Antibiotic resistance among bacterial pathogens in central africa: a review of the published literature between 1955 and 2008


ARTICLE IN PRESS
International Journal of Antimicrobial Agents xxx (2009) xxx–xxx
Contents lists available at
International Journal of Antimicrobial Agents
Antibiotic resistance among bacterial pathogens in Central Africa: a reviewof the published literature between 1955 and 2008
E. Vlieghe , M.F. Phoba , J.J. Muyembe Tamfun , J. Jacobs
a Department of Clinical Sciences, Institute of Tropical Medicine, Nationalestraat 155, 2000 Antwerp, Belgiumb Institut National de Recherche Biomédicale (INRB), Avenue de la Démocratie (ex Huileries), Kinshasa/Gombe B.P. 1197 Kinshasa 1, Democratic Republic of the Congo
Article history:
A systematic review of the published literature on bacterial resistance in Central Africa between 1955
Received 2 February 2009
and 2008 was performed. Eighty-three publications from seven countries were retrieved, the majority
Accepted 30 April 2009
presenting data on enteric and other Gram-negative pathogens. Despite methodological limitations inmany studies, alarming resistance rates are noted in nearly all pathogens. Of special concern are mul-
tidrug resistance in Shigella and Salmonella spp. and the emergence of meticillin-resistant Staphylococcus
aureus, high-level penicillin-resistant Streptococcus pneumoniae and extended-spectrum -lactamases
Antibacterial agents
among Gram-negative pathogens. These findings make clear that the Central African region shares the
worldwide trend of increasing antimicrobial resistance and is in urgent need of sound surveillance based
Surveillance review
on competent and affordable microbiology to provide clear data on antimicrobial resistance. These datacould enable redaction of local treatment guidelines and fuel national and regional policies to containantimicrobial resistance.
2009 Elsevier B.V. and the International Society of Chemotherapy. All rights reserved.
tuberculosis and agents causing sexually transmitted infections.
Relevant literature was identified from the PubMed online database
Developing countries face substantial problems of antimicrobial
of the US National Library of Medicine. Search terms included ‘bac-
resistance. Contributing factors include those involving the public
terial resistance', ‘antibiotic use', ‘antimicrobial resistance' and
(e.g. self-medication), prescriber (e.g. misinformation, absence of
‘bacterial surveillance' combined with the different countries
diagnostic tools) and dispenser (e.g. over-the-counter use, inad-
and ‘Central Africa'. Bibliographies of all relevant papers were
equate storage, use of expired drugs) ect knowledge of
searched to identify further papers. A similar search was also
actual resistance patterns is the cornerstone of successful contain-
performed in the Medical Literature on Central Africa database at
ment of antimicrobial resistance. Good-quality data are available
the library of the Institute of Tropical Medicine Antwerp (Belgium).
from more affluent and transitory countries (e.g. in Latin America
In addition, relevant websites providing medical literature for use
Asia but there is little information available from
in developing countries were accessed.
many countries in sub-Saharan Africa, especially from the Central
All articles were reviewed by at least two authors. Only publi-
African region. Thus, a systematic review was performed to compile
cations listing original data on bacterial resistance in humans were
the available information on bacterial resistance in this region.
included. Studies were further assessed for design, setting, demo-graphic data and microbiological methodology. Studies reporting
2. Materials and methods
archived results of susceptibility testing as part of routine clini-cal care were categorised as retrospective laboratory studies, and
The region of Central Africa was considered as described in
studies addressing a particular clinical condition (e.g. meningitis)
the United Nation's geoscheme comprising the countries
were termed clinical studies. Surveillance studies were considered
Cameroon, Chad, Gabon, São Tomé e Príncipe, Congo-Brazzaville,
as those complying with the World Health Organization (WHO)
Democratic Republic of the Congo (DRC) (formerly Zaire), the
surveillance standards i.e. studies focusing on a particular
Central African Republic (CAR), Angola and Equatorial Guinea. The
pathogen carried out by a reference laboratory and/or a network
study focused on bacterial pathogens, excluding Mycobacterium
of sentinel laboratories.
The WHO criterion of 48 h hospital admission was used for the
distinction between community- and hospital-acquired (nosoco-
∗ Corresponding author. Tel.: +32 3 821 39 77/47 582 15 83.
mial) infections. If no data on duration of hospital admission were
E-mail address: (E. Vlieghe).
mentioned, the categories as mentioned by the authors were used.
0924-8579/$ – see front matter 2009 Elsevier B.V. and the International Society of Chemotherapy. All rights reserved.
Please cite this article in press as: Vlieghe E, et al. Antibiotic resistance among bacterial pathogens in Central Africa: a review of thepublished literature between 1955 and 2008. Int J Antimicrob Agents (2009), doi:
ARTICLE IN PRESS
E. Vlieghe et al. / International Journal of Antimicrobial Agents xxx (2009) xxx–xxx
Relevant microorganisms and antibiotics were primarily those
external quality programmes were mentioned in only 14 and 3
listed by the WHO Surveillance Standards. Key organisms included
studies, respectively. Only 5 (6.0%) of 83 studies considered only
Staphylococcus aureus, Streptococcus pneumoniae, the enteric
one sample per patient and per episode as recommended
pathogens (i.e. Campylobacter, Vibrio, Shigella and Salmonellaspp.), Klebsiella pneumoniae, Escherichia coli, Pseudomonas aerugi-
4. Resistance rates for different species
nosa, Haemophilus influenzae and Neisseria meningitidis. Antibioticsincluded the first-line antibiotics [benzyl penicillin, ampi-
4.1. Gram-positive bacteria
cillin and/or amoxicillin, oxacillin, trimethoprim/sulfamethoxazole(SXT), tetracycline, chloramphenicol, erythromycin, clindamycin
4.1.1. Staphylococcus aureus
and nalidixic acid] as well as representatives of the different gen-
Resistance data for S. aureus were reported in nine studies,
erations of cephalosporins, aminoglycosides and fluoroquinolones.
including three surveillance studies and six clinical studies from
Unless otherwise stated, intermediate-resistant strains were
urban hospitals. Although a wide range of samples (pus, wounds,
grouped together with resistant strains. Mean resistance rates for
urine and CSF) was addressed from mixed origins (community,
data from different studies combined were calculated by dividing
nosocomial), only one study considered blood cultures f note
the sum of all resistant organisms (nominator) over the sum of all
is that in some studies panels for susceptibility testing were not in
organisms tested (denominator). The minimum number of isolates
accordance with WHO surveillance standards, e.g. did not contain
per species for separate reporting was set at 10, in line with the
oxacillin or an equivalent antibiotic
European Society of Clinical Microbiology and Infectious Diseases
As can be seen in the presence of meticillin-resistant
Study Group for Antimicrobial Resistance Surveillance (ESGARS)
S. aureus (MRSA) was examined in six studies. Kesah et al.
the Clinical and Laboratory Standards Institute (CLSI)
surveyed MRSA prevalence in eight African countries, including
Cameroon as the only country from Central Africa. They foundMRSA in 27 (21.3%) of 127 Cameronese samples. This was amongthe top three highest prevalence figures in the study, only after
3. Results
Kenya (38/137; 27.7%) and Nigeria (42/142; 29.6%). A recent Con-golese study nasal staphylococcal carriers reported an
3.1. Overview of study design, study setting and microbiological
MRSA prevalence of 63.1%, 66.7% and 23.3% in hospitalised patients,
hospital staff and outpatients, respectively.
A total of 173 articles published between 1954 and 2008 were
4.1.2. Streptococcus pneumoniae
retrieved, of which 83 were eligible for inclusion in the review.
Data were found in 11 studies conducted between 1990
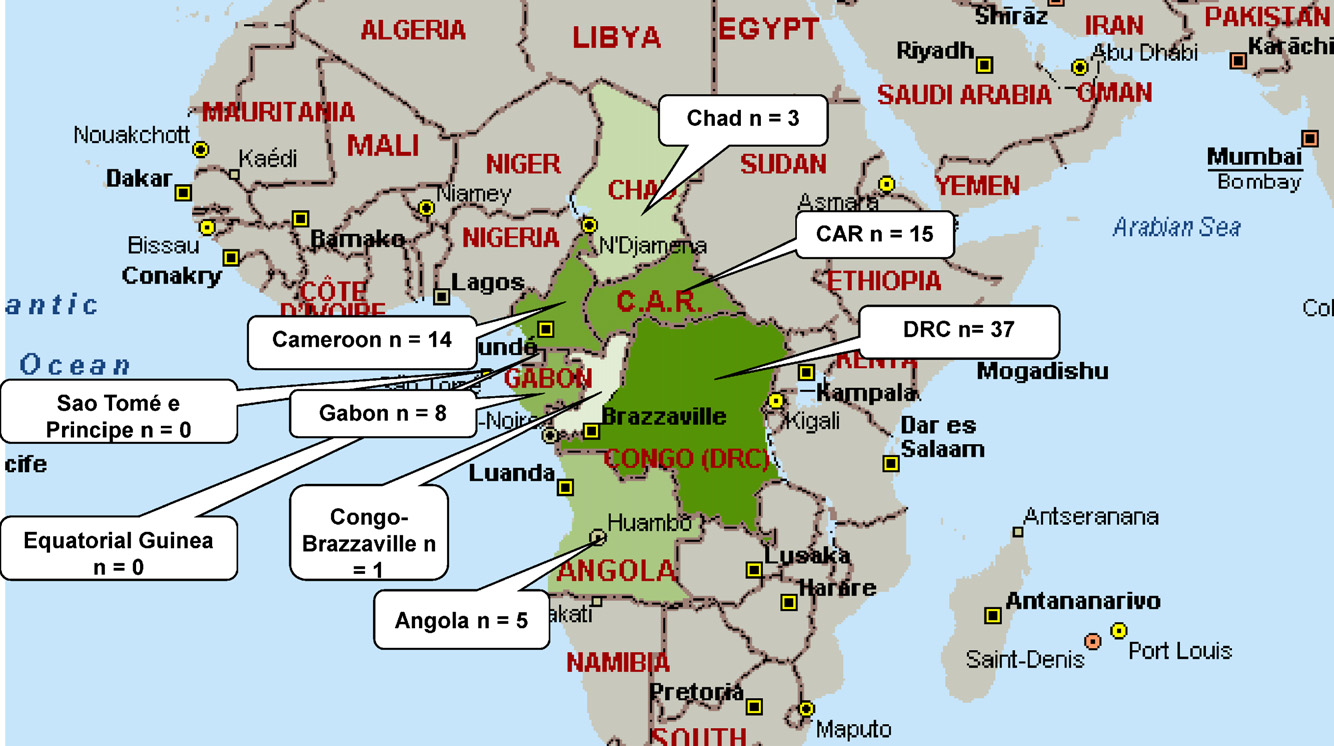
The majority of these publications originated from DRC (n = 37,
and 2008, mainly in urban children. Clinical samples were pre-
including 3 publications on data from Rwandese and Congolese
dominantly from CSF (8/11; 72.7%); one study described blood
patients during cross-border epidemics), followed by CAR (n = 15)
culture isolates Another two studies described surveil-
and Cameroon (n = 14) (More than one-half (43/83; 51.8%) of
lance in nasopharyngeal isolates from healthy and sick children
the studies were surveillance studies, the majority of them study-
ing enteric pathogens (30/43; 69.8%) in DRC (21/43; 48.8%). Another
Overall, low numbers of isolates were studied (range 7–272;
large part of the studies (34/83; 41.0%) were retrospective labora-
median 34); only 4/11 studies tested more than 50 isolates. Peni-
tory studies or short-term clinical studies.
cillin was tested in eight studies, of which only four distinguished
Most studies (50/83; 60.2%) were carried out in urban settings.
between ‘intermediate' and ‘high-level' resistance. Although rates
Of the rural studies, nearly three-quarters (18/25; 72.0%) were per-
of intermediate resistance to penicillin were reported up to 67.0%,
formed in DCR, mainly on Shigella spp. and other enteric pathogens.
high-level resistance remained <6% overall. Notable resistance
The median duration of studies was 16.5 months (range 1–133
rates were those for SXT (up to 69%) and chloramphenicol (up to
Among the studies that mentioned the patients' age groups,
there was a predominance of studies on or involving children
4.2. Gram-negative bacteria
(40/50; 80.0%). The origin of infection was clearly retrievable in56 studies, most of which (52/56; 92.9%) described community-
4.2.1. Shigella spp.
acquired infections, and only 4 studies explicitly addressed
Eighteen studies were found, mainly from rural DRC (12/18;
nosocomial infections was stated in only five
66.7%) and published between 1955 and 2006. Two-thirds reported
studies, mainly human immunodeficiency virus (HIV) and sickle
on outbreak investigations, and Shigella dysenteriae type 1 was the
predominant serotype (15/18 studies). Occasionally, samples from
A research or reference laboratory was involved in 58 (69.9%) of
blood, CSF or pus were included ultiresistant Shigella iso-
the 83 studies, the vast majority of which addressed Vibrio cholerae
lates emerged in the late 1970s. A survey from Kinshasa (DRC) in
and Shigella spp. Faeces were the most frequently studied clini-
1964 demonstrated resistance to two or more antibiotics in 10% of
cal sample (42/83; 50.6%), followed by blood (19/83; 22.9%) and
368 strains. In 1979, spread of a clone with plasmid-mediated resis-
cerebrospinal fluid (CSF) (18/83; 21.7%).
tance to ampicillin, chloramphenicol, streptomycin, sulphonamides
The methodologies of antibiotic susceptibility testing were
and tetracycline was noted in Eastern Zaire (now DRC)
described in 71 studies (85.5%), including mainly the disk diffusion
and 2 years later 75% of the strains showed additional trimethoprim
method (60/83; 72.3%) and the agar dilution method (17/83;
resistance through a newly acquired plasmid. Dysentery treat-
20.5%). Broth dilution and Etest were performed in four and three
ment consequently shifted to nalidixic acid, to which resistance
studies, respectively. Batch testing with the agar dilution method
developed in May 1982, disappearing after the discontinuation of
was mainly used by reference laboratories assessing outbreaks of V.
mass treatment with nalidixic acid everal reports described
cholerae and Shigella spp. Only 31 (37.3%) of 83 studies mentioned
the large outbreak of S. dysenteriae dysentery among Rwandese
a reference method such as the Kirby–Bauer method (or its mod-
refugees after the 1994 civil war reporting simi-
ifications) or the methods of the CLSI the French Society
lar patterns of multidrug resistance including nalidixic acid and
for Microbiology Use of control stains and participation in
trimethoprim, leaving the newer fluoroquinolones as a treatment
Please cite this article in press as: Vlieghe E, et al. Antibiotic resistance among bacterial pathogens in Central Africa: a review of thepublished literature between 1955 and 2008. Int J Antimicrob Agents (2009),
ARTICLE IN PRESS
E. Vlieghe et al. / International Journal of Antimicrobial Agents xxx (2009) xxx–xxx
Fig. 1. Map of the Central African region, with numbers of articles retrieved per country. DRC, Democratic Republic of the Congo; CAR, Central African Republic.
option. Bercion et al. Cunin et al. ed similar
4.2.2. Salmonella spp.
outbreaks in CAR and Cameroon, respectively. Even outside the epi-
demic context, the prevalence of multidrug-resistant strains was
(Thirteen studies between 1973 and 2005 addressed
found to be >50% in recent reports from Kinshasa (DRC)
S. Typhi, mainly in urban settings in DRC and Gabon (10/13;
76.9%). Six of them were surveillance studies f
Table 1
Antimicrobial resistance rates of Staphylococcus aureus, Central Africa
Antimicrobial agent
Resistance rate (%) as reported in different studies
Mean resistance rate (%)
Penicillin (or ampicillin, amoxicillin)
0.0 (2 studies), 53.6, 68.0, 62.8, 82.9, 89.5
0.0 (2 studies), 5.3, 21.3, 35.7, 63.1
0.0 (2 studies), 2.8, 33.3, 75.0
0.0 (2 studies), 5.4, 10.0
0.0 (2 studies), 1.4, 5.3
a Countries and years of publication: Democratic Republic of the Congo (1954, 2001, 2004, 2004), Cameroon (1990, 2003), Congo Brazzaville (2006), Gabon (1985) and
Central African Republic (2007).
b Number of resistant strains/total number of strains in each study, expressed as %.
c Sum of all resistant organisms/sum of all organisms tested.
Table 2
Antimicrobial resistance rates of Streptococcus pneumoniae, Central Africa
Antimicrobial agent
Resistance rate (%) as reported in different studies
Mean resistance rate (%)
Penicillin (intermediate resistance)
8.8, 6.0, 28.6, 29.6, 67.0
Penicillin (high-level resistance)
0.0 (2 studies), 4.2, 5.6 (2 studies), 5.7
0.0 (2 studies), 1.4
14.3, 32.4, 36.0, 62.0
0.0, 6.2, 61.1, 69.0
4.0, 5.5, 9.2, 11.1, 20.7, 25.0, 25.9, 43.5, 44.0, 60.6
a Countries and years of publication: Democratic Republic of the Congo (2005), Cameroon (1990, 1991, 2001), Gabon (2002, 2004) and Central African Republic (1997, 2000,
2003, 2006, 2008).
b Number of resistant strains/total number of strains in each study, expressed as %.
c Sum of all resistant organisms/sum of all organisms tested.
d No clear specification of intermediate or resistant by author ed intermediate resistance.
Please cite this article in press as: Vlieghe E, et al. Antibiotic resistance among bacterial pathogens in Central Africa: a review of thepublished literature between 1955 and 2008. Int J Antimicrob Agents (2009), doi:
ARTICLE IN PRESS
E. Vlieghe et al. / International Journal of Antimicrobial Agents xxx (2009) xxx–xxx
Table 3
Antimicrobial resistance rates of Salmonella enterica serovar Typhi, Central Africa
Antimicrobial agent
Resistance rate (%) as reported in different studies
Mean resistance rate (%)
Ampicillin and amoxicillin
0.0 (5 studies), 5.5, 21.0, 55.5
0.0 (4 studies), 4.0, 23.5, 28.0, 41.0, 50, 66.6
0.0 (3 studies), 8.0 (2 studies), 37.5
Fluoroquinolones (ofloxacin, ciprofloxacin)
0.0 (3 studies), 98.4
0.0 (5 studies), 44.4
First-generation (cefalothin, cefazolin)
Second-generation (cefamandole, cefoxitin)
Third-generation (cefotaxime, ceftriaxone)
ND, no data.
a Countries and years of publication: Democratic Republic of the Congo (1973, 1977, 1984, 1991, 1999, 2005); Gabon (1997, 1999, 2000, 2001); Central African Republic
(1978, 1984) and Cameroon (2001).
b Number of resistant strains/total number of strains in each study, expressed as %.
c Sum of all resistant organisms/sum of all organisms tested.
which five were conducted in DRC. Isolates were recovered from
noted to cefotaxime. Unlike S .Typhi, there was no clear temporal
blood and faeces. Although mean resistance rates to amoxicillin,
trend, except for SXT and the fluoroquinolones to which resistance
chloramphenicol and SXT were relatively low, there was a steeply
emerged from 1999 onwards.
increasing upward trend towards 50% resistance rates since the
Multiresistance was reported frequently. In a 1973 study of a
mid 1990s. Overall susceptibility for fluoroquinolones remained
hospital outbreak in DRC, Kashemwa et al. ted 24% of all NTS
high. Only a single study ested resistance for nalidixic acid
isolates to have combined resistance to ampicillin, streptomycin,
as a predictor for fluoroquinolone resistance: all 37 samples were
kanamycin, tetracycline, chloramphenicol and sulfamethoxazole.
sensitive. Combined resistance was described in at least three
Also in DRC, in 1977 Muyembe et al. ed multiresistance
studies. In a paediatric bacteraemia study in the DRC, Kariuki et al.
in 76% of 1070 NTS isolates, of which >90% were co-resistant to
resistance to five or more first-line antibiotics in >95%
at least five commonly used antibiotics. In a 1993 Congolese bac-
of the isolates.
teraemia study, multiresistance was noted in 30% of all NTS; 90%of isolates were co-resistant to ampicillin, chloramphenicol, tetra-
4.2.2.2. Non-typhoid Salmonella (NTS)
cycline, streptomycin, sulphonamides, streptomycin and SXT
(The general study characteristics were similar to those
Similar findings were described in Bangui (CAR)
reporting on S. Typhi, with five studies also addressing nosocomialinfections. High mean resistance rates were noted for ampicillin
4.2.3. Vibrio cholerae
(63.0%), chloramphenicol (42.9%) and the fluoroquinolones (16.8%).
Eleven reports from nearly all countries in the region were avail-
Resistance to SXT was relatively low (14.6%) and no resistance was
able. Most were published in the second half of the 1990s, often
Table 4
Antimicrobial resistance rates of non-typhoid Salmonella spp., Central Africa
Antimicrobial agent
Resistance rate (%) as reported in different studies
Mean resistance rate (%)
Ampicillin and amoxicillin
5.4, 62.9, 69.0, 92.7, 100 (3 studies)
5.7, 31.3, 35.4, 54.3, 67, 75, 100 (2 studies)
0.0, 1.1, 7.0, 16.0, 42.9, 53.9, 100
Fluoroquinolones (ofloxacin, ciprofloxacin)
0.0 (2 studies), 1.6, 85.7
0.0 (3 studies), 15.0
First-generation (cefalothin, cefazolin)
Third-generation (cefotaxime, ceftriaxone)
ND, no data.
a Countries and years of publication: Democratic Republic of the Congo (1973, 1977, 1984, 1984, 1991, 2001, 2004, 2005), Central African Republic (1984, 1990, 2008), Gabon
(1986) and Cameroon (2001).
b Number of resistant strains/total number of strains in each study, expressed as %.
c Sum of all resistant organisms/sum of all organisms tested.
Please cite this article in press as: Vlieghe E, et al. Antibiotic resistance among bacterial pathogens in Central Africa: a review of thepublished literature between 1955 and 2008. Int J Antimicrob Agents (2009),
ARTICLE IN PRESS
E. Vlieghe et al. / International Journal of Antimicrobial Agents xxx (2009) xxx–xxx
Table 5
Antimicrobial resistance rates of Escherichia coli, Central Africa
Antimicrobial agent
Resistance rate (%) as reported in different studies
Mean resistance rate (%)
Ampicillin and amoxicillin
Median 82.1 (range 64.0–100) in 12 studies
2184/2834 (77.1%)
Median 64.5 (range 15.0–100) in 7 studies
1753/2363 (74.2%)
Nitrofurantoin (urinary tract isolates)
Trimethoprim (urinary tract isolates)
Median 63.3 (range 0.0–87.1) in 11 studies
1264/1839 (68.7%)
Fluoroquinolones (ofloxacin, ciprofloxacin)
0.0 (2 studies), 9.0, 10.0, 43.5
0.0 (2 studies), 6.0, 11.2, 16.0, 20.0, 21.0, 91.1
First-generation (cefalothin, cefazolin)
Second-generation (cefamandole, cefoxitin)
Third-generation (cefotaxime, ceftriaxone)
0.0 (4 studies), 9.0
a Countries and years of publication: Democratic Republic of the Congo (1958, 1976, 1979, 1990, 1998, 2001, 2004, 2005), Cameroon (1999, 2006), Angola (1992), Gabon
(1985, 2003) and Central African Republic (2003).
b Number of resistant strains/total number of strains in each study, expressed as %.
c Sum of all resistant organisms/sum of all organisms tested.
describing outbreaks among refugees, all by V. cholerae O1 El Tor.
resistance rates were noted for amoxicillin/clavulanic acid (52.9%),
Three studies from Chad, Cameroon and DRC noted little or no resis-
chloramphenicol (43.2%), gentamicin (47.7%) and SXT (80.3%). For
tance owever, extensive and multidrug resistance was
fluoroquinolones and third-generation cephalosporins, resistance
described from outbreaks in DRC (1995–1997) with strains resistant
is emerging (7.4% and 3.3%, respectively). Susceptibility to car-
to first-line drugs including ampicillin, tetracycline, doxycycline,
bapenems remained preserved. Ndip et al. (Cameroon)
SXT, nalidixic acid and chloramphenicol. In the same period, sim-
Burchard and Wolff (Gabon) ed multiresistance for
ilar findings were reported from neighbouring Angola
(combinations of) ampicillin, doxycycline, SXT and chlorampheni-
Of nine studies testing ampicillin, seven showed high (≥84%) rates
col at 77.3% and 33%, respectively.
of resistance. For tetracycline and chloramphenicol, four of eightstudies revealed very high (>90%) resistance rates. Isolates were
4.2.6. Presence of extended-spectrum ˇ-lactamase (ESBL)
resistant to sulphonamides or SXT in six and five of eight studies,
Two authors focused on the presence of ESBLs in Gram-negative
bacteria Both used the double-disk method and stud-ied miscellaneous Enterobacteriaceae, mostly E. coli and Klebsiella
4.2.4. Escherichia coli
spp. Frank et al. in Bangui (CAR) ed an ESBL prevalence
Most data on E. coli were retrieved from clinical or labora-
of 4% among 450 Enterobacteriaceae isolates. In addition, nearly
tory archives, predominantly from urine and faeces, and rarely
65% of the ESBL-producing isolates showed combined resistance to
from blood or pus. Two publications focused on enteroaggrega-
aminoglycosides, tetracycline and fluoroquinolones. In Cameroon,
tive E. coli in children and Cunin et al. ed a
Gangoué-Pieboji et al. ed the presence of ESBL in 13
dysentery outbreak with E. coli 0157 in Cameroon. Overall, broad
(14.3%) of 91 E. coli isolates and in 12 (18.8%) of 64 Klebsiella spp.
ranges of antibiotics were used for susceptibility testing and several
tested. A large part (47–84%) of these isolates was of nosocomial
inappropriate drugs (e.g. clindamycin, erythromycin, tetracycline
origin. Most of them carried CTX-M-15 and SVH-12 and to a lesser
and doxycycline) were tested The reported and mean
extent CTX-M-3 and SHV 2a as the responsible gene.
resistance rates were very high for all commonly used antibi-otics but also for amoxicillin/clavulanic acid and first-generation
4.2.7. Pseudomonas aeruginosa (
cephalosporins. Resistance remained much lower for aminoglyco-
Four studies described resistance data from urine, pus and res-
sides, fluoroquinolones and nitrofurantoin, and no resistance to
piratory tract samples and the hospital environment; they included
third-generation cephalosporins was reported by the cited authors
few invasive (blood) samples. At least one study included nosoco-
with the exception of Gangoue-Pieboji et al. on multire-
mial samples esistance was not limited to gentamicin and
sistance were recorded from DRC and Gabon by Jalaluddin et al.
piperacillin but also extended to fluoroquinolones and ceftazidime
and Burchard and Wolff espectively. The former described 10
(15.5% and 32.2% mean resistance rates, respectively). Resistance to
different resistance patterns, with the most frequent pattern being
carbapenems was looked for in a single study and remained low
combined resistance to tetracycline, ampicillin, chloramphenicol,
streptomycin and chloramphenicol. Similarly, 26.7% of 86 isolates in
Multiresistance to clinically relevant antibiotics was assessed by
the latter study combined resistance to ampicillin with doxycycline,
Ndip et al. in Cameroon noted combined resistance to at
SXT and chloramphenicol.
least two antibiotics in all strains. Multidrug resistance to at leastfour antibiotics was as frequent as 41.2%.
4.2.5. Klebsiella spp.
Eleven authors described resistance patterns in Klebsiella spp.
4.3. Gram-negative cocci
from a variety of samples (blood, urine, respiratory, pus and fae-ces). Similar to E. coli, the source of information was archived
4.3.1. Neisseria meningitidis
clinical or laboratory data in large urban centres from a hetero-
Data between 1968 and 2008 were found, mainly from coun-
geneous group of patients, covering the period 1976–2006. High
tries near to the meningitis belt (i.e. Chad, Cameroon). Unlike most
Please cite this article in press as: Vlieghe E, et al. Antibiotic resistance among bacterial pathogens in Central Africa: a review of thepublished literature between 1955 and 2008. Int J Antimicrob Agents (2009), doi:
ARTICLE IN PRESS
E. Vlieghe et al. / International Journal of Antimicrobial Agents xxx (2009) xxx–xxx
Table 6
Antimicrobial resistance rates of Klebsiella spp., Central Africa
Antimicrobial agent
Resistance rate (%) as reported in different studies
Mean resistance rate (%)
Ampicillin and amoxicillin
Median 97.6 (range 6.0–100) in 10 studies
Median 42.6 (range 25.0–95.7) in 6 studies
Median 84.2 (range 12.7–100) in 10 studies
1159/1444 (80.3%)
0.0, 2.0, 4.5, 42.9
Median 43.0 (4.8–94.4) in 7 studies
First-generation (cefalexin, cefazolin)
Second-generation (cefoxitin, cefuroxime)
a Countries and years of publication: Democratic Republic of the Congo (1976, 1979, 1990, 2001, 2004, 2005), Cameroon (2001, 2006), Angola (1992) and Gabon (1985, 2004).
b Number of resistant strains/total number of strains in each study, expressed as %.
c Sum of all resistant organisms/sum of all organisms tested.
Cameroon were most represented; for Equatorial Guinea and São
Tomé e Príncipe, no usable data were found. Second, at present we
did not consider agents of mycobacterial and sexually transmitted
Antimicrobial agent
Resistance rate (%) as reported
in different studies
Regarding the retrieved studies, several issues regarding epi-
demiological and microbiological methods must be mentioned.
First, it should be noted that few studies were designed as prospec-
tive surveillance studies. Furthermore, many studies performed
43.4, 51.0, 66.7, 93.0
susceptibility testing on only a fraction of isolates recovered, and
few studies mentioned selection of a single strain per patient and
episode. Enteric pathogens (Shigella, Salmonella, Vibrio spp.) were
far more represented than the other key pathogens. Only a few
reports essed genuine nosocomial and/or invasive iso-
lates, which are notorious for their antimicrobial resistance even
a Countries and years of publication: Democratic Republic of the Congo (2003),
in developing countries n the other hand, urine, respiratory
Cameroon (2005, 2006) and Gabon (1985).
and wound samples from clinical studies might include a selec-
Number of resistant strains/total number of strains in each study, expressed as
tion of difficult-to-treat infections and hence possibly present a
c Sum of all resistant organisms/sum of all organisms tested.
bias toward resistant strains ith regard to microbiologicalmethods, few studies mentioned application of quality assurancein identification and susceptibility testing, and quantitative suscep-
other pathogens studied, resistance to all commonly used antibi-
tibility data (e.g. MICs or inhibition diameters) were only rarely
otics was not demonstrated or was very low, although minimal
available. Likewise, distinction between intermediate and high-
inhibitory concentration (MIC) values were not reported and num-
level resistance in case of S. pneumoniae was not always reported,
bers studied were low. Most authors reported resistance data for
and in several studies panels of antibiotics selected for testing were
penicillin [mean resistance rate 1/76 (1.3%)] and chloramphenicol
heterogeneous (making comparisons difficult) or even inappropri-
[mean resistance rate 2/91 (2.2%)]. Some authors gave also resis-
tance figures for rifampicin (no resistance noted in seven isolates
Despite the limitations, several interesting observations can be
tested) third-generation cephalosporins (2/61; 3.3%)
made. The studies on Shigella and Vibrio spp. give a good impres-
sion on the evolution of their epidemiology and resistance patterns,particularly in the eastern part of DRC. The resistance patterns
of Shigella spp. are in line with those found in the neighbour-ing countries of Rwanda and Burundi, which have historically
4.4.1. Haemophilus influenzae
been investigated together with DRC iven the fact that
Moraxella catarrhalis
these pathogens often act in outbreaks among weakened peo-
Seven studies reported on H. influenzae strains, with the
ple, these very extensive resistance patterns urge for very tight
following mean resistance rates: ampicillin 34.3% (68/198); third-
surveillance and frequently updated treatment policies in case of
generation cephalosporins 6.2% (6/97); chloramphenicol 11.6%
(23/198); gentamicin 41.3% (19/46); and SXT 32.7% (37/113). Screen-
Resistance data on the other pathogens were much more scarce
ing of respiratory samples from 19 rural Angolan children for the
and heterogeneous and displayed wide ranges in resistance rates.
presence of -lactamase in M. catarrhalis revealed a prevalence of
Nevertheless, worrying trends are to be noted, especially among
Gram-negative enteric organisms. The actual multiresistance pat-terns of NTS and the emerging resistance trends among S. Typhi
5. Discussion and conclusion
isolates are of concern, as many of the widely used cheap first-line antibiotics are no longer active for the treatment of these
In this study we reviewed published data on bacterial resistance
endemic organisms. In particular, emerging fluoroquinolone resis-
in Central Africa. There are several limitations to this study. First, the
tance is of concern. With regard to other Enterobacteriaceae and
exploration of data was problematic. For logistical reasons we did
non-fermentative organisms such as P. aeruginosa, only estimations
not search the ‘grey' literature. Owing to historical collaboration and
of antimicrobial resistance patterns can be given, but it is clear that
institutional and private networks, some countries such as DRC and
multiresistant isolates including those with ESBL are present.
Please cite this article in press as: Vlieghe E, et al. Antibiotic resistance among bacterial pathogens in Central Africa: a review of thepublished literature between 1955 and 2008. Int J Antimicrob Agents (2009),
ARTICLE IN PRESS
E. Vlieghe et al. / International Journal of Antimicrobial Agents xxx (2009) xxx–xxx
In Gram-positive pathogens data are even more scarce and het-
[4] Song JH, Chang HH, Suh JY, Ko KS, Jung SI, Oh WS, et al. Macrolide resistance and
erogeneous. The real prevalence of colonising and invasive MRSA
genotypic characterization of Streptococcus pneumoniae in Asian countries: astudy of the Asian Network for Surveillance of Resistant Pathogens (ANSORP).
infections in this region remains unclear. The problem is under-
J Antimicrob Chemother 2004;53:457–63.
screened and possibly underestimated.
[5] Bell J, Turnidge J. SENTRY Antimicrobial Surveillance Program Asia–Pacific
Penicillin resistance in S. pneumoniae was more consistently
region and South Africa. Commun Dis Intell 2003;27(Suppl):S61–6.
[6] United Nations Statistics Division. Standard country and area codes clas-
tested but was rarely expressed as intermediate or high-level
sification (M49).
resistance despite its relevance for future empirical antibiotic
[accessed 7 May 2009].
guidelines. High-level resistance was seen rarely but an increase
[7] World Health Organization. Surveillance standards for antimicrobial resis-
in intermediate-level resistance was noted.
tance. Geneva: WHO; 2002. WHO/CDS/CSR/DRS/2001.5. drugresistance/publications/WHO CDS CSR DRS 2001 5/en/ [accessed 7 May
The present data should be a matter of concern and increased
awareness, especially in view of their entanglement with other
[8] Cornaglia G, Hryniewicz W, Jarlier V, Kahlmeter G, Mittermayer H, Stratchoun-
major health problems highly prevalent in the Central African
ski L, et al. European recommendations for antimicrobial resistancesurveillance. Clin Microbiol Infect 2004;10:349–83.
region such as HIV/acquired immune deficiency syndrome (AIDS),
[9] Clinical and Laboratory Standards Institute. Principles and procedures for
tuberculosis, malnutrition and malaria. Bacterial infections often
blood cultures; approved guideline. Document M47-A. Wayne, PA: CLSI; 2007.
coincide or complicate the course of other health problems. Shifts
[10] Kesah C, Ben Redjeb S, Odugbemi TO, Boye CS, Dosso M, Ndinya Achola JO, et
al. Prevalence of methicillin-resistant Staphylococcus aureus in eight African
in antibiotic use for one infection can jeopardise treatment options
hospitals and Malta. Clin Microbiol Infect 2003;9:153–6.
[11] Ndip RN, Titanji VP, Akenji TN, Mutanga AM, Mbacham WF, Ndip LM. Antibi-
Therefore, there is need for genuine and integrated surveillance.
ogram of Klebsiella pneumoniae isolates from Buea, Cameroon. Cent Afr J Med2001;47:173–6.
Bacteriology as a diagnostic tool in individual patient management
[12] Gangoué-Pieboji J, Bedenic B, Koulla-Shiro S, Randegger C, Adiogo D, Ngas-
is probably not cost effective, but cohort-based surveys tudy-
sam P, et al. Extended-spectrum--lactamase-producing Enterobacteriaceae
ing a limited set of key pathogens in selected locations, with limited
in Yaounde, Cameroon. J Clin Microbiol 2005;43:3273–7.
[13] Bercion R, Gaudeuille A, Mapouka PA, Behounde T, Guetahoun Y. Surgical site
duration and repeated over time, may provide valuable information
infection survey in the orthopaedic surgery department of the "Hôpital com-
for the redaction and adaptation of standard treatment guidelines.
munautaire de Bangui," Central African Republic [in French]. Bull Soc Pathol
Prerequisites for any type of laboratory surveillance are the imple-
mentation of quality-assured procedures and the availability of
[14] Bekondi C, Bernede C, Passone N, Minssart P, Kamalo C, Mbolidi D, et al.
Primary and opportunistic pathogens associated with meningitis in adults
culture media, reagents and control strains of correct quality and at
in Bangui, Central African Republic, in relation to human immunodeficiency
a reasonable cost. Most important, competent and dedicated lab-
virus serostatus. Int J Infect Dis 2006;10:387–95.
oratory staff collaborating closely with the medical and nursing
[15] Kassa-Kelembho E, Mbolidi CD, Service YB, Morvan J, Minssart P. Bacteremia
in adults admitted to the Department of Medicine of Bangui Community Hos-
staff are needed. Partnerships with laboratories in resource-rich
pital (Central African Republic). Acta Trop 2003;89:67–72.
countries y be helpful for local capacity building through
[16] Okome-Nkoumou M, Elsa NJ, Kombila M. Causes of adult acute bacterial diar-
teaching, providing feedback and performing more expensive or
rhea in an internal medicine department in Libreville, Gabon [in French]. MedTrop (Mars) 2001;61:143–7.
technically complex investigations on selected strains.
[17] Bileckot RR, Miakoundoba RC, Yala F. Microbiology and prognosis of septic
In conclusion, although the present data only give a glimpse of
arthritis in Brazzaville. Joint Bone Spine 2006;73:575–6.
the antimicrobial resistance rates of bacteria in Central Africa, it
[18] Okome-Nkoumou M, Ayo NE, Bekale J, Kombila M. Typhoid and paratyphoid
fever in adults in the Internal Medicine Department at Libreville (Gabon).
is clear that this region shares the worldwide trend of increasing
antimicrobial resistance. Sound surveillance based on competent
[19] World Health Organization. Basic laboratory procedures in clinical bac-
and affordable microbiology is required to provide clear data on
tmp/export/who/who01e.pdf [accessed 7 May 2009].
antimicrobial resistance, which in turn can enable redaction of local
[20] Soussy CJ, Carret G, Cavallo JD, Chardon H, Chidiac C, Choutet P, et al. Antibi-
treatment guidelines and fuel national and regional policies to con-
ogram Committee of the French Microbiology Society. Report 2000–2001 [in
tain antimicrobial resistance.
French]. Pathol Biol (Paris) 2000;48:832–71.
[21] Peterson LR, Brossette SE. Hunting health care-associated infections from the
clinical microbiology laboratory: passive, active, and virtual surveillance. J Clin
[22] Tetanye E, Yondo D, Bernard-Bonnin AC, Tchokoteu PF, Kago I, Ndayo M, et al.
EV and JJ performed the research, retrieval and assessment of
Initial treatment of bacterial meningitis in Yaounde, Cameroon: theoreticalbenefits of the ampicillin–chloramphenicol combination versus chloram-
English language literature, and analysed the data; MFP performed
phenicol alone. Ann Trop Paediatr 1990;10:285–91.
the research, retrieval and assessment of French language litera-
[23] Takaïsi K, Kandolo DK, Musema MG, Sadiki NH. Résistance aux antibiotiques
ture, and performed analysis of the English literature. All authors
des Entérobactéries et des Staphylococques isolés en milieu hospitalier de laville de Kinshasa. Congo Médical 2003;3:1367–71.
contributed to the revision of the results and the discussion.
[24] Bahwere P, Levy J, Hennart P, Donnen P, Lomoyo W, Dramaix-Wilmet M, et al.
Community-acquired bacteremia among hospitalized children in rural central
Africa. Int J Infect Dis 2001;5:180–8.
[25] Burchard GD, Wolff T. Study of bacterial sensitivity in a Lambaréné hospital
(Gabon) [in French]. Med Trop (Mars) 1985;45:265–9.
The authors wish to thank Diane Stessens and Lies Huyskens for
[26] Nyembwe KM, Manienga KJ, Manyebwa KJD, Mulumba MP, Verhaegen J,
their assistance.
Myembe TJJ. Profil de la méthicillinorésistance de Staphylococcus à Kinshasaet implications dans l'hygiène hospitalière. Congo Médical 2004;3:1262–5.
Funding: No funding sources.
[27] Delcour G. Staphylococci and antibiotics. Ann Soc Belg Med Trop (1920)
Competing interests: None declared.
Ethical approval: Not required.
[28] Bercion R, Bobossi-Serengbe G, Gody JC, Beyam EN, Manirakiza A, Le Faou A.
Acute bacterial meningitis at the ‘Complexe Pédiatrique' of Bangui, CentralAfrican Republic. J Trop Pediatr 2008;54:125–8.
[29] Fonkoua MC, Cunin P, Sorlin P, Musi J, Martin PM. Bacterial meningitis in
Yaounde (Cameroon) in 1999–2000. Bull Soc Pathol Exot 2001;94:300–3.
[1] Byarugaba DK. A view on antimicrobial resistance in developing countries and
[30] Kokindombo PO, Nko'o AS, Ndjitoyap Ndam EC, Wouaffo NM, Tangam OT,
responsible risk factors. Int J Antimicrob Agents 2004;24:105–10.
Tietche F, et al. Purulent meningitis due to Flavobacterium meningosepticum
[2] Okeke IN, Lamikanra A, Edelman R. Socioeconomic and behavioral factors
in Cameroonian children [in French]. Ann Pediatr (Paris) 1991;38:491–5.
leading to acquired bacterial resistance to antibiotics in developing countries.
[31] Mumba ND, Verhaegen J, Tshilolo L, Kabeya A, Kabulu AJ, Muyembe TJJ.
Emerg Infect Dis 1999;5:18–27.
Sérotypes et pharmacorésistance des pneumocoques isolés chez les porteurs
[3] Sader HS, Jones RN, Gales AC, Silva JB, Pignatari AC; SENTRY Participants
sains: étude préliminaire. Congo Médical 2005;4:135–8.
Group (Latin America). SENTRY antimicrobial surveillance program report:
[32] Ndoyo J. Antibiotic resistance among nasopharyngeal isolates of Streptococcus
Latin American and Brazilian results for 1997 through 2001. Braz J Infect Dis
pneumoniae and Haemophilus influenzae—Bangui, Central African Republic,
1995. MMWR Morb Mortal Wkly Rep 1997;46:62–4.
Please cite this article in press as: Vlieghe E, et al. Antibiotic resistance among bacterial pathogens in Central Africa: a review of thepublished literature between 1955 and 2008. Int J Antimicrob Agents (2009), doi:
ARTICLE IN PRESS
E. Vlieghe et al. / International Journal of Antimicrobial Agents xxx (2009) xxx–xxx
[33] Okome-Nkoumou M, Mahoumbou J, Ngaka-Safou D. Data on the etiology of
[62] Muyembe TL, Maes L, Makulu MU, Ghysels G, Vandeven J, Vandepitte J. Epi-
bacterial infection in Libreville between 1992 and 1996 [in French]. Med Trop
demiology and drug resistance of Salmonella infections in Kinshasa 1974–1975
[in French]. Ann Soc Belg Med Trop 1977;57:545–56.
[34] Sile Mefo H, Sile H, Mbonda E, Fezeu R, Fonkoua D. Les meningitis puru-
[63] Georges-Courbot MC, Wachsmuth IK, Bouquety JC, Siopathis MR, Cameron
lentes de l'enfant au Nord-Cameroun: aspects épidemiologiques, diagnostics
DN, Georges AJ. Cluster of antibiotic-resistant Salmonella enteritidis infections
et évolutifs. Med Afr Noire 1999;46:83–6.
in the Central African Republic. J Clin Microbiol 1990;28:771–3.
[35] Koko J, Dufillot D, Kani F, Seilhan C, Batsielili S, Gahouma D, et al. Antibiotic
[64] Green SD, Cheesbrough JS. Salmonella bacteraemia among young children at
sensitivity of bacteria responsible for acute meningitis in children in Libreville
a rural hospital in western Zaire. Ann Trop Paediatr 1993;13:45–53.
(Gabon). Arch Pediatr 2002;9:445–6.
[65] Omanga U, Mashako M, Ntihinyurwa M. Indications for antibiotic therapy and
[36] Bitwe MR, Vandenberg O, Donnen Ph, Nsibu NC. Epidémies de dysen-
stool culture during acute diarrhea in the child [in French]. Ann Pediatr (Paris)
térie bacillaire dans la région de Lwiro au Sud-Kivu (1993–1994): étude de
la chimiorésistance de Shigella aux antibiotiques. Congo Médical 2000;2:
[66] Georges-Courbot MC, Baya C, Georges AJ. Serotypes, lysotypes and antibio-
types of 127 strains of Salmonella isolated in the Central African Republic:
[37] Frost JA, Willshaw GA, Barclay EA, Rowe B, Lemmens P, Vandepitte J. Plasmid
evaluation of 3378 samples taken in Bangui [in French]. Bull Soc Pathol Exot
characterization of drug-resistant Shigella dysenteriae 1 from an epidemic in
Central Africa. J Hyg (Lond) 1985;94:163–72.
[67] Ceccarelli D, Salvia AM, Sami J, Cappuccinelli P, Colombo MM. New cluster of
[38] Bercion R, Demartin M, Recio C, Massamba PM, Frank T, Escriba JM, et al.
plasmid-located class 1 integrons in Vibrio cholerae O1 and a dfrA15 cassette-
Molecular epidemiology of multidrug-resistant Shigella dysenteriae type 1
containing integron in Vibrio parahaemolyticus isolated in Angola. Antimicrob
causing dysentery outbreaks in Central African Republic, 2003–2004. Trans
Agents Chemother 2006;50:2493–9.
R Soc Trop Med Hyg 2006;100:1151–8.
[68] Garrigue GP, Ndayo M, Sicard JM, Fonkoua MC, Lemao G, Durand JP,
[39] Ebright JR, Moore EC, Sanborn WR, Schaberg D, Kyle J, Ishida K. Epidemic Shiga
et al. Antibiotic resistance of strains of Vibrio cholerae eltor isolated in
bacillus dysentery in Central Africa. Am J Trop Med Hyg 1984;33:1192–7.
Douala (Cameroon) [in French]. Bull Soc Pathol Exot Filiales 1986;79:305–
[40] Cavallo JD, Niel L, Talarmin A, Dubrous P. Antibiotic sensitivity to epidemic
strains of Vibrio cholerae and Shigella dysenteriae 1 isolated in Rwandan refugee
[69] Germani Y, Quilici ML, Glaziou P, Mattera D, Morvan J, Fournier JM. Emer-
camps in Zaire [in French]. Med Trop (Mars) 1995;55:351–3.
gence of cholera in the Central African Republic. Eur J Clin Microbiol Infect Dis
[41] Frost JA, Rowe B, Vandepitte J, Threlfall EJ. Plasmid characterisation in the
investigation of an epidemic caused by multiply resistant Shigella dysenteriae
[70] Colaert J, van Dyck E, Ursi JP, Piot P. Antimicrobial susceptibility of Vibrio
type 1 in Central Africa. Lancet 1981;2:1074–6.
cholerae from Zaire and Rwanda. Lancet 1979;2:849.
[42] Islam MS, Siddique AK, Salam A, Akram K, Majumdar RN, Zaman K, et al. Micro-
[71] Aldighieri S, Kamoso P, Suermondt G, Rugimbanya P, Ngabonziza C, Kayi-
biological investigation of diarrhoea epidemics among Rwandan refugees in
rangwa E. The adaptation of therapeutic standards as a function of antibiotic
Zaire. Trans R Soc Trop Med Hyg 1995;89:506.
sensitivity of strains of Shigella spp. isolated in Rwanda in 1997 [in French].
[43] Malengreau M, Molima-Kaba, Gillieaux M, de Feyter M, Kyele-Duibone,
Med Trop (Mars) 1997;57:412.
Mukolo-Ndjolo. Outbreak of Shigella dysentery in Eastern Zaire, 1980-1982.
[72] Colombo MM, Mastrandrea S, Leite F, Santona A, Uzzau S, Rappelli P, et al.
Ann Soc Belg Med Trop 1983;63:59–67.
Tracking of clinical and environmental Vibrio cholerae O1 strains by combined
[44] Cunin P, Tedjouka E, Germani Y, Ncharre C, Bercion R, Morvan J, et al. An epi-
analysis of the presence of toxin cassette, plasmid content and ERIC PCR. FEMS
demic of bloody diarrhea: Escherichia coli O157 emerging in Cameroon? Emerg
Immunol Med Microbiol 1997;19:33–45.
Infect Dis 1999;5:285–90.
[73] Massenet D, Djerane L, Gamougane K, Fournier JM. Bacteriological aspects of
[45] Kashemwa C, Isebaert A, Vieu JF, Vandepitte J. Shigella and Salmonella of
cholera in Chad (1991 and 1994 epidemics) [in French]. Bull Soc Pathol Exot
the Kivu region (Zaire) [in French]. Ann Soc Belg Med Trop 1973;53:595–
[74] Ferreira E, Costa M, Vaz Pato MV. Resistance to antibiotics of Vibrio cholerae
[46] Omanga U, Muganga N, Kapepela M. Bacterial septicemias in children with
strains isolated in Angola [in French]. Pathol Biol (Paris) 1992;40:561–5.
homozygous sickle cell anemia. Analysis of 69 cases [in French]. Ann Pediatr
[75] Guevart E, Noeske J, Solle J, Mouangue A, Bikoti JM. Large-scale selec-
tive antibiotic prophylaxis during the 2004 cholera outbreak in Douala
[47] Frost JA, Rowe B, Vandepitte J. Acquisition of trimethoprim resistance in
(Cameroon) [in French]. Sante 2007;17:63–8.
epidemic strain of Shigella dysenteriae type 1 from Zaire. Lancet 1982;1:
[76] Gangoue-Pieboji J, Koulla-Shiro S, Ngassam P, Adiogo D, Ndumbe P. Antimi-
crobial activity against Gram negative bacilli from Yaounde Central Hospital,
[48] World Health Organization. Shigellosis surveillance; drug resistant shiga
Cameroon. Afr Health Sci 2006;6:232–5.
strains. Wkly Epidemiol Rec 1983;12:87.
[77] Hima-Lerible H, Menard D, Talarmin A. Antimicrobial resistance among
[49] Lunguya O, Asuni M, Mumba D, Mabwa L, Nsungu M, Manienga J, et al.
uropathogens that cause community-acquired urinary tract infections in Ban-
Sérotypes et pharmacorésistance des Salmonella et Shigella à Kinshasa
gui, Central African Republic. J Antimicrob Chemother 2003;51:192–4.
(1994–1999). Congo Médical 2005;4:227–33.
[78] Jalaluddin S, de Mol P, Hemelhof W, Bauma N, Brasseur D, Hennart P, et al. Iso-
[50] Vandepitte J, Gatti F. Shigellosis at Leopoldville. Bacteriologic and epidemio-
lation and characterization of enteroaggregative Escherichia coli (EAggEC) by
logic aspects [in French]. Ann Soc Belg Med Trop 1964;44:549–65.
genotypic and phenotypic markers, isolated from diarrheal children in Congo.
[51] Verselder R, Courtois G, Imbos P. Bacillary dysentery in Stanleyville; clini-
Clin Microbiol Infect 1998;4:213–9.
cal and epidemiological importance of the therapeutic resistance of certain
[79] Presterl E, Zwick RH, Reichmann S, Aichelburg A, Winkler S, Kremsner PG,
Shigella cultures [in French]. Bull Soc Pathol Exot Filiales 1955;48:892–
et al. Frequency and virulence properties of diarrheagenic Escherichia coli in
children with diarrhea in Gabon. Am J Trop Med Hyg 2003;69:406–10.
[52] Milleliri JM, Soares JL, Signoret J, Bechen R, Lamarque D, Boutin JP, et al.
[80] Eggert W, Eggert S, Ferreira E, Bernardino L. The pathogen spectrum and its
Epidemic of bacillary dysentery in the Rwanda refugee camps of the Goma
resistance behavior in children with urinary tract infections in Angola [in
region (Zaire, North Kivu) in August 1994 [in French]. Ann Soc Belg Med Trop
German]. Kinderarztl Prax 1992;60:46–8.
[81] Tshiendenda SA, Mutombo KL, Musuasua M, Mulowayi KW. Etude "in vitro"
[53] Bercion R, Njuimo SP, Boudjeka PM, Manirakiza A. Distribution and antibiotic
de la sensibilité et de la résistance de Escherichia coli et Klebsiella pneumoniae
susceptibility of Shigella isolates in Bangui, Central African Republic. Trop Med
au cours de la diarrhée infantile. Congo Médical 2005;4:121–5.
Int Health 2008;13:468–71.
[82] Osterrieth P, Doupagne P. Note sur l'incidence des différents germes dans
[54] Nkuo-Akenji TK, Ntemgwa ML, Ndip RN. Asymptomatic salmonellosis
les urinocultures. Leur sensibilité aux antibiotiques. Ann Inst Pasteur (Paris)
and drug susceptibility in the Buea District, Cameroon. Cent Afr J Med
[83] Tshiani K, Nyomba B, Bidingija M, Nkurikiyinfura JB. Infections urinaires en
[55] Laroche R, Sirol J, Peghin M. Typhoid fever at Bangui (Centrafrican Empire).
milieu tropical hospitalier. Une étude épidémiologique d'une bactériurie sig-
100 cases [in French]. Bull Soc Pathol Exot Filiales 1978;71:54–63.
nificative. Med Afr Noire 1997;26:243–9.
[56] Georges MC, Wachsmuth IK, Meunier DM, Nebout N, Didier F, Siopathis MR,
[84] Binda ki Muaka P, Kanda T, Ngiyulu Makuaka R, Mbensa Massabi L. Etude
et al. Parasitic, bacterial, and viral enteric pathogens associated with diarrhea
clinique de l'infection des voies urinaires chez l'enfant en milieu hospitalier
in the Central African Republic. J Clin Microbiol 1984;19:571–5.
tropical. Med Afr Noire 1990;37:19–26.
[57] Koko J, Dufillot D, Kani F, Gahouma D, Reymond-Yeni A. Salmonella meningitis
[85] Zawisza-Zenkteler W, Muyembe TL, Makulu MU. Etude de l'activité in vitro
in children in Libreville. Retrospective study of 9 cases [in French]. Arch Pediatr
de l'association sulfamethoxazole–triméthoprime sur les bactéries isolées
d'infections urinaires à Kinshasa. Med Mal Infect 1976;6:287–91.
[58] Okome-Kouakou M, Bekale J, Kombila M. Salmonellosis in HIV infection in a
[86] Gangoue-Pieboji J, Miriagou V, Vourli S, Tzelepi E, Ngassam P, Tzouvelekis
hospital setting in Gabon [in French]. Med Trop (Mars) 1999;59:46–50.
LS. Emergence of CTX-M-15-producing enterobacteria in Cameroon and char-
[59] Mashako MN, Luki N, Nsibu N, Kapongo N, Bankoto M, Mupuala M, et al.
acterization of a blaCTX-M-15-carrying element. Antimicrob Agents Chemother
Salmonella infections in Kinshasa: the species involved and sensitivity to
antibiotics [in French]. Pediatrie 1991;46:691–6.
[87] Frank T, Arlet G, Gautier V, Talarmin A, Bercion R. Extended-spectrum -
[60] Kariuki S, Cheesbrough J, Mavridis AK, Hart CA. Typing of Salmonella enterica
lactamase-producing Enterobacteriaceae, Central African Republic. Emerg
serotype Paratyphi C isolates from various countries by plasmid profiles and
Infect Dis 2006;12:863–5.
pulsed-field gel electrophoresis. J Clin Microbiol 1999;37:2058–60.
[88] Ndip RN, Dilonga HM, Ndip LM, Akoachere JF, Nkuo AT. Pseudomonas aerug-
[61] Goossens H, Vanhoof R, de Mol P, Grados O, Ghysels G, Butzler JP. In-vitro
inosa isolates recovered from clinical and environmental samples in Buea,
susceptibility of salmonellae to antimicrobial agents. J Antimicrob Chemother
Cameroon: current status on biotyping and antibiogram. Trop Med Int Health
Please cite this article in press as: Vlieghe E, et al. Antibiotic resistance among bacterial pathogens in Central Africa: a review of thepublished literature between 1955 and 2008. Int J Antimicrob Agents (2009),
ARTICLE IN PRESS
E. Vlieghe et al. / International Journal of Antimicrobial Agents xxx (2009) xxx–xxx
[89] Nyembue DT, Tshiswaka JM, Sabue MJ, Muyunga CK. Bacteriology of chronic
[97] Rogerie F, Vimont-Vicary P. Bacteriological study of shigellosis in the Lake
suppurative otitis media in Congolese children. Acta Otorhinolaryngol Belg
Kivu area (Central Africa). Developments in the last 15 years (1968-1983) [in
French]. Bull Soc Pathol Exot Filiales 1986;79:435–46.
[90] Lefevre M, Sirol J. Moraxella (Acinetobacter) and meningeal syndrome. Isolation
[98] Piot P. Eternal return. . The reappearance of Shiga bacillus in Central Africa
in Fort-Lamy (Chad) of 17 strains from the cerebrospinal fluid [in French].
[in French]. Ann Soc Belg Med Trop 1983;63:1–3.
Presse Med 1969;77:1899–902.
[99] Bogaerts J, Verhaegen J, Munyabikali JP, Mukantabana B, Lemmens P, Van-
[91] World Health Organization. Meningococcal disease, Chad. Wkly Epidemiol
deven J, et al. Antimicrobial resistance and serotypes of Shigella isolates in
Kigali, Rwanda (1983 to 1993): increasing frequency of multiple resistance.
[92] Niel L, Lamarque D, Coue JC, Soares JL, Milleliri JM, Boutin JP, et al. Chronicle
Diagn Microbiol Infect Dis 1997;28:165–71.
of a declared meningococcal meningitis epidemic (Goma, Zaire, August 1994)
[100] Ndihokubwayo JB, Baribwira C, Ndayiragije A, Poste B. Antibiotic sensitivity
[in French]. Bull Soc Pathol Exot 1997;90:299–302.
of 299 strains of Shigella isolated in Burundi [in French]. Med Trop (Mars)
[93] Rowe AK, Schwartz B, Wasas A, Klugman KP. Evaluation of the Etest as a
means of determining the antibiotic susceptibilities of isolates of Streptococcus
[101] Ries AA, Wells JG, Olivola D, Ntakibirora M, Nyandwi S, Ntibakivayo M, et al.
pneumoniae and Haemophilus influenzae from children in the Central African
Epidemic Shigella dysenteriae type 1 in Burundi: panresistance and implica-
Republic. J Antimicrob Chemother 2000;45:132–3.
tions for prevention. J Infect Dis 1994;169:1035–41.
[94] Wolf B, Kools-Sijmons M, Verduin C, Rey LC, Gama A, Roord J, et al. Genetic
[102] von Gottberg A, Klugman KP, Cohen C, Wolter N, de Gouveia L, du Plessis M, et
diversity among strains of Moraxella catarrhalis cultured from the nasophar-
al. Emergence of levofloxacin-non-susceptible Streptococcus pneumoniae and
ynx of young and healthy Brazilian, Angolan and Dutch children. Eur J Clin
treatment for multidrug-resistant tuberculosis in children in South Africa: a
Microbiol Infect Dis 2000;19:759–64.
cohort observational surveillance study. Lancet 2008;371:1108–13.
[95] Zaidi AK, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA.
[103] Archibald LK, Reller LB. Clinical microbiology in developing countries. Emerg
Hospital-acquired neonatal infections in developing countries. Lancet
Infect Dis 2001;7:302–5.
[104] World Health Organization. Developing laboratory partnerships to detect
[96] Blomberg B, Olsen BE, Hinderaker SG, Langeland N, Gasheka P, Jureen R,
infections and prevent epidemics. Lyon: WHO; 2005. WHO/CDS/CSR/LYO/
et al. Antimicrobial resistance in urinary bacterial isolates from pregnant
women in rural Tanzania: implications for public health. Scand J Infect Dis
2005 19.pdf. [accessed 8 May 2009].
Please cite this article in press as: Vlieghe E, et al. Antibiotic resistance among bacterial pathogens in Central Africa: a review of thepublished literature between 1955 and 2008. Int J Antimicrob Agents (2009), doi:
Source: http://www.labquality.be/documents/ANALYSIS/BACTERIOLOGY/Vlieghe_2009_Ab%20resitance%20review.pdf
Intraindividual aqueous flare comparison after implantation of hydrophobic intraocular lenses with or without a heparin-coated surface Eva M. Krall, MD, Eva-M. Arlt, MD, Gerlinde Jell, MD, Clemens Strohmaier, MD, Alexander Bachernegg, MD, Martin Emesz, MD, G€ unther Grabner, MD, Alois K. Dexl, MD, MSc PURPOSE: To assess the efficacy of a heparin-surface-modified (HSM) hydrophobic acrylicintraocular lens (IOL) (EC-1YH PAL) and the same IOL without heparin coating (EC-1Y-PAL) bythe flare and cell intensity in the anterior chamber after uneventful cataract surgery.
YTAAP-12785; No. of pages: 10; 4C: 3 J. Hamdam et al. / Toxicology and Applied Pharmacology xxx (2013) xxx–xxx Contents lists available at Toxicology and Applied Pharmacology Invited Review Article Safety pharmacology — Current and emerging concepts Junnat Hamdam , Swaminathan Sethu , Trevor Smith , Ana Alfirevic Mohammad Alhaidari , Jeffrey Atkinson , Mimieveshiofou Ayala Helen Box Michael Cross Annie Delaunois Ailsa Dermody ,


