Pebbleweb.co.uk
Exertional myopathy post fox attack in an agile wallaby (Macropus agilis).
1.1 Introduction
Exertional myopathy or ‘capture myopathy' refers to a non-infectious, metabolic syndrome
characterized by damage to skeletal or cardiac muscle following intense physical activity.
Skeletal muscle necrosis and acute renal failure are common sequelae (Hartup
et al. 1999).
In mammals, the disease has been primarily documented in cervids, (Wallace
et al. 1987)
macropods, cetaceans, crocodilians, pinnipeds, rodents and primate species (Hartup
et al.
1999). Elevated environmental temperature may increase the likelihood of the disease and in
macropods the disease is most commonly associated with capture or restraint procedures
(Chalmers & Barrett 1982, Spraker 1993, Williams & Thorne 1996), repeated handling, close
confinement or exposure to predators (Vogelnest & Portas 2008). In severe cases, death
may occur, acutely within hours, or more commonly a sub-acute syndrome of mortality
between two and four days after the onset of clinical signs is reported (Carpenter 1993).
Chronically affected animals may experience a delayed-peracute syndrome causing death
weeks later, and individuals if only mildly affected then exposed to repeated stress may
relapse and develop the acute form of disease (Hartup
et al. 1999).
Clinical history
A five year old, female, Agile wallaby (
Macropus agilis) A00035 in a zoological collection
presented with wet forearms from overgrooming, restlessness, frequent head movements, a
stumbling gait, and appeared anxious and agitated in demeanor. A carcass of a bridle nail
tailed wallaby
(Onychogalea fraenata) was found in close proximity which appeared to have
been predated upon by a fox owing to the primary wound and lack of musculature
surrounding the jugular vein and lateral neck (Figure 1). A fox had also been sighted exiting
the zoo grounds the night prior. This wallaby was housed in an open bushwalk enclosure,
approximately 200m by 200m in diameter. Five agile wallabies, and ten red (
Macropus rufus)
and Western grey (
Macropus fulginosus) kangaroos also resided in this non-fenced area.
Two other male agile wallabies, appeared skittish, but did not appear as agitated as A00035.

Figure 1. Typical lesions of predation by the introduced European red fox
(Vulpes vulpes). The site of attack is the lateral neck surrounding the jugular,
and bite wounds and tearing through the muscular regions of the neck is noted
with a 22 mm inter-canine distance.
Clinical examination
The gait and demeanor of this wallaby were observed from behind a tree with binoculars as to
not initiate a further flight response. Increased activity with associated stumbling and
unsteadiness was seen. Wide dilated pupils with ears pointed in a backward direction were
evident and respiratory rate was increased (40rpm). Given the keepers had noticed this
behaviour over the past hour prior to calling for veterinary attention a decision was made to
anaethetise the wallaby immediately for diagnosis, treatment and cooling.
Supportive care
The sprinklers were immediately turned on to cool the wallaby, and the bushwalk area closed
to the public to minimize any further excitation. The two other affected agile wallabies of an
estimated 11.5kg were darted with the neuroleptic azaperone (Stresnil® Boehringer
Ingelheim Pty. Ltd, NSW Australia) 2mg/kg IM and fluphenazine (Modecate® Bristol-Myers
Squibb, NSW Australia) 2.5mg/kg IM. (Appendix 2). This was to ensure rapid and sustained
tranquilisation. Keepers conducted hourly checks of all the macropods in the bushwalk.
Diagnostic procedures
The wallaby was darted with a CO2 powered pistol (Tel-Inject Australasia®, Victoria Australia)
from an approximate distance of 8 meters with 7mg/kg of tiletamine/zolazepam (Zoletil®
Virbac, Australia) (Appendix 1). Eight minutes post darting this wallaby was in lateral
recumbency and a hand towel was placed over the eyes to minimize further excitation. At 10
minutes post darting the wallaby was judged to be adequately sedated for transport, there
was no corneal reflex or spontaneous movement elicited on palpation with a broom handle. A
stretcher was slid beneath the wallaby's body for transport to the veterinary hospital.

Once at the veterinary department the wallaby was placed on a face mask delivering 5%
isoflurane and 2L/minute oxygen and her temperature taken (38'C) then intubated as soon as
jaw tone had decreased. (Figure 2).
Figure 2.
Intubation of an agile wallaby with the aid of a laryngoscope and stylet
A 22G catheter was inserted into the lateral caudal vein and 5mL of blood drawn. Packed cell
volume (PCV) and total protein (TP) was measured on a refractometer. The remaining blood
was submitted for a complete blood count (CBC), biochemistry, and serum storage.
Intravenous fluids (0.9% NaCl solutiom, Baxter healthcare, NSW Australia) were commenced
at 10ml/kg/hr. An elevated PCV and TP indicated likely haemoconcentration from
Despite being anaethetised spontaneous muscle activity and fasciculations were noted in the
hindlimbs. However jaw tone and cloacal tone was absent indicative of deep sedation, the
muscle fasciculation's were more likely related to the myonecrotic state. There was no
evidence of trauma on clinical exam.
Temperature was beginning to decrease (37'C) and the respiratory rate and heart rate had
stabilized at 20rpm and120bpm respectively. Mucous membranes were tacky.
The bladder was palpated and a patch of hair shaved directly above the bladder. This site
was aseptically prepared and cystocentesis was conducted. The urine obtained was pink in
colouration with +++ blood found on urine dipstix (Figure 3).
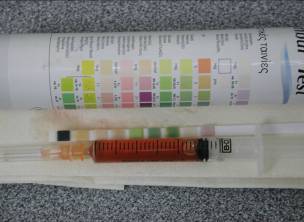
Figure 3.
Blood was evident on cystocentesis.
Radiographs were taken of the pelvis and hindlimbs for the evidence of fractures or bony
pathology, no abnormalities were detected.
Differential diagnoses included; nutritional, toxic, infectious and parasitic myopathies however
given the history the most likely diagnoses was that of an exertional myopathy occurring in
response to a fox sighting.
Treatment
Once anaethetised the isoflurane was dropped to 1.5%, however the oxygen remained at
2L/min to promote oxygenation of muscle and enhance tissue perfusion. Diuresis and cooling
was undertaken through IV fluid therapy.
Body temperature was closely monitored and controlled with cool packs, fans, mist and IV
fluids. Muscle fasciculation's and myonecrosis was reduced with the skeletal muscle relaxant
diazepam (Pamlin®, Parnell Laboratories, NSW Australia) administered IV 0.5-1 mg/kg (Rose
1999). Dantrolene (Dantrium powder for injection®, Pfizer, NSW, Australia) a skeletal muscle
relaxant used in humans for treatment of malignant hyperthermia was administered slowly IV
at 1mg/kg then 1mg/kg PO SID 3-5 days. Prednisolone sodium succinate (Solu-Delta Cortef
powder®, Pharmacia & Upjohn Company, Michigan USA) (5-10mg/kg IV) was administered
followed by Vitamin E/Selenium (Selevite®, Troy Laboratories Pty Ltd, NSW Australia)
1ml/50kg body weight.
The isoflurane saturation was slowly reduced and the endo tracheal tube removed once
swallowing evident. Azaperone was administered 0.5mg/kg IM to provide short term
tranquilisation and sedation. The wallaby was placed in a hessian sack in lateral recumbency
in a large dog pet pack with a maintenance dose of IV fluids being delivered through the
catheterized lateral tail vein. The wallaby was then moved to a quiet, dark, recovery room
within the veterinary hospital for observation. The wallaby was rolled every two hours to
prevent respiratory deficits and pressure necrosis. Recovery was prolonged and there was
little attempt to stand until six hours post-anaesthetic. Blood results revealed massively

elevated muscle enzymes, and evidence of severe renal compromise (CK > 20 000 U/L,
significantly elevated ALP, AST, urea and creatinine). The wallaby was severely depressed,
had minimal response to stimuli, had developed a fever (38°C) and gurgly respiration possibly
indicative of pulmonary oedema and was given a poor prognosis.
1.3 Outcome
A decision was made to euthanse this animal on medical and welfare grounds post
consultation with curatorial staff. Given the wallaby was already deeply sedated 5mL of IV
pentobarbitone (Lethabarb euthanasia injection®, Virbac Australia Pty Ltd, NSW Australia)
was delivered through the lateral caudal vein and death shortly followed. A post mortem
conducted revealed a paleness of the skeletal muscle especially of the femoral and gluteal
muscles, and muscular haemorrhage (Figure 5). On histopathology haemorrhage and
oedema throughout the interstitium of the hindlimb adductor muscles and multifocal myofibre
degeneration was reported. Renal changes consisted of hydropic change, and necrosis of
renal tubule epithelial cells, with pulmonary congestion and oedema (S.Wong, personal
communication, 2009). No infectious causes were found on culture of routine tissues and
histopathology. The body was disposed of in the quarantine bins then incinenerated to
prevent the potential for residue and secondary poisonings.
Figure 5.
Typical lesions of an exertional myopathy were evident including
paleness of the femoral muscles and muscular haemorrhage.
Discussion
Capture myopathy occurs as a result of prolonged sympathetic nervous system stimulation
causing ischaemia as a result of reduced tissue perfusion, lactic acidosis and muscular
adenosisne triphosphate reserve depletion. Which in turn may lead to cardiovascular and
circulatory collapse, muscular compartment syndrome and acute renal failure subsequent to
ischaemia and myoglobinuric nephrosis (Vogelnest & Portas 2008).
In this case the most likely stimulator was the sighting of a predator which led to the above
cascade of events and eventual demise. The blood in the urine most likely occurred due to
myoglobinuric nephrosis. Whereby the severe muscle breakdown lead to a circulating
myoglobinemia. Myoglobin is toxic to the proximal tubules and loop of Henle (Rose 2005)
and this combined with the associated circulatory collapse contributes to the acute tubular
necrosis seen in this condition.
Fluid therapy with 0.9% NaCl is a mainstay of treatment for many reasons, to improve
perfusion to the kidney, dilute the damage that myoglobin causes to the kidney, dilute the
lactic acid in the blood stream, and so improve heart function, expand the blood volume and
so address the mechanisms of shock, and reverse the hyperthermia. Steroids were
administered as they may contribute to stabilising cell membranes to prevent ongoing or
irreversible cell degeneration and Vitamin E and Selenium were administered as it has been
proven in some species to reduce the risk of subsequent myopathy (Williams & Thorne 1996).
The biochemical changes that occurred are related to metabolic acidosis and include
elevation in muscle enzymes (CK, AST & LDH). The CK level increases rapidly in response
to cardiac or skeletal muscle damage, yet owing to the enzyme's short half life will only
remain highly elevated if ongoing muscle damage is occurring (Fowler 1993). AST has a
longer half life than CK. As such we conducted serial monitoring of AST & CK to provide
insight into the duration and degree of muscle damage which was sub-acute and had most
likely had begun the night before presentation in response to the fox sighting.
The prevention of capture myopathy is far more effective than the cure, and prophylactic
measures should be considered every time a susceptible species is handled or anaesthetised.
In this case, the stimulus was difficult to prevent, although fencing has subsequently been
improved by adding further height and digging the wire three feet beneath the ground to
prevent tunneling of predators. When anaethetising a susceptible species, noise and
movement should be kept to a minimum during induction and elective procedures should be
conducted in the cool of the day (early morning) and appropriate restraint for the size of the
animal should be considered. In the event of a stressful situation, it may be useful to
administer neuroleptic drugs prophylactically.
Conclusion
This agile wallaby presented with clinical signs of overgrooming, agitated behaviour and a
stumbling gait. CBC and biochemistry indicated haemoconcentration, severe myonecrosis
and azotaemia and cystocentesis found blood in the urine, all consistent with a diagnosis of
an exertional myopathy when combined with the history of a fox attack and sighting the night
previously. Treatment was attempted to reverse concurrent shock and hyperthermia, reverse
metabolic acidosis, and stabilise cellular membranes. However owing to the severity of the
myopathy, on repeated blood sampling and advancing clinical deterioration a poor prognosis
for recovery was given and the wallaby euthansed. Lesions consistent with acute tubular
nephrosis, and skeletal muscle necrosis were evident histologically. Fencing upgrades to
minimize further predation and the provision of neuroleptics to other exposed macropods
have prevented further deaths.
References
Chalmers GA, & Barrett MW, Capture myopathy In: Noninfectious diseases of Wildlife. GL. Hoff & JW. Davis (eds.). Iowa State University Press, Ames, Iowa, Pp. 84–94.
Fowler MF (1993) Zoo and wild animal medicine, current therapy 3, W.B Saunders. Pennsylvania USA.
Hartup BK, Koillas GV, Jacobsen MC, Valentine BA, & Kimber KK. Exertional myopathy in
translocated river otters from New York. Journal of Wildlife Diseases. 1999; 35(3): 542–547
Rose K. (2005) Common disease of Urban Wildlife. Wildlife in Australia: Healthcare & Management, General diseases – myopathy & trauma. Australian Registry of Wildlife Health Field Manual. Pp. 1-11.
Spraker TR. Stress and capture myopathy in artiodactylids. In: Zoo and wild animal medicine, current therapy 3, ME. Fowler (ed.). W. B. Saunders Company, Philadelphia, Pennsylvania. Pp. 481–488.
Vogelnest L, & Portas T (2008) Macropods. In: Medicine of Australian Mammals. L. Vogelnest, R. Woods (eds). CSIRO Publishing, Sydney, Australia.
Wallace RS, Bush M, & Montali RJ Deaths from exertional myopathy at the National
Zoological Park from 1975 to 1985. Journal of Wildlife Diseases. 1987; 23(3): 454-462
Williams ES, & Thorne ET (1996) Exertional myopathy. In: Non-infectious diseases of
wildlife. 2nd Ed. A. Fairbrother, LN. Locke, GL Hoff (eds). Iowa State Press, Ames, Iowa.
Pp. 181 - 193.
Appendix 1:
Agile Wallaby A00035
EUA, suspected exertional
Procedure
Veterinary:
Drug Conc
Total dose
Total dart
volume ml
Zolazepam (Zoletil) 1mg/mL
Drug Conc
Total dose
volume ml
Drug Conc
Total dose
Route Notes
volume ml
Adrenaline 1:1,000
0.447 0.745 IV/SC
Diazepam 0.5-1mg/kg
CV/Resp:
Integument:
Urogenital:
cystocentesis express
Blood tests:
ZP1, serum banking, toxoplasmosis
Rectal swab:
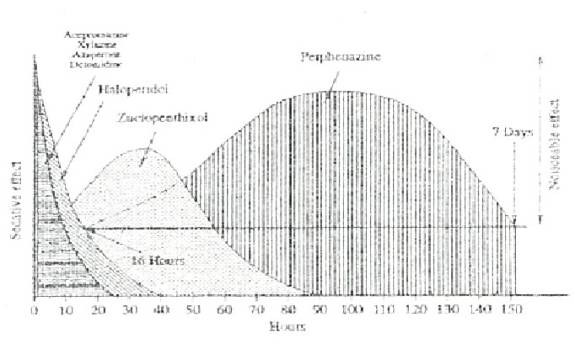
Appendix 2: Neuroleptic drug combinations
Generic Name
Duration
(Stresnil® Boehringher
Haloperidol B.P.
(Serenace® Searl)
Duration up to 4 weeks
Duration 3-4 weeks
From: Vogelnest, L. (1998) Chemical Restraint of Native Fauna Wildlife Proceedings 1998. pp.150-187
Source: http://www.pebbleweb.co.uk/edinburgh/download.aspx?oid=47579&useroid=0
"Charging Lithium-Ion Batteries: Not All Charging Systems Are Created Equal" By Scott Dearborn Principal Applications Engineer Microchip Technology Inc. 2355 West Chandler Blvd Chandler, AZ 85224 INTRODUCTION Powering today's portable world poses many challenges for system designers. The use of batteries as a prime power source is on the rise. As a result, a burden has been placed on the system designer to create sophisticated systems utilizing the battery's full potential. Each application is unique, but one common theme rings through: maximize battery capacity usage. This theme directly relates to how energy is properly restored to rechargeable batteries. No single method is ideal for all applications. An understanding of the charging characteristics of the battery and the application's requirements is essential in order to design an appropriate and reliable battery charging system. Each method has its associated advantages and disadvantages. It is the particular application with its individual requirements that determines which method will be the best to use. Far too often, the charging system is given low priority, especially in cost-sensitive applications. The quality of the charging system, however, plays a key role in the life and reliability of the battery. In this article, the fundamentals of charging Lithium-Ion (Li-Ion) batteries are explored. In particular, linear charging solutions and a microcontroller-based, switch-mode solution shall be explored. Microchip's MCP73843 and MCP73861 linear charge management controllers and PIC16F684 microcontroller along with a MCP1630 pulse width modulator (PWM), shall be used as examples. LI-ION CHARGING The rate of charge or discharge is often expressed in relation to the capacity of the battery. This rate is known as the C-Rate. The C-Rate equates to a charge or discharge current and is defined as:
LABORATOIRE DE BIOLOGIE MÉDICALE BIOMEDYS 509 avenue du 8 Mai 1945 69300 CALUIRE ET CUIRE Le MANUEL DE PRELEVEMENT regroupe les indications, consignes et informations nécessaires à la prise en charge des patients lors de l'acte de prélèvement. Il est destiné aux préleveurs du laboratoire, aux préleveurs externes et aux laboratoires transmetteurs.




