Virtualplant.bio.puc.cl
MPMI Vol. 26, No. 5, 2013, pp. 546–553. http://dx.doi.org/10.1094/MPMI-10-12-0241-R.
Quorum Sensing and Indole-3-Acetic Acid Degradation
Play a Role in Colonization and Plant Growth Promotion
of Arabidopsis thaliana by Burkholderia phytofirmans PsJN
Ana Zúñiga,1,2 María Josefina Poupin,1,2 Raúl Donoso,1,2 Thomas Ledger,1,2 Nicolás Guiliani,3
Rodrigo A. Gutiérrez,2 and Bernardo González1,2
1Facultad de Ingeniería y Ciencias. Universidad Adolfo Ibáñez. Santiago, Chile; 2Millennium Nucleus-PFG. FONDAP Center for Genome Regulation. Pontificia Universidad Católica de Chile. Santiago, Chile; 3Departamento de Biología, Facultad de Ciencias, Universidad de Chile. Santiago, Chile
Submitted 8 October 2012. Accepted 28 December 2012.
Although not fully understood, molecular communication
hosts plays a fundamental role in pathogenesis, and in the estab-
in the rhizosphere plays an important role regulating
lishment of beneficial interactions (Mark et al. 2005).
traits involved in plant–bacteria association. Burkholderia
Plant-growth-promoting rhizobacteria (PGPR) produce bene-
phytofirmans PsJN is a well-known plant-growth-promot-
ficial effects on plant growth through several mechanisms such
ing bacterium, which establishes rhizospheric and endo-
as nitrogen fixation (Hurek et al. 2002), improved nutrient up-
phytic colonization in different plants. A competent colo-
take (Kraiser et al. 2011), phytohormone production (Idris et
nization is essential for plant-growth-promoting effects
al. 2007), and induction of systemic resistance (ISR) (Bakker
produced by bacteria. Using appropriate mutant strains
et al. 2007). A single bacterium may possess more than one of
of B. phytofirmans, we obtained evidence for the impor-
these mechanisms (Ahmad et al. 2008). Recent studies show
tance of N-acyl homoserine lactone-mediated (quorum
the importance of bacteria-to-bacteria communication in plant
sensing) cell-to-cell communication in efficient coloniza-
colonization by PGPR (Bais et al. 2004; Compant et al. 2005;
tion of Arabidopsis thaliana plants and the establishment
Steindler et al. 2009). Bacteria can communicate by sensing
of a beneficial interaction. We also observed that bacte-
and responding to small signaling molecules that make them
rial degradation of the auxin indole-3-acetic acid (IAA)
responsive to neighboring bacteria (Whitehead et al. 2001). A
plays a key role in plant-growth-promoting traits and is
variety of signaling molecules have been identified. Among
necessary for efficient rhizosphere colonization. Wild-
them,
N-acyl-homoserine lactones (AHL) are frequently syn-
type B. phytofirmans but not the iacC mutant in IAA min-
thesized by gram-negative bacteria that communicate through
eralization is able to restore promotion effects in roots of
quorum sensing (QS) (Whitehead et al. 2001). Bacteria defec-
A. thaliana in the presence of exogenously added IAA,
tive in QS signaling are less effective in host colonization
indicating the importance of this trait for promoting pri-
(Bauer and Mathesius 2004; Ortíz-Castro et al. 2009; Quiñones
mary root length. Using a transgenic A. thaliana line with
et al. 2005), and the role of QS regulating rice growth promo-
suppressed auxin signaling (miR393) and analyzing the
tion by
Pseudomonas aeruginosa PUPa3 has been reported
expression of auxin receptors in wild-type inoculated
(Steindler et al. 2009).
plants, we provide evidence that auxin signaling in plants
In addition to QS molecules, other signal molecules may
is necessary for the growth promotion effects produced
also play a role in plant-bacterial signaling. Among them, the
by B. phytofirmans. The interplay between ethylene and
auxin phytohormone indole-3-acetic acid (IAA) has been
auxin signaling was also confirmed by the response of the
detected in culture supernatants of several rhizobacteria (Idris
plant to a 1-aminocyclopropane-1-carboxylate deaminase
et al. 2007; Loper and Schroth 1986; Phi et al. 2008), suggest-
bacterial mutant strain.
ing that IAA may be a relevant signaling molecule in microor-ganisms (Bianco et al. 2006; Liu and Nester 2006; Spaepen et al. 2007, 2009; Van Puyvelde et al. 2011; Yang et al. 2007).
The rhizosphere represents a highly dynamic space for inter-
For example, IAA triggers a broad gene-expression response
actions between plant roots and pathogenic and beneficial soil
in
Azospirillum brasilense (Van Puyvelde et al. 2011), and IAA
microorganisms (Bais et al. 2006). Nutrient availability in the
synthesis is controlled by a positive feedback transcriptional
rhizosphere is higher than in the bulk soil and the presence of
mechanism (Vande Broek et al. 1999). IAA has been also
plant exudates creates a suitable environment for growth of
reported as a signaling molecule in
Escherichia coli (Bianco et
microorganisms (Costa et al. 2007). In the rhizosphere, molec-
al. 2006),
Agrobacterium tumefaciens (Liu and Nester, 2006;
ular communication between microorganisms and their plant
Yuan et al. 2008),
Erwinia chrysanthemi (Yang et al. 2007), and
Rhizobium etli (Spaepen et al. 2009). The production of IAA by PGPR has been involved in plant growth and root
Corresponding author: B. González; E-mail:
[email protected];
proliferation (Idris et al. 2007; Vande Broek et al. 2005). Nota-
Telephone: +56-2-3311619; Fax: +56-2-3311906.
bly, five different biosynthetic pathways and one pathway for IAA mineralization or transformation have been described in
* The
e-
Xtra logo stands for "electronic extra" and indicates that three
supplementary figures are published online.
bacteria (Idris et al. 2007; Leveau and Gerards 2008).
The PGPR
Burkholderia phytofirmans PsJN promotes
2013 The American Phytopathological Society
growth of horticultural crops such as tomato, potato, and grape
546 / Molecular Plant-Microbe Interactions
(Genin and Boucher 2004; Spaepen et al. 2007; Theocharis et
with the wild-type strain, plants inoculated with this mutant
al. 2012). To date, only one plant-growth-promotion molecular
did not increase primary root length, chlorophyll content, or
mechanism has been proved experimentally in
B. phytofirmans
the number of root hairs and only partially increased FW
PsJN: the reduction of the plant ethylene hormone levels by 1-
(126%) (Fig. 1A), indicating that AcdS activity and, therefore,
aminocyclopropane-1-carboxylate ACC deaminase (AcdS)
ethylene levels, play a role in
A. thaliana growth promotion
(Compant et al. 2005; Vadassery et al. 2008).
B. phytofirmans
produced by
B. phytofirmans PsJN.
PsJN mutants in the
acdS gene, lacking AcdS activity, are unable to promote the elongation of canola roots (Sun et al.
Effects of B. phytofirmans PsJN QS mutants
2009). Genome sequence analysis of
B. phytofirmans PsJN
on growth promotion and colonization of A. thaliana.
shows the presence of two putative IAA synthesis pathways
To test the role of QS in the ability of
B. phytofirmans PsJN
(the indole-3-acetamide and the tryptophan side chain oxidase
to promote plant growth, we used strain PsJN
bpI.1 and
bpI.2
pathways) and two putative QS systems associated with AHL
AHL synthase mutants. In comparison with the wild-type
production (Weilharter et al. 2011). In addition, it encodes one
strain,
bpI.1 and
bpI.2 mutants produced significantly lower
iac operon putatively involved in degradation of IAA, as re-
levels of 3-hydroxy-C8-homoserine lactone, and the
bpI.2 mu-
ported in
Pseudomonas spp. (Leveau and Gerards 2008).
tant did not produce C14-3-oxo-homoserine lactone (Supple-
In this work, we studied the importance of molecular signal-
mentary Fig. S1). In addition, the
bpI.1 mutant displayed a
ing for
B. phytofirmans PsJN colonization and growth promo-
decreased swimming motility (Supplementary Fig. S2). These
tion of
Arabidopsis thaliana plants, comparing the effects of
mutant strains grow on several carbon sources, such as fruc-
wild-type
B. phytofirmans PsJN and four mutants on this plant
tose, 4-hydroxybenzoate, or benzoate, at the same levels as the
model. We found that AHL molecular signaling is required for
wild-type strain (optical density of 600 nm [OD600nm] = 1, after
root colonization and plant-growth-promoting effects of this
12 h of culture), indicating that their general metabolism is not
bacterium. Furthermore, we demonstrated that degradation of
affected. Concerning turnover of IAA (see next section), the
IAA is important in plant growth promotion by
B. phytofir-
bpI.1 and
bpI.2 mutants synthesized IAA (19.6 and 19.9
mans PsJN. The importance of plant auxin signaling for bacte-
µg/ml, respectively) to an amount similar to the wild-type strain
rial promotion of plant growth was supported by the use of an
(20.0 µg/ml), and the three strains grow with 2.5 mM IAA as
A. thaliana line with reduced expression of the auxin receptors
the sole carbon and energy source at the same level: OD600nm =
and by analysis of the expression of genes encoding those re-
0.6, after 26 h of growth.
After 3 weeks of inoculation, the
B.
ceptors in wild-type plants inoculated with strain PsJN.
phytofirmans PsJN
bpI.1 mutant was unable to increase pri-mary root length (89%), FW (87%), and chlorophyll content
(82%) of
A. thaliana, in contrast with the wild-type strain (Fig. 1A). Only the number of root hairs increased (150%) in plants
Effect of B. phytofirmans PsJN on growth of A. thaliana.
inoculated with this mutant, although to a lower extent com-
Based on its effects on other plant species (Ait Barka et al.
pared with the wild-type strain. In contrast, plants inoculated
2000; Compant et al. 2005; Frommel et al. 1991; Pillay and
with the
bpI.2 mutant increased primary root length, number
Nowak 1997), it was supposed that
B. phytofirmans PsJN
of roots hairs, chlorophyll content, and number of lateral roots
would also promote growth of
A. thaliana. Different growth
to an extent similar to that of plants inoculated with the wild-
parameters were evaluated in plants inoculated with strain
type strain (Fig. 1A).
PsJN, as well as in mock-inoculated controls. After 3 weeks of
To determine whether differences in growth promotion of
A.
inoculation, the presence of strain PsJN increased fresh weight
thaliana by
B. phytofirmans PsJN and the
bpI.1 mutant were
(FW) (169%), primary root length (121%), root hair number
due to different capabilities for rhizospheric and endophytic
(197%), and chlorophyll content (130%) of
A. thaliana as
colonization, bacterial colonization of this plant was evaluated
compared with noninoculated plants (Fig. 1A). The number of
in gnotobiotic culture systems. Strain PsJN
bpI.1 mutant
lateral roots, however, remained unchanged. AcdS has been
showed a reduced ability for rhizospheric colonization of
A.
reported as required for canola growth promotion by this bac-
thaliana (8 ± 0.1 log CFU/mg FW) compared with the wild-
terium (Sun et al. 2009); therefore, we tested the effect of a
type strain and the
bpI.2 mutant (9.84 ± 0.01 and 9 ± 0.3 log
strain PsJN
acdS mutant on
A. thaliana growth. In contrast
CFU/mg FW, respectively). Epifluorescence and confocal
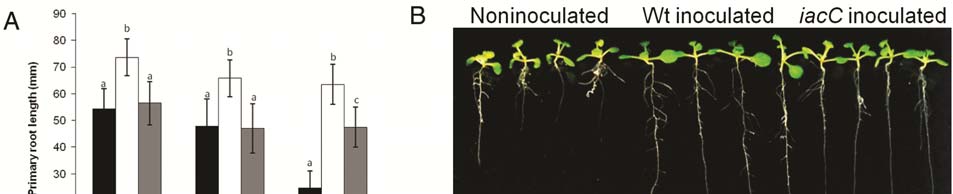
Fig. 1. Plant growth and metabolic parameters in
A, gnotobiotic
Arabidopsis thaliana col-0 and
B, A. thaliana miR393 overexpressor lines, culture systems
inoculated with
Burkholderia phytofirmans PsJN, its
bpI.1, bpI.2, iacC, and
acdS mutants, or noninoculated (control). Growth parameters were measured 3
weeks after inoculation. Bars show mean percentage values with respect to control plants, and the error bars indicate standard deviations from average of
three biological replicate experiments for each treatment. Different letters indicate statistically significant differences between treatments (one-way analysis
of variance Tukey's honestly significant difference tests,
P < 0.05).
Vol. 26, No. 5, 2013 /
547

microscopy analysis of A. thaliana roots inoculated with the
strain completely prevented the primary root length inhibition
wild-type PsJN or the bpI.1 mutant green fluorescent protein
effect of such concentration of IAA, whereas the iacC mutant
(GFP)-tagged strains showed green fluorescent bacterial cells
only partially reverted such inhibition (Fig. 2A and B).
mainly attached to lateral root emergences and root tips and highly spread on hair roots; however, strong adherence to tis-
Effect of B. phytofirmans PsJN and its mutants
sues was observed only with the wild-type strain (Supplemen-
in growth promotion of a transgenic A. thaliana
tary Fig. S3) and the bpI.2 mutant (data not shown), whereas the
with reduced auxin signaling.
bpI.1 mutant cells are present but seem to move loosely on the
To determine whether IAA signaling in the plant is required
root surface. Endophytic colonization of A. thaliana by both
for plant growth promotion by B. phytofirmans PsJN, a trans-
bpI.1 and bpI.2 mutant strains was statistically lower (5 ± 0.9
genic line of A. thaliana overexpressing miR393 (ox-miR393)
and 4.9 ± 0.2 log CFU/mg FW, respectively) than with the wild-
was used. These plants have reduced expression of the auxin
type strain (7.9 ± 0.5 log CFU/mg FW). These results indicate
receptor genes TIR1, AFB1, AFB2, and AFB3 and reduced
that QS signaling is required for rhizospheric and endophytic
auxin signaling (Navarro et al. 2006). Gnotobiotic cultures of
colonization of strain PsJN in A. thaliana roots.
ox-miR393 plants were inoculated with the wild-type PsJN strain or the iacC, bpI.1, and acdS mutants. Three weeks after
Effect of an IAA degradation mutant of B. phytofirmans
the inoculation, the wild-type strain and some of its mutants
on growth promotion and colonization of A. thaliana.
failed to produce an increase in primary root length, FW, or to-
To study whether IAA degradation by B. phytofirmans PsJN
tal chlorophyll content, as seen in wild-type plants (Fig. 1B).
has a role on plant growth promotion, the mutant iacC, com-
Only the number of root hairs increased in the transgenic plant
pletely unable to grow on IAA because it lacks a functional
inoculated with the wild-type strain and the iacC mutant (Fig.
aromatic ring hydroxylating dioxygenase involved in the IAA
1B). B. phytofirmans colonized the rhizosphere of gnotobiotic
degradation pathway (R. A. Donoso, unpublished results), was
cultures of ox-miR393 plants at normal levels (9.8 ± 0.1 log
constructed and analyzed. This mutant grows on different car-
CFU/mg FW). However, no B. phytofirmans endophytes were
bon sources such as fructose, 4-hydroxybenzoate, or benzoate
found in ox-miR393 plants. The transcript levels of the auxin
at the same yields and rates as the wild-type strain, and pro-
receptor genes TIR1, AFB1, AFB2, and AFB3 were analyzed
duces normal extracellular levels of IAA (18 µg/ml). After 3
in wild-type plants inoculated with strain PsJN using quantita-
weeks of inoculation of a gnotobiotic A. thaliana culture with
tive real time polymerase chain reaction (qRT-PCR) (Fig. 3).
the iacC mutant, no differences were observed in number of
Inoculation did not affect the expression of these genes in
root hairs, chlorophyll content, and number of lateral roots
plants of four leaves (13 days after sowing). However, AFB1
with respect to plants inoculated with the wild-type PsJN (Fig.
and AFB3 genes were up regulated in PsJN-inoculated plants
1A). However, the growth promotion effects of the wild-type
at the stage of six leaves (19 days after sowing).
strain on FW and primary root length were not observed with the iacC mutant (Fig. 1A), indicating that degradation of IAA
DISCUSSION
by B. phytofirmans PsJN is required for full plant growth pro-motion. Neither the rhizospheric (9.4 ± 0.1 versus 9.84 ± 0.01
B. phytofirmans colonizes the rhizosphere and internal tissues
log CFU/mg FW) nor the endophytic (6.8 ± 0.6 versus 7.9 ±
of its plant hosts (Compant et al. 2005; Sessitsch et al. 2005). In
0.5 log CFU/mg FW) colonization levels in A. thaliana were
this work, we showed that the plant model A. thaliana may also
affected in this mutant compared with the wild-type PsJN. To
be colonized by strain PsJN. We also found that AcdS activity
further study the effect of IAA degradation on growth promo-
and QS are required for plant growth promotion and rhizosphere
tion by B. phytofirmans, the abilities of the wild-type strain
and endophytic colonization in A. thaliana. We report, for the
and iacC mutant to abolish the effects of exogenously added
first time, the involvement of IAA in plant growth promotion by
IAA on root development were compared. Two concentrations
this well-known PGPR. An IAA degradation mutant is not effec-
of IAA were exogenously added when seed were placed on
tive in promoting plant growth and in preventing adverse effects
plates. A sharp decrease in primary root lengths was verified at
of exogenously added IAA; the plant growth promotion effects
1 µM IAA (Fig. 2A and B). The inoculation with the wild-type
are partially abolished in A. thaliana plants with reduced IAA
Fig. 2. A, Effect of exogenous addition of indole-3-acetic acid (IAA) on the elongation of primary roots in plants of Arabidopsis thaliana inoculated with
Burkholderia phytofirmans PsJN or its iacC mutant. Different letters indicate statistically significant differences between root lengths (one-way analysis of
variance Tukey's honestly significant difference tests, P < 0.05). B, Photograph of 10-day-old seedlings grown exposed to 1 µM exogenous IAA.
548 / Molecular Plant-Microbe Interactions
signaling, and the expression of some auxin receptors is upregu-
genes increasing IAA levels at the root tip (Růzicka et al.
lated in inoculated plants. In this context, it is worth mentioning
2007; Strader et al. 2010; Swarup et al. 2007). Therefore, high
that batch cultures of B. phytofirmans degrading IAA present
concentrations of IAA lead to high levels of ethylene, decreas-
twofold longer lag phases compared with other aromatic carbon
ing root cell length (Strader et al. 2010). Consistently, we
sources (data not shown), and the synthesis of IAA by B. phy-
found that strain PsJN abolishes the effect of exogenous IAA
tofirmans occurs before IAA degradation, suggesting that IAA
that decreases primary root length and that A. thaliana plants
turnover is carefully controlled in this bacterium (R. A. Donoso,
inoculated with the iacC mutant showed only a partial rever-
unpublished results). Furthermore, qRT-PCR detection of iacC
sion of the effect of exogenous IAA. Although we cannot dis-
gene transcripts and iacC gene promoter transcriptional fusion
card the possibility that the iacC mutant partially transforms
analysis clearly demonstrated that IAA regulates its degradation
exogenously added IAA, this partial effect may be also ex-
and is able to significantly induce iacC gene expression (R. A.
plained through cross talk with the AcdS effect, which is still
Donoso, unpublished results).
operative in the iacC mutant, that reduces levels of ethylene
Although some hints of the mechanism or mechanisms used
and stimulates root elongation. These observations suggest that
by B. phytofirmans to promote plants growth have been pro-
both AcdS and IAA degradation activities are important in pri-
posed (Ait Barka et al. 2000; Sessitsch et al. 2005), thus far,
mary root growth promotion by B. phytofirmans PsJN.
only the involvement of AcdS in primary root length stimula-
The use of an A. thaliana transgenic line overexpressing
tion in canola had been described (Sun et al. 2009). In our
miR393 provided additional support for the involvement of
model plant (Fig. 1), the effect of PsJN inoculation on root
plant IAA signaling in plant growth promotion by B. phytofir-
elongation is certainly lesser than the elongation observed with
mans PsJN. The positive effects of bacterial inoculation almost
other plant models such as canola (Sun et al. 2009); however,
completely disappeared in ox-mi393 plants. Although rhizo-
we have shown it to be reproducible and statistically signifi-
spheric colonization still takes place in this transgenic line, en-
cant, which allows us to differentiate the effects of the wild-
dophytic colonization by B. phytofirmans was grossly impaired.
type strain and the acdS mutant strain. Interestingly, tests with
Similar results were found with ox-miR393 plants inoculated
the IAA degradation mutant strain (iacC) also showed no im-
with pathogen P. syringae DC 3000, where repression of auxin
provement of primary root lengths. It has been described that
signaling restricted its growth inside plants (Navarro et al.
root growth can be stimulated or inhibited depending on the
2006). The results obtained with this A. thaliana transgenic
concentration of IAA produced by bacteria (López-Bucio et al.
line which, among other effects in auxin signaling, has reduced
2007; Persello-Cartieux et al. 2001). In plants inoculated with
expression of auxin receptor genes (Navarro et al. 2006),
the iacC mutant, IAA levels in roots should be higher than in
prompted us to test the effect of inoculation with strain PsJN
roots inoculated with the wild-type PsJN strain, because this
on the expression of these genes, finding upregulation of the
mutant cannot degrade IAA and, thus, the plant would be more
expression of AFB1 and AFB3 genes in a developmental time-
susceptible to primary root shortening. Leveau and Lindow
controlled manner (Fig. 3). Furthermore, our lab has analyzed
(2005) have described similar effects in radish plants inocu-
recently global transcription changes in Arabidopsis plants
lated with P. putida 1290, a strain that catabolizes IAA. This
inoculated with strain PsJN, finding that genes implicated in
can be explained through the cross-talk between auxin and eth-
auxin pathway were significantly regulated (M. J. Poupin and
ylene synthesis in plants, where auxin increases ACC synthase
T. Timmermann unpublished results).
gene transcription, stimulating ethylene synthesis and, con-
Bacterial IAA synthesis has been proposed as an important
versely, ethylene promotes expression of IAA biosynthetic
feature in pathogenic bacteria-plant interactions (Comai and
Fig. 3. Effect of PsJN inoculation in the gene expression of auxin receptors in wild-type plants. Quantitative real-time polymerase chain reaction determina-
tions of relative levels of gene expression in complete plants at four rosette leaves (4L) or six rosette leaves (6L) stages. Data are means ± standard
errors of three biological replicates. Asterisk indicates statistical significance (AFB1: Mann-Whitney U test, Z = –1,963, P < 0.05; AFB3: Mann-Whitney U
test, Z = –1,727, P < 0.05).
Vol. 26, No. 5, 2013 / 549
Kosuge 1982; Navarro et al. 2006) as well as in phytostimula-
MATERIALS AND METHODS
tion processes (Patten and Glick 2002a and b) and, conse-quently, should play a relevant role in plant growth promotion
Bacterial strains and growth conditions.
mechanisms in B. phytofirmans and other PGPR. The results
B. phytofirmans PsJN was obtained from A. Sessitsch. Wild-
reported here support that possibility; unfortunately, the con-
type PsJN and the four mutants were grown at 30°C on Dorn
struction of IAA synthesis strain PsJN mutants, which may
mineral salts medium (Dorn et al. 1974) containing 10 mM
allow testing the effects on plant growth promotion, is not sim-
fructose, 5 mM 4-hydroxybenzoate or benzoate, or 2.5 mM
ple because this strain possesses at least two putative IAA bio-
IAA as the sole carbon and energy source and, if required,
synthetic pathways: the indole-3-acetamide pathway and the
kanamycin (50 µg ml–1) or spectinomycin (100 µg ml–1) for
tryptophan side chain oxidase pathway (Weilharter et al.
12 h. For growth tests of strains on 2.5 mM IAA, cells were
2011). In addition, we carried out homology searches, in the
grown for 36 h, biomass measured at OD600nm, and three repli-
strain PsJN genome, of additional IAA synthesis pathways and
cates were performed for each growth measurement.
we found putative marker genes for the indole-3-pyruvate, indole-3-acetonitrile, and tryptamine pathway. We have gener-
Construction of B. phytofirmans PsJN mutants.
ated single mutants for all these marker genes but these mu-
Internal fragments of the bpI.1 gene (locus Bphyt_0126,
tants still synthesize IAA (R. A. Donoso, unpublished results),
AHL synthase of chromosome 1 QS system), bpI.2 gene (locus
indicating that strain PsJN carries multiple pathways to syn-
Bphyt_4275, AHL synthase of chromosome 2 QS system), iacC
thesize this compound. This is similar to the case of Azospiril-
gene (locus Bphyt_2156, aromatic ring hydroxylating dioxy-
lum brasilense, in which at least three different pathways of
genase involved in catabolism of IAA), and acdS gene (locus
IAA synthesis are functional (Prinsen et al. 1993).
Bphyt_5397, 1-aminocyclopropane-1-carboxylic acid deamin-
Other molecular signaling processes may contribute to PGPR
ase) sequences were amplified by PCR, using the primer pairs
performance. Recently, it has been described that the PGPR A.
bpi.1mutFW (GACGGAGGCCAGCAATATAA) and bpi.1-
lipoferum cell-to-cell communication QS system mediated by
mutRV (GTATGGGAGATGTCGCGATT), bpI.2mutFW (GA
AHL is implicated in rhizosphere competence and adaptation to
ACGTCACCAGTTCGTGAAT) and bpI.2mutRV (ATGGAGA
plant roots (Boyer et al. 2008). Furthermore, a QS system has
TCGACGGCTATGA), iacCmutFW (GGTCAACGTCTTGCA
been involved in the regulation of plant-growth-promoting traits
GAACC) and iacCmutRV (GTTTCGTCGTCGATCGATTT),
of P. aeruginosa PUPa3 (Steindler et al. 2009). We reported here
and acdSmutFW (CGAATATCTGATCCCCGAAG) and acdS-
that a bpI.1 mutant in the QS system of B. phytofirmans, which
mutRV (AAGCCGATGTCGAAACCAT), respectively. The
produces lower AHL levels than the wild-type strain, is unable
PCR products were cloned using the pCR2.1-TOPO system
to promote growth on Arabidopsis thaliana. This is probably
(Invitrogen, Carlsbad, CA, U.S.A.) to generate plasmids
due to the lower rhizosphere colonization and null endophytic
pCR2.1bpi.1, pCR2.1iacC, and pCR2.1acdS. The bpI.2 PCR
colonization ability of the mutant compared with the wild-type
product was cloned using the pCR8/GW/TOPO system (In-
strain. Consistently, less biofilm formation on plates fed with
vitrogen), to generate plasmid pCR8bpI.2. These plasmids were
root exudates (A. Zúñiga, unpublished results), low motility, and
electroporated in B. phytofirmans PsJN to get one recombina-
grossly impaired adherence to A. thaliana root surface are ob-
tion event disruption of the target gene, and recombinants were
served in the bpI.1 mutant compared with the wild-type strain.
selected on Luria-Bertani (LB) agar containing kanamycin at
Furthermore, analysis of transcript levels of genes involved in
50 µg/ml to obtain strain PsJN bpi.1, strain PsJN iacC, and
swimming motility (Bphyt_3794, Bphyt_3820, Bphy_3804, and
PsJN acdS mutants and spectinomycin 100 µg/ml to obtain
Bphyt_3770) and exopolysaccharide production (Bphyt_4056,
strain PsJN bpI.2 mutant. Correct insertions in mutant strains
Bphyt_6264, Bphyt_0818, and Bphyt-1955) showed, for most
were confirmed by PCR and sequencing.
of them, downregulated or repressed expression in the PsJN bpI.1 mutant compared with expression in wild-type PsJN (A.
RNA extraction, cDNA synthesis, and qRT-PCR analysis.
Zúñiga, unpublished results). Motility, attachment to roots, and
For RNA extraction, plants of LP.04 stage (Boyes et al.
biofilm formation are important traits for root surface coloniza-
2001) (four rosette leaves visible, corresponding to 13 days
tion (Danhorn and Fuqua 2007; Steindler et al. 2009), because
after sowing) or LP.06 stage (Boyes et al. 2001) (six rosette
PGPR have been reported to colonize developing small biofilms
leaves visible, corresponding to 19 days after sowing) were
along epidermal fissures (Ramey et al. 2004). Regulation by a
used. Ten plantlets for each treatment were collected in liquid
QS system may influence key steps leading to biofilm formation
nitrogen and ground with a pestle in an Eppendorf tube. Then,
required for plant growth promotion. This may explain low rhi-
RNA was obtained using the Trizol method following the
zosphere and endophytic colonization and lack of plant-growth-
manufacturer's instructions (Invitrogen). For cDNA synthesis,
promoting traits observed with the PsJN bpI.1 mutant, and low
1 µg of total RNA treated with DNAse I (RQ1; Promega
endophytic levels of the bpI.2 mutant. The production of exopol-
Corp., Madison, WI, U.S.A.) was reverse transcribed with ran-
ysaccharide polymer has been reported in strain PsJN and in
dom hexamer primers using the Improm II reverse transcrip-
other plant-associated Burkholderia spp., and it is believed to be
tase (Promega Corp.), according to the manufacturer's instruc-
involved in the plant–bacterium interaction (Ferreira et al.
tions. RT-PCR was performed using the Brilliant SYBR Green
2010). Interestingly, impaired exopolysaccharide production
QPCR Master Reagent Kit (Agilent Technologies, Santa Clara,
affected endophytic colonization by a QS mutant in B. kururi-
CA, U.S.A.) and the Eco Real-Time PCR detection system (Il-
ensis (Suárez-Moreno et al. 2010). Although the production of
lumina, San Diego, CA, U.S.A.). The PCR mixture (15 µl)
cell-wall-degrading endoglucanases by strain PsJN plays a role
contained 2.0 µl of template cDNA (diluted 1:10) and 140 nM
in grapevine endophytic colonization (Compant et al. 2005),
each primer. Amplification was performed under the following
both bpI mutants and the wild-type PsJN strain showed no dif-
conditions: 95°C for 10 min; followed by 40 cycles of 94°C
ferences in endoglucanase levels (data not shown), indicating
for 30 s, 57°C for 30 s, and 72°C for 30 s; followed by a melt-
that lower endophytic colonization found with A. thaliana is not
ing cycle from 55 to 95°C. Relative gene expression calcula-
due to the lack of this cell-wall-degrading activity. Additional
tions were conducted as described in the software manufac-
studies with QS mutants are required for full understanding of
turer's instructions: an accurate ratio between the expression of
the role of a QS system in endophytic and rhizospheric coloniza-
the gene of interest (GOI) and the housekeeping (HK) gene
tion and, ultimately, plant growth promotion.
was calculated according to the following 2–(ΔCtGOI – HK). Then,
550 / Molecular Plant-Microbe Interactions
gene expression levels were normalized to the average value of
Determination of rhizospheric and
the expressions in the control treatment. AtSAND (AT2G28390)
endophytic bacterial colonization.
was used as the HK gene using previously described primer
For rhizospheric colonization tests, 3-week-old plants were
pairs (Czechowski et al. 2005). Primer pairs for TIR1
removed from inoculated MS media agar and washed in phos-
(AT3G62980), AFB1 (AT4G03190), AFB2 (AT3G26810), and
phate buffer solution, with vortex agitation. Extracted liquid
AFB3 (AT1G12820) have been used elsewhere (Vidal et al.
material was serially diluted with Dorn mineral salts medium
2010). All experiments were performed in three biological and
before plating on Dorn medium plates supplemented with
two technical replicates.
benzoate as the sole carbon and energy source. The CFU/mg FW was determined after 48 h of incubation at 30°C. For en-
Green fluorescence protein labeling.
dophytic colonization tests, 3-week-old plantlets inoculated
Strain PsJN and its mutants were tagged with the GFP
with GFP-labeled PsJN strains were removed from the agar
marker gene using a mini-Tn5 system (Mathysse et al. 1996),
plates, surface sterilized with 70% ethanol for 1 min followed
which forms stable genomic insertions. Wild-type PsJN and
by 1% commercial chlorine bleach and a 0.01% Tween 20
PsJN bpI.1 and PsJN bpI.2 mutants were conjugated with
solution for 1 min, then washed three times in sterile distilled
Escherichia coli PRK2073, as helper in a triparental mating,
water (adapted from Compant and associates [2005]). Plating
and E. coli S17, which contains the plasmid with mini-
the distilled water from a final wash on R2A medium rou-
Tn5GFP construct carrying tetracycline resistance. Transcon-
tinely controlled sterility on these plants. Then, the sterilized
jugants carrying the GFP marker were selected on LB con-
plant material was macerated in sterile mortars and the dis-
taining tetracycline at 10 µg/ml. GFP-labeled cells were
rupted tissue was resuspended in 1 ml of sterile 50 mM phos-
examined by an Optical Fluorescence microscope in Model
phate buffer to obtain an aqueous extract. CFU/mg FW was
Nikon Eclipse 50i (Nikon, Tokyo) equipped with GFP HYQ
determined by serial dilutions of these extracts in R2A agar
and G-2E/C filters.
plates after 48 h of incubation at 30°C and plates were exam-ined under UV light using an Optical Epi-fluorescence Nikon
Measurement of IAA synthesis.
Eclipse 50i microscope (Nikon). Experiments were conducted
Bacterial cells were grown with 5 mM fructose at OD600nm =
with six plants analyzed for each treatment, in two biological
1, which corresponds to the logarithmic phase of growth. ATtR
this point, bacterial cells were incubated with 2.5 mM trypto-phan (as inducer of IAA synthesis) for 3 h; then, IAA synthe-
Microscopy analyses.
sis was measured on supernatants of cultures using Salkowski
To determine rhizosphere colonization by GFP-marked
reagent, as described previously (Glickmann and Dessaux
strains, treated and untreated plant root surfaces were exam-
1995). Three replicates were performed for each IAA synthesis
ined by optical epifluorescence and confocal microscopy.
Epifluorescence images were taken using a Nikon Eclipse 50i microscope (Nikon) equipped with GFP HYQ and G-
Preparation of bacterial inoculants.
2E/C filters, and photographs were taken with a DS Fi1 digi-
For plant colonization and growth effect experiments, B.
tal camera (Nikon). Confocal microscope images were ob-
phytofirmans PsJN and its four mutant derivative strains
tained using Olympus FluoView 1000 confocal laser scanning
(PsJN bpI.1, PsJN bpI.2, PsJN acdS, and PsJN iacC) were
(Olympus, Tokyo) equipped with high-performance sputtered
routinely grown in Dorn mineral salts medium (Dorn et al.
1974) with 2 mM fructose in an orbital shaker (150 rpm) for 24 h at 30°C. Cell suspensions from each inoculum were
Determination of the plant growth parameters.
then obtained and adjusted to approximately 108 CFU/ml, as
In all, 25 plantlets from each inoculated treatment as well
determined by plate counting. Then, each strain at 104 CFU/ml
as 25 noninoculated plantlets were analyzed. Root length and
was homogeneously inoculated on 1% agar plates containing
lateral roots number were measured directly in harvested
Murashige and Skoog (MS) basal salt mixture (Sigma-Aldrich,
plants, and FW was recorded as previously described
(Compant et al. 2005; Nowak et al. 1995; Sessitsch et al. 2005). The chlorophyll contents were also determined fol-
Plant growth.
lowing a published procedure (Porra et al. 1989). Chlo-
A. thaliana ecotype Col-0 and the A. thaliana transgenic
rophyll was extracted from leaves of A. thaliana in N,N-9-
line ox-miR393 (Navarro et al. 2006; Vidal et al. 2010) were
dimethylformamide for 24 h at 4°C in the dark, and
used. Seed were surface sterilized with 50% (vol/vol) com-
chlorophyll a and chlorophyll b concentrations were meas-
mercial chlorine bleach for 7 min and washed three times in
ured simultaneously by spectrophotometry. For root hair
sterile distilled water. Then, seed were kept at 4°C for 2 days
number measurements, segments at a distance of 1 cm from
in the absence of light to produce stratification. After that,
the root tip of the primary root were analyzed using light
seed were sown in sterile plastic petri dishes with 1% agar
plates containing MS basal salt mixture (Sigma-Aldrich) in-oculated or not with bacteria. Eight seeds were sown in each
Statistical analysis.
plate and six plates were used for each treatment: control
Data for plant growth parameters and population density
without bacteria, wild type, and bpI.1, bpI.2, iacC, and acdS
(CFU/mg FW) were statistically analyzed using one-way analy-
mutant strains. To perform exogenous IAA degradation assays,
sis of variance. The chlorophyll contents and CFU/mg FW were
seed were germinated and grown for 2 weeks on plates with
subjected to logarithmic transformation before data analysis.
MS medium containing 0.001 or 1 µM IAA inoculated or not
When analysis of variance showed significant treatment
with either wild-type or iacC mutant strains. Plates were
effects, Tukey's honestly significant difference (P < 0.05) test
placed vertically, sealed with parafilm, and arranged in a com-
was applied to make comparisons between treatments. Statistical
pletely randomized design. The plant growth chamber was
analyses of plant gene expression were performed using the
run with a cycle of 12 h of light and 12 h of darkness and a
Mann-Whitney Test for nonparametrics data in the statistical
temperature of 22 ± 2°C. Three biological replicates were
software package STATISTICA (version 6.0; StatSoft Inc.,
Tulsa, OK, U.S.A.).
Vol. 26, No. 5, 2013 / 551
Idris, E. E., Iglesias, D. J., Talon, M., and Borriss, R. 2007. Tryptophan-
dependent production of indole-3-acetic acid (IAA) affects level of
We thank J. Jones for providing the A. thaliana miR393 overexpressor
plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol.
lines and G. León from Universidad Andres Bello for technical and inter-
Plant-Microbe Interact. 20:619-626.
pretation advice in confocal microscopy. This work was funded by the
Kraiser, T., Gras, D. E., Gutiérrez, A. G., González, B., and Gutiérrez, R.
FONDECYT grants 3100040 and 3090051, the Millennium Nuclei in
A. 2011. A holistic view of nitrogen acquisition in plants. J. Exp. Bot.
"Microbial Ecology and Environmental Microbiology and Biotechnology"
grant P/04-007-F, and "Plant Functional Genomics" grant P/06-009-F.
Leveau, J. H., and Gerards, S. 2008. Discovery of a bacterial gene cluster
Additional support from CONICYT grant 79090016 is acknowledged. A.
for catabolism of the plant hormone indole 3-acetic acid. FEMS (Fed.
Zúñiga and R. Donoso are CONICYT-PhD fellows.
Eur. Microbiol. Soc.) Microbiol. Ecol. 65:238-250.
Leveau, J. H., and Lindow, S. E. 2005. Utilization of the plant hormone
indole-3-acetic acid for growth by Pseudomonas putida strain 1290.
LITERATURE CITED
Appl. Environ. Microbiol. 75:2365-2371.
Liu, P., and Nester, E. W. 2006. Indoleacetic acid, a product of transferred
Ahmad, F., Ahmad, I., and Khan, M. S. 2008. Screening of free-living rhi-
DNA, inhibits vir gene expression and growth of Agrobacterium tume-
zospheric bacteria for their multiple plant growth promoting activities.
faciens C58. Proc. Natl. Acad. Sci. U.S.A. 103:4658-4662.
Microbiol. Res. 163:173-181.
Loper, J. E., and Schroth, M. N. 1986. Influence of bacterial sources of
Ait Barka, E., Belarbi, A., Hachet, C., Nowak, J., and Audran, J.-C. 2000.
indole-3-acetic acid biosynthetic on root elongation of sugar beet. Phy-
Enhancement of in vitro growth and resistance to gray mould of Vitis
topathology 76:386-389.
vinifera co-cultured with plant growth-promoting rhizobacteria. FEMS
López-Bucio, J., Campos-Cuevas, J. C., Hernández-Calderón, E.,
(Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 186:91-95.
Velásquez-Becerra, C., Farías-Rodríguez, R., Macías-Rodríguez, L. I.,
Bais, H. P., Park, S. W., Weir, T. L., Callaway, R. M., and Vivanco, J. M.
and Valencia-Cantero, E. 2007. Bacillus megaterium rhizobacteria
2004. How plants communicate using the underground information
promote growth and alter root-system architecture through an auxin and
superhighway. Trends Plant Sci. 9:26-32.
ethylene-independent signaling mechanism in Arabidopsis thaliana.
Bais, H. P., Weir, T. L., Perry, L. G., Gilroy, S., and Vivanco, J. M. 2006.
Mol. Plant-Microbe Interact. 20:207-217.
The role of root exudates in rhizosphere interactions with plants and
Mark, G. L., Dow, J. M., Kiely, P. D., Higgins, H., Haynes, J., Baysse, C.,
other organisms. Annu. Rev. Plant Biol. 57:233-266.
Abbas, A., Foley, T., Franks, A., Morrissey, J., and O'Gara, F. 2005.
Bakker, P. A., Pieterse, C. M., and van Loon, L. C. 2007. Induced sys-
Transcriptome profiling of bacterial responses to root exudates identi-
temic resistance by fluorescent Pseudomonas spp. Phytopathology
fies genes involved in microbe–plant interactions. Proc. Natl. Acad. Sci.
U.S.A. 102:17454-17459.
Bauer, W. D., and Mathesius, U. 2004. Plant responses to bacterial quorum
Mathysse, A. G., Stretton, S., Dandie, C., McClure, N. C., and Goodman,
sensing signals. Curr. Opin. Plant Biol. 7:429-433.
A. E. 1996. Construction of GFP vectors for use in gram-negative bac-
Bianco, C., Imperlini, E., Calogero, R., Senatore, B., Amoresano, A.,
teria other than Escherichia coli. FEMS (Fed. Eur. Microbiol. Soc.)
Carpentieri, A., Pucci, P., and Defez, R. 2006. Indole-3-acetic acid
Microbiol. Lett. 145:87-94.
improves Escherichia coli's defenses to stress. Arch. Microbiol.
Navarro, L., Dunoyer, P., Jay, F., Arnold, B., Dharmasiri, N., Estelle, M.,
Voinnet, O., and Jones, J. D. G. 2006. A plant miRNA contributes to
Boyer, M., Bally, R., Perrotto, S., Chaintreuil, C., and Wisniewski-Dye, F.
antibacterial resistance by repressing auxin signaling. Science 312:436-
2008. A quorum-quenching approach to identify quorum sensing-regu-
lated functions in Azospirillum lipoferum. Res. Microbiol. 159:699-708.
Nowak, J., Asiedu, S. K., Lazarovits, G., Pillay, V., Stewart, A., Smith, C.,
Boyes, D. C., Zayed, A. M., Ascenzi, R., McCaskill, A. J., Hoffman, N. E.,
and Liu, Z. 1995. Enhancement of in vitro growth and transplant stress
Davis, K. R., and Gorlach, J. 2001. Growth stage-based phenotypic
tolerance of potato and vegetable plants co-cultured with a plant growth
analysis of Arabidopsis: A model for high throughput functional
promoting rhizobacterium. Pages 173-180 in: Ecophysiology and Pho-
genomics in plants. Plant Cell 13:1499-1510.
tosynthetic In Vitro Cultures. CEA, Aix-en-Provence, France.
Comai, L., and Kosuge, T. 1982. Cloning characterization of iaaM, a viru-
Ortíz-Castro, R., Contreras-Cornejo, H. A., Macías-Rodríguez, L., and
lence determinant of Pseudomonas savastanoi. J. Bacteriol. 149:40-46.
López-Bucio, J. 2009. The role of microbial signals in plant growth and
Compant, S., Reiter, B., Sessitsch, A., Nowak, J., Clement, C., and Ait
development. Plant Signal. Behav. 4:701-712.
Barka, E. 2005. Endophytic colonization of Vitis vinifera L. by a plant
Patten, C. L., and Glick, B. R. 2002a. Regulation of indoleacetic acid pro-
growth-promoting bacterium, Burkholderia sp. strain PsJN. Appl. Envi-
duction in Pseudomonas putida GR12-2 by tryptophan and the station-
ron. Microbiol. 71:1685-1693.
ary-phase sigma factor RpoS. Can. J. Microbiol. 48:635-642.
Costa, R., Gomes, N. C. M., Krögerrecklenfort, E., Opelt, K., Berg, G.,
Patten, C. L., and Glick, B. R. 2002b. Role of Pseudomonas putida indole-
and Smalla, K. 2007. Pseudomonas community structure and antago-
acetic acid in development of the host plant root system. Appl. Environ.
nistic potential in the rhizosphere: Insights gained by combining phylo-
Microbiol. 68:3795-3801.
genetic and functional gene-based analyses. Environ. Microbiol.
Persello-Cartieaux, F., David, P., Sarrobert, C., Thibaud, M. C., Achouak,
W., Robaglia, C., and Nussaume, L. 2001. Utilization of mutants to an-
Czechowski, T., Stitt, M., Altmann, T., Udvardi, M. K., and Scheible, W.
alyze the interaction between Arabidopsis thaliana and its naturally
R. 2005. Genome-wide identification and testing of superior reference
root-associated Pseudomonas. Planta 212:190-198.
genes for transcript normalization in Arabidopsis. Plant Physiol. 139:5-
Phi, Q. T., Park, Y. M., Ryu, C. M., Park, S. H., and Ghim, S. Y. 2008.
Functional identification and expression of indole-3-pyruvate decar-
Danhorn, T., and Fuqua, C. 2007. Biofilm formation by plant-associated
boxylase from Paenibacillus polymyxa E681. J. Microbiol. Biotechnol.
bacteria. Annu. Rev. Microbiol. 61:401-422.
Dorn, E., Hellwig, M., Reineke, W., and Knackmuss, H.-J. 1974. Isolation
Pillay, V. K., and Nowak, J. 1997. Inoculum density, temperature and
and characterization of a 3-chlorobenzoate degrading pseudomonad.
genotype effects on epiphytic and endophytic colonization and in vitro
Arch. Microbiol. 99:61-70.
growth promotion of tomato (Lycopersicon esculentum L.) by a pseudo-
Ferreira, A., Leitao, J., Silva, I., Pinheiro, P., Sousa, S., Ramos, C., and
monad bacterium. Can. J. Microbiol. 43:354-361.
Moreira, L. 2010. Distribution of cepacian biosynthesis genes among
Porra, R., Thompson, W., and Kriedmann, P. 1989. Determination of accu-
environmental and clinical Burkholderia strains and role of cepacian
rate extinction coefficients and simultaneous equations for assaying
exopolysaccharide in resistance to stress conditions. Appl. Environ.
chlorophyll a and b extracted with four different solvents: Verification
Microbiol. 76:441-450.
of the concentration of chlorophyll standards by atomic absorption
Frommel, M. I., Nowak, J., and Lazarovits, G. 1991. Growth enhancement
spectroscopy. Biochem. Biophys. Acta 975:384-394.
and developmental modifications of in vitro grown potato (Solanum
Prinsen, E., Costacurta, A., Michiels, K., Vanderleyden, J., and Van
tuberosum ssp. tuberosum) as affected by a nonfluorescent Pseudomo-
Onckelen, H. 1993. Azospirillum brasilense indole-3-acetic acid bio-
nas sp. Plant Physiol. 96:928-936.
synthesis: Evidence for a non-tryptophan dependent pathway. Mol.
Genin, S., and Boucher, C. 2004. Lessons learned from the genome analy-
Plant-Microbe Interact. 6:609-615.
sis of Ralstonia solanacearum. Annu. Rev. Phytopathol. 42:107-134.
Quiñones, B., Dulla, G., and Lindow, S. E. 2005. Quorum sensing regu-
Glickmann, E., and Dessaux, Y. 1995. A critical examination of the speci-
lates exopolysaccharide production, motility, and virulence in Pseudo-
ficity of the Salkowski reagent for indolic compounds produced by phy-
monas syringae. Mol. Plant-Microbe Interact. 18:682-693.
topathogenic bacteria. Appl. Environ. Microbiol. 61:793-796.
Ramey, B. E., Koutsoudis, M., von Bodman, S. B., and Fuqua, C. 2004.
Hurek, T., Handley, L. L., Reinhold-Hurek, B., and Piché, Y. 2002.
Biofilm formation in plant–microbe associations. Curr. Opin. Micro-
Azoarcus grass endophytes contribute fixed nitrogen to the plant in an
biol. 7:602-609.
unculturable state. Mol. Plant-Microbe Interact. 15:233-242.
Růzicka, K., Ljung, K., Vanneste, S., Podhorská, R., Beeckman, T., Friml,
552 / Molecular Plant-Microbe Interactions
J., and Benková, E. 2007. Ethylene regulates root growth through
Vadassery, J., Ritter, C., Venus, Y., Camehl, I., Varma, A., Shahollari, B.,
effects on auxin biosynthesis and transport-dependent auxin distribu-
Novák, O., Strnad, M., Ludwig-Müller, J., and Oelmüller, R. 2008. The
tion. Plant Cell 19:2197-2212.
role of auxins and cytokinins in the mutualistic interaction between
Sessitsch, A., Coenye, T., Sturz, A. V., Vandamme, P., Ait Barka, E.,
Arabidopsis and Piriformospora indica. Mol. Plant-Microbe Interact.
Salles, J. F., Van Elsas, J. D., Faure, D., Reiter, B., Glick, B. R., Wang-
Pruski, G., and Nowak, J. 2005. Burkholderia phytofirmans sp. nov., a
Vande Broek, A., Lambrecht, M., Eggermont, K., and Vanderleyden, J.
novel plant-associated bacterium with plant-beneficial properties. Int. J.
1999. Auxins upregulate expression of the indole-3-pyruvate decar-
Syst. Evol. Microbiol. 55:1187-1192.
boxylase gene in Azospirillum brasilense. J. Bacteriol. 181:1338-1342.
Spaepen, S., Vanderleyden, J., and Remans, R. 2007. Indole-3-acetic acid
Vande Broek, A., Gysegom, P., Ona, O., Hendrickx, N., Prinsen, E., Van
in microbial and microorganism-plant signaling. FEMS (Fed. Eur. Mi-
Impe, J., and Vanderleyden, J. 2005. Transcriptional analysis of the
crobiol. Soc.) Microbiol. Rev. 31:425-448.
Azospirillum brasilense indole-3-pyruvate decarboxylase gene and
Spaepen, S., Das, F., Luyten, E., Michiels, J., and Vanderleyden, J. 2009.
identification of a cis-acting sequence involved in auxin responsive ex-
Indole-3-acetic acid-regulated genes in Rhizobium etli CNPAF512.
pression. Mol. Plant-Microbe Interact. 18:311-323.
FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 291:195-200.
Van Puyvelde, S., Cloots, L., Engelen, K., Das, F., Marchal, K., Vanderleyden,
Steindler, L., Bertani, I., De Sordi, L., Schwager, S., Eberl, L., and Venturi,
J., and Spaepen, S. 2011. Transcriptome analysis of the rhizosphere
V. 2009. LasI/R and RhlI/R quorum sensing in a strain of Pseudomonas
bacterium Azospirillum brasilense reveals an extensive auxin response.
aeruginosa beneficial to plants. Appl. Environ. Microbiol. 75:5131-5140.
Microb. Ecol. 61:723-728.
Strader, L. C., Chen, G. L., and Bartel, B. 2010. Ethylene directs auxin to
Vidal, E. A., Araus, V., Lu, C., Parry, G., Green, P. J., Coruzzi, G. M., and
control root cell expansion. Plant J. 64:874-884.
Gutiérrez, R. A. 2010. Nitrate-responsive miR393/AFB3 regulatory
Suárez-Moreno, Z. R., Devescovi, G., Myers, M., Hallack, L., Mendonça-
module controls root system architecture in Arabidopsis thaliana. Proc.
Previato, L., Caballero-Mellado, J., and Venturi, V. 2010. Commonali-
Natl. Acad. Sci. U.S.A. 107:4477-4482.
ties and differences in regulation of N-acyl homoserine lactone quorum
Weilharter, A., Mitter, B., Shin, M. V., Chain, P. S., Nowak, J., and Sessitsch,
sensing in the beneficial plant-associated Burkholderia species cluster.
A. 2011. Complete genome sequence of the plant growth-promoting
Appl. Environ. Microbiol. 76:4302-4317.
endophyte Burkholderia phytofirmans strain PsJN. J. Bacteriol. 193:3383-
Sun, Y., Cheng, Z., and Glick, B. R. 2009. The presence of a 1-aminocy-
clopropane-1-carboxylate (ACC) deaminase deletion mutation alters
Whitehead, N., Barnard, A., Slater, H., Simpson, N., and Salmond, G.
the physiology of the endophytic plant growth-promoting bacterium
2001. Quorum-sensing in Gram-negative bacteria. FEMS (Fed. Eur.
Burkholderia phytofirmans PsJN. FEMS (Fed. Eur. Microbiol. Soc.)
Microbiol. Soc.) Microbiol. Rev. 25:365-404.
Microbiol. Lett. 296:131-136.
Yang, S., Zhang, Q., Guo, J., Charkowski, A. O., Glick, B. R., Ibekwe, A.
Swarup, R., Perry, P., Hagenbeek, D., Van Der Straeten, D., Beemster, G.
M., Cooksey, D. A., and Yang, C. H. 2007. Global effect of indole-3-
T., Sandberg, G., Bhalerao, R., Ljung, K., and Bennett, M. J. 2007. Eth-
acetic acid biosynthesis on multiple virulence factors of Erwinia chry-
ylene upregulates auxin biosynthesis in Arabidopsis seedlings to
santhemi 3937. Appl. Environ. Microbiol. 73:1079-1088.
enhance inhibition of root cell elongation. Plant Cell 19:2186-2196.
Yuan, Z., Haudecoeur, E., Faure, D., Kerr, K., and Nester, E. 2008. Com-
Theocharis, A., Bordiec, S., Fernandez, O., Paquis, S., Dhondt-Cordelier, S.,
parative transcriptome analysis of Agrobacterium tumefaciens in
Baillieul, F., Clément, C., and Barka, E. A. 2012. Burkholderia phyto-
response to plant signal salicylic acid, indole-3-acetic acid and gamma-
firmans PsJN primes Vitis vinifera L. and confers a better tolerance to
amino butyric acid reveals signaling cross talk and Agrobacterium-plant
low nonfreezing temperatures. Mol. Plant-Microbe Interact. 25:241-249.
co-evolution. Cell Microbiol. 10:2339-2354.
Vol. 26, No. 5, 2013 / 553
Source: http://virtualplant.bio.puc.cl/Lab/doc/23301615.pdf
mals for use as tools, without extensive prior experience, is almost unknown. In experiments Shaping of Hooks in New by Povinelli [experiments 24 to 26 in (2)], chim-panzees (Pan troglodytes) repeatedly failed to unbend piping and insert it through a hole toobtain an apple, unless they received explicitcoaching. Further experiments [exp. 27 in (2)]
Im Auftrag der Stadtgemeinde Saalfelden seit 1996 Kinder & Jugendzentrum Saalfelden TREFFPUNKT · Berglandstraße 28 Gefördert durch die Stadtgemeinde Saalfelden und Mitteln des Land Salzburg Im Auftrag der Stadtgemeinde Saalfelden seit 1996 Im Wandel der Zeit! Das Kinder und Jugendzentrum Saalfelden „Treffpunkt", befi ndet sich ständig im Wandel. Viele der „älteren" Jugendlichen in der Altersgruppe von 15 bis 18 Jah-ren, haben nicht nur neue Interessen, sondern stehen auch vor neuen Herausfor-derungen wie dem Schulabschluss, den Beginn einer Lehre oder weiterführende Schule, der Führerscheinprüfung, dem Bundesheer oder Zivildienst und so wei-ter. Sie treffen sich im Jugendzentrum um sich diesbezüglich bei gleichaltrigen Alexander Houtman BEdauszutauschen und auch um Erfahrungswerte von BetreuerInnen anzuhören. Leiter des Kinder & Auffallend sind dabei die „kurzen" Besuche im Jugendzentrum - die Gespräche Jugendzentrum Saalfeldenmit den BetreuerInnen sind dafür aber intensiver.

