto, Akimasa Matsuyama; Division of Cardiology, Seki
Hospital—Kiyofumi Suzuki, Takashi Ito, Jun Koga, Mamoru Kunou; Division of Cardiology, Tsunan Metropolitan Hospital—Shinichiro Ishikawa,
Yusaku Hayashi; Division of Cardiology, Tokyo Musashino Hospital—Takuya
Jikei University School of Medicine—Seibu Mochizuki, Mitsuyuki Shimizu,
Sakamoto, Akihisa Tomaru; Division of Cardiology, Kanoiwa Metropolitan
Ikuo Taniguchi, Katsunori Ikewaki, Kenichi Sugimoto, Kazuhiko Ogawa,
Hospital—Takeshi Sato; Division of Cardiology, Shonan Memorial
Tsuneo Mizokami, Takahiro Shibata, Satoru Yoshida, Kenichi Hongo,
Hospital—Hisao Nakamura; Division of Cardiology, Mitaka
Hideki Sasaki, Naofumi Aoyama, Hidenori Yagi, Takayuki Ogawa,
Hospital—Tatsuo Yamazaki; Division of Cardiology, Higashiyama Takeda
Syunrou Minami, Fumiko Okazaki, Kiyoshi Kanae, Masayuki Taniguchi,
Hospital—Izuru Masuda; Division of Cardiology, Sagamino Central
Shingo Seki, Makoto Yoshikawa, Tatsuo Yamazaki, Taku Yamada,
Hospital—Takaaki Iwai; Division of Cardiology, Seirei-Mikatagahara—
Mie Kawai, Hidetoshi Kajiwara, Kenji Noma; West-Saitama Central
Sousuke Miyazawa, Hideki Kajiwara, Tohru Sugiura.
Hospital—Tatsuyuki Onodera; Tsunan Metropolitan Hospital—Shinichiro Ishikawa, Yusaku Hayashi; Fuji City Central
Confl ict of interest statement
Hospital—Hidefumi Mikawa; Atsugi Metropolitan Hospital—Kenichi Maie,
SM has received lecture fees from Novartis and Daiichi-Sankyo; BD has
Nobunori Tominaga; Saitama Cardiovascular and Respiratory Centre—
served as a consultant for and received lecture fees from
Makoto Muto; Shonan Hospital—Noriaki Yoshitake, Hideaki Suzuki;
Boehringer-Ingelheim, Novartis, Merck, and Pfi zer and lecture fees from
Oarai-kaigan Hospital—Osamu Aizawa; Seki Hospital—Kiyofumi Suzuki;
Servier and Astra; MS has received lecture fees from Shionogi; KI has
Sakuragaoka General Hospital—Tetsushi Ito.
served as a consultant for Japan Tobacco and received lecture fees from Kissei, Sankyo, Astellas, Kowa, and Novartis; NT has served as a consultant
for Eli Lilly, Novo Nordisk Pharma, Sanofi -Aventis, and Takeda, and
Ehime University—Masatsugu Horiuchi; Toho University—
received lecture fees from Astellas, Banyu, Dainippon Sumitomo, Novartis,
Junichi Yamazaki; Osaka University—Hiromi Rakugi.
and Sankyo. None of the other authors had any potential confl ict of
Jikei University School of Medicine—Shigeru Kageyama, Tetsuo Sato,
Masato Matsushima, Shigeto Murakami .
The study was funded by the Jikei University School of Medicine, with
an unrestricted grant from Novartis Pharma KK, Japan. We thank all
Jikei University School of Medicine—Mitsuyuki Shimizu, Naoko Tajima,
trial physicians and nurses in all participating hospitals, and most of all
Ikuo Taniguchi, Kiyoshi Kanae, Kazunori Utsunomiya, Kenichi Sugimoto,
the patients, for their important contribution to the study. We also thank
Katsunori Ikewaki, Satoru Yoshida, Hideaki Kurata.
Anita Holmner at A+ Science AB, Göteborg, Sweden, and Yoko Takeda and Maki Tsukiori at Jikei University, Tokyo, Japan for help in typing
and collating the report.
Jikei University School of Medicine—Seibu Mochizuki, Mitsuyuki Shimizu, Katsunori Ikewaki; Sahlgrenska University Hospital/Östra—Björn Dahlöf.
www.thelancet.com Vol 369 April 28, 2007
References
19 Munakata M, Nagasaki A, Nunokawa T, et al. Eff ect of valsartan and
Bonow RO, Smaha LA, Smith SC Jr, Mensah GA, Lenfant C. World
nifedipine coat-core on systemic arterial stiff ness in hypertensive
Heart Day 2002: the international burden of cardiovascular disease:
patients. Am J Hypertens 2004; 17: 1050–55.
responding to the emerging global epidemic. Circulation 2002; 106:
20 Kawano H, Toda G, Nakamizo R, Koide Y, Seto S, Yano K. Valsartan
decreases type I collagen synthesis in patients with hypertrophic
Kubo M, Kiyohara Y, Kato I, et al. Trends in the incidence, mortality,
cardiomyopathy. Circ J 2005; 69: 124 4–48.
and survival rate of cardiovascular disease in a Japanese
21 Suzuki H, Kanno Y; Effi
cacy of Candesartan on Outcome in
community: the Hisayama study. Stroke 2003; 34: 2349–54.
Saitama Trial (E-COST) Group. Eff ects of candesartan on
Sadoshima J, Izumo S. The cellular and molecular response of cardiac
cardiovascular outcomes in Japanese hypertensive patients.
myocytes to mechanical stress. Annu Rev Physiol 1997; 59: 551–71.
Hypertens Res 2005; 28: 307–14.
Brunner HR. Experimental and clinical evidence that angiotensin II
22 Dzau V. The cardiovascular continuum and renin–angiotensin–
is an independent risk factor for cardiovascular disease.
aldosterone system blockade. J Hypertens 2005; 23 (suppl 1): 1–17.
Am J Cardiol 2001; 87: 3–9.
23 Mochizuki S, Shimuzu M, Taniguchi I, et al. for the JIKEI HEART
Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II
Study Group. JIKEI HEART Study – A morbid-mortality and
mediated cardiovascular and renal diseases. Pharmacol Rev 2000; 52:
remodeling study with valsartan in Japanese patients with
hypertension and cardiovascular disease. Cardiovasc Drugs Ther 2004;
18:
Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A
randomized trial of the angiotensin receptor blocker valsartan in
24 Hansson L, Hedner T, Dahlöf B. Prospective randomized open
chronic heart failure. N Engl J Med 2001; 345: 1667–75.
blind endpoint (PROBE) study. A novel design for intervention
trials. Blood Press 1992; 1: 113–19.
7 Pfeff er MA, Swedberg K, Granger CB, et al. Eff ects of candesartan
on mortality and morbidity in patients with chronic heart failure:
25 Pocock SJ, Simon R. Sequential treatment assignment with
the CHARM-Overall programme. Lancet 2003; 362: 759–66.
balancing for prognostic factors in the controlled clinical trial.
Biometrics 1975; 31: 103–15.
Pitt B, Poole-Wilson PA, Segal R, et al. Eff ect of losartan compared with captopril on mortality in patients with symptomatic Heart
26 Goldman, L, Hashimoto B, Cook EF, Loscalzo A. Comparative
failure: randomised trial—the Losartan Heart Failure Survival Study
reproducibility and validity of systems for assessing cardiovascular
ELITE II. Lancet 2000; 355: 1582–87.
functional class: advantages of a new specifi c activity scale.
Circulation 1981; 64: 1227–34.
9 Pfeff er MA, McMurray JJ, Velazques EJ et al. Valsartan, captopril or
both in myocardial infarction complicated by heart failure, left
27 O'Brien PC, Fleming TR. A multiple testing procedure for clinical
ventricular dysfunction or both. N Engl J Med 2003; 349: 1893–1906.
trials. Biometrics 1979; 35: 549–56.
10 Dickstein K, Kjekshus J, OPTIMAAL Steering Committee. Eff ects
28 Munakata M, Nagasaki A, Nunokawa T, et al. Eff ects of valsartan
of losartan and captopril on mortality and morbidity in high-risk
and nifedipine coat-core on systemic arterial stiff ness in
patients after acute myocardial infarction: the OPTIMAAL
hypertensive patients. Am J Hypertens 2004; 17: 1050–55.
randomised trial. Optimal Trial in Myocardial Infarction with
29 Yasunari K, Maeda K, Watanabe T, Nakamura M, Yoshikawa J,
Angiotensin II Antagonist Losartan. Lancet 2002; 360: 752–60.
Asada A. Comparative eff ects of valsartan versus amlodipine on left
11 Dahlöf B, Devereux RB, Kjeldsen SE, et al. Cardiovascular
ventricular mass and reactive oxygen species formation by
morbidity and mortality in the Losartan Intervention For Endpoint
monocytes in hypertensive patients with left ventricular
reduction in hypertension study (LIFE) a randomised trial against
hypertrophy. J Am Coll Cardiol 2004; 43: 2116–23.
atenolol. Lancet 2002; 359: 995–1003.
30 Saruta T. [The Japanese Society of Hypertension Guidelines for the
12 Schrader J, Luders S, Kulschewski A, et al. Morbidity and mortality
Management of Hypertension (JSH 2004)]. Nippon Rinsho 2005; 63:
after stroke. Eprosartan compared to nitrendipine for secondary
prevention: principal results of a prospective randomized controlled
31 Lindholm LH, Carlberg B, Samuelsson O. Should beta-blockers
study (MOSES). Stroke 2005; 36: 1218–26.
remain fi rst choice in the treatment of primary hypertension? A
13 Viberti F, Wheelden NM. Microalbuminuria reduction with
meta-analysis. Lancet 2005; 366: 1545–53.
valsartan in patients with type 2 diabetes mellitus: a blood
32 Staessen JA, Wang JG, Thijs L. Cardiovascular prevention and blood
pressure-independent eff ect. Circulation 2002; 106: 672–78.
pressure reduction: a quantitative overview updated until 1 March
14 Brenner BM, Cooper ME, de Zeeuw D, et al. Eff ects of losartan on
2003. J Hypertens 2003; 21: 1055–76.
renal and cardiovascular outcomes in patients with type 2 diabetes
33 Kimura Y, Takishita S, Muratani H, et al. Demographic study of
and nephropathy. N Engl J Med 2001; 345: 861–69.
fi rst-ever stroke and acute myocardial infarction in Okinawa, Japan.
15 Parving HH, Lehnert H, Bröchner-Mortensen J, et al. The eff ect of
Intern Med 1998; 37: 736–45.
irbesartan on the development of diabetic nephropathy in patients
34 Dzau V, Braunwald E. Resolved and unresolved issues in the
with type 2 diabetes. N Engl J Med 2001; 345: 870–78.
prevention and treatment of coronary artery disease: a workshop
16 Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive
consensus statement. Am Heart J 1991; 121: 1244–63.
patients at high cardiovascular risk treated with regimens based on
35 Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective eff ect of
valsartan or amlodipine: the VALUE randomised trial. Lancet 2004;
the angiotensin-receptor antagonist irbesartan in patients with
nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–60.
17 Suzuki K, Souda S, Ikarashi T, et al. Renoprotective eff ects of
low-dose valsartan in type 2 diabetic patients with diabetic
nephropathy. Diabetes Res Clin Pract 2002; 57: 179–83.
18 Kasama S, Toyama T, Kumakura H, et al. Addition of valsartan to an
angiotensin converting enzyme inhibitor improves cardiac
sympathetic nerve activity and left ventricular function in patients
with congestive heart failure. J Nucl Med 2003; 44: 884–90.
www.thelancet.com Vol 369 April 28, 2007
Source: http://www.icirculation.com/special/KHS/images/5.pdf
AM P: SLTOPHO Acknowledgments The content of this booklet was researched and written by Dr. Janet McKeown (MD, CCFP, DipSportsMed), Cristina Sutter (Registered Sport Dietitian) and Susan Boegman (Registered Sport Dietitian) with input from Dr. Penny Miller (Clinical Pharmacologist), Dr. Susan Hollenberg (Family & Travel Medicine Physician), and Dr. Reka Gustafson (Medical Health Officer).
Preventing Drug Overdoses in Oklahoma Drug overdose deaths in Oklahoma have risen sharply during the past decade, according to Oklahoma City Addiction Medicine physicians Hal Vorse, MD, and Billy Stout, MD. Between 2002 and 2010, the Oklahoma Bureau of Narcotics and Dangerous Drugs Control (OBN) reports the number of overdose deaths in the state rose from 470 to 814 per year. Preliminary data indicates there were at least 795 drug overdose deaths in Oklahoma in 2011. Seventy-seven percent of the deaths were due to the use of prescription drugs in combination with other prescription drugs or alcohol.
