Doi:10.1016/j.joms.2007.09.019
J Oral Maxillofac Surg66:223-230, 2008
Outcomes of Placing Dental Implants in
Patients Taking Oral Bisphosphonates:
A Review of 115 Cases
Bao-Thy Grant, DDS,* Christopher Amenedo, DDS,†
Katherine Freeman, DrPH,‡ and Richard A. Kraut, DDS§
Purpose: In recent years, numerous cases of bisphosphonate-associated osteonecrosis of the jaw have
been reported involving both intravenous and oral therapy regimens. The majority of these cases have
involved intravenous bisphosphonates. Subsequently, drug manufacturers and the US Food and Drug
Administration issued warnings about possible bisphosphonate-associated osteonecrosis of the jaw. The
American Dental Association and the American Association of Oral and Maxillofacial Surgeons assembled
expert panels to formulate treatment guidelines. Both panels differentiated between patients receiving
bisphosphonates intravenously and those receiving the drugs orally. However, the recommendations
were based on limited data, especially with regard to patients taking oral bisphosphonates. We wanted
to ascertain the extent to which bisphosphonate-associated necrosis of the jaw has occurred in our dental
implant patients. We also wanted to determine whether there was any indication that the bisphospho-
nate therapy affected the overall success of the implants as defined by Albrektsson and Zarb.
Patients and Methods: We identified 1,319 female patients over the age of 40 who had received dental
implants at Montefiore Medical Center between January 1998 and December 2006. A survey about bisphos-
phonate therapy was mailed to all 1,319 patients. Responses were received from 458 patients of whom 115
reported that they had taken oral bisphosphonates. None had received intravenous bisphosphonates. All 115
patients were contacted and informed about the risk of bisphosphonate-associated osteonecrosis of the jaw.
Seventy-two patients returned to the clinic for follow-up clinical and radiological evaluation.
Results: A total of 468 implants were placed in the 115 patients who reported that they had received
oral bisphosphonate therapy. There is no evidence of bisphosphonate-associated osteonecrosis of the
jaw in any of the patients evaluated in the clinic and those contacted by phone or e-mail reported no
symptoms. Of the 468 implants, all but 2 integrated fully and meet criteria for establishing implant
success. Implant success rates were comparable for patients receiving oral bisphosphonate therapy and
those not receiving oral bisphosphonate therapy.
Conclusions: Guidelines for treatment of dental patients receiving intravenous bisphosphonate treatments
should be different than for patients taking the oral formulations of these medications. In this study, oral
bisphosphonate therapy did not appear to significantly affect implant success. Implant surgery on patients
receiving bisphosphonate therapy did not result in bisphosphonate-associated osteonecrosis of the jaw.
Nevertheless, sufficient evidence exists to suggest that all patients undergoing implant placement should be
questioned about bisphosphonate therapy including the drug taken, the dosage, and length of treatment prior
to surgery. For patients having a history of oral bisphosphonate treatment exceeding 3 years and those having
concomitant treatment with prednisone, additional testing and alternate treatment options should be con-
sidered.
2008 American Association of Oral and Maxillofacial Surgeons
J Oral Maxillofac Surg 66:223-230, 2008
Received from Albert Einstein College of Medicine/Montefiore
Address correspondence and reprint requests to Dr Kraut:
Medical Center, Bronx, NY.
Montefiore Medical Center, Dentistry/Oral and Maxillofacial Sur-
*Chief Resident, Department of Oral and Maxillofacial Surgery.
gery, 111 East 210th Street, Bronx, NY 10467-2460; e-mail:
†Attending, Department of Oral and Maxillofacial Surgery.
‡Professor, Department of Epidemiology and Population Health.
2008 American Association of Oral and Maxillofacial Surgeons
§Chairman of Department of Dentistry, Director of Oral and Maxil-
lofacial Surgery, and Professor.
IMPLANTS AND ORAL BISPHOSPHONATES: 115 CASES
Table 1. ORAL AND INTRAVENOUS BISPHOSPHONATE MEDICATIONS AVAILABLE IN THE UNITED STATES
Roche Pharmaceutical
Aredia (intravenous)
Bonefos (intravenous)
Zometa (intravenous)
Adapted with permission from American Dental Association Council on Scientific Affairs: Dental management of patients receiving oral
bisphosphonate therapy: Expert panel recommendations. J Am Dent Assoc 137:1144, 2006.
Grant et al. Implants and Oral Bisphosphonates: 115 Cases. J Oral Maxillofac Surg 2008.
According to the American Society of Clinical Oncol-
thors noted that during the 3 years covered by the
ogy, the use of intravenous bisphosphonates for the
study, the number of patients presenting at the med-
reduction of bone pain, hypercalcemia of malignancy,
ical center with necrotic lesions of the jaw had dra-
and skeletal complications in patients with multiple
matically increased. The necrosis was typical of that
myeloma, lung, breast and other cancers is the cur-
seen in patients receiving radiation therapy. How-
rent standard of The 2 drugs associated with
ever, these patients were not being treated with radi-
these treatments are pamidronate (Aredia; Novartis
ation; rather they were receiving bisphosphonate
Pharmaceuticals Corp, East Hanover, NJ) and zoledronic
acid (Zometa; Novartis Pharmaceuticals
Soon after publication of the Ruggerio article, in
Oral bisphosphonates are used to treat osteoporo-
September 2004, Novartis, manufacturer of Aredia
sis, Paget's disease, and osteogenesis
and Zometa, notified health care professionals of the
They are most widely used for treatment of osteopo-
possible relationship between intravenous bisphos-
rosis; in the United States, some 22 million prescrip-
phonate therapy and necrosis of the jaws. Thereafter
tions were written for Fosamax (Merck Co, West
the US Food and Drug Administration issued an alert
Point, VA) between May 2003 and April It is
that included not only the intravenous bisphospho-
estimated that the number of hip fractures in the
nate formulations but the oral ones as
United States will triple by 2020 and that 1 of every 2
Additional articles and case studies have been pub-
women will sustain an osteoporosis fracture in her
lished though all have limited sample sizes and all are
Twenty-four percent of the women who
retrospective studies. Incidence of bisphosphonate-
fracture a hip will die within a As the pop-
associated osteonecrosis of the jaw is estimated to
ulation ages, the number of people receiving bisphos-
range from 0.8% to 12% for patients receiving intra-
phonate therapy is likely to rise. In addition, many of
venous For patients taking oral
those treated will receive medication for an extended
medication, the incidence is estimated to be 0.7 per
number of years. Oral and intravenous bisphospho-
100,000 person years of Nationwide,
nate medications available in the United States are
there have been over 200 reported cases of possible
bisphosphonate-associated osteonecrosis of the jaw
In a letter to the editor of the
Journal of Oral and
in patients taking Fosamax or Actonel (Procter and
Maxillofacial Surgery published in September 2003,
Gamble, Cincinnati, OH). So far there are no docu-
Marx alerted the dental community to the possible
mented cases for patients taking Boniva (Hoffman-
relationship between intravenous bisphosphonate
LaRoche, Nutley, NY) although there are some anec-
therapy and necrosis of the He reported on 36
dotal Results for patients taking other oral
cases of bone exposure that were not responsive to
bisphosphonates are expected to be comparable.
surgical or medical treatments. All 36 patients were
Risks associated with intravenous therapy appear to
receiving intravenous bisphosphonate in the form of
be substantially higher than for the oral medications.
either Aredia or Zometa. His letter prompted others to
A conclusive cause and effect relationship between
review patient records and to make reports of similar
bisphosphonate therapy and osteonecrosis of the jaw
has not been established. But evidence suggests that
The following year, Ruggerio et al published a re-
such a link may in fact exist. Unfortunately there is
view of 56 cases of osteonecrosis associated with the
limited data to aid in the identification of other risk
use of intravenous bisphosphonate The au-
factors for development of the disease. Some evi-
dence suggests that those risk factors may include the
If dental implants are to be placed, the panel sug-
potency of the drug used, the duration of therapy,
gests contacting the physician who prescribed the
being Caucasian, being older than 65, having chronic
oral bisphosphonate prior to surgery to suggest an
periodontitis, ongoing corticosteroid therapy, having
alternate dosing schedule, a drug holiday, or an alter-
diabetes, smoking, and the use of
native to bisphosphonate therapy. These recommen-
It appears important to make a distinction between
dations were made by 2 of the Task Force members
osteonecrosis of the jaw induced by oral bisphospho-
based on their clinical experience with 50 such pa-
nates versus that induced by intravenous bisphospho-
nates. Oral bisphosphonate-induced necrosis appears
For years dentists have routinely performed surgi-
to be less frequent, less severe, more responsive to
cal procedures and placed implants in patients receiv-
discontinuation of the drug, and curable with surgical
ing bisphosphonate therapy. Prior to widespread
debridement. Marx states that osteonecrosis from oral
awareness of the risk of bisphosphonate-associated
bisphosphonates differs significantly from intrave-
osteonecrosis and publication of treatment guidelines,
nous bisphosphonate-associated osteonecrosis in 3
these patients were treated without modification of stan-
major ways: Patients taking oral bisphosphonates
1)
dard treatment procedures. Additional research is re-
require a longer period of drug therapy before bone is
quired to determine if additional diagnostic testing or
exposed,
2) manifest less bone exposure and symp-
treatment modifications are actually necessary. Because
toms are less severe, and
3) have a chance of symp-
implant surgery is a major part of our practice, we
toms improving or exposed bone healing after taking
wanted to determine the necessity of making wholesale
a drug holiday.
modifications in our treatment of patients receiving oral
Nevertheless, the Council of Scientific Affairs of the
bisphosphonate therapy based on our past clinical ex-
American Dental Association assembled a panel of
perience. Specifically we wanted to determine whether
experts to provide dentists with guidelines for treat-
any patients had developed osteonecrosis of the jaw and
ing patients who are receiving bisphosphonate ther-
whether there was any indication that bisphosphonate
apy. The American Association of Oral and Maxillofa-
therapy affected the overall success of the implants as
cial Surgeons convened an expert Task Force with
defined by Albrektsson and
similar goals. The resulting guidelines suggested avery cautious approach to implant surgery and extrac-tions for patients receiving bisphosphonate therapy
Patients and Methods
either intravenously or
The American Dental Association Expert Panel rec-
We identified 1,319 female patients over the age of 40
ommends that patients taking oral bisphosphonates be
who had implant surgery in the Department of Oral and
informed about the risks and benefits. They further
Maxillofacial Surgery at Montefiore Medical Center be-
recommend that nonsurgical and less invasive treatment
tween January 1998 and December 2006. A survey
alternatives be used when possible. The panel cautions
asking about current and past oral bisphosphonate
that patients may be at increased risk when extensive
therapy was mailed to those patients. Thirty-five percent
implant placement or guided bone regeneration is nec-
(458) of the surveys were returned. Of those, 115 pa-
essary. When the treatment plan involves the medullary
tients reported taking oral bisphosphonates before or
bone and/or periosteum in multiple sextants, the panel
after implant surgery. None reported receiving intrave-
recommends treating 1 sextant or tooth at a time. They
nous bisphosphonate. A total of 468 implants had been
recommend treatment with an antimicrobial mouth
placed in these patients.
rinse and a 2-month disease-free follow-up before other
The patients who responded to our survey that
sextants are treated.
reported a history of bisphosphonate use were com-
The Task Force appointed by the American Associ-
pared with a random sample of nonresponders with
ation of Oral and Maxillofacial Surgeons also recom-
regard to age and number of implants. The difference
mends that patients taking oral bisphosphonates be
between the groups with regard to number of im-
informed of the small risk of compromised bone heal-
plants was not significant, whereas there was a signif-
The Task Force states that elective dentoalveo-
icant difference in age between the groups. In addi-
lar surgery does not appear to be contraindicated in
tion, 115 of the 458 responders, whereas only 5
patients without known risk factors who have been
among 100 nonresponders, had a history of bisphos-
taking oral bisphosphonates for less than 3 years. A
phonate use (
P ⬍ .0001).
drug holiday of at least 3 months prior to surgery is
The remaining 343 patients indicated that they had
suggested for patients who have taken an oral
not received bisphosphonate therapy. A total of 1,450
bisphosphonate for more than 3 years and those that
implants had been placed in these patients; 1,436
have taken corticosteroids concomitantly.
implants integrated successfully.
IMPLANTS AND ORAL BISPHOSPHONATES: 115 CASES
FIGURE 1. Patient survey.
Grant et al. Implants and Oral Bisphosphonates: 115 Cases. J Oral Maxillofac Surg 2008.
We used the criteria developed by Albrektsson and
The mean duration of oral bisphosphonate therapy
Zarb to determine implant success. Those criteria are
was 38 months. Twenty-six patients started oral
bisphosphonate therapy after implant surgery andsubsequent healing. The remaining 89 patients started
bisphosphonate therapy before implant placement.
Sixty-six patients reported taking Fosamax prior to
The 115 patients who reported having bisphospho-
implant placement; 21 patients were taking Actonel;
nate therapy were contacted and informed that there
and 2 patients were taking Boniva. Out of the 89
was a slight risk of bisphosphonate-associated osteone-
patients taking oral bisphosphonates prior to surgery,
crosis of the jaw. All 115 patients had been treated using
33 patients reported taking an oral bisphosphonate
standard implant surgery techniques without modifica-
for more than 3 years prior to surgery: 27 patients
tion due to the bisphosphonate therapy. Sixty-three per-
reported taking Fosamax, 5 patients reported taking
cent of the patients (72 out of 115) were seen for a
Actonel, and 1 patient reported taking Boniva. The
follow-up clinical and radiographic examination.
remaining 56 patients reported taking oral bisphos-
teonecrosis, then the upper bound of a 1-sided confi-
Table 2. CRITERIA FOR IMPLANT SUCCESS
dence interval for 0 events for N ⫽ 115 is 0.026. Thus,
An individual, unattached implant is immobile when
we would not expect that the rate of osteonecrosis is
tested clinically.
greater than 2.6%. However, none of the 72 patients
A radiograph does not show any evidence of peri-implant
who were examined in the clinic after reporting a his-
tory of bisphosphonate therapy had evidence of osteo-
Vertical bone loss is less than 0.2 mm annually after the
implant's first year of service.
necrosis. We have no reports of osteonecrosis from any
Individual implant performance is characterized by an
of the 861 patients who did not return the survey. Nor
absence of persistent and/or irreversible signs and
do we have reports of osteonecrosis from any of the
symptoms such as pain, infection, neuropathies,
referring restorative dentists. We cannot say definitively
paresthesia, or violation of the mandibular canal.
that none of these patients has developed bisphospho-
To be considered successful, the dental implant should
provide functional service for 5 years in 75% of the
nate-associated necrosis. However, it is our experience
that we hear rather quickly from implant patients whohave problems such as losing an implant or develop-
Adapted with permission from Albrektsson et
ment of oral lesions.
Grant et al. Implants and Oral Bisphosphonates: 115 Cases. J Oral
Patients taking bisphosphonates who reported to
Maxillofac Surg 2008.
the clinic or who contacted us by phone or e-mailwere asked about known risk factors such as age over
phonates for less than 3 years prior to dental implant
65, having diabetes or taking prednisone concomitantly
with bisphosphonate therapy. The mean age of the
A total of 468 dental implants were placed in pa-
patients who had bisphosphonate therapy was 67.4
tients receiving bisphosphonate therapy. Four hun-
years; 51 of the 115 were over the age of 65. Three of
dred sixty-six implants are in function and are suc-
the patients reported taking prednisone concomitantly
cessful according to criteria for success defined by
with bisphosphonate therapy; 2 patients had diabetes.
Albrektsson and Zarb. Two implants failed. In the first
More invasive surgical procedures have been iden-
case, the patient had 2 implants placed in the maxilla
tified as risk factors for developing bisphosphonate-
to restore the upper left first and second molars, and
associated osteonecrosis. In the group of 115 pa-
a simultaneous sinus lift. In the next year, an addi-
tients, 32 had maxillary sinus augmentation. Six of the
tional 2 implants were placed to restore the upper left
32 were taking an oral bisphosphonate for at least 3
first and second bicuspids. The following year the
years prior to sinus augmentation.
implant in the area of the upper left second bicuspid
The ADA panel recommended that surgical treat-
failed to integrate. This was discovered prior to res-
ments be limited to a single sextant with a substan-
toration. The implant was subsequently removed and
tial interval for healing before proceeding to treat-
replaced several months later. This implant success-
ment of another sextant.4 At Montefiore, single
fully integrated and was definitively restored 6
session, multiple sextant surgery was performed on
months later. The patient did report taking oral
29 patients who had been taking oral bisphospho-
bisphosphonates for 3 years prior to implant place-
nates prior to surgery.
ment. The patient was no longer taking the oral
The most interesting of these patients is a 67-year-
bisphosphonate at the time of implant placement or
old female who had been taking Actonel for more
thereafter. To date, all implants are successful and
than 5 years prior to dental implant placement. Two
have been in function for more than 4 years.
implants were placed into the upper right quadrant
In the second case, the patient started taking oral
with a simultaneous maxillary bone graft. The follow-
bisphosphonates more than 4 years prior to implant
ing year, 5 additional implants were placed in the
placement. The patient had 6 implants placed in the
right and left mandible. To date, the patient is still
maxilla. Two months later, 7 implants were placed in
taking Actonel. All 7 dental implants are in function
the mandible. The most posterior implant replacing
and show no evidence of osteonecrosis
the lower left second molar did not integrate and wasremoved 1 month later. That implant was not re-
placed and the area healed uneventfully. All 12 im-plants are integrated and in function. The patient
Ruggerio's study that included 7 patients taking
remains on oral bisphosphonates and reports over 8
oral bisphosphonates for treatment of osteoporosis
consecutive years of oral bisphosphonate therapy.
contains the single most compelling evidence of a link
None of the 458 patients who responded to the sur-
between oral bisphosphonate treatment and osteone-
vey reported symptoms of bisphosphonate-associated
None of the 7 patients had a history of malig-
osteonecrosis of the jaw. If it is assumed that none of the
nant disease or chemotherapy. He did not report on
115 responders treated with bisphosphonates had os-
the duration of the bisphosphonate therapy. The re-
IMPLANTS AND ORAL BISPHOSPHONATES: 115 CASES
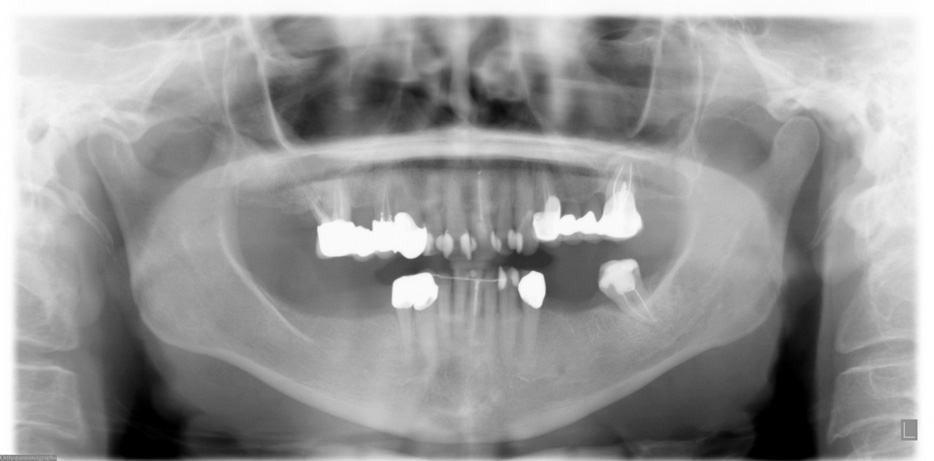
FIGURE 2. Preoperative panoramic x-ray taken in September 2005.
Grant et al. Implants and Oral Bisphosphonates: 115 Cases. J Oral Maxillofac Surg 2008.
maining 56 patients in the study were all taking intra-
the jaw. Marx speculates that Didronel does not cause
venous bisphosphonate in conjunction with treat-
osteonecrosis because it contains no nitrogen and
ment for multiple myeloma, breast cancer, or some
therapy is not constant; patients are treated with a
other form of malignant disease.
cycle of on-off doses.
Despite the widespread use of oral bisphospho-
According to the case report, 5 implants were
nates, a review of the literature found only 1 case of
placed and successfully integrated in the anterior
dental implant failure associated specifically with oral
mandible. The patient was restored and had an
bisphosphonate use. A case report from 1995 sug-
uneventful postoperative course. The patient began
gested that failure of 5 implants was caused by
etidronate disodium (Didronel) therapy 28 months
bisphosphonate therapy.The drug discussed in this
after implant placement. This drug can be adminis-
case report was etidronate disodium (Didronel; Procter
tered intravenously or orally; however, the route of
and Gamble), a non-nitrogen containing bisphospho-
drug administration for the patient was not speci-
nate that is not the drug of choice for treatment of
fied by the authors. After taking the drug for 4
osteoporosis. Didronel is 1,000 times less potent than
months, the patient presented with pain in the
alendronate (Fosamax) and is used to treat fibrous
mandible. A panoramic radiograph revealed exten-
dysplasia and Paget's disease.Didronel does not
sive osteolysis around all implants and all 5 im-
contain nitrogen. Other than this report, only nitro-
plants were removed 1 month later. The bisphos-
gen containing bisphosphonates have been found to
phonate treatment was discontinued. The authors
produce bisphosphonate-associated osteonecrosis of
make no mention of poor or delayed healing fol-
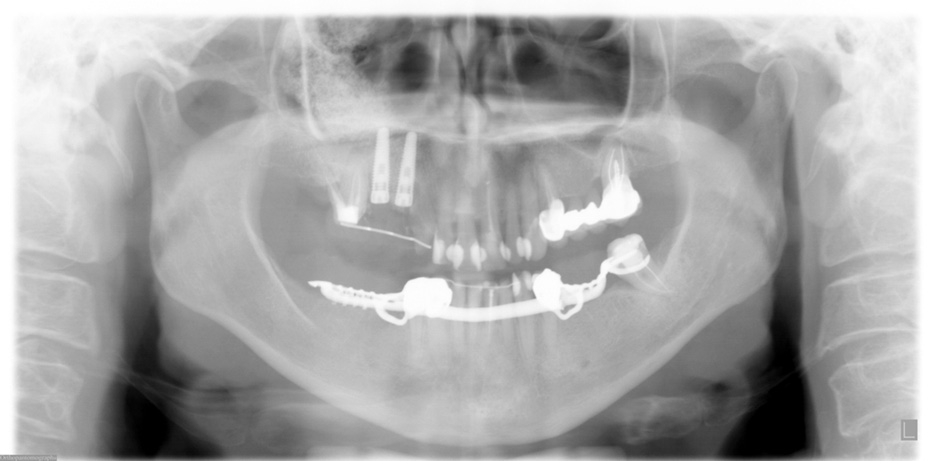
FIGURE 3. Postoperative panoramic x-ray after 2 dental implants were placed in the upper right quadrant with a simultaneous maxillary sinus lift
in October 2005.
Grant et al. Implants and Oral Bisphosphonates: 115 Cases. J Oral Maxillofac Surg 2008.
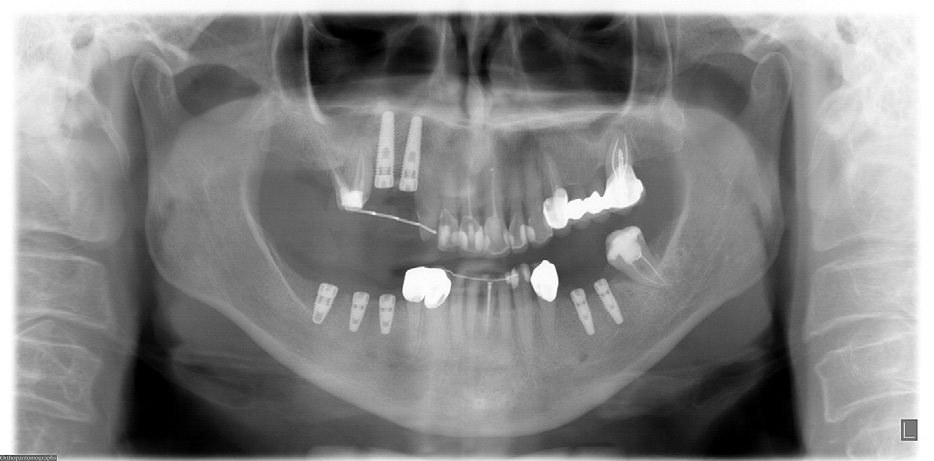
FIGURE 4. Panoramic x-ray after mandibular dental implants were placed in May 2006.
Grant et al. Implants and Oral Bisphosphonates: 115 Cases. J Oral Maxillofac Surg 2008.
lowing removal of the implants as might be ex-
that the implant failure and osteolysis were caused
pected with bisphosphonate-induced necrosis.
by the Didronel They recommend that
The authors reported that the patient developed
patients who have previously undergone implant
a parafunctional clenching habit subsequent to im-
placement forego bisphosphonate therapy. They
plant placement and loading. It is possible that
further recommend that dentists avoid implant
increased physiologic loads were the cause of im-
placement in patients who require bisphosphonate
plant failure. Nevertheless, the authors concluded
FIGURE 5. Clinical photos show no evidence of biphosphonate-associated osteonecrosis.
Grant et al. Implants and Oral Bisphosphonates: 115 Cases. J Oral Maxillofac Surg 2008.
IMPLANTS AND ORAL BISPHOSPHONATES: 115 CASES
In 2006, Jeffcoat reported the results of a single-
2. Hillner BE, Ingle JN, Berenson JR, et al: American Society of
blind controlled study of 50 postmenopausal female
Clinical Oncology guideline on the role of bisphosphonates inbreast cancer. J Clin Oncol 18:1378, 2000
dental implant patients, all of whom had bone mineral
3. Berenson JR, Hillner BE, Kyle RA, et al: American Society of
density scores indicative of Twenty-
Clinical Oncology clinical practice guidelines: The role of
five patients had taken oral bisphosphonates (alen-
bisphosphonates in multiple myeloma. J Clin Oncol 20:3719,2002
dronate or risendronate) for 1 to 4 years prior to in-
4. Migliorati CA. Bisphosphonates and oral cavity avascular bone
clusion in the study. The mean duration of bisphos-
necrosis. J Clin Oncol 21:4253, 2003
phonate therapy prior to the study was 3 years. The
5. Marx RE. Pamidromate (Aredia) and zoledronate (Zometa) in-
duced avascular necrosis of the jaws: A growing epidemic.
other 25 patients did not take oral bisphosphonates
J Oral Maxillofac Surg 61:1115, 2003
prior to or during the study. One patient in each
6. Glorieux FH, Bishop NJ, Plotkin H, et al: Cyclic administration
group smoked. A total of 102 implants were placed in
of pamidronate in children with severe osteogenesis imper-
the group taking bisphosphonates; 108 dental im-
fecta. N Engl J Med 339:947, 1998
7. Marx RE: Oral and Intravenous Bisphosphonate-Induced Osteo-
plants were placed in the nonbisphosphonate group.
necrosis of the Jaws. Hanover Park, IL, Quintessence, 2007
After 3 years, there was a 100% success rate with no
8. American Dental Association Council on Scientific Affairs: Den-
clinical evidence of infection, pain, or necrosis in the
tal management of patients receiving oral bisphosphonate ther-apy: Expert panel recommendations. J Am Dent Assoc 137:
patients who received oral bisphosphonates. There
was a 99.2% success rate in the group who did not
9. US Department of Health and Human Services. Bone health and
receive oral bisphosphonates.
osteoporosis: A report of the Surgeon General. Rockville, MD:US Department of Health and Human Services, Office of the
We found similar results in our study. Of the 115
Surgeon General, 2004
patients taking oral bisphosphonates, none show ev-
10. American Dental Association Council on Scientific Affairs: Den-
idence or have symptoms of necrosis. All have had
tal management of patients receiving oral bisphosphonate ther-apy: Expert panel recommendations. J Am Dent Assoc 137:
successful implant restorations. Had recommenda-
tions of the ADA and AAOMS panels been followed,
11. Advisory Task Force on Bisphosphonate-Related Ostenonecro-
treatment for at least 30 of these patients would have
sis of the Jaws, American Association of Oral and Maxillofacial
been modified. Possible treatment modifications in-
Surgeons: American Association of Oral and Maxillofacial Sur-geons position paper on bisphosphonate-related osteonecrosis
clude a drug holiday, obtaining a C-terminal cross-
of the jaws. J Oral Maxillofac Surg 65:369, 2007
linking telopeptide serum test, treating individual
12. Durie BGM, Katz M, Crowley J: Osteonecrosis of the jaws and
quadrants separately, or even use of nonimplant-sup-
bisphosphonates. N Engl J Med 353:99, 2005
13. Bamias A, Kastritis E, Bamia C, et al: Osteonecrosis of the jaw
ported Management of patients re-
in cancer after treatment with bisphosphonates: Incidence and
ceiving oral bisphosphonates should be separated and
risk factors. J Clin Oncol 23:8580, 2005
distinguished from the management of patients re-
14. Dimopoulos MA, Kastritis E, Anagnostopoulos A, et al: Osteo-
necrosis of the jaw in patients with multiple myeloma treated
ceiving intravenous bisphosphonates. All patients
with bisphosphonates: Evidence of increased risk after treat-
considering surgical treatments should be asked
ment with zoledronic acid. Haematologica 91:968, 2006
about bisphosphonate use and should be advised of
15. Zavras AI, Zhu S: Bisphosphonates are associated with in-
creased risk for jaw surgery in medical claims data; Is it osteo-
possible risks. Dental records should be revised to
necrosis? J Oral Maxillofac Surg 64:917, 2006
include this information. Additional research is re-
16. Marx RE, Sawatari Y, Fortin M, et al: Bisphosphonate-induced
quired to guide the management of patients taking oral
exposed bone (osteonecrosis/psteopetrosis) of the jaws. Risk
bisphosphonates. Some evidence suggests that duration
factors, recognition, prevention, and treatment. J Oral Maxillo-fac Surg 63:1567, 2005
of oral bisphosphonate therapy correlates with the de-
17. Ruggiero SL, Mehrotra B: Ten years of alendronate treatment
velopment and severity of osteonecrosis. More data are
for osteoporosis in postmenopausal women. N Engl J Med
needed to determine at what point invasive dental treat-
18. Schwartz HC: Osteonecrosis and bisphosphonates: Correlation
ment should be routinely modified. In addition, more
versus causation. J Oral Maxillofac Surg 62:763, 2004
data is needed to determine whether the serum C-ter-
19. Albrektsson T, Zarb G, Worthington P, et al: The long-term effi-
minal cross-linking telopeptide serum test is valid for
cacy of currently used dental implants: A review and proposedcriteria of success. Int J Oral Maxillofac Implants 1:11, 1986
assessing There is insufficient evidence to suggest
20. Stark WJ, Epker BN: Failure of osteointegrated dental implants
that implant placement, tooth extraction, and other sur-
after diphosphonate therapy for osteoporosis: A case report.
gical treatments should be routinely avoided for patients
Int J Oral Maxillofac Implants 10:74, 1995
21. Chapurlat R, Meunier PJ: Bisphosphonates and bone remodel-
receiving oral bisphosphonate therapy. Instead, evi-
ing: Effectiveness in Paget's disease, fibrous dysplasia, and
dence suggests that frequent clinical and radiological
osteoporosis [in French]. Rev Chir Orthop Reparatrice Appar
examinations with prompt treatment of problems will
minimize potential risks.
22. Jeffcoat MK: Safety of oral bisphosphonates: Controlled
studies on alveolar bone. Int J Oral Maxillofac Implants21:349, 2006
23. Rosen HN, Moses AC, Garber J, et al: Serum CTX. A new
marker of bone resorption that shows treatment effect more
1. Ruggerio SI, Mekrotra B, Engroff SL: Osteonecrosis of the jaws
often than other markers because of low coefficient of variabil-
associated with the use of bisphosphonates. A review of 63
ity and large changes with bisphosphonate therapy. Calcif
cases. J Oral Maxillofac Surg 62:527, 2004
Tissue Int 66:100, 2000
Source: http://www.occore.com/files/2011/08/bisphimp.pdf
American Journal of Clinical Hypnosis, 55: 272–290, 2013Copyright © American Society of Clinical HypnosisISSN: 0002-9157 print / 2160-0562 onlineDOI: 10.1080/00029157.2012.707156 Treating Depression With Antidepressants: Drug-Placebo Efficacy Debates Limit Broader Considerations Private Practice, Fallbrook, California, USA The core issue regarding antidepressants for many clinicians is whether they perform significantly bet-ter than placebos. However, this article suggests eight additional concerns beyond drug efficacy aloneto consider regarding antidepressants including: (1) formulating only a one-dimensional, biologicalview of depression; (2) defining the client's role as passive in treatment; (3) economic corruptionof the research and reporting; (4) false or misleading consumer advertising; (5) conflicting data thatconfuse practitioners and consumers alike; (6) over- and under-prescription of medications; (7) drugside-effects; and (8) harm to the environment. The enhanced effects of psychotherapy utilizing hypno-sis offer a means of avoiding most, if not all, of the problems associated with the use of antidepressantsas a primary form of treatment.
LA LETTRE D'ACTUALITÉS N°143 - Septembre 2014 SOMMAIRE 1) Les infections respiratoires hautes Le mot de la rédaction 2) Les infections urinaires 3) Documentation Les aspects épidémiologiques concernant les maladies in-fectieuses pédiatriques sont de manière irrégulière et in- complète soumis à investigation.


