Ukcia.org
Vol 435 23 June 2005 doi:10.1038/nature03658
An endocannabinoid mechanism for stress-inducedanalgesia
Andrea G. Hohmann1, Richard L. Suplita1, Nathan M. Bolton1, Mark H. Neely1, Darren Fegley2, Regina Mangieri2,Jocelyn F. Krey3, J. Michael Walker3, Philip V. Holmes1, Jonathon D. Crystal1, Andrea Duranti4, Andrea Tontini4,Marco Mor5, Giorgio Tarzia4 & Daniele Piomelli2
Acute stress suppresses pain by activating brain pathways that
antagonist SR144528 (5 mg kg21 i.p.) (Fig. 1a). The effects of CB1
engage opioid or non-opioid mechanisms. Here we show that an
antagonists cannot be attributed to changes in basal nociceptive
opioid-independent form of this phenomenon, termed stress-
threshold because, in the absence of the stressor, the drugs failed to
induced analgesia1, is mediated by the release of endogenous
alter tail-flick latencies (Supplementary Figs 1 and 2).
marijuana-like (cannabinoid) compounds in the brain. Blockade
If CB1 activation is required for the expression of non-opioid SIA,
of cannabinoid CB1 receptors in the periaqueductal grey matter of
the latter should be lower in animals rendered tolerant to the
the midbrain prevents non-opioid stress-induced analgesia. Inthis region, stress elicits the rapid formation of two endogenouscannabinoids, the lipids 2-arachidonoylglycerol2 (2-AG) andanandamide3. A newly developed inhibitor of the 2-AG-deactivat-ing enzyme, monoacylglycerol lipase4,5, selectively increases 2-AGconcentrations and, when injected into the periaqueductal greymatter, enhances stress-induced analgesia in a CB1-dependentmanner. Inhibitors of the anandamide-deactivating enzymefatty-acid amide hydrolase6, which selectively elevate anandamideconcentrations, exert similar effects. Our results indicate that thecoordinated release of 2-AG and anandamide in the periaqueduc-tal grey matter might mediate opioid-independent stress-inducedanalgesia. These studies also identify monoacylglycerol lipase as apreviously unrecognized therapeutic target.
Stress activates neural systems that inhibit pain sensation. This
adaptive response, referred to as stress-induced analgesia (SIA),depends on the recruitment of brain pathways that project fromthe amygdala to the midbrain periaqueductal grey matter (PAG) anddescend to the brainstem rostroventromedial medulla and dorsalhorn of the spinal cord7. Endogenous opioid peptides have keyfunctions in this process1,8, but other as yet unidentified neurotrans-mitters are also known to be involved1. We proposed that endo-cannabinoids might be implicated in stress analgesia for two reasons.
First, agonists of CB1 receptors—the predominant cannabinoidreceptor subtype present in the brain9,10—exert profound antinoci-ceptive effects7 and suppress activity in nociceptive neurons11–14.
Figure 1 CB1 receptors mediate non-opioid stress-induced analgesia.
Second, CB1 antagonists increase the activity of nociceptive rostro-
a, CB1 antagonist rimonabant (Ri, filled circles) blocks stress
ventromedial medulla neurons14 and enhance sensitivity to noxious
antinociception. Opiate antagonist naltrexone (N, open diamonds) and CB2
stimuli15, indicating that an intrinsic endocannabinoid tone might
antagonist SR144528 (S, filled diamonds) have no effect. Open circles,
regulate descending antinociceptive pathways7.
vehicle (V). Inset: drug effects (F
5.99, P , 0.003). The dotted line
To study non-opioid SIA we delivered brief, continuous electric
indicates the nociceptive threshold. Analgesia index was measured as the
foot shock to rats and quantified their sensitivity to pain after stress
tail-flick latency. b, Stress antinociception is attenuated in WIN55212-2-
by using the tail-flick test. As demonstrated previously1,16, this
tolerant rats (filled squares) compared with controls (open squares)(F
¼ 16.74, P , 0.0007). Inset: non-stress cannabinoid antinociception
stimulation protocol caused a profound antinociceptive effect that
is attenuated in WIN55212-2-tolerant rats (F
¼ 35.11, P , 0.0002).
was not affected by intraperitoneal (i.p.) injection of the opiate
c, Rimonabant in dorsolateral PAG suppresses stress antinociception
antagonist naltrexone (14 mg kg21) (Fig. 1a). However, the response
20.01, P , 0.0002). Inset: drug effects (F 1,17
was almost abolished by administration of the CB1 antagonist
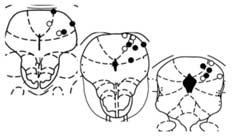
P , 0.0002). d, PAG injection sites. Error bars, where visible, indicate s.e.m.;
rimonabant (SR141617A, 5 mg kg21 i.p.) (Fig. 1a) or its analogue
n ¼ 6–11 per group. Asterisk, P , 0.05; two asterisks, P , 0.01 (analysis of
AM251 (5 mg kg21 i.p.) (Supplementary Fig. 1), but not by the CB
variance, Fisher's PLSD test).
1Neuroscience and Behavior Program, Department of Psychology, The University of Georgia, Athens, Georgia 30602-3013, USA. 2Department of Pharmacology and Center forDrug Discovery, University of California, Irvine, California 92697-4260, USA. 3Schrier Research Laboratory, Departments of Psychology and Neuroscience, Brown University,Providence, Rhode Island 02912, USA. 4Institute of Medicinal Chemistry, University of Urbino Carlo Bo, I-61029 Urbino, Italy. 5Pharmaceutical Department, University of Parma,I-43100 Parma, Italy.
2005 Nature Publishing Group
NATURE Vol 435 23 June 2005
antinociceptive effects of cannabinoids. Consistent with this predic-
peaked 7–15 min after foot shock (Fig. 2a). No such changes were
tion, rats chronically treated with the cannabinoid agonist
observed in the occipital cortex (Fig. 2b), a brain region that contains
WIN55212-2 (10 mg kg21 i.p., once daily for 14 days) displayed,
CB1 receptors9 but is not considered part of the SIA circuit.
along with the expected blunting of acute CB1-dependent antinoci-
The rapid accumulation of 2-AG in the midbrain after stress
ception (Fig. 1b, inset), a marked decrease in stress antinociception
indicates that endocannabinoid release, rather than intrinsic CB1
(Fig. 1b). The possibility that this decrement might be due to changes
activity, might be responsible for SIA. If this is so, selective inhibitors
in opioid tone is unlikely for two reasons: first, rats tolerant to
of the 2-AG-hydrolysing enzyme monoacylglycerol lipase (MGL)
WIN55212-2 showed no deficit in their antinociceptive response to
should heighten the intrinsic actions of 2-AG and enhance its
morphine (2.5 mg kg21 subcutaneously) (data not shown); and
analgesic effects. The absence of selective MGL inhibitors prompted
second, in accord with previous results16, rats tolerant to morphine
us to develop such an agent. To develop MGL-specific inhibitors we
(10 mg kg21 subcutaneously once daily for 7 days) showed normal
started from the assumption that similarities should exist between
non-opioid stress antinociception (Supplementary Fig. 3).
the substrate-binding site of MGL and that of the anandamide-
The PAG serves key functions in both the descending control of
hydrolysing enzyme fatty-acid amide hydrolase (FAAH)6: the fact
pain7,17 and the antinociceptive actions of cannabinoid agonists18. We
that both hydrolases cleave arachidonic-acid derivatives indicates
therefore asked whether blockade of CB1 receptors in this structure
that their binding pockets might accommodate inhibitors of similar
could affect SIA. Rimonabant (2 nmol) reduced stress antinocicep-
bulk and hydrophobicity. We therefore examined a collection of
tion when microinjected into the dorsolateral PAG (Fig. 1c, d), a
carbamate derivatives in which selective FAAH inhibition had been
structure linked to non-opioid stimulation-produced analgesia17,19,
achieved by mimicking the flexible acyl chain of anandamide with
but was inactive after injection into the lateral and ventrolateral PAG
the isosteric, but more rigid, biphenyl group (Fig. 3a)21,22. This
(Supplementary Fig. 4). The antagonist was also ineffective when
screening revealed that although O-biphenyl carbamates (Fig. 3a:
administered into the lateral ventricle, indicating that its actions were
1, URB597; 2, URB524) inhibit the activity of FAAH but not that of
not due to diffusion to distal sites (Supplementary Fig. 5). These
MGL, N-biphenyl carbamates (Fig. 3a, 3, URB602) display an
results are consistent with the presence of CB1 receptors throughout
opposite selectivity (Fig. 3b, c). URB602 inhibited rat brain MGL
the dorsal midbrain9,20 and indicate that endocannabinoid release
with a half-maximal concentration (IC50) of 28 4 mM (Fig. 3b)
and/or intrinsic CB1 receptor activity in the PAG might contribute to
through a noncompetitive mechanism. Without URB602, the appar-
ent Michaelis constant (K m) of MGL for 2-AG was 24.0 1.7 mM
To determine whether endocannabinoid release participates in this
and the maximum velocity (V max) was 1814 51 nmol min per mg
response, we measured anandamide and 2-AG concentrations in the
protein; with URB602, the K m was 20.0 0.4 mM and the Vmax was
dorsal midbrain of rats killed before (nonshock) or at various times
541 20 nmol min per mg protein (n ¼ 4). When organotypic slice
after foot shock (Supplementary Fig. 6). Liquid chromatography/mass spectrometry (LC–MS) analyses revealed that midbrain 2-AGconcentrations were markedly increased 2 min after shock terminationand returned to baseline about 15 min later (Fig. 2a). This responsepreceded a sustained increase in anandamide concentration, which
Figure 3 URB602 is a selective MGL inhibitor. a, Structures ofO-biphenyl-substituted FAAH inhibitors (1, URB597; 2, URB524) and theN-biphenyl-substituted MGL inhibitor URB602 (3). b, URB602 (circles)inhibits rat brain MGL activity, whereas URB597 (squares) and URB524
Figure 2 Stress stimulates the formation of 2-AG and anandamide in
(triangles) have no such effect. c, URB602 does not affect rat brain FAAH
dorsal midbrain. Non-stress (open squares) and post-stress (filled circles)
activity, which is suppressed by URB597 and URB524. d, e, URB602
concentrations of 2-AG and anandamide in dorsal midbrain samples
(100 mM) increases the concentration of 2-AG (d) but not of anandamide (e)
containing the entire PAG (a) and in occipital cortex samples (b). Error bars
in rat brain slice cultures. Effects of ionomycin (2 mM) and URB597 (1 mM)
indicate s.e.m.; n ¼ 10 per group. Asterisk, P , 0.05 compared with non-
are also shown. Asterisk, P , 0.05; two asterisks, P , 0.01 versus control,
stressed controls; two asterisks, P , 0.01.
t-test (n ¼ 4). Error bars indicate s.e.m.
2005 Nature Publishing Group
NATURE Vol 435 23 June 2005
URB602 did not affect the activities of lipid-metabolizing enzymessuch as diacylglycerol lipase23 and cyclooxygenase-2 (ref. 24) and didnot significantly influence binding of [3H]WIN55212-2 to CB1 or CB2receptors (IC50 $ 5 mM) or [35S]GTP-gS to rat cerebellar membranes(half-maximal effective concentration (EC50) . 50 mM) (Sup-plementary Table 1, and data not shown).
Because of its relatively low potency, URB602 is not suitable for
systemic administration. Nevertheless, microinjections of theMGL inhibitor (0.1 nmol) into the dorsolateral PAG (SupplementaryFig. 7a) or lateral/ventrolateral PAG (Supplementary Fig. 7b)enhanced stress-induced antinociception (Fig. 4a, b). Basal nocicep-tive thresholds in non-shocked rats were unaffected (Fig. 4c; Sup-plementary Fig. 7c). This effect was probably due to theaccumulation of 2-AG in the PAG, for three reasons. First, it wasprevented by the simultaneous administration of rimonabant(0.2 nmol) (Fig. 4a, b). Second, it was mimicked by the non-selectiveMGL inhibitor methyl arachidonyl fluorophosphonate5 (2.6 nmol),whose effects also were blocked by rimonabant (SupplementaryFig. 9). Last, it was accompanied by an increase in midbrain 2-AGconcentration: 25 min after foot shock, when the antinociceptiveeffect of URB602 was at its peak (Fig. 4a, b), 2-AG content was
Figure 4 The MGL inhibitor URB602 enhances non-opioid stress-induced
significantly higher in midbrain fragments of URB602-treated rats
analgesia. a, b, URB602 (602) increases stress antinociception when
relative to vehicle-treated controls (Fig. 4d). Anandamide concentra-
microinjected in dorsolateral PAG (a) and lateral/ventrolateral PAG (b), but
tions were identical in the two groups (Fig. 4d), further highlighting
does not cause antinociception in non-stressed rats (lateral/ventrolateral
the selectivity of URB602 for MGL. These results indicate that
PAG) (c). Rimonabant blocks these effects. Open circles, vehicle; filled
URB602 is a selective MGL inhibitor that enhances stress
triangles, URB602; filled circles, rimonabant; open triangles, URB602/
rimonabant. Analgesia index was measured as the tail-flick latency. Insets:
To examine the possible role of anandamide in SIA, we adminis-
drug effects (a, F
7.39, P , 0.002; b, F3,25
9.15, P , 0.0004). Dotted
tered the FAAH inhibitor URB597 (ref. 21) either by systemic
lines indicate nociceptive thresholds. d, URB602 in the ventrolateral PAG
(0.3 mg kg21, i.p) (Fig. 5a) or local (0.1 nmol) (Fig. 5b; Supplemen-
increases the concentration of 2-AG, but not of anandamide, measured25 min after shock. Error bars indicate s.e.m.; n ¼ 6–10 per group. Asterisk,
tary Fig. 8) injection into the dorsolateral PAG. In both cases URB597
P , 0.05 compared with all groups; two asterisks, P , 0.01; cross, P , 0.05
caused a potentiation of stress antinociception, which was prevented
compared with vehicle; two crosses, P , 0.01; hash, P , 0.05 compared
by rimonabant (1 mg kg21 i.p.; 0.2 nmol in the PAG) (Fig. 5a, b). The
with URB602/rimonabant; ANOVA, Fisher's PLSD post-hoc test.
FAAH inhibitor did not modify basal nociceptive thresholds(Fig. 5a, b). Furthermore, administration of the anandamidetransport inhibitor VDM11 (10 mg kg21 i.p.)25 exerted similar
cultures of rat forebrain were incubated with URB602 (100 mM),
effects, which also were blocked by rimonabant (2 mg kg21 i.p.)
both baseline and Ca2þ-ionophore-stimulated 2-AG concentrations
were increased (Fig. 3d). In contrast, URB602 did not change
Our results indicate that the concerted release of 2-AG and
anandamide content (Fig. 3e), which was markedly elevated by the
anandamide in the PAG might mediate non-opioid SIA. The two
FAAH inhibitor URB597 (ref. 21) at 1 mM (Fig. 3e). Moreover,
endocannabinoids might act on local CB1 receptors9,20,26 to regulate
Figure 5 Inhibitors of anandamide hydrolysis (URB597) and transport
measured as the tail-flick latency. Insets: effects of URB597 (a, F
(VDM11) enhance non-opioid stress-induced analgesia. URB597 (filled
33.69, P , 0.0002) and VDM11 (c, F 3,26
squares) administered systemically (a) or in dorsolateral PAG (b) and
P , 0.0002). Error bars indicate s.e.m.; n ¼ 5–9 per group. Asterisk,
VDM11 (filled diamonds) administered systemically (c) potentiate stress
P , 0.05 compared with all groups; two asterisks, P , 0.01; cross, P , 0.05
antinociception. Rimonabant blocks these effects after systemic
compared with vehicle; two crosses, P , 0.01; hash, P , 0.05 compared
administration (a, 1 mg kg21; c, 2 mg kg21) or administration in PAG (b).
with rimonabant (ANOVA, Fisher's PLSD post-hoc test). Dotted lines
Vehicle, open circles; rimonabant, filled circles, URB597/rimonabant, open
indicate nociceptive thresholds.
squares; VDM11/rimonabant, open diamonds. Analgesia index was
2005 Nature Publishing Group
NATURE Vol 435 23 June 2005
glutamate- and GABA-mediated transmission, ultimately disinhibit-
heat source terminated the application of thermal stimulation. Tail-flick
ing descending pain control pathways. Three points are noteworthy.
latencies were monitored for 4 min immediately before exposure to the
First, endocannabinoid-dependent stress antinociception is not
stressor to evaluate changes in nociceptive thresholds induced by pharmaco-
affected by opioid antagonists or morphine tolerance, implying
logical manipulations. Ceiling tail-flick latencies were 10 s except where noted.
that it might not require opioid activity. The reverse may not be
Tail-flick latencies, measured at baseline or before administration of the stressor,did not differ between groups in any study.
true, however, because mutant CB1-null mice have reduced opioid-
Data analyses. We analysed results with analysis of variance (ANOVA),
mediated responses to stress27. Second, the residual antinociception
repeated-measures ANOVA and Fisher's protected least-significant-difference
observed in the presence of CB1 antagonists leaves open the possibility
post-hoc tests. P , 0.05 was considered significant.
that additional mediators of SIA remain to be discovered. Last, stresstriggers the formation of both 2-AG and anandamide in the midbrain,
Received 23 February; accepted 18 April 2005.
but these two endocannabinoids are released with strikingly dissimilar
Lewis, J. W., Cannon, J. T. & Liebeskind, J. C. Opioid and nonopioid
time-courses. This observation underscores the existence of functional
mechanisms of stress analgesia. Science 208, 623–-625 (1980).
differences between these signalling molecules28, pointing to the
Mechoulam, R. et al. Identification of an endogenous 2-monoglyceride, present
possibility that they might act in a coordinated manner to modulate
in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 50,
temporally and/or spatially distinct processes in the PAG and other
83–-90 (1995).
Devane, W. A. et al. Isolation and structure of a brain constituent that binds to
brain regions. The ability of both MGL and FAAH inhibitors to
the cannabinoid receptor. Science 258, 1946–-1949 (1992).
magnify endocannabinoid-dependent SIA also highlights the signifi-
Dinh, T. P. et al. Brain monoglyceride lipase participating in endocannabinoid
cance of these enzymes as previously undescribed targets for the
inactivation. Proc. Natl Acad. Sci. USA 99, 10819–-10824 (2002).
treatment of pain and stress-related disorders.
Dinh, T. P., Kathuria, S. & Piomelli, D. RNA interference suggests a primary rolefor monoacylglycerol lipase in the degradation of the endocannabinoid2-arachidonoylglycerol. Mol. Pharmacol. 66, 1260–-1264 (2004).
Cravatt, B. F. et al. Molecular characterization of an enzyme that degrades
Chemicals. We synthesized URB597, URB524 and [2H4]anandamide as
neuromodulatory fatty-acid amides. Nature 384, 83–-87 (1996).
described21,22,29,30, and URB602 (biphenyl-3-yl carbamic acid cyclohexyl ester)
Walker, J. M. & Hohmann, A. G. in Cannabinoids—Handbook of Experimental
by reacting diimidazole-1-ylmethanone with biphenyl-3-yl amine in acetonitrile
Pharmacology (ed. Pertwee, R.) 509–-554 (Springer, Berlin, 2005).
in the presence of 4-dimethylaminopyridine and subsequently with cyclo-
Akil, H., Young, E., Walker, J. M. & Watson, S. J. The many possible roles of
hexanol. Other chemicals were obtained and administered as described in
opioids and related peptides in stress-induced analgesia. Ann. NY Acad. Sci.
467, 140–-153 (1986).
Animals. We used adult male Sprague–Dawley rats for in vivo experiments and
Herkenham, M. et al. Characterization and localization of cannabinoid
Wistar rats for enzyme assays and tissue cultures. All procedures were approved
receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci.
11, 563–-583 (1991).
by the institutional animal care and use committee and followed guidelines of
10. Zimmer, A., Zimmer, A. M., Hohmann, A. G., Herkenham, M. & Bonner, T. I.
the International Association for the Study of Pain.
Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor
Brain slice cultures. We cultured brain slices from Wistar rats. Pups were killed
knockout mice. Proc. Natl Acad. Sci. USA 96, 5780–-5785 (1999).
on postnatal day 5 by decapitation after cryo-anaesthesia. Brains were removed
Hohmann, A. G., Martin, W. J., Tsou, K. & Walker, J. M. Inhibition of noxious
and cut (0.4-mm-thick coronal slices) with a vibratome in a bath of ice-cold
stimulus-evoked activity of spinal cord dorsal horn neurons by the cannabinoid
high-glucose DMEM (Gibco). Hemispheres were placed on Millicell culture
WIN 55,212–-2. Life Sci. 56, 2111–-2118 (1995).
inserts (Millipore) in six-well plates with serum-based culture medium (1.5 ml)
12. Hohmann, A. G., Tsou, K. & Walker, J. M. Cannabinoid suppression of noxious
composed of basal Eagle's medium with Earle's salts (100 ml), Earle's balanced
heat-evoked activity in wide dynamic range neurons in the lumbar dorsal horn
salt solution (50 ml), heat-inactivated horse serum (50 ml),
of the rat. J. Neurophysiol. 81, 575–-583 (1999).
13. Martin, W. J., Hohmann, A. G. & Walker, J. M. Suppression of noxious
(0.2 mM, 1 ml) and 50% glucose (2 ml) (all from Gibco). Slices were maintained
stimulus-evoked activity in the ventral posterolateral nucleus of the thalamus
at 37 8C with 5% CO2 for 7 days before use.
by a cannabinoid agonist: correlation between electrophysiological and
Lipid extractions and LC–MS analyses. For ex vivo experiments, we habituated
antinociceptive effects. J. Neurosci. 16, 6601–-6611 (1996).
rats to the guillotine for at least 7 days before the experiment and killed them
14. Meng, I. D., Manning, B. H., Martin, W. J. & Fields, H. L. An analgesia circuit
either before or at various times (2, 7, 15 and 25 min) after a 3-min foot shock
activated by cannabinoids. Nature 395, 381–-383 (1998).
(n ¼ 10 per group). The brains were rapidly removed, dissected and stored
15. Calignano, A., La Rana, G., Giuffrida, A. & Piomelli, D. Control of pain initiation
frozen (280 8C) until lipid extraction. For in vitro experiments, we removed the
by endogenous cannabinoids. Nature 394, 277–-281 (1998).
medium of slice cultures and replaced it with DMEM (1 ml) containing URB602
16. Terman, G. W., Lewis, J. W. & Liebeskind, J. C. Two opioid forms of stress
(100 mM), URB597 (1 mM) or vehicle (0.1% dimethylsulphoxide) and incubated
analgesia: studies of tolerance and cross-tolerance. Brain Res. 368, 101–-106(1986).
the slices at 25 8C for 10 min. In some experiments, slices were incubated with
17. Walker, J. M., Huang, S. M., Strangman, N. M., Tsou, K. & San
ionomycin (2 mM) in DMEM for a further 15 min. Reactions were stopped and
Pain modulation by release of the endogenous cannabinoid anandamide. Proc.
washed with ice-cold 50% methanol (1 ml). Slices were collected in the same
Natl Acad. Sci. USA 96, 12198–-12203 (1999).
medium (0.2 ml) and homogenized. Brain tissue (about 50 mg) and slice
18. Martin, W. J., Patrick, S. L., Coffin, P. O., Tsou, K. & Walker, J. M. An
homogenates were suspended in methanol (2 ml) including 2H-containing
examination of the central sites of action of cannabinoid-induced
internal standards (25 pmol). Lipids were extracted in methanol/chloroform/
antinociception in the rat. Life Sci. 56, 2103–-2109 (1995).
water (1:2:0.25). The organic phase was recovered, evaporated to dryness,
19. Cannon, J. T., Prieto, G. J., Lee, A. & Liebeskind, J. C. Evidence for opioid and
reconstituted in chloroform/methanol (1:3, 80 ml) and subjected to LC–MS
non-opioid forms of stimulation-produced analgesia in the rat. Brain Res. 243,
analysis as described30.
315–-321 (1982).
Enzyme assays. We prepared cell fractions from Wistar rat brain homogenates,
20. Tsou, K., Brown, S., San
˜a, M. C., Mackie, K. & Walker, J. M.
Immunohistochemical distribution of cannabinoid CB1 receptors in the rat
and assayed cytosol MGL activity and membrane FAAH activity with 2-
central nervous system. Neuroscience 83, 393–-411 (1998).
monooleoyl[1,2,3-3H]glycerol (ARC; 20 Ci mmol21), and [ethanolamine-3H]
21. Kathuria, S. et al. Modulation of anxiety through blockade of anandamide
anandamide (ARC; 60 Ci mmol21), respectively, as substrates4,21.
hydrolysis. Nature Med. 1, 76–-81 (2003).
Surgery and tolerance induction. We implanted stainless-steel guide cannulae
22. Mor, M. et al. Cyclohexylcarbamic acid 3 0 - or 4 0 -substituted biphenyl-3-yl
in the PAG (dorsolateral or lateral/ventrolateral) or left lateral ventricle under
esters as fatty acid amide hydrolase inhibitors: synthesis, quantitative
pentobarbital/ketamine anaesthesia 3–7 days before testing. Placements of
structure–-activity relationships, and molecular modeling studies. J. Med. Chem.
cannulae were verified in Nissl-stained sections or by post-mortem injection
47, 4998–-5008 (2004).
of fast green dye. Analyses were restricted to animals exhibiting dye spread
23. Stella, N., Schweitzer, P. & Piomelli, D. A second endogenous cannabinoid that
throughout the ventricular system. The induction of tolerance to WIN55212-2
modulates long-term potentiation. Nature 388, 773–-778 (1997).
24. Kozak, K. R., Prusakiewicz, J. J. & Marnett, L. J. Oxidative metabolism of
and morphine is described in Supplementary Methods.
endocannabinoids by COX-2. Curr. Pharm. Des. 10, 659–-667 (2004).
Assessment of antinociception. We administered foot shock (0.9 mA, alternat-
25. De Petrocellis, L., Bisogno, T., Davis, J. B., Pertwee, R. G. & Di Marzo, V.
ing current, for 3 min) to Sprague–Dawley rats with a Lafayette grid-shock
Overlap between the ligand recognition properties of the anandamide
apparatus. Withdrawal latencies in the radiant-heat tail-flick test13,17 were
transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake
measured at 2-min intervals before (baseline) and after foot shock, and
with negligible capsaicin-like activity. FEBS Lett. 483, 52–-56 (2000).
calculated for each subject in two-trial blocks. Removal of the tail from the
26. Vaughan, C. W., Connor, M., Bagley, E. E. & Christie, M. J. Actions of
2005 Nature Publishing Group
NATURE Vol 435 23 June 2005
cannabinoids on membrane properties and synaptic transmission in rat
Supplementary Information is linked to the online version of the paper at
periaqueductal gray neurons in vitro. Mol. Pharmacol. 57, 288–-295 (2000).
27. Valverde, O., Ledent, C., Beslot, F., Parmentier, M. & Roques, B. P. Reduction of
stress-induced analgesia but not of exogenous opioid effects in mice lacking
Acknowledgements The assistance of the Centro di Calcolo at the University of
CB1 receptors. Eur. J. Neurosci. 12, 533–-539 (2000).
Parma is gratefully acknowledged. This research was supported by grants from
28. Piomelli, D. The molecular logic of endocannabinoid signalling. Nature Rev.
the National Institute on Drug Abuse (A.G.H., D.P.) and from the MIUR and the
Neurosci. 4, 873–-884 (2003).
Universities of Parma and Urbino ‘Carlo Bo'.
29. Tarzia, G. et al. Design, synthesis, and structure–-activity relationships of
alkylcarbamic acid aryl esters, a new class of fatty acid amide hydrolase
Author Information Reprints and permissions information is available at
inhibitors. J. Med. Chem. 46, 2352–-2360 (2003).
npg.nature.com/reprintsandpermissions. The authors declare competing
30. Giuffrida, A., Rodrı´guez de Fonseca, F. & Piomelli, D. Quantification of bioactive
financial interests: details accompany the paper on www.nature.com/nature.
acylethanolamides in rat plasma by electrospray mass spectrometry. Anal.
Correspondence and requests for materials should be addressed to A.G.H.
Biochem. 280, 87–-93 (2000).
([email protected]) or D.P. ([email protected]).
2005 Nature Publishing Group
Source: http://www.ukcia.org/research/EndocannabinoidMechanismForAnalgesia.pdf
Periodico edito dal Centro Servizi per ilVolontariato - Ferrara Sped. in A.P. Comma 20/c art.2 Legge 662/96 Filiale di Ferrara 4 Un convegno suletteratura e diversità CSV Ferrara - Settore Documentazione Questo numero spe- versità, emarginazione. I e Volontariato', tenutosi il ciale di "Mosaico" generi, le esperienze", il 31 maggio 2003 a Ferra-
Tilladelse til fremstilling og indførsel af lægemidler og mellemprodukter til human brug Manufacturer's / Importer's Authorisation regarding Human Medicinal Products Lægemiddelstyrelsen godkender hermed, at The Danish Medicines Agency hereby authorises 1. Autorisationsnummer Authorisation No. 22843 Virksomhed/fremstiller Name of manufacturer
