Akimov_et_al.i.qxd
Vestnik zoologii, 38(5): 57–66, 2004 I. A. Akimov, S. V. Benedyk, L. M. Zaloznaya, 2004
COMPLEX ANALYSIS OF MORPHOLOGICALCHARACTERS OF GAMASID MITEVARROA DESTRUCTOR (PARASITIFORMES, VARROIDAE)
I. A. Akimov, S. V. Benedyk, L. M. Zaloznaya
Schmalhausen Institute of Zoology NAS Ukraine,vul. B. Khmelnits'kogo, 15, Kyiv, 01601 Ukraine
Accepted 23 October 2003
Complex Analysis of Morphological Characters of Gamasid Mite Varroa destructor (Parasitiformes, Var-roidae). Akimov I. A., Benedyk S. V., Zaloznaya L. M. — The study of seasonal variability of miteV. destructor was carried out. The summer generation of mites appears to be characterized by the largestmorphological variability whereas the winter one has stable characters. We failed to evolve the complexof morphological characters that would allow us to identify, with high level of reliability, certainphenotype of the mite. Significant stability of morphological characters of V. destructor in the course oftime was determined. The mean values of the length and width of the body allow to consider theUkrainian population of Varroa mite, which parasitize the honey bee Apis mellifera Linnaeus, as theKorean haplotype of Varroa destructor Anderson et Trueman, 2000.
K e y w o r d s: Varroa destructor, gamasid mites, morphology, seasonal variability.
Êîìïëåêñíûé àíàëèç ìîðôîëîãè÷åñêèõ ïðèçíàêîâ ãàìàçîâîãî êëåùà Varroa destructor (Parasitifor-mes, Varroidae). Àêèìîâ È. À., Áåíåäèê Ñ. Â., Çàëîçíàÿ Ë. Ì. — Ïðîâåäåíî èññëåäîâàíèå ñåçîí-íîé èçìåí÷èâîñòè êëåùà V. destructor. Óñòàíîâëåíî, ÷òî ëåòíÿÿ ãåíåðàöèÿ êëåùåé õàðàêòåðèçè-ðóåòñÿ íàèáîëüøåé äèñïåðñèåé ïðèçíàêîâ, à çèìíÿÿ — íàèáîëüøåé èõ ñòàáèëüíîñòüþ. Íåóäàëîñü âûäåëèòü êîìïëåêñ ìîðôîëîãè÷åñêèõ ïðèçíàêîâ, êîòîðûé áû ïîçâîëÿë ñ âûñîêèìóðîâíåì äîñòîâåðíîñòè èäåíòèôèöèðîâàòü îïðåäåëåííûé ôåíîòèï êëåùà. Âûÿâëåíà çíà÷èòåëü-íàÿ ñòàáèëüíîñòü ìîðôîëîãè÷åñêèõ ïðèçíàêîâ V. destructor âî âðåìåíè. Ïîäòâåðæäåíî, ÷òî ïîñðåäíåìó çíà÷åíèþ äëèíû è øèðèíû òåëà, ïàðàçèòèðóþùèé íà ìåäîíîñíîé ï÷åëå Apis melliferaLinnaeus â Óêðàèíå, êëåù âàððîà îòíîñèòñÿ ê îïèñàííîìó êîðåéñêîìó ãàïëîòèïó Varroa des-tructor Anderson et Trueman, 2000.
Ê ë þ ÷ å â û å ñ ë î â à: Varroa destructor, ãàìàçîâûå êëåùè, ìîðôîëîãèÿ, ñåçîííàÿ èçìåí÷èâîñòü.
The ectoparasitic mite Varroa destructor Anderson and Trueman, 2000 (V. jacobsoni in our works) has
tropical origin and initially parasitized the waxy bee Apis cerana Fabricius. The shift to the honey bee Apismellifera Linnaeus happened likely in the late 1960s, when A. cerana and A. mellifera were kept jointly in EastAsia (Delfinado, 1963). Then V. jacobsoni successfully acclimatized in the wide and different areas ofdistribution of its new host, and became cosmopolitan species (Griffiths, Bowman, 1981; Akimov et al., 1993;Zhang, 2000). Elucidating the question how, in the expansion, the mite reveals its genetic and phenotypicpotential of variability, resulted in a number of study works on intraspecific morphological differentiation ofthe parasite (Grobov, Shabanov, 1979; Akimov, Yastrebtsov, 1985; Akimov, Zaloznaya, 1986; Zaloznaya,1988; Delfinado-Baker, Houck, 1989). It is proved that morphological characters of varroa mite characterizedby geographic variability that is connected with not only different natural climatic conditions, but also withdistinct host species (Delfinado-Baker, Houck, 1989; Anderson, 2000). In addition, there are periods ofintensive reproduction of mite (in summer) and non-reproduction (in winter) under temperate climateconditions were observed that is an abnormal phenomenon of varroa, because it is reproducing all the yearround in the area of its origin (Malaysia–Indonesia–New Guinea region). That is why the study ofmorphological adaptation of the parasite to season change is of significant interest. The first studiesconcerning this issue were carried out by I. A. Akimov, L. M. Zaloznaya, V. M. Efimov and Yu. K. Galak-tionov (1988, 1989, 1990).
The purpose of this study was the complex analysis of seasonal morphological differentiation of females
V. destructor using the multivariate statistics method.
I. A. Akimov, S. V. Benedyk, L. M. Zaloznaya
Material and methods
The study of morphological variability of Varroa destructor was carried out on adult females, collected
in two bee colonies of private apiary in Radomyshl of Zhitomyr Region in different seasons of 2001–2003.
Fifteen samples, each of 40–70 mites, were examined. The samples G, J (the X month), H (the II month),I, K, L (the III–V months), M (the VI–VII months) were collected in the colony {1, and the samples A,B, E, (the IX–X months), Ñ (the II month), D (the III month), N, O (the VI month) from the colony {2. Total amount is 741 mites.
At first, every mite was characterized by 22 quantitative characters; 13 of them were bilaterally symmetric.
Later, some of characters depending on the position of mites on slide, were excluded from consideration. Theseare the length of the anal setae, the length of the anal shield, the length of the peritremal tubes, the number ofthe setae on the second segment of the palps, the number of the pores on the sternal shield.
As a result, 17 morphological characters of females were treated statistically, as follows: 1 — length of
dorsal shield; 2 — width of dorsal shield; 3 — width of pleyral shield; 4 — length of pleyral shield; 5 — widthof lateral shield; 6 — larger width of sternal shield; 7 — length of genital shield; 8 — width of genital shield;9 — width of the basis of gnathosoma; 10 — distance between 1st pair of sternal setae; 11 — distance between1st and 2nd of sternal setae; 12 — number of setae on sternal shield; 13 — distance between anal setae; 14 —length of macrochaeta of trochanter of IV leg; 15 — length of tarsus of IV leg; 16 — distance between 1stand 2nd hypostomal setae; 17 — distance between 2nd and 3rd hypostomal setae.
In addition to absolute measurements of mite, the length to the width ratios of the same organ were
used that allow to draw conclusions about variability of organ shape (Filippova, Musatov, 1996). The mea-surements are as follows: 18 — length / width ratio of dorsal shield; 19 — length / width ratio of pleyralshield; 20 — length / width ratio of genital shield.
The principal component method, analysis of variance and linear discriminant analysis were performed
to evaluate variability and compare samples. All characters were standardized prior to the principal compo-nent method analysis (Zhivotovskij, l991). Pictorial model of eight samples (two samples of each season,collected from two colonies at the same time) was used to establish significant dependency of characters onthe seasonal factor (Plochinskij, 1961). These samples from all seasons were analyzed by the discriminantanalysis method.
Calculations were made using IBM PC, by the use of the Statistica. 6 for Windows (StatSoft, Inc.,
USA) and SPSS. 11 for Windows (DiaSoft, Inc., USA) software.
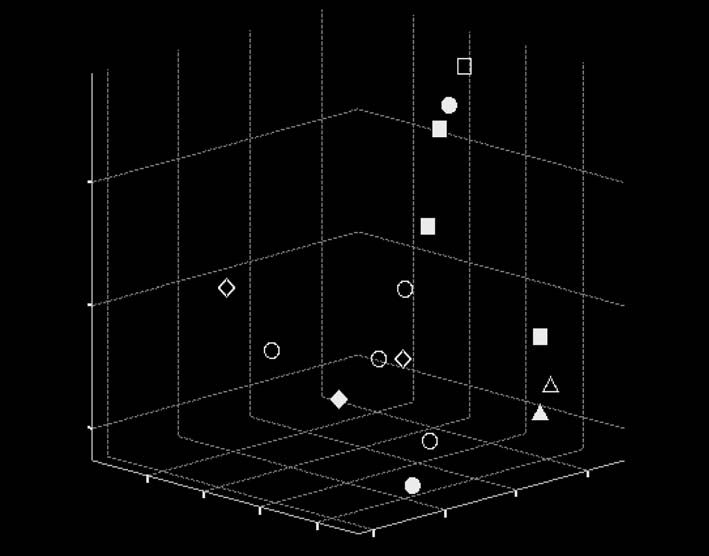
Performed analysis resulted in discrimination of 5 principal components that de-
scribe 61% of the total variance. The scatter of seasonal samples occurred in space ofthe first three principal components (fig. 1). Significant contributions of characters 1,2, 3, 4, 5, 6, 7, 8 in the first principal component (30.7% of the total variance) (tabl. 1)indicate that its establishment is connected with the variability of shields, and it maybe interpreted as dimensional. Characters 10, 11 that put significant contributions (butwith opposite signs) in the second principal component (8.8%) characterize the dis-tance between sternal setae, i. e., the oblongness of its front part. So that, they may beregarded as female characters that indicate body size oscillations in longitudinal-trans-verse direction. That is why the second principal component may be considered a com-ponent of variation of longitudinal-transverse axis of mite. The third principal compo-nent (8.1%) that was established by characters 15, 16, connected with variability of thesize of legs and the distance between the 1st and 2nd hypostomal setae, i. e., also withthe size of the coxa of I leg (chelicera).
Scatter plot of seasonal samples in space of three principal component (fig. 1) indi-
cates that the groups of summer and spring samples are the most detached from eachother, where separation is on the second principal component. When centers of thesamples groups projected on its axis, the summer samples are disposed at the beginningof the axis, with the spring samples on the end. Hence we may affirm that summermites are characterized by less sizes of the body in its longitudinal-transverse directionif compared with spring mites. The winter and autumn samples stand on intermediateposition, the winter samples are situated closer to spring ones.
The analysis of ranges of standard deviation and mean values of the absolute mea-
surements of shields and their proportion (fig. 2–4) indicates that the summer mitesare characterized by the maximum variability of characters in regard to specimens of
Complex Analysis of Morphological Characters of Gamasid Mite.
— winter— spring— summer— autumn
Fig. 1. Scatter plot of seasonal samples of female mite in space of three principal components (blank signsrefer colony N 8, black signs to colony N 1).
Ðèñ. 1. Äèàãðàììà ðàññåÿíèÿ ñåçîííûõ âûáîðîê ñàìîê êëåùà â ïðîñòðàíñòâå òðåõ ãëàâíûõ êîìïîíåíò(íåçàêðàøåííûå ìàðêåðû îòíîñÿòñÿ ê ï÷åëîñåìüå ¹ 8, çàêðàøåííûå — ê ï÷åëîñåìüå ¹ 1).
other seasonal samples. Moreover, they have the largest mean value of the shield lengthto width ratio with lesser mean value of the width and insignificantly larger mean valueof the length in comparison with the other seasonal samples. The winter and spring mitesshow enlargement of the width of the genital and pleural shields with almost the samevalue of the length in regard to the other samples. So, the summer mites are character-ized by diminution of measurements of shields in transverse direction as compared withthe winter and spring mites that is verified by the results of aforesaid analysis as well.
T a b l e 1. Contributions of characters of seasonal samples' objects in the I, II and III principal components
Ò à á ë è ö à 1. Âêëàäû ïðèçíàêîâ îáúåêòîâ ñåçîííûõ âûáîðîê â I, II è III ãëàâíûå êîìïîíåíòû
Principal components
Principal components
Cumulative variability, %
N o t e. l — percent of variance; * — factor loading = or > 0,6 .
I. A. Akimov, S. V. Benedyk, L. M. Zaloznaya
Fig. 2. Mean value (Mean), standard error of mean (SE) and standard deviation (SD) of length (v1) and width(v2) of the dorsal shield and their ratios (v18). By the axis X: I — autumn; II — winter; III — spring; IV — summer.
Ðèñ. 2. Ñðåäíåå çíà÷åíèå (Mean), ñòàíäàðòíàÿ îøèáêà ñðåäíåãî (SE) è ñòàíäàðòíîå îòêëîíåíèå (SD)äëèíû (v1) è øèðèíû (v2) äîðñàëüíîãî ùèòà è èõ ñîîòíîøåíèå (v18); ïî îñè X: I — îñåíü; II —çèìà; III — âåñíà; IV — ëåòî.
Fig. 3. Mean value (Mean), standard error of mean (SE) and standard deviation (SD) of length (v4) andwidth (v3) of the pleyral shield and their ratios (v19). By the axis X: I — autumn; II — winter; III — spring;IV — summer.
Ðèñ. 3. Ñðåäíåå çíà÷åíèå (Mean), ñòàíäàðòíàÿ îøèáêà ñðåäíåãî (SE) è ñòàíäàðòíîå îòêëîíåíèå (SD)äëèíû (v4) è øèðèíû (v3) ïëåéðàëüíîãî ùèòà è èõ ñîîòíîøåíèå (v19); ïî îñè X: I — îñåíü; II —çèìà; III — âåñíà; IV — ëåòî.
Fig. 4. Mean value (Mean), standard error of mean (SE) and standard deviation (SD) of length (v7) andwidth (v8) of the genitoventral shield and their ratios (v20). By the axis X: I — autumn; II — winter; III —spring; IV — summer.
Ðèñ. 4. Ñðåäíåå çíà÷åíèå (Mean), ñòàíäàðòíàÿ îøèáêà ñðåäíåãî (SE) è ñòàíäàðòíîå îòêëîíåíèå (SD)äëèíû (v7) è øèðèíû (v8) ãåíèòîâåíòðàëüíîãî ùèòà è èõ ñîîòíîøåíèå (v20); ïî îñè X: I — îñåíü;II — çèìà; III — âåñíà; IV — ëåòî.







Complex Analysis of Morphological Characters of Gamasid Mite.
T a b l e 2. Characters and the shield length/width ratio by which the distinction of seasonal samples is statisti-cally significant (P < 0.01) (one-way ANOVA)
Ò à á ë è ö à 2. Ïðèçíàêè è ñîîòíîøåíèÿ äëèíû è øèðèíû ùèòîâ, ïî êîòîðûì ñòàòèñòè÷åñêè äîñòîâåðíî(P < 0,01) îòëè÷àþòñÿ ñåçîííûå âûáîðêè (one-way ANOVA)
summer 2, 3, 5, 2, 3, 5,
9, 5, 15, 2, 3, 11,
summer 9, 5, 6 9, 15, 16 9, 11, 16
5, 15, 16 3, 9, 14,
N o t e. The left of diagonal — characters, the right of diagonal — ratios.
When pairs of studied seasonal samples are compared (tabl. 2), the summer sam-
ples proved to have the largest number of statistically significant distinction in charac-ters as compared with all other samples, though the distribution of these characters iswidely overlapped (fig. 2–5). They significantly differ from the spring and winter sam-ples in the width of dorsal, pleyral and lateral shields, width of the basis of the gnatho-soma, distance between the 1st and 2nd sternal setae, length of the of the IV leg tar-sus, distance between the 1st and 2nd hypostomal setae. They significantly differ fromthe autumn samples in the length and width of the dorsal shield, width of the pleyralshield, distance between the 1st and 2nd sternal setae, the length of the macrochaetaof the IV leg trochanter, the distance between the 1st and 2nd hypostomal setae. Thesummer samples also significantly differ from the other in length to width ratio of thedorsal, pleyral and genital shields. The winter and spring mites are most similar
Fig. 5. Distribution frequency of length (A) and width (B) of the dorsal shield of summer and winter samples.
Ðèñ. 5. ×àñòîòà ðàñïðåäåëåíèÿ äëèíû (À) è øèðèíû (Â) äîðñàëüíîãî ùèòà êëåùåé ëåòíèõ è çèìíèõâûáîðîê.
I. A. Akimov, S. V. Benedyk, L. M. Zaloznaya
T a b l e 3. Correlation coefficients of variables with discriminant functions (P<0.01)
Ò à á ë è ö à 3. Êîýôôèöèåíòû êîððåëÿöèè ïðèçíàêîâ ñ äèñêðèìèíàíòíûìè ôóíêöèÿìè (P < 0,01)
Characters and ratios
Cumulative variability, %
Cumulative variability, %
morphologically and differ significantly from each other in the width of the basis of thegnathosoma, the length of the macrochaeta of the IV leg trochanter, and length towidth ratio of the dorsal shield. The exception is the pair of samples Ñ–L that does notsignificantly differ in any character. Concerning the autumn samples, they are lying inintermediate position between the summer and the spring and winter samples.
Certain distinction is shown to exist between the pairs of the summer, autumn and
spring samples, but not winter samples from different colonies. This may indicate sig-nificant stability of characters of the winter mites.
The results of discriminant analysis (tabl. 3, beginning from r = 0.4 , P < 0.01)
indicate that the length of the IV leg tarsus, distance between the 1st and 2nd hypos-tomal setae (on the first discriminant function (D 1), length of the dorsal shield andlength to width ratio of the dorsal shield (on the second discriminant function (DF 2),length of the IV leg tarsus, distance between the 1st and 2nd hypostomal setae (onDF 1), length of the dorsal shield, and length to width ratio of the dorsal shield (onD 2) mostly contribute to establishment of distinction among seasonal samples.
Simultaneous analysis of the absolute values of the length and width of shields and theirproportion was not carried out, for in this case the length and width of shields wouldhave been in use twice, and would have resulted in artificial increase in weight of char-
acters (Filippova et al., 1990).
T a b l e 4. Squared Mahalanobis Distances
In the two-dimensional distribution
Ò à á ë è ö à 4. Ðàññòîÿíèå Ìàõàëàíîáèñà
of discriminant functions (fig. 6, A, B),
clear separation of seasonal samples doesnot occur, though the summer specimens
appear to be quite detached.
The analysis of values of squared
Mahalanobis distances (tabl 4) also indi-cate that the summer samples significant-
ly differ from all others, namely they dif-
Complex Analysis of Morphological Characters of Gamasid Mite.
Fig. 6. Distribution of specimens of seasonal samples on area of two discriminant functions by the complexof characters and rations of length and width of shields.
Ðèñ. 6. Ðàñïðåäåëåíèå ýêçåìïëÿðîâ ñåçîííûõ âûáîðîê â ïëîñêîñòè äâóõ äèñêðèìèíàíòíèõ ôóíêöèéïî êîìïëåêñó ïðèçíàêîâ è ñîîòíîøåíèé äëèíû è øèðèíû ùèòîâ.
fer most considerably from the spring samples, and are the least different from theautumn ones. There was insignificant morphological distinction between the winter andspring mites.
A search for the complex of characters that would significantly improve diagnostic
capability of morphological characters, both on the group as a whole and on individu-al seasonal samples (Magowski et al., 2000) by the means of discriminant analysis, doesnot bring an expected result. The removal of the characters 12, 8, 2 from the analysisimproves the accuracy of identification only by 2% (62% in comparison with 60% whenall 17 characters are used). Further diminution of the number of characters in differ-ent combinations and change of absolute values of the length and width of shields totheir ratio is resulted in decrease of diagnostic capabilities of discriminant analysis. Sucha low level of forecasting ability of the given combination of characters may beexplained by the fact that every seasonal sample is quite variable by characters that donot allow to collect similar samples for analysis.
Studied seasonal samples of V. destructor differ from each other statistically reli-
ably. The summer generation of mites is particularly distinguishable in the degree ofcharacters variability. On the contrary, the winter generation is characterized by the sta-bility of morphological characters.
The maximum similarity of characters of the winter and spring generations found
in the course of study can be explained by the fact that qualitative structure of mitepopulation during this period changes insignificantly. The point is that the mite mor-tality does not exceed 6–8% during the wintering (Sal'chenko, 1977; Lange et al., 1977,Fries, Perez-Escala, 2001), and the emergence of sealed brood in February–Marchdoes not result in successful reproduction of the parasite (Piletskaya, 1992; Akimov etal, 1993; Martin, 2001).
During intensive reproduction (late spring and summer) maximum diversity of
mites may be observed, that explains the highest distinction of the summer samplesfrom the other ones. Variability of most characters of females increases simultaneouslyfrom spring till autumn, whereas in winter period it decreases. In particular, the sum-mer generation of mites differs from the winter generation in general decrease of thebody measurements and decrease of the measurements of the shields on its ventricularsurface, i. e., by decrease in the degree of its sclerotization, decrease of transversal
I. A. Akimov, S. V. Benedyk, L. M. Zaloznaya
measurements of the body, length of the IV leg tarsus, distance between the 1st and2nd hypostomal setae. However, ranges of these characters widely overlap. That is whywe failed to distinguish clearly between the summer and winter phenotypes (morpho-types) of the mite and pick out the complex of characters that would allow us to iden-tify the definite morphotype with high level of reliability.
Like the other authors (Milushev et al., 1979; Rademacher, 1985), we also noted
the mass natural mortality of varroa during the end of August–September that was pos-sibly connected with the completion of life cycle of the summer generation and selec-tion of the wintering generation. This phenomenon is assumed to be connected withdifferent viability of the summer and winter mites during different seasons. During thewintering selection is directed upon elimination of individuals that worse adapted tophoresia on bee, and during the summer selection upholds individuals that better adapt-ed to reproduction (Zaloznaya, 1988; Akimov et al., 1989; 1990; 1993). It may also beassumed that mass natural mortality of the summer generation of mites connected withthe fact that, because of intensive reproduction, the summer females exhaust theirresources more quickly than the winter females. As a result, their lifetime becomes 2–3months shorter than that of the winter mites (Lange et al., 1976; Petrova et al., 1982).
One of the main features of the mite biology is the strict synchronization of its
reproductive cycle with vital functions of the host, first of all with brood development.
The time of its appearance depends, apart from climate conditions, on the microcli-mate conditions of a colony, race peculiarities, and the age of a queen (Akimov et al.,1993). This fact, to a considerable extent, clarifies why in some colonies (even at thesame apiary) some difference in the dynamic of their development may be observed.
That is why both the dynamic of mite population development and the increase in itsvariability may considerably differ in different colonies at the same time. This causesthe distinction of pairs of the same seasonal samples, namely those of the summer,autumn, and spring from different colonies. On the contrary, similarity of the wintersamples shows that minimum variance of characters is typical for the winter phenotypeof the mite. It means that the winter form may figure as a model sample for the com-parison of various populations of varroa mites from distinct colonies or ever local pop-ulation of the parasite.
In general, it is worth noting the considerable conservatism of the morphological
characters of V. destructor in the course of time. Twenty-five years after the latest studyof seasonal variability of mite more then 250 generations of the parasite changed, butits general statistic indexes remain stable.
Hence we may assume that Varroa keeps on evolving functionally, adjusting itself
to different races of bees and to the new climate conditions. This assumption isstrengthen by the interest in study of biochemical interactions between the parasite andhost (Hanel, Koeniger, 1986; Rosenkranz et al., 1993; Martin et al., 2001; Colin et al.,2001). It is possible that not only morphologically distinct forms but also the adapta-tion of outwardly similar forms on the physiological level may serve as a mobilizingreserve.
The obtained results also suggest that the studied mite is identified as V. destructor
Anderson & Trueman, 2000, in particular, its Korea haplotype by the mean value ofthe length and width of the body (1149.46 mem. (± 0.8 mem.) and 1692.6 mem.
(± 1.70 mem.) accordingly). However, in the course of the study we came across themites with the least mean value of the length and width of the body 1037.5 mem.,1400.0 mem. accordingly that is typical of V. jacobsoni (Anderson, Trueman, 2000).
This variation of characters may be considered as a display of the reaction norm undercertain conditions (Yablokov, Yusufov, 1989), since the frequency of occurrence of theindividuals has made 0.1% from total amount of sample. However, as this study demon-strated, the length and width of the body significantly differ from each other even in
Complex Analysis of Morphological Characters of Gamasid Mite.
the same population of mite during different seasons. That is why we assume that onlytwo morphological characters are not enough for a valid description. The complexapproach with use of morphological, genetic and physiological studies is necessary.
Authors express their deep gratitude to I. V. Piletskaya (Schmalhausen Institute of Zoology, Kiev) for
her assistance in collecting materials and O. E. Zykov (Ukrainian Sanitary and Epidemiological Service,Ministry of Health of Ukraine) for his kind assistance in mastering statistic software SPSS. 11 for Windows.
Akimov I. A., Yastrebtsov A. V. Variability of some characters of the mite Varroa jacobsoni Oudemans, 1904-a
parasite of the honey bee // Dokl. AN USSR. Ser. Â. Geol., ehem., biol. nauki. — 1985. — N 9. —P. 58–60. — Russian.
Akimov I. A., Zaloznaya L. M. Variability of morphological characters of males and females of the mite
Varroa // Pchelovodstvo. — 1986. — N 17. — P. 42–46. — Russian.
Akimov I. A., Zaloznaya L. M., Efimov V. M., Galaktionov Yu. K. Seasonal polimorphism of the mite Varroa //
Pchelovodstvo. — 1988. — N 7. — P. 16–17. — Russian.
Akimov I. A., Zaloznaya L. M., Efimov V. M., Galaktionov Yu. K. Seasonal polimorphism of the mite Varroa
jacobsoni Oudem., 1904 (Parasitiformes, Varroidae) // Zhurn. Obsch. Biol. — 1989. — 50, N 6. —P. 819–823. — Russian.
Akimov I. A., Zaloznaya L. M., Efimov V. M., Galaktionov Yu. K. Seasonal and geographical variation of the
morphological characters of the mite Varroa jacobsoni (Parasitiformes, Varroidae): moving of meanvalues, standard deviations and coefficients of the fluctuating asymmetry // Zool. Zhurn. — 1990. —69, N 9. — P. 27–37. — Russian.
Akimov I. A., Grobov O. P., Piletskaya I. V. et al. Honeybee mite Varroa jacobsoni. — Kyiv : Nauk. dumka. —
1993. — 256 p. — Russian.
Anderson D. L., Trueman J. W. H. Varroa jacobsoni (Acari: Varroidae) is more than one species // Exp. Appl.
Acarol. — 2000. — 24. — P. 165–189.
Anderson D. L. Variation in the parasitic bee mite Varroa jacobsoni Oud. // Apidologie. — 2000. — 31. —
P. 281–292.
Colin M., Tchamitchian M., Bonmatin JM., Di Pasquale S. Presente of chitinase in adult Varroa destructor,
an ectoparasitic mite of Apis mellifera // Exp. Appl. Acarol. — 2001. — 25, N 12. — P. 947–955.
Delfinado M. D. Mites of the honeybees in South-East Asia // J. Apicult. Res. — 1963. — N 2. — P. 113–114.
Delfinado-Baker M. D., Houck M. A. Geographic variation in Varroa jacobsoni (Acari, Varroidae): application
of multivariate morphometric techniques // Apidologie. — 1989. — 20. — P. 345–358.
Filippova N. A., Musatov S. A. Geographic variation of adults of Ixodes persulcatus (Ixodidae). Experience of
morphometric data bases application // Parazitologia. — 1996. — 30, N 3. — P. 205–215. — Russian.
Filippova N. A., Petrov A. V., Lobanov A. L. First experience in the discriminant analysis application for
differentiation of close spesies of the genus Dermacentor (Ixodidae) at the larval phase on the basis ofmorphometric data // Parazitologia. — 1990. — 24, N 6. — P. 480–484. — Russian.
Fries I., Perez-Escala S. Mortality of Varroa destructor in honey bee (Apis mellifera) colonies during winter //
Apidologie. — 2001. — 32. — P. 223–229.
Griffiths D. A., Bowman C. E. World distridution of the mite Varroa jacobsoni, a parasite of honeybees // Bee
World. — 1981. — 62, N 4. — P. 154–163.
Grobov O. P., Shabanov M. The influence of climatic factors on the mite Varroa jacobsoni Oud. // Dokl.
Bolg. AN. — 1979. — 32, N 12. — P. 1701–1703. — Russian.
Hanel H., Koeniger N. Possible regulation of the reproduction of the honey bee Varroa jacobsoni
(Mesostigmata: Acari) by a host's hormone: juvenile hormone III // J. Insect Physiol. — 1986. — 32,N 9. — P. 791–798.
Lange À. Â., Natsgij K. V., Tatsij V. M. The Varroa mite and working methods of control against it //
Pchelovodstvo. — 1976. — N 3. — P. 16–20. — Russian.
Lange À. Â., Natsgij K. V., Tatsij V. M. Regarding some features of biology of the mite Varroa jacobsoni —
a parasite of the honey bee // Varroatoz pchel. — M. : Nauka, 1977. — P. 13–18. — Russian.
Magowski W. L, Dolata M., Kuczynski L. Zmiennosc mierzalnych cech morfologicznych w pieciu populacjach
Tetranychus urticae Koch (Acari, Tetranychidae) z Polski i Holandii // Akarologia polska u progu XXIwieku Ed. Ignatowicz. — Warszawa : Wydawnictwo SGGW, 2000. — P. 38–49.
Martin S. Varroa destructor reproduction during the winter in Apis mellifera colonies in UK // Exp. Appl.
Acarol. — 2001. — 25. — P. 321–325.
Martin C., Salvy M., Provost E. et al. Variations in chemical mimicry by the ectoparasitic mite Varroa
jacobsoni according to the developmental stage of the host honey-bee Apis mellifera II Insect Biochem.
Mol. Biol. — 2001. — 31, N 4–5. — P. 365–379.
Milushev M., Petrov I., Stoimenov A. Seasonal dynamics of contrasting varroatosis of honey bees //
Pchelarstvo. — 1979. — 77, N 8. — P. 20–22. — Bulgarian.
Petrova A. D., Bizova Yu. Â., Tatsij V. M., Emel'yanova O. Yu. Waste on the exchange interchange of the
mite Varroa jacobsoni Oudemans, 1904 (Mesostigmata, Varroidae) — ectoparasite of a honey bee //Dokl. Akad. Nauk SSSR. — 1982. — 262, N 2. — P. 499–502. — Russian.
I. A. Akimov, S. V. Benedyk, L. M. Zaloznaya
Piletskaya I. V. Seasonal changes of Varroa jacobsoni reproductive indices in a bee colony // Vestn.
Zoologii. — 1992. — N 1. — P. 70–74. — Russian.
Plochinskij N. A. Biometrics. — Novosibirsk : Izd-vo Sibir. otd. AN SSSR., 1961. — 364 p. — Russian.
Rademacher E. Ist eine Befallsprognose aus dem naturlichen Totenfall von Varrja Jacobsone moglich? //
Apidologie. — 1985. — 6, N 4. — P. 395–405.
Rosenkranz P., Tewarson NC., Rachinsky A. et al. Juvenile hormone titer and reproduction of Varroa jacobsoni
in capped brood strages of Apis cerana indica in comparison to Apis mellifera ligustica // Apidologie. —1993. — 24. — P. 375–382.
Salchenko V. L. Biology of the agent of varroatosis of the mite Varroa jacobsoni and the search for materials
in its control // Varroatoz pchel. — M. : Nauka, 1977. — P. 16–18. — Russian.
YablokovA. V., Yusufov A. G. Doctrine of evolution. — M. : Vys. shk., 1989. — 335 p.
Zaloznaya L. M. Morphological variability of the mite Varroa jacobsoni Oud. 1904 in connection with the
expansion of its habitat and distribution in the territory of the USSR : Abstr. of thesis. candidate ofbiol. sciences. — K., 1988. — 26 p. — Russian.
Zhang Z.-O. Notes on Varroa destructor (Acari: Varroidae) parasitis on honeybees in New Zealand //
Systematic and Applied Acarology Special Publications. — 2000. — 5. — P. 9–14.
Zhivotovskij L. A. Population biometrics. — M. : Nauka, 1991. — 271 p. — Russian.
Source: http://www.v-zool.kiev.ua/pdfs/2004/5/07.pdf
BENZON SYMPOSIUM No. 47 MOLECULAR PHARMACOLOGY OF ION CHANNELS AUGUST 13-17, 2000, COPENHAGEN, DENMARK Organizing committee: Jan Egebjerg, Søren-P. Olesen and Povl Krogsgaard-Larsen Abstracts - MONDAY, August 14, 2000 Kainate Receptor-Deficient Mice Stephen Heinemann, Molecular Neurobiology Laboratory, Salk Institute, La Jolla, Ca, U.S.A Abstract not received Importance of AMPA Receptors in synaptic plasticity Sprengel R., Borchardt T., Mack V., Jensen V., Ovind H. 1, Feldmeyer D., Burnashev N.2& P.H. Seeburg Depts. of Molecular Neuroscience and 2Cell Physiology of the Max-Planck Institute for Medical Research, Heidelberg, Germany 1Dept. of Physiology, Institute of Basic Medical Sciences, University of Oslo, Norway. We have adopted genetic strategies to determine the function of AMPA receptors in the formation of synaptic plasticity i.e long term potentiation (LTP) at CA3/CA1 synapses in the hippocampus of adult and juvenile mice. Using a variety of different mouse lines with perturbations in AMPA receptor expression, the involvement of the AMPA receptor subunits GluR-A and GluR-B in long term LTP was dissected. The GluR-B subunit was found to have its major function during development of a mouse brain. Up to postnatal day 15 to 25 the GluR-B subunit has to maintain distinct AMPA receptor channel properties, such as Ca2+-impermeability and linear current/voltage relationship. Mice with disturbed AMPA receptor mediated Ca2+-flux in principal neurones develop neurological phenotypes which can result in epileptic attacks and premature death of the carriers. At all ages examined LTP was present at CA3/CA1 connections indicating that GluR-B is not essential for synaptic plasticity. In contrast, abolished GluR-A subunit expression had minor effects on mouse development but on LTP formation, which was diminished completely in adult mice. In the absence of the GluR-A subunit the total amount of functional AMPA receptors was strongly reduced in CA1 pyramidal cells, and most of the remaining receptors were localised in synapses. In wild-type mice the majority of AMPA receptors are in extra-synaptic sites, which might indicate that the presence of extra-synaptic receptors is important for the formation of LTP. This is supported by the presence of CA3-CA1 LTP in young GluR-A-/- mice. At this age the ratio of extra-synaptic versus synaptic receptor is more in favour of extra-synaptic receptors. In summary, our mouse models might indicate that the AMPA-receptor level in the postsynaptic neuron is more important for LTP than the presence of individual AMPA receptor subunits. Regulation of Glutamate Receptor Function and Synaptic Plasticity Hey-Kyoung Lee, HHMI, Johns Hopkins University, Baltimore, MD, USA. Neurotransmitter receptors mediate signal transduction at the postsynaptic membrane of synaptic connections between neurons in both the central and peripheral nervous systems. We have been studying the molecular mechanisms in the regulation of neurotransmitter receptor function. Recently we have focused on glutamate receptors, the major excitatory receptors in the brain. Glutamate receptors can be divided into two major classes: non- NMDA and NMDA receptors. Non-NMDA receptors mediate rapid excitatory synaptic transmission while NMDA receptors play important roles in neuronal plasticity and development. Studies in our laboratory have found that both non-NMDA and NMDA receptors are multiply phosphorylated by a variety of protein kinases. Phosphorylation regulates several functional properties of these receptors including conductance and membrane targeting. For example, phosphorylation of the GluR1 subunit of non-NMDA receptors by multiple kinases including PKA, PKC and CaM kinase II regulates its ion channel function. Recent studies have demonstrated that the phosphorylation of AMPA receptors is regulated during cellular models of learning and memory such as long term potentiation (LTP) and long term depression (LTD). We have also been examining the mechanisms of the subcellular targeting and clustering of glutamate receptors at synapses. We have recently identified a variety of proteins that directly or indirectly interact with non-NMDA and NMDA receptors. We have found a novel family of proteins that we call GRIPs (Glutamate Receptor Interacting Proteins) that directly bind to the C-termini of the GluR2/3 subunits of non-NMDA receptors. GRIPs contain seven PDZ domains, protein-protein interaction motifs, which appear to crosslink non-NMDA receptors or link them to other proteins. In addition, we have recently found that the C-termini of GluR2 also interacts with the PDZ domain of
Contents lists available at Practice Parameter Emergency department diagnosis and treatment of anaphylaxis:a practice parameter Ronna L. Campbell, MD, PhD; James T.C. Li, MD, PhD; Richard A. Nicklas, MD; Annie T. Sadosty, MDMembers of the Joint Task Force: David Bernstein, MD; Joann Blessing-Moore, MD;David Khan, MD; David Lang, MD; Richard Nicklas, MD; John Oppenheimer, MD; Jay Portnoy, MD;Christopher Randolph, MD; Diane Schuller, MD; Sheldon Spector, MD; Stephen Tilles, MD;Dana Wallace, MDPractice Parameter Workgroup: Ronna L. Campbell, MD, PhD; James T.C. Li, MD, PhD; Annie T. Sadosty, MD







